Abstract
Non-thermal technologies allow for the nutritional and sensory properties of foods to be preserved, something that consumers demand. Combining their use with antimicrobial peptides (AMPs) provides potential methods for food preservation that could have advantages over the use of chemical preservatives and thermal technologies. The aim of this review was to discuss the advances in the application of non-thermal technologies in combination with AMPs as a method for microbial inactivation. Published papers reporting studies on the combined use of power ultrasound (US), pulsed electrical fields (PEF), and high hydrostatic pressure (HHP) with AMPs were reviewed. All three technologies show a possibility of being combined with AMPs, generally demonstrating higher efficiency than the application of US, PEF, HHP, and AMPs separately. The most studied AMP used in combination with the three technologies was nisin, probably due to the fact that it is already officially regulated. However, the combination of these non-thermal technologies with other AMPs also shows promising results for microbial inactivation, as does the combination of AMPs with other novel non-thermal technologies. The effectiveness of the combined treatment depends on several factors; in particular, the characteristics of the food matrix, the conditions of the non-thermal treatment, and the conditions of AMP application.
1. Introduction
Recently, the development of new food products has been directed toward producing healthier foods that have characteristics similar to those observed in fresh products, with increased nutritional and functional properties, and no additives [1,2]. Consumers are showing a lower preference for food products that have been subjected to conventional thermal technologies, such as pasteurization and sterilization [3]. These technologies can involve temperatures higher than 80 °C, which can deteriorate food quality by affecting the organoleptic properties of the product and destroying its nutritional compounds [4,5]. Therefore, non-thermal processing technologies, which are considered less aggressive than thermal treatments, have been developed. These technologies preserve the functional and organoleptic characteristics of processed foods [6].
Non-thermal technologies include ultrasound (US), high-intensity pulsed electric fields (PEF), and high hydrostatic pressure (HHP) processes. The potential of these technologies to guarantee food safety lies in their ability to rupture microbial membranes through physical forces. However, when applied at the levels required to preserve food properties, these technologies may not be enough to inhibit microbial growth [5,7]. Thus, non-thermal technologies have been coupled with antimicrobial agents, mainly derived from bacteria, known as antimicrobial peptides (AMPs) [8]. AMPs are broad-spectrum biological preservatives that can inactivate bacteria, fungi, and viruses [9]. Previous studies have shown that the preservative activity of AMPs increases when they are combined with other preservative agents, such as chemical preservatives [10,11,12,13]. AMPs have also been combined with US, PEF, and HHP technologies to avoid the excessive use of chemical preservatives, which have been associated with food toxicity and the development of cancer and other degenerative diseases [14].
It is essential to understand the current use of non-thermal and AMP technologies to promote their future industrial upscaling and the development of new products. The combination of non-thermal technologies with AMPs aims to (1) amplify the individual effect of both food preservative methods, and (2) replace the use of chemical preservatives to reduce health risks, while securing food safety. To our knowledge, no previous review has covered these topics. Therefore, we aimed to review and discuss the current published knowledge on non-thermal technologies combined with AMPs as an alternative method for microbial inactivation. In Section 2, we review the current information on AMPs, including their classification, mechanisms of action, and potential use in food products. We also discuss both the acceptable daily intake and the antimicrobial effects of the most commonly used chemical preservatives in the food industry, and compare them with those resulting from their combination with AMPs. Section 3 compiles information about the use of the three non-thermal technologies (US, PEF, and HHP) and their combination with AMPs, including the effect of the AMPs on microbial inactivation in different food matrices. In Section 4, we review the current applications and regulation of the three non-thermal technologies and the AMPs used in the food industry as methods of microbial inactivation. Here, we also discuss the challenges of industrial upscaling. Finally, Section 5 offers the main conclusions regarding the progress achieved in this field of study.
2. Antimicrobial Peptides
AMPs, also known as bacteriocins, are mainly produced by lactic acid bacteria. These peptides are synthesized in the ribosomes and released into the extracellular space [15]. Different AMPs have different inhibition spectra, biochemical characteristics, and mechanisms of action. These functional differences are mainly due to their disparate structures [16].
Some of the AMPs derived from lactic acid bacteria have been evaluated in specific food matrices, such as the use of nisin in fruit juices [17,18], pediocin in meat products [19,20], and thurincin H in orange juice and cow’s milk [8], among others [21,22]. These AMPs have been proven to be effective against the growth of microorganisms such as Listeria monocytogenes, Escherichia coli, Shigella flexneri, and other pathogens of great importance to food safety [17,22,23,24]. Because they are innocuous, these AMPs are considered to be of food-grade quality, which is of great interest to the food industry. Additionally, because AMPs have similar effects to those of chemical preservatives, they represent an alternative for food preservation that both avoids adverse health effects and preserves organoleptic properties.
2.1. Antimicrobial Peptide Classification
AMPs are classified into three main classes according to their structure and molecular weight (Table 1). The first AMP classification, proposed by Klaenhammer [25] and adjusted by Duraisamy et al. [26], includes post-translationally modified peptides with 19-50 amino acid residues; nisin being the best-known bacteriocin in this class. Class II includes non-modified, heat-stable peptides with molecular weights from 4 to 8 kDa, such as pediocin. Finally, large and heat-labile proteins with molecular weights higher than 30 kDa make up Class III. Little is known about the mechanism of action of these bacteriocins [27]. Class III includes streptococcin A-M57, which is produced by S. pyogenes [27].

Table 1.
Antimicrobial Peptide Classification *.
2.2. Antimicrobial Peptide Mechanisms of Action
AMPs interact with bacterial membranes, causing the release of intracellular material and, eventually, cell death [15]. The first interaction occurs due to the electrostatic attraction between the cationic AMPs and the anionic nature of the cell membrane surface [40]. This attraction results in the formation of transmembrane pores [41]. Bacterial membrane permeabilization due to AMPs is described by barrel-stave, carpet-like, and toroidal-pore models [42].
The barrel-stave model states that the AMP inserts into the membrane and adopts a perpendicular orientation. The amphipathic characteristics of the AMPs promote their interaction with the cell membrane lipids, which results in the formation of a helical-shaped transmembrane pore, the release of intracellular material, and, finally, cell death [42].
The toroidal-pore model describes the interaction between the hydrophobic segment of the AMP molecule and the polar end of the phospholipids in the cell membrane. This interaction induces monolayer curvature and marks the spot where the AMP will form a pore, which, as is also described in the barrel-stave model, results in the release of intracellular material and cell death [42,43].
Lastly, the carpet-like model states that the AMPs cover the surface of the cell membrane, simulating a carpet. After reaching a specific threshold, AMPs enter the membrane by forming micelles with phospholipids; this induces lipid bilayer curvature, membrane denaturation, and cell death [43].
2.3. The Use of Antimicrobial Peptides in Food Preservation
As food preservatives, AMPs can provide food safety benefits mainly by reducing the use of chemical preservatives [15]. AMPs preserve the organoleptic characteristics of processed foods, which is desirable for consumers [44]. A relatively new preservation technique, AMPs contribute to the clean labeling of food products, that is, the provision of food products without chemical preservatives [45].
Among the AMPs that have been recognized as safe by the Food and Drug Administration (FDA) is nisin. This AMP is used in the food industry to avoid microbial contamination of food products [28]. Pediocin PA-1, effective against contamination of vegetables by L. monocytogenes, is also used commercially [13], and enterocin AS-48 and CCM4231 effectively prevent microbial growth in fruit and vegetable juices and canned food [46]. In the following section, we review the use of different chemical preservatives and how AMPs could represent viable alternatives.
2.4. The Use of Antimicrobial Peptides with Chemical Preservatives
Due to their low cost and high accessibility, chemical preservatives are widely used in the food industry to increase the shelf life of food products that are particularly susceptible to contamination by pathogenic microorganisms [47]. Chemical preservatives must be non-toxic and readily soluble in the food product. Additionally, these chemicals must not transfer unpleasant flavors when used to decrease microbial growth. Most approved chemical preservatives are considered safe, but in uncontrolled amounts they can have adverse health effects, such as cancer, Alzheimer’s disease, type 2 diabetes, and other degenerative diseases [14,48]. Therefore, usage criteria and specific concentrations (i.e., maximum permissible doses) have been established as international standards for each preservative and specific food matrix [49]. Table 2 shows the most commonly used chemical preservatives in the food industry, including some AMPs already regulated for use in food products.

Table 2.
Maximum doses of some preservatives commonly used in the food industry.
In the food industry, the most widely used chemical preservatives are benzoic acid and sorbic acid, and their salts [50]. However, their long-term excessive use has been associated with degenerative diseases, such as cardiovascular disease, and food poisoning [14,48]. Therefore, alternative options have been sought to reduce their use, including by partially replacing them with AMPs. Table 3 shows the effects of different chemical preservatives when they are combined with AMPs in food matrices.

Table 3.
Effect of chemical preservatives and antimicrobial peptides (AMPs) on microbial inactivation in food matrices.
Bari et al. [13] studied pediocin and nisin in combination with sodium lactate, citric acid, phytic acid, potassium sorbate, and EDTA in cabbage, broccoli, and mung bean sprouts (Table 3). The authors observed that the combinations of phytic acid with both bacteriocins were most effective in reducing the growth of Listeria monocytogenes in cabbage and broccoli.
Cobo et al. [12] reported maximum inactivation of Listeria monocytogenes in Russian-type salad using a mixture of enterocin AS-48 with different essential oils (Table 3). Improved inactivation of microbial growth in this product was obtained when each of the tested natural preservatives was combined with enterocin AS-48, compared to the chemical preservatives or AMPs alone. The most effective combinations were thymol, terpineol, tyrosol, or isopropyl methyl phenol with enterocin AS-48, all of which resulted in viable cells being not detected in Russian type salad. In ready-to-eat products, Anacarso et al. [11] evaluated the effect of enterocin 416K1 and chitosan on Listeria monocytogenes. They reported that the antimicrobial activity of the combined preservatives in apples, grapes, mixed salad, carrots, and zucchini was more significant than that observed for the individual preservatives. Kashani et al. [10] reported a more significant inactivation of Staphylococcus aureus ATCC 1112 in a Mueller–Hinton nutrient broth using benzoic acid and sodium nitrite when in combination with nisin. These results favor the reduction of chemical preservatives in the food industry.
It is important to consider the maximum permissible doses for AMPs. One way to enhance the antimicrobial effect of AMPs is through combination with other technologies. Based on the studies summarized in Table 3, bacteriocins can enhance microbial inhibition when combined with organic acids, essential oils, and other chemical preservatives. However, one way to avoid using potentially harmful substances is to combine AMPs with non-thermal technologies, such as US, PEF, or HHP. The microbial inactivation of these technologies alone, and in combination with AMPs, is reviewed in the following section.
3. Non-Thermal Technologies Combined with Antimicrobial Peptides
This section compiles information about three non-thermal technologies (US, PEF, and HHP) and their combined use with AMPs in different food matrices.
3.1. Ultrasound
Ultrasound (US) is a non-thermal technology used for food preservation. This technology generates acoustic waves that propagate in liquid media and create a vibration composed of compression and expansion cycles. Under optimal temperature (5000 K) and pressure (>199 MPa), these vibrations produce microbubbles that, upon reaching a critical size, implode, leading to cavitation. The collapse of these bubbles generates shear forces that favor mass transfer and particle displacement [51].
Gao et al. [52] mention some specific advantages of US, including its efficiency in inactivating vegetative cells, spores, and enzymes. This technology reduces processing temperature and time without having any adverse effects on the quality of the final product. However, the effects of US on processed food depend on the parameters used, such as temperature, intensity, power, and time, and on the properties of the food matrix [51]. For example, US treatment with a frequency of 20 kHz can improve the stability of fruit juices, even after only a few minutes of treatment, and this can be attributed to a decrease in particle size as a result of cavitation [7]; while in milk, US treatment at 24 kHz can oxidize unsaturated fatty acids due to the hydroperoxide free radicals produced during cavitation [53].
3.1.1. Effect of US on Microbial Inactivation in Food Matrices
Microbial inactivation by US in different food matrices occurs through various mechanisms [51]. As shown in the first mechanism in Figure 1, changes in cell permeability occur due to the progressive heating of the microbial plasma membrane that results from the absorption of US-derived acoustic energy. The second mechanism involves the formation of free radicals that can damage microbial DNA. Additionally, peroxides formed during cavitation have bactericidal and bacteriostatic properties [54]. However, microbial inactivation (or stimulation) depends on the particular US parameters [51]. More specifically, microbial inactivation by US mainly depends on treatment intensity and duration. For example, high-intensity US treatments can cause irreversible cell damage, while at low intensities the US can stimulate microbial proliferation and metabolism [55]. For instance, low-intensity US treatment can accelerate the proliferation of Saccharomyces cerevisiae due to an increase in cytoplasmic Ca2+ concentration [56].
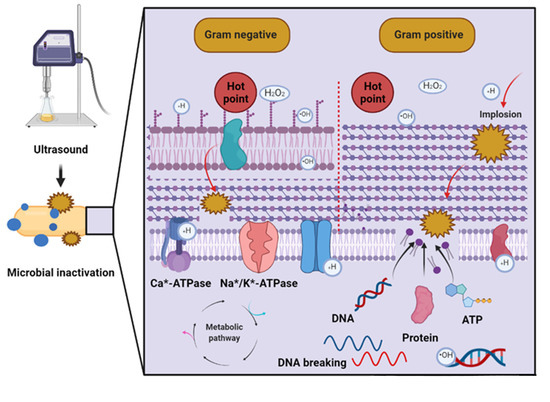
Figure 1.
Mechanisms of microbial inactivation by ultrasound [51,54] (Created with BioRender.com; accessed on 30 March 2022). DNA: Deoxyribonucleic acid, ATP: Adenosine triphosphate.
3.1.2. Effect of US and AMPs on Microbial Inactivation in Food Matrices
US alone is insufficient to achieve adequate microbial inactivation [7]. Since AMPs can inhibit microorganisms by forming pores in the cell membrane, their combined use with US, which also induces pore formation, can increase its antimicrobial activity. Table 4 reviews the most recent studies investigating the combined use of US and AMPs in food matrices.
As shown in Table 4, the AMP most studied in combination with US is nisin. Wang et al. [44] studied the inactivation of E. coli in either nutrient broth, phosphate buffered saline, or milk using US and nisin under controlled conditions (Table 4). Their results suggest that the microbial inactivation is mainly due to the effects of the US treatment, since nisin has a weak effect on E. coli. Subsequently, Liao et al. [18] reported that the combination of US and nisin at temperatures higher than 37 °C had an effect on the growth of aerobic mesophilic bacteria in apple juice (Table 4); but this combination did not affect the growth of molds or yeasts. The authors highlight the potential use of this combination to produce preserved apple juice with characteristics similar to the fresh product. Freitas et al. [57] optimized the inactivation of S. flexneri in a growth medium using US and nisin at different pH values. The authors observed that microbial inactivation was more effective at lower pH values. Importantly, nisin remained active after US treatment under refrigerated conditions. The combined effect of US and different concentrations of nisin on microbial growth has also been evaluated in carrot juice [58], grape juice [59], milk [60], and orange juice [61]. Combined treatment was more effective than either US or nisin alone. Together, these studies suggest that the combined use of nisin with US is not equally effective for all microorganisms. Therefore, it is essential to also review the use of US combined with other AMPs.
In addition to nisin, other AMPs have been studied in combination with US (Table 4). Wu & Narsimhan [23] found that the combination of US with melittin has a synergistic effect on the inactivation of L. monocytogenes due to the transient pores formed after sonication. Fitriyanti & Narsimhan [22] investigated the combined effect of cecropin P1 and US on E. coli O157:H7. These authors reported that the combined treatment is more effective against the studied microorganism than either the US or AMP alone. Yikmiş [21] studied the combined use and effect of US and natamycin on aerobic mesophilic bacteria, molds, and yeasts in pomegranate juice. The author found a significant reduction in the microbial load to levels acceptable for general quality parameters, and highlighted the potential use of this combination to increase the nutritional value of the juice. Recently, Ruiz-De Anda et al. [8] reported the synergistic effect of US and thurincin H on the growth of L. innocua and E. coli in orange juice and milk, observing that US augmented the effect of thurincin H, demonstrating their potential use for antimicrobial applications in the food industry. Together, these studies show that, besides nisin, other AMPs could be combined with US to improve microbial inactivation in food matrices.
In conclusion, although several studies have evaluated the combined use of US and different AMPs for microbial inactivation in different matrices, several combinations have not yet been explored. Notably, most of the reviewed studies employed an ultrasonic probe. The use of a probe implies the use of higher power and intensity, in addition to being more expensive than an ultrasonic bath, which, unlike the probe, allows batch treatment and provides less severe treatment conditions. For future studies, it would also be interesting to evaluate the combination of US with several AMPs simultaneously.

Table 4.
Effect of ultrasound (US) and antimicrobial peptides (AMPs) on microbial inactivation in food matrices.
Table 4.
Effect of ultrasound (US) and antimicrobial peptides (AMPs) on microbial inactivation in food matrices.
| Media | US Device | US Parameters | AMP | AMP Concentration | Microorganism | Maximal Inactivation | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| US | AMP | US + AMP | |||||||
| Milk | Ultrasonic probe, UCD-150, BIOBASE, Jinan, China | 20–25 kHz; 150 W, 16–30 min, 30 ± 5 °C | Thurincin H | 40 ppm | L. innocua | 0.4 log CFU/mL | <0.1 log CFU/mL | 0.7 log CFU/mL | [8] |
| E. coli | 1.1 log CFU/mL | <0.1 log CFU/mL | 2.8 log CFU/mL | ||||||
| Orange juice | Ultrasonic probe, UCD-150, BIOBASE, Jinan, China | 20–25 kHz; 150 W (B); 16–30 min; 30 ± 5 °C | Thurincin H | 40 ppm | L. innocua | 0.8 log CFU/mL | 5.5 log CFU/mL | 5.5 log CFU/mL | [8] |
| E. coli | 1.5 log CFU/mL | 1 log CFU/mL | 3.4 log CFU/mL | ||||||
| Orange juice | Ultrasonic probe, ATPIO-1000D Xianou Co., Nanjing, China | 20-25 kHz; 7000 W; 10 min; 50 °C | Nisin | 200 ppm | Aerobic bacteria | 2.3 log CFU/mL * | Not reported | 1.4 log CFU/mL * | [61] |
| E. coli | n.d. * | n.d. * | |||||||
| Molds | n.d. * | n.d. * | |||||||
| Xanthan gum | Ultrasonic device designed by Costello et al. [60] | 500, kHz; 30 W; 30 min; 20 °C | Nisin | 35 UI/mL | L. innocua | 6 CFU/mL * | 6 CFU/mL * | 5 log CFU/mL * | [60] |
| E. coli | 4.8 log CFU/mL | 6 CFU/mL * | 4 log CFU/mL * | ||||||
| Grape juice | Ultrasonic probe, ATPIO-1000D, Xianou Co., Nanjing, China | 20–25 kHz; 7000 W; 10 min; 55 °C | Nisin | 200 ppm | Aerobic bacteria | 1.9 log CFU/mL * | Not reported | 1.7 log CFU/mL * | [59] |
| E. coli | n.d. * | n.d. * | |||||||
| Yeast and mold | 2.2 log CFU/mL * | 2.1 log CFU/mL * | |||||||
| Carrot juice | Ultrasonic probe, SCIENTZ-IID, Scientz Biotech Co., Ningbo, China | 20 kHz; 271,321 W; 10 min; 3 °C | Nisin | 80 ppm | Aerobic bacteria | 3 log cycles | Not reported | 3.7 log cycles | [58] |
| Yeast and mold | 3.6 log cycles | 3.7 log cycles | |||||||
| Pomegranate juice | Ultrasonic probe, UP200St, Hielscher Ultrasonics, Teltow, Germany | 26 kHz; 48 W, 5 min | Natamycin | 12.5 ppm | Aerobic bacteria | n.d. * | 4.2 log CFU/mL * | n.d. * | [21] |
| Yeast and mold | n.d. * | n.d. * | n.d. * | ||||||
| Lactic acid bacteria | n.d. * | 3.9 log CFU/mL | n.d. * | ||||||
| Growth medium | Ultrasonic probe, VCX 130 Cell, Sonics, Oklahoma City, OK, USA | 20 kHz; 130 W; 20 min; <52 °C; pH 4.5 | Nisin | 175 μM | S. flexneri | <1 log CFU/mL | 1.3 log CFU/mL | 3.5 log CFU/mL | [57] |
| Milk (100%) | Ultrasonic probe, Sonifier 450, Branson Ultrasonics, Brookfield, CT, USA | 20 kHz; 160 W; 60 min; 25 °C; pH 4 | Cecropin P1 | 20 μg/mL | E. coli | 106 CFU/mL * | 107 CFU/mL * | 105 CFU/mL * | [22] |
| Orange juice (100%) | Ultrasonic probe, Sonifier 450, Branson Ultrasonics, Brookfield, CT, USA | 20 kHz; 160 W; 60 min; 25 °C; pH 4 | Cecropin P1 | 20 μg/mL | E. coli | 105 CFU/mL * | 106 CFU/mL * | 103 CFU/mL * | [22] |
| Phosphate buffered saline | Ultrasonic probe, Sonifier 450, Branson Ultrasonics, Brookfield, CT, USA | 20 kHz; 160 W; 60 min; 25 °C; pH 4 | Cecropin P1 | 20 μg/mL | E. coli | 107 CFU/mL * | 106 CFU/mL * | 103 CFU/mL * | [22] |
| Apple juice | Ultrasonic probe, Scientz-IID, Scientz Biotech Co., Ningbo, China | 20–25 kHz; 950 W; 40 min; 52 °C | Nisin | 100 ppm | Aerobic bateria | 2.8 log cycles | Not reported | 3.6 log cycles | [18] |
| Yeast and mold | 3.1 log cycles | 1.5 log cycles | |||||||
| Nutrient broth | Ultrasonic probe, Scientz-IID, Scientz Biotech Co., Ningbo, China | 968 W/cm2; 5 min; 0 °C | Nisin | 20 ppm | E. coli | 0.7 log cycles | Not reported | 0.8 log cycles | [44] |
| Phosphate buffered saline | Ultrasonic probe, Scientz-IID, Scientz Biotech Co., Ningbo, China | 968 W/cm2; 5 min; 0 °C | Nisin | 20 ppm | E. coli | 0.9 log cycles | Not reported | 1 log cycles | [44] |
| Milk | Ultrasonic probe, Scientz-IID, Scientz Biotech Co., Ningbo, China | 968 W/cm2; 5 min; 0 °C | Nisin | 20 ppm | E. coli | 0.9 log cycles | Not reported | 1 log cycles | [44] |
| Growth medium | Ultrasonic bath | 20–100 kHz; 60 W; 60 min; 25 °C | Melittin | 0.78 μg/mL | L. monocytogenes | 4 log CFU/mL * | 6.5 log CFU/mL * | n.d. * | [23] |
US parameters: frequency (kHz), power (W or W/cm2), treatment times (min), temperature (°C), pH, maximal inactivation (log CFU/mL or log cycles); n.d., no viable cells detected; *, viable cell count.
3.2. Pulsed Electric Fields
Pulsed electric fields (PEF) technology is a promising non-thermal method used in the food industry to process liquid products such as fruit juices, dairy products, liquid eggs, and alcoholic beverages. The effectiveness of this technology relies on the concentration of ions in these food products. These ions act as electrical charge carriers [62]. During this treatment, food products receive high-intensity PEF for microseconds, passing through a processing chamber where an electric field above 10 kV/cm is applied [63]. In these chambers, high-voltage pulses are supplied to the system by a generator with the desired strength, shape, and duration.
PEF provide treated food products with some advantages related to their functionality, extractability, and retention of compounds with beneficial nutritional properties [64]. However, some disadvantages include a change in taste and pH that occurs when electrode-derived components are released into the food products due to the electrode corrosion produced by the electrochemical reactions that take place inside the PEF chamber [65].
3.2.1. Effect of PEF on Microbial Inactivation in Food Matrices
To achieve microbial inactivation, PEF rupture or change the cell membrane structure, thus increasing permeabilization, which may or may not be reversible. These events can lead to physiological and biochemical changes, and even cell death [66]. During treatment with PEF, a membrane pore is formed through a process known as electroporation, which is the main mechanism of microbial inactivation. This mechanism is divided into four phases [66]. The first phase consists of inducing a transmembrane potential that leads to the formation of different sizes and numbers of hydrophilic pores. These pores favor the leakage of intracellular material and entry of extracellular substances, as shown in Figure 2.
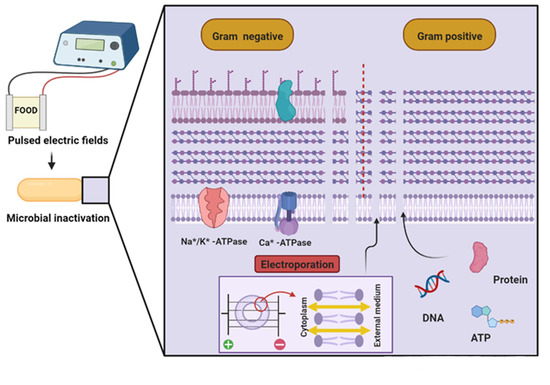
Figure 2.
Mechanisms of microbial inactivation by pulsed electric fields [66] (Created with BioRender.com; accessed on 30 March 2022). DNA: Deoxyribonucleic acid, ATP: Adenosine triphosphate.
3.2.2. Effect of PEF and AMPs on Microbial Inactivation in Food Matrices
To achieve the electroporation of bacterial cells, it is necessary to apply an electric field intensity of 10-14 kV/cm. However, PEF are not effective against gram positive bacteria, yeasts, and spores under these conditions [64]. Therefore, to increase their efficiency, PEF have been studied in conjunction with AMPs such as nisin and enterocin AS-48 [67,68,69]. Table 5 reviews the studies that have evaluated the combined effects of PEF and AMPs on microbial inactivation in food matrices.
According to Table 5, nisin and enterocin AS-48 are the AMPs most studied for combination with PEF. Terebiznik et al. [70] studied the combined effect of PEF and nisin on E. coli in simulated milk ultrafiltrate. The authors reported that PEF inhibited the effect of nisin. This inhibition was a possible consequence of the non-specific binding of nisin to cell debris released from E. coli through the pores formed by the PEF. Furthermore, the authors observed that nisin seems to have a protective effect against PEF effects on bacterial cells. However, by adding NaCl, the authors modified the non-specific binding of nisin and improved the lethality of the combined treatment [71].
Sobrino-López & Martín-Belloso [72], using high-intensity PEF and nisin in skimmed milk, demonstrated the synergistic effect of both technologies on S. aureus, with the highest efficiency observed at neutral pH values. This synergism could result from increased cell permeability induced by PEF, promoting nisin sensitization. The authors highlight the potential use of this combination as a preservative method for milk and other dairy products.
Bermúdez-Aguirre & Barbosa-Cánovas [73] studied the inactivation of Bacillus cereus spores in milk using PEF combined with nisin at temperatures of 65 °C. The authors managed to almost completely inactivate the spores of B. cereus using nisin. However, resistance to the combined treatment observed in B. cereus could be due to the fat present in the milk, which could affect nisin activity.
It appears that nisin is sensitive to extreme conditions and that the food matrix composition is an important factor in the activity of nisin against specific microorganisms. Therefore, it is important to study other AMPs which may be resistant to more severe PEF conditions.
In addition to nisin, the combined treatment of PEF with enterocin AS-48 has also been studied in different food matrices [69,74,75,76]. The matrices were subjected to the same PEF processing conditions, but with different concentrations of enterocin AS-48. These combined treatments were evaluated against S. enterica, S. aureus, L. diolivorans, and P. parvulus [76]. The authors reported that the combined treatment was more effective for microbial inhibition than the individual treatments (Table 5). The highest inactivation was observed for P. parvulus [76], achieving at the same time, better microbial stability of the product. Interestingly, enterocin AS-48 was more effective before treatment with PEF than after.
In conclusion, microbial inactivation depends on the processing time, the concentrations of AMPs, the pH of the medium, and the sequence in which the treatments are applied.
Different types of electric fields have been established and used under different working conditions to manipulate bacterial viability based on the nature of the cells [77]. In addition to PEF, there is moderate electric field (MEF) treatment. MEF technology is characterized by a lower electric field (1 V/cm to 1 kV/cm) than that generally used for PEF (10 to 14 kV/cm) [77]. Depending on the intensity, frequency, and electric field waveform, cell membrane permeabilization and pore formation can be controlled. Additionally, MEF treatment seems to have fewer adverse effects on the organoleptic properties of the food product compared to the aftertaste that PEF can cause [77].
Mok et al. [78] reported a synergistic inhibitory effect on the growth of E. coli K12 and L. innocua in apple juice and kale using shear-stress-MEF (SS-MEF) and nisin. SS-MEF alone achieved 1.1 and 2.9 log inactivation in five minutes, respectively. In contrast, the combination of SS-MEF with nisin reached a 5-log reduction in less than five minutes. These combined technologies were effective against the growth of both microorganisms without changing the color or pH of the juice. The individual use of SS-MEF also did not affect these two parameters. However, sensory studies are needed in order to develop preserved products that retain their original flavors while being safe.

Table 5.
Effect of pulsed electric fields (PEF) and antimicrobial peptides (AMPs) on microbial inactivation in food matrices.
Table 5.
Effect of pulsed electric fields (PEF) and antimicrobial peptides (AMPs) on microbial inactivation in food matrices.
| Media | PEF Device | PEF Parameters | AMP | AMP Concentration | Microorganism | Maximal Inactivation | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| PEF | AMP | PEF + AMP | |||||||
| Whole and skim milk | Pilot plant, Physics International, USA | 40 kV/cm; 2.5 µs (p.w.); 10 Hz; 65 °C; 276, 144 n.p. | Nisin | 50 IU/mL | Bacillus cereus | 2.5 log spores/mL | Not reported | 3.6 log spores/mL | [73] |
| Apple juice | PEF unit, OSU-4F HIPEF, Ohio State University, USA | 35 kV/cm; 1000 µs (t); 4 µs (p.w.); 150 Hz; 20 °C | Enterocin AS-48 | 0.613 AU/mL | Pediococcus parvulus | 3.1 log CFU/mL | 3.7 log CFU/mL | 6.6 log CFU/mL | [76] |
| Apple juice | PEF unit, OSU-4F HIPEF, Ohio State University, USA | 35 kV/cm; 1000 µs (t); 4 µs (p.w.); 150 Hz; 20 °C | Enterocin AS-48 | 2 µg/mL | Lactobacillus diolivorans | 3 log | < 1 log | 4.9 log | [74] |
| Skim milk | PEF unit, OSU-4F HIPEF, Ohio State University, USA | 35 kV/cm; 1200 µs (t); 6 µs (p.w.); 75 Hz; 25 °C | Enterocin AS-48 | 28 AU/mL | Staphylococcus aureus | 3.5 log | Values not reported | 4.5 log | [75] |
| Apple juice | PEF unit, OSU-4F HIPEF, Ohio State University, USA | 35 kV/cm; 1000 µs (t); 4 µs (p.w.); 150 Hz; 40 °C | Enterocin AS-48 | 60 μg/mL | Salmonella enterica | 3 log cycles | <1 Log | 4.5 log cycles | [69] |
| Skim milk | PEF unit, OSU-4F HIPEF, Ohio State University, USA | 35 kV/cm; 2400 µs (t); 4 µs (p.w.); 100 Hz; 25 °C | Nisin | 20 ppm | Staphylococcus aureus | 1 log units | <1 Log | 6 log units | [72] |
| Growth medium | PEF system, Gene Pulser II Electroporation, Bio-Rad, Hercules, CA, USA | 5 kV/cm; 3 n.p.; 95 (w.a.) | Nisin | 1500 IU/mL | Escherichia coli | 0.2 log cycles | 3.2 log cycles | 5 log cycles | [71] |
| Simulated milk ultrafiltrate | Gene Pulser II Electroporation system (Bio-Rad) | 11.25 kV/cm; 3 n.p. | Nisin | 7.15 µM | Escherichia coli | 1.5 log cycles | 3 log cycles | 4 log cycles | [70] |
PEF parameters: field strength (kV/cm); treatment times (t, µs); pulse width (p.w., µs); pulse frequency (Hz); temperature (°C); number pulses (n.p.); water activity (w.a.).
3.3. High Hydrostatic Pressure
The high hydrostatic pressure (HHP) process, also known as cold pasteurization, is a non-thermal technology capable of producing preserved food with sensory characteristics similar to fresh products; it also extends shelf-life [79]. During HHP food processing, the product is placed in a container of water and subjected to pressures ranging from 100 to 1000 MPa and temperatures of 60–65 °C, which ensures a decrease in microbial counts [80]. Two basic principles dictate the mechanism of action of the HHP process. The first of these is Le Chatelier’s principle, which states that any process that is in equilibrium will evolve, if disturbed, to counteract the disturbance. If there is a pressure variation in the system, it will modify its volume to reestablish equilibrium [81]. The second principle that dictates the mechanism of action of the HHP process is that of isostatic pressing. This principle states that during the compaction of a system, the pressure is distributed uniformly in every direction, regardless of its geometry; this is why a food product can be subjected to HHP in its final packaging, regardless of its shape [80].
The advantages of HHP include the inactivation of enzymes that affect the quality of the final product, which extends its shelf life with minimal degradation of vitamins, color, and taste [82]. However, being an expensive technology, its use at an industrial level requires a significant investment.
3.3.1. Effect of HHP on Microbial Inactivation in Food Matrices
The antimicrobial effect of HHP processing is related to its simultaneous effects on cell permeability, cell morphology, and biochemical reactions. However, the exact mechanism by which HHP induces cell death is still unknown. The current hypothesis is that HHP acts on proteins, ribosomes, and DNA simultaneously [80], as shown in Figure 3. This treatment is an alternative to thermal preservation because it inactivates bacteria, yeasts, and molds. However, its ability to inactivate viruses and bacterial spores is limited [82].
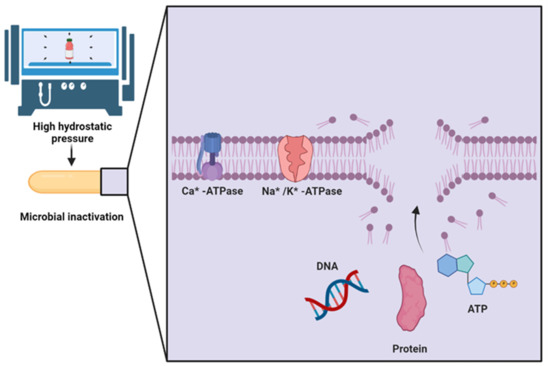
Figure 3.
Mechanisms of microbial inactivation by high hydrostatic pressure [80] (Created with BioRender.com; accessed on 30 March 2022). DNA: Deoxyribonucleic acid, ATP: Adenosine triphosphate.
3.3.2. Effect of HHP and AMPs on Microbial Inactivation in Food Matrices
During HHP treatment, the food matrix is an important factor to consider due to the protection it can provide to the microorganisms. It is also essential to consider the processing time and temperature, since these parameters can cause changes in the composition of the food product, the predominant microbiota, the pH, and the water activity [80].
Some bacterial species are more resistant to HHP than others; for example, S. aureus is more resistant than Salmonella enterica. The most influential factors for this resistance are species, applied pressure, and pH [83]. Therefore, it has been hypothesized that the combined use of HHP and AMPs could increase cell death, due to the damage caused to the cell membrane by HHP resulting in sensitization to AMPs [84]. Table 6 reviews the studies that have evaluated the combined effects of HHP and AMPs on microbial inactivation in food matrices.
Based on the information in Table 6, the combination of HHP with nisin has been widely studied in different food matrices. Black et al. [85] studied the combined effect of HPP and nisin on the inactivation of Bacillus spores in milk. The authors reported that nisin and HHP act synergistically to retard germination and improve inactivation of Bacillus spores, compared to HHP and nisin alone.
Gao & Ju [86] studied the combination of HHP and nisin on the inactivation of Clostridium botulinum spores in milk using a response surface methodology. The authors reported that, by optimizing the treatment, it is possible to completely inhibit C. botulinum [86].
Furthermore, Hereu et al. [87] studied the combined effect of HHP and nisin on L. monocytogenes in slices of cured ham. The authors observed a greater inhibition of L. monocytogenes during storage after treatment with HHP and nisin, compared to the samples that were only treated with HHP. Therefore, this combination could control contamination by L. monocytogenes in ready-to-eat meat products [87].
Aouadhi et al. [24] studied the effect of HHP and nisin on the inactivation of Bacillus sporothermodurans spores in distilled water. The authors reported that the combination of HHP and nisin at moderate temperature had a synergistic effect against B. sporothermodurans spores. Using response surface modelling, they described how spore inactivation increased with increasing pressure, temperature, and nisin concentration [24].
Pokhrel et al. [88] studied the combined effect of HHP and nisin on L. innocua and E. coli in carrot juice. The authors observed a synergistic effect on the inactivation of L. innocua and E. coli when applying HHP in combination with nisin at mild temperatures.
Finally, Oner [17] studied the effect of HHP combined with nisin on E. coli and L. innocua in green juice. The author observed that the combined treatment was more effective against E. coli than against L. innocua, and that there were no changes in pH or total soluble solids, although color changes were detected. The combination of HHP with nisin proved to be a viable alternative to pasteurization for green juice [17].
In summary, the combination of nisin with HPP increases the effectiveness of microbial inhibition, including that for spores. In some cases, it is possible to optimize conditions and use shorter processing times, which could lead to savings in operating costs.
The combined use of HHP with AMPs other than nisin has also been studied (Table 6). According to the results of these studies, it seems that the combination of HHP with nisin has a stronger antimicrobial effect than the combination HHP with other AMPs.
The combined treatment of HHP with a mixture of nisin and pediocin AcH [89] caused cell membrane collapse and cell lysis in L. monocytogenes, E. coli, and S. typhimunum.
Masschalck et al. [90] studied the inactivation of E. coli using a combination of HHP, nisin, and lysozyme. They reported that the inactivation of E. coli was significantly higher with the combined treatment than with the individual treatments, because HHP improves the efficiency of both nisin and lysozyme; however, an inactivation limit can be reached under mild pressures and temperatures.
Morgan et al. [91] studied the combined effect of HHP and lacticin 3147 on the inactivation of S. aureus and L. innocua in milk. The authors reported a synergistic effect in the inactivation of both microorganisms using the combined treatment; the activity of lacticin 3147 increased after treatment with HHP.
Garriga et al. [92] studied the combined effect of HHP and AMPs (nisin, enterocin A and B, sakacin K, or pediocin AcH) on E. coli CTC1018 and CTC1023, S. enterica CTC1003 and Schwarzengrund CTC1015, S. aureus CTC1008 and CTC1019, L. monocytogenes CTC1010 and CTC1034, Lactobacillus sakei CTC746, Leuconostoc carnosum CTC747, and Staphylococcus carnosus LTH2102 in cooked ham stored for 61 days. The authors observed that Staphylococcus was somewhat resistant to HHP and nisin but this combination significantly inactivated E. coli, showing no change in inactivation after 61 days. Treatment with sakacin, pediocin, or enterocin maintained the same microbial count of L. monocytogenes throughout storage. Meanwhile, S. enterica CTC1003 and Schwarzengrund CTC1015 remained below the detection limit. The authors also reported the inactivation of L. sakei and Lc. carnosum after treatment with HHP and AMPs, both in combination and individually. L. sakei recovered from the combined treatment with HHP and AMPs (sakacin and pediocin) during storage, while HHP combined with nisin successfully inactivated Lc. carnosum.
Furthermore, López-Pedemonte et al. [93] studied the combined effect of HHP with nisin or lysozyme on B. cereus spores in cheese. The authors obtained better results with HHP combined with nisin than for HHP combined with lysozyme. The latter was not able to increase bacterial sensitivity to HHP treatment.
More recently, Lee et al. [94] studied the inactivation of Salmonella Enteriditis using HHP and nisin in TSYB culture medium. In this study, the combined treatment of HHP and nisin was more effective against bacteria than with either HHP or nisin alone. Individual treatments had no effect on viability or bacterial cell components.
Pérez-Pulido et al. [95] studied the combined effect of HHP and AMPs (nisin, enterocin AS-48, and cinnamon and clove essential oils) on S. aureus in rice pudding. Of all the combined treatments, the one with clove essential oil was most effective.
De Alba et al. [96] studied the effect of combined treatment with HHP and AMPs (nisin or pediocin) on E. coli in slices of cured ham. The authors reported that the combined treatment with HHP and nisin was more effective than individual treatments, with no changes in texture or color during 60 days of storage.
Pérez-Pulido et al. [97] and Toledo del Árbol et al. [98] reported the combined use of HHP and enterocin AS-48 in cherimoya pulp. The authors suggest that the HHP and AMP combined treatment can be useful to inactivate Leuconostoc and epiphytic microbiota in this type of product.
Dallagnol et al. [84] reported that using HHP alone or in combination with lactocin AL705 in cured pork loin is effective for the total inhibition of L. innocua. Furthermore, Castro et al. [19,20] reported that pediocin bacHA-6111-2 in combination with HPP effectively controlled the growth of L. innocua in meat products.
Komora et al. [99] studied the potential use of phage P100 and pediocin PA-1 in combination with HHP to control L. monocytogenes in milk, using pressures lower than commonly used in HHP. The authors reported a synergistic effect against L. monocytogenes immediately after treatment, which was not observed when using P100, pediocin PA-1, or HHP alone. However, in terms of shelf-life, this treatment was not as effective as the thermal treatment.
In conclusion, it is crucial to select the specific AMP for use in combined treatment with HHP based on the target bacterial species. It is also important to consider the characteristics of the food matrix in order to achieve the desired microbial inactivation. Another important factor is ensuring the working pressure of the HHP process is able to prevent bacteria from being able to recover during storage.

Table 6.
Effect of high hydrostatic pressure (HHP) and antimicrobial peptides (AMP) on microbial inactivation in food matrices.
Table 6.
Effect of high hydrostatic pressure (HHP) and antimicrobial peptides (AMP) on microbial inactivation in food matrices.
| Media | HHP Device | HHP Parameters | AMP | AMP Concentration | Microorganism | Maximal Inactivation | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| HHP | AMP | HHP + AMP | |||||||
| Green juice | HHP equipment, 914-100 Engineered Pressure Systems Inc., Haverhill, MA, USA | 400 MPa, 3 min, 20 °C | Nisin | 100 mg/L | E. coli | 1.9 log CFU/mL | 0.1 log CFU/mL | 7.5 log CFU/mL | [17] |
| 200 mg/L | L. innocua | 0.7 log CFU/mL | Values not reported | 6.4 log CFU/mL | |||||
| Milk | HHP equipment, Hiperbaric 55, Burgos, Spain | 300 MPa, 5 min, 10 °C | Pediocin PA-1 | 1280 AU/mL | L. monocytogenes | 0.3 log/CFU | Values not reported | 4.5 log CFU/mL | [99] |
| Phage P100 | |||||||||
| Carrot juice | HHP equipment, Engineering Pressure Systems Inc., Haverhill, MA, USA | 500 MPa, 2 min, 20 °C | Nisin | 25 ppm | L. innocua | 4 log CFU/mL | Not reported | 7 log CFU/mL | [88] |
| E. coli | 5 log CFU/mL | 7 log CFU/mL | |||||||
| Fermented meat product | HHP equipment, Unipress U33, Poland | 300 MPa, 5 min, 10 °C | Pediocin bacHA-6111-2 | 320 AU/g | L. innocua | 1.0 log CFU/g | 1.2 log CFU/g | 2 log CFU/g | [19] |
| Ready-to-eat meat slices | HHP equipment, Unipress U33, Poland | 300 MPa, 5 min, 10 °C | Pediocin bacHA-6111-2 | 6400 AU/mL | L. innocua | 0.5 log CFU/g | 0.5 log CFU/g | 1.0 log CFU/g | [20] |
| Cured cooked pork loins | HHP equipment, Stansted Fluid Power Ltd. System, Harlow, UK | 600 MPa, 5 min, 5 °C (C) | Lactocin AL705 | 105 AU/cm2 | L. innocua | n.d. * | 5.5 log CFU/cm2 | n.d. * | [84] |
| Aerobic bacteria | n.d. * | 2 log CFU/cm2 | n.d. * | ||||||
| Lactic acid bacteria | n.d. * | 7 log CFU/cm2 | n.d. * | ||||||
| Cherimoya pulp | HHP equipment, Stansted Fluid Power LTD, Harlow, UK | 600 MPa, 8 min, 23–27 °C | EnterocinAS-48 | 35 mg/g | Cocktail of Leuconostoc; L. mesenteroides, L. gasicomitatum and L. gelidum | 0.5 log CFU/g * | 4.5 log CFU/g * | n.d. * | [98] |
| Cherimoya pulp | HHP equipment, Stansted Fluid Power LTD, UK | 600 MPa, 8 min, 23–27 °C | EnterocinAS-48 | 50 μg/g | Epiphytic microbiota | 2 log CFU/g | 6.5 log CFU/g | n.d. * | [97] |
| Laboratory solution | HHP equipment, ACB Pressure Systems, France | 472 MPa, 5 min, 53 °C | Nisin | 121 UI/mL | B. sporothermodurans spores | Values not reported | 1 log spores/mL | 5 log spores/mL | [24] |
| Dry-cured ham | HHP prototype, ACIP 6000, France | 500 MPa, 10 min, 12 °C | Nisin | 100 IU/g | E. coli | 1.3 log CFU/g | Not reported | 1.8 log CFU/g | [96] |
| Dry-cured ham | HHP equipment, Wave 6000/120, Hyperbaric, Spain | 600 MPa, 5 min, 15 °C, 0.92 (a.w.) | Nisin | 200 AU/cm2 | L.monocytogenes | 3.5 log CFU/g * | 6.5 log CFU/g * | 1 log CFU/g * | [87] |
| Rice puddings | HHP equipment, Stansted Fluid Power LTD, Harlow, UK | 500 MPa, 5 min | Nisin | 500 IU/g | S. aureus cocktail | 2.9 log cycles | Not reported | 3.7 log cycles | [95] |
| Enterocin AS-48 | 50 µg/g | 3.7 log cycles | |||||||
| Cinnamon oil | 0.2% | 4.5 log cycles | |||||||
| Clove oil | 0.25% | 5 log cycles | |||||||
| Growth medium | ABB Quintus Food Processor, QFP-6 Cold Isostatic Press, USA). | 400 MPa, 10 min, 25 °C | Nisin | 200 IU/mL | S. Enteritidis FDA | 5 log CFU/mL | <1 log CFU/mL | 8 log CFU/mL | [94] |
| Skim milk | HHP equipment, Stansted Foodlab 900, UK | 500 MPa, 5 min, 40 °C | Nisin | 500 IU/mL | B. subtilis spores | 2.5 log CFU/mL | Not reported | 5.9 log CFU/mL | [85] |
| Milk | High pressure unit 200 mL, 52 Institute, China | 545 MPa, 13 min, 51 °C | Nisin | 129 IU/mL | C. botulinum spores | Values not reported | Not reported | 6 log cycles | [86] |
| Cheese | Discontinuous HHP equipment, ALSTOM, France | 60 + 400 MPa, 210 + 15 min, 30 °C | Nisin | 1.56 mg/L | B. cereus spores | 1.4 log CFU/g | Not reported | 2.4 log CFU/g | [93] |
| Lysozyme | 22.4 mg/L | 1.9 log CFU/g | |||||||
| Cooked ham | Industrial hydrostatic pressurization unit, Alstom, France | 400 MPa, 10 min, 17 °C | Nisin A | 1280 AU/g | E. coli | 4.5 log CFU/g | Not reported | >6 log CFU/g | [92] |
| Potassium phosphate buffer | Pressure vessel, Resato, The Netherlands | 450 MPa, 15 min, 20 °C | Nisin | 100 IU/mL | E. coli | 6.3 log CFU/mL | Not reported | >8.3 log CFU/mL | [90] |
| Lysozyme | 50 µg/mL | ||||||||
| Milk | Pressure vessel, Stansted Fluid Power Ltd., Harlow, UK | 250 MPa, 30 min, 25 °C | Lacticina 3147 | 15000 AU/mL | S. aureus | 2.2 log CFU/mL | 1 log CFU/mL | >6 log CFU/mL | [91] |
| L. innocua | 2.5 log CFU/mL | 1.2 log CFU/mL | >6.4 log CFU/mL | ||||||
| Growth medium | Hydrostatic pressure unit, Harwood, USA | 345 MPa, 5 min, 25 °C | Nisin A and pediocin AcH mixture | 5000 AU/mL | L. monocytogenes | 7.8 log CFU/g * | Not reported | 4.5 log CFU/g * | [89] |
| E. coli | 9.3 log CFU/g * | 5.7 log CFU/g * | |||||||
| S. typhimurium Ml | 7.6 log CFU/g * | 4 log CFU/g * | |||||||
HHP parameters: pressure (MPa), treatment times (min), temperature (°C), water activity (w.a.); n.d., no viable cells detected; *, viable cell count.
3.4. Other Non-Thermal Technologies Used with AMPs for Microbial Inactivation
In addition to US, PEF, and HHP, other non-thermal technologies have been studied in combination with AMPs.
Costello et al. [100] studied the effect of cold atmospheric plasma (CAP, Figure 4a) in combination with nisin on L. innocua in food models based on tryptic soy broth containing yeast extract (TSBYE) and xanthan gum. Combining CAP with an AMP was more effective than the individual treatments.
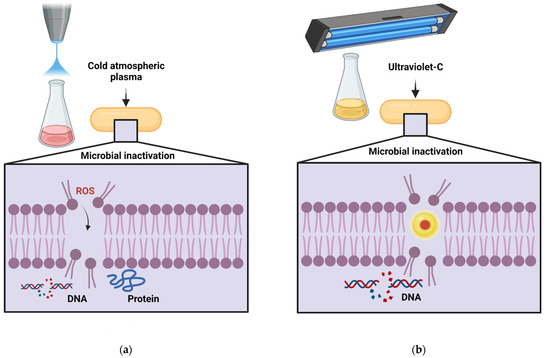
Figure 4.
Mechanisms of microbial inactivation of (a) cold atmospheric plasma and (b) ultraviolet-C light on microbial inactivation [100,101] (Created with BioRender.com; accessed on 30 March 2022). DNA: Deoxyribonucleic acid, ROS: Reactive oxygen species.
Combined treatment of ultraviolet-C light (UV-C, Figure 4b) and AMPs has also been studied. Specifically, Ferreira et al. [101] studied the effect of combining UV-C and nisin on the inactivation of Alicyclobacillus acidoterrestris spores in orange juice. The combined treatment of UV-C with low doses of nisin eliminated A. acidoterrestris spores without degrading the vitamins in the juice.
Thus, AMPs are a promising alternative means to increase the efficiency of other antimicrobial non-thermal technologies without affecting the sensory and functional properties of the final product.
Despite there being only two published studies investigating CAP and UV-C in combination with AMPs, nisin in particular appears to be a promising co-treatment for inactivating microorganisms in food matrices while retaining the sensory and functional properties of the product. However, more research on these technologies is needed to better understand the factors involved in their inactivation of microorganisms.
4. Applications and Prospects of AMP Use in the Food Industry in Combination with Non-Thermal Technologies
This section reviews the current food industry applications of the three non-thermal technologies, and the challenges that these applications present, either individually or in combination with AMPs. Finally, the regulatory requirements of the FDA for the use of US, PEF, HHP, and AMPs is also included.
In the food industry, US has been used as a unit operation in various processes, such as fermentation, emulsification, crystallization, and extraction [4]. Combined with other technologies, such as AMPs, US can guarantee food safety. However, the use of US still faces particular challenges in upscaling, due to its complex physicochemical mechanisms. For example, US alone has a low efficiency in inactivating the microbial load and guaranteeing food safety, which occurs due to the resistance of some microorganisms to cavitation. US treatment can also stimulate spore formation, and spores are more difficult to inactivate than vegetative cells. Additionally, it is necessary to identify the factors that influence the transmission of the acoustic energy generated by the US within the food matrix in order to ensure microbiological quality.
PEF have potential uses in the food industry, such as the extraction of bioactive compounds or protein modification, which require low or moderate-intensity electric fields [64] but microbial inactivation requires high-intensity electric fields. For preservation purposes, it is preferable to combine them with other technologies such as AMPs to avoid the adverse effects caused by PEF on food matrices. The industrial application of PEF has various limitations, mainly due to the complexity of the electric pulse generator, which must be safe and affordable. The main challenge is in reducing the average number of electrochemical reactions by both selecting more stable materials for the electrode and considering the physicochemical properties of the food product that will be subjected to the treatment. It is necessary to further study the scalability of PEF in terms of optimization of the desired processing parameters, including field intensity, specific energy, and the selection of AMPs for different food matrices [65].
As for HHP, this technology is used in the food industry to preserve various food products, such as milk, juices, sauces, guacamole, and cheeses, with results equivalent to those obtained with thermal pasteurization. HHP has been used with other technologies including ultrafiltration, CO2 and US. However, HHP, either alone or combined with these other technologies, raises production costs, thus increasing the price of products. Therefore, the combined use of AMPs and HHP could make this technology more affordable and safer for the consumer by increasing its antimicrobial efficiency. It is necessary to focus on process optimization and to define its microbial inactivation mechanisms in order to achieve minimal processing while ensuring the safety of processed foods.
With regard to the regulation of US, PEF, and HHP, the FDA requires a 5-log reduction process for liquid food preservation [64]. US alone is not enough to achieve the FDA requirement, so its combination with other technologies, such as AMPs, is being explored. The combination of US and AMPs shows potential to meet the FDA requirements and become an analog of thermal pasteurization, while for PEF, the minimum 5-log reduction has already been verified. The FDA has also approved using HHP technology as an analogous to thermal pasteurization for commercial products. In Canada, there are no legal impediments because it is not considered new technology and it is already regulated. In Europe, there are different legal requirements for using HHP in each country [102].
Regarding the regulation of AMPs, the FDA considers the use of nisin, natamycin, and lysozyme to be safe at specific concentrations. However, other AMPs have not yet been regulated by the FDA, but they do show potential antimicrobial effects individually and in combination with US, PEF, and HHP.
5. Conclusions
This study aimed to compile and review information about three non-thermal technologies (US, PEF, and HHP) and their combination with AMPs as alternative technologies for microbial inactivation in different food matrices.
Combined treatments are more effective than US, PEF, HHP, or AMPs alone. Nisin is the AMP that has been most studied in combination with the three non-thermal technologies, probably because it has already been officially regulated. However, combining these non-thermal technologies with other AMPs also shows promise as alternative microbial inactivation methods. The same is true for the combination of AMPs with other non-thermal technologies, such as CAP and UV-C.
The combined use of US and AMPs has been studied with both ultrasonic probes and ultrasonic baths, generating different results. The probe is more effective for microbial inactivation, both for individual and combined treatments. AMPs are not adversely affected by either US application method. Combining HHP treatment with AMPs shows similar results to those observed with US; this differs from those observed with PEF, which can inhibit the effect of some AMPs, especially under a high-intensity electric field.
The efficacy of the combined treatments depends on several factors. First, the characteristics of the food matrix, such as its physicochemical properties and composition, are essential considerations. Additionally, the non-thermal treatment parameters (i.e., application time, sequence, and intensity) and the AMP application conditions (i.e., concentration, combination with various other AMPs, and time of application with the other technology) are also crucial factors.
Based on the studies reviewed herein, we can conclude that, under mild conditions, the different combinations of non-thermal technologies and AMPs can differ in their specific action toward each type of microorganism. Therefore, future research must focus on developing non-thermal technologies in combination with various AMPs, while simultaneously substituting for the extreme conditions of non-thermal technologies that can decrease the quality of the treated food product. The idea is to develop less intensive but more effective food preservation methods, that is, methods that guarantee both the preservation of properties similar to those in fresh food, and the safety of the final product.
In addition to AMPs, non-thermal technologies can also be combined with essential oils, which were more effective than AMPs in some studies. Since essential oils also represent an excellent alternative to chemical preservatives and thermal treatments, their use must continue to be studied, either alone or combined with AMPs.
Author Contributions
Conceptualization, C.O.; methodology, C.O.; investigation, L.A.-M. and D.R.-D.A.; data curation, L.A.-M. and D.R.-D.A.; writing—original draft preparation, L.A.-M.; writing—review and editing, C.O.; supervision, C.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Consejo Nacional de Ciencia y Tecnología in Mexico (CONACYT) for the funding granted to L.A.-M. and D.R.-D.A. to carry out their Master’s degree studies in the Postgraduate Program of Biosciences of the Universidad de Guanajuato. The authors would also like to thank Stanislav Mulík, for his valuable contribution in the writing process of this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martinho, V.J.P.D.; Bartkiene, E.; Djekic, I.; Tarcea, M.; Barić, C.; Černelič-bizjak, M.; Szűcs, V.; Sarcona, A.; Ferreira, V.; Klava, D.; et al. Determinants of economic motivations for food choice: Insights for the understanding of consumer behaviour. Int. J. Food Sci. Nutr. 2022, 73, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, A.; Wijaya, T.; Ishak, A.; Rejeki Ekasasi, S.; Zalzalah, G.G. Model of the Consumer Switching Behavior Related to Healthy Food Products. Sustainability 2021, 13, 3555. [Google Scholar] [CrossRef]
- Putnik, P.; Pavlić, B.; Šojić, B.; Zavadlav, S.; Žuntar, I.; Kao, L.; Kitonić, D.; Kovačević, D.B. Innovative hurdle technologies for the preservation of functional fruit juices. Foods 2020, 9, 699. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef]
- Yang, S.; Yuan, Z.; Aweya, J.J.; Huang, S.; Deng, S.; Shi, L.; Zheng, M.; Zhang, Y.; Liu, G. Low-intensity ultrasound enhances the antimicrobial activity of neutral peptide TGH2 against Escherichia coli. Ultrason. Sonochem. 2021, 77, 105676. [Google Scholar] [CrossRef]
- Yildiz, S.; Pokhrel, P.R.; Unluturk, S.; Barbosa-Cánovas, G.V. Identification of equivalent processing conditions for pasteurization of strawberry juice by high pressure, ultrasound, and pulsed electric fields processing. Innov. Food Sci. Emerg. Technol. 2019, 57, 102195. [Google Scholar] [CrossRef]
- Ruiz-De Anda, D.; Ventura-Lara, M.G.; Rodríguez-Hernández, G.; Ozuna, C. The impact of power ultrasound application on physicochemical, antioxidant, and microbiological properties of fresh orange and celery juice blend. J. Food Meas. Charact. 2019, 13, 3140–3148. [Google Scholar] [CrossRef]
- Ruiz-De Anda, D.; Casados-Vázquez, L.E.; Ozuna, C. The synergistic effect of thurincin H and power ultrasound: An alternative for the inactivation of Listeria innocua ATCC 33090 and Escherichia coli K-12 in liquid food matrices. Food Control 2022, 135, 108778. [Google Scholar] [CrossRef]
- Anumudu, C.; Hart, A.; Miri, T. Recent Advances in the Application of the Antimicrobial Peptide Nisin in the Inactivation of Spore-Forming Bacteria in Foods. Molecules 2021, 26, 5552. [Google Scholar] [CrossRef]
- Kashani, H.H.; Nikzad, H.; Mobaseri, S.; Hoseini, E.S. Synergism Effect of Nisin Peptide in Reducing Chemical Preservatives in Food Industry. Life Sci. J. 2012, 9, 1–8. [Google Scholar]
- Anacarso, I.; Niederhäusern, S.; Iseppi, R.; Sabia, C.; Bondi, M.; Messi, P. Anti-listerial activity of chitosan and Enterocin 416K1 in artificially contaminated RTE products. Food Control 2011, 22, 2076–2080. [Google Scholar] [CrossRef]
- Cobo, A.; Abriouel, H.; Lucas, R.; Ben, N.; Valdivia, E.; Gálvez, A. Enhanced bactericidal activity of enterocin AS-48 in combination with essential oils, natural bioactive compounds and chemical preservatives against Listeria monocytogenes in ready-to-eat salad. Food Chem. Toxicol. 2009, 47, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.I.; Ukuku, D.O.; Kawasaki, T.; Inatsu, Y.; Isshiki, K.; Kawamoto, S. Combined Efficacy of Nisin and Pediocin with Sodium Lactate, Citric Acid, Phytic Acid, and Potassium Sorbate and EDTA in Reducing the Listeria monocytogenes Population of Inoculated. J. Food Prot. 2005, 68, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.P.; Sati, N. Stability Indicating Rp–Hplc Method for Combination of Ambroxol Hydrochloride and Cefadroxil Monohydrate in Pharmaceutical Formulation. Int. J. Pharm. Sci. Res. 2013, 4, 2496–2501. [Google Scholar] [CrossRef]
- Ahmed, T.A.E.; Hammami, R. Recent insights into structure–function relationships of antimicrobial peptides. J. Food Biochem. 2019, 43, e12546. [Google Scholar] [CrossRef]
- Barbosa, A.A.T.; Mantovani, H.C.; Jain, S. Bacteriocins from lactic acid bacteria and their potential in the preservation of fruit products. Crit. Rev. Biotechnol. 2017, 37, 852–864. [Google Scholar] [CrossRef]
- Oner, M.E. The effect of high-pressure processing or thermosonication in combination with nisin on microbial inactivation and quality of green juice. J. Food Process. Preserv. 2020, 44, e14830. [Google Scholar] [CrossRef]
- Liao, H.; Jiang, L.; Cheng, Y.; Liao, X.; Zhang, R. Application of nisin-assisted thermosonication processing for preservation and quality retention of fresh apple juice. Ultrason. Sonochem. 2018, 42, 244–249. [Google Scholar] [CrossRef]
- Castro, S.M.; Silva, J.; Casquete, R.; Queirós, R.; Saraiva, J.A.; Teixeira, P. Combined effect of pediocin bacHA-6111-2 and high hydrostatic pressure to control Listeria innocua in fermented meat sausage. Int. Food Res. J. 2018, 25, 553–560. [Google Scholar]
- Castro, S.M.; Kolomeytseva, M.; Casquete, R.; Silva, J.; Teixeira, P.; Castro, S.M.; Queirós, R.; Saraiva, J.A. Biopreservation strategies in combination with mild high pressure treatments in traditional Portuguese ready-to-eat meat sausage. Food Biosci. 2017, 19, 65–72. [Google Scholar] [CrossRef]
- Yikmiş, S. Investigation of the effects of non-thermal, combined and thermal treatments on the physicochemical parameters of pomegranate (Punica granatum L.) Juice. Food Sci. Technol. Res. 2019, 25, 341–350. [Google Scholar] [CrossRef]
- Fitriyanti, M.; Narsimhan, G. Synergistic effect of low power ultrasonication on antimicrobial activity of cecropin P1 against E. coli in food systems. LWT 2018, 96, 175–181. [Google Scholar] [CrossRef]
- Wu, X.; Narsimhan, G. Synergistic effect of low power ultrasonication on antimicrobial activity of melittin against Listeria monocytogenes. LWT–Food Sci. Technol. 2017, 75, 578–581. [Google Scholar] [CrossRef]
- Aouadhi, C.; Simonin, H.; Mejri, S.; Maaroufi, A. The combined effect of nisin, moderate heating and high hydrostatic pressure on the inactivation of Bacillus sporothermodurans spores. J. Appl. Microbiol. 2013, 115, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Klaenhammer, T.R. Bacteriocins of lactic acid bacteria. Biochimie 1988, 70, 337–349. [Google Scholar] [CrossRef]
- Duraisamy, S.; Balakrishnan, S.; Ranjith, S.; Husain, F.; Sathyan, A. Bacteriocin—A potential antimicrobial peptide towards disrupting and preventing biofilm formation in the clinical and environmental locales. Environ. Sci. Pollut. Res. 2020, 27, 44922–44936. [Google Scholar] [CrossRef]
- Vogel, V.; Spellerberg, B. Bacteriocin Production by Beta-Hemolytic Streptococci. Pathogens 2021, 10, 867. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Wirawan, R.E.; Klesse, N.A.; Jack, R.W.; Tagg, J.R. Molecular and genetic characterization of a novel nisin variant produced by Streptococcus uberis. Appl. Environ. Microbiol. 2006, 72, 1148–1156. [Google Scholar] [CrossRef]
- Meindl, K.; Schmiederer, T.; Schneider, K.; Reicke, A.; Butz, D.; Keller, S.; Gühring, H.; Vértesy, L.; Wink, J.; Hoffmann, H.; et al. Labyrinthopeptins: A new class of carbacyclic lantibiotics. Angew. Chemie Int. Ed. 2010, 49, 1151–1154. [Google Scholar] [CrossRef]
- Kawulka, K.E.; Sprules, T.; Diaper, C.M.; Whittal, R.M.; McKay, R.T.; Mercier, P.; Zuber, P.; Vederas, J.C. Structure of Subtilosin A, A Cyclic Antimicrobial Peptide from Bacillus subtilis with Unusual Sulfur to α-Carbon Cross-Links: Formation and Reduction of α-Thio-α-Amino Acid Derivatives. Biochemistry 2004, 43, 3385–3395. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.T.; Chopko, A.L.; van Wassenaar, P.D. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC-1.0. Arch. Biochem. Biophys. 1992, 295, 5–12. [Google Scholar] [CrossRef]
- Abee, T.; Klaenhammer, T.R.; Letellier, L. Kinetic studies of the action of lactacin F, a bacteriocin produced by Lactobacillus johnsonii that forms poration complexes in the cytoplasmic membrane. Appl. Environ. Microbiol. 1994, 60, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Martin-Visscher, L.A.; Yoganathan, S.; Sit, C.S.; Lohans, C.T.; Vederas, J.C. The activity of bacteriocins from Carnobacterium maltaromaticum UAL307 against Gram-negative bacteria in combination with EDTA treatment. FEMS Microbiol. Lett. 2011, 317, 152–159. [Google Scholar] [CrossRef]
- Ovchinnikov, K.V.; Kristiansen, P.E.; Straume, D.; Jensen, M.S.; Aleksandrzak-Piekarczyk, T.; Nes, I.F.; Diep, D.B. The leaderless bacteriocin enterocin K1 is highly potent against Enterococcus faecium: A study on structure, target spectrum and receptor. Front. Microbiol. 2017, 8, 774. [Google Scholar] [CrossRef]
- De Lorenzo, V.; Pugsley, A.P. Microcin E492, a low-molecular-weight peptide antibiotic which causes depolarization of the Escherichia coli cytoplasmic membrane. Antimicrob. Agents Chemother. 1985, 27, 666–669. [Google Scholar] [CrossRef][Green Version]
- Müller, E.; Radler, F. Caseicin, a bacteriocin from Lactobacillus casei. Folia Microbiol. 1993, 38, 441–446. [Google Scholar] [CrossRef]
- Joerger, M.C.; Klaenhammer, T.R. Characterization and purification of helveticin J and evidence for a chromosomally determined bacteriocin produced by Lactobacillus helveticus 481. J. Bacteriol. 1986, 167, 439–446. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Zhang, X.; Wu, H.; Zou, Y.; Li, P.; Sun, C.; Xu, W.; Liu, F.; Wang, D. Class III bacteriocin Helveticin-M causes sublethal damage on target cells through impairment of cell wall and membrane. J. Ind. Microbiol. Biotechnol. 2018, 45, 213–227. [Google Scholar] [CrossRef]
- Zhao, H.; Mattila, J.; Holopainen, J.M.; Kinnunen, P.K.J. Comparison of the Membrane Association of Two Antimicrobial Peptides, Magainin 2 and Indolicidin. Biophys. J. 2001, 81, 2979–2991. [Google Scholar] [CrossRef]
- Lee, M.; Chen, F.; Huang, H.W. Energetics of Pore Formation Induced by Membrane Active Peptides. Biochemistry 2004, 43, 3590–3599. [Google Scholar] [CrossRef]
- Melo, M.N.; Ferre, R.; Castanho, M.A.R.B. Antimicrobial peptides: Linking partition, activity and high membrane-bound concentrations. Nat. Rev. Microbiol. 2009, 7, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bi, X.; Xiang, R.; Chen, L.; Feng, X.; Zhou, M.; Che, Z. Inactivation of Escherichia coli by ultrasound combined with nisin. J. Food Prot. 2018, 81, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Streletskaya, N.A.; Lim, J. Clean label: Why this ingredient but not that one? Food Qual. Prefer. 2021, 87, 104062. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- Jia, W.; Wu, X.; Li, R.; Liu, S.; Shi, L. Effect of nisin and potassium sorbate additions on lipids and nutritional quality of Tan sheep meat. Food Chem. 2021, 365, 130535. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D.; et al. Application of natural antimicrobials in food preservation: Recent views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- FAO. Organización de las Naciones Unidas para la Alimentación y la Agricultura Codex Alimentarius Norma Internacional De Los Alimentos Norma General Para Los Aditivos Alimentarios Codex Stan 192-1995. Fao Omg 2019, 53, 3–4. [Google Scholar]
- Taylor, T.M.; Ravishankar, S.; Bhargava, K.; Juneja, V.K. Chemical preservatives and natural food antimicrobials. Food Microbiol. Fundam. Front. 2019, 705–731. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Scudino, H.; Ramos, G.L.; Oliveira, G.A.; Margalho, L.P.; Costa, L.E.; Freitas, M.Q.; Duarte, M.C.K.; Sant’Ana, A.S.; Cruz, A.G. Current applications of high-intensity ultrasound with microbial inactivation or stimulation purposes in dairy products. Curr. Opin. Food Sci. 2021, 42, 140–147. [Google Scholar] [CrossRef]
- Gao, S.; Lewis, G.D.; Ashokkumar, M.; Hemar, Y. Inactivation of microorganisms by low-frequency high-power ultrasound: 1. Effect of growth phase and capsule properties of the bacteria. Ultrason. Sonochem. 2014, 21, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Riener, J.; Noci, F.; Cronin, D.A.; Morgan, D.J.; Lyng, J.G. Characterisation of volatile compounds generated in milk by high intensity ultrasound. Int. Dairy J. 2009, 19, 269–272. [Google Scholar] [CrossRef]
- Dolas, R.; Saravanan, C.; Kaur, B.P. Emergence and era of ultrasonic’s in fruit juice preservation: A review. Ultrason. Sonochem. 2019, 58, 104609. [Google Scholar] [CrossRef] [PubMed]
- Starek, A.; Kobus, Z.; Sagan, A.; Chudzik, B.; Pawłat, J.; Kwiatkowski, M.; Terebun, P.; Andrejko, D. Influence of ultrasound on selected microorganisms, chemical and structural changes in fresh tomato juice. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Bochu, W.; Lanchun, S.; Jing, Z.; Yuanyuan, Y.; Yanhong, Y. The influence of Ca2+ on the proliferation of S. cerevisiae and low ultrasonic on the concentration of Ca2+ in the S. cerevisiae cells. Colloids Surf. B Biointerfaces 2003, 32, 35–42. [Google Scholar] [CrossRef]
- Freitas, L.L.D.; Prudêncio, C.V.; Peña, W.E.L.; Vanetti, M.C.D. Modeling of Shigella flexneri inactivation by combination of ultrasound, pH and nisin. LWT 2019, 109, 40–46. [Google Scholar] [CrossRef]
- Bi, X.; Zhou, Z.; Wang, X.; Jiang, X.; Chen, L.; Xing, Y.; Che, Z. Changes in the Microbial Content and Quality Attributes of Carrot Juice Treated by a Combination of Ultrasound and Nisin During Storage. Food Bioprocess Technol. 2020, 13, 1556–1565. [Google Scholar] [CrossRef]
- Ma, T.; Wang, J.; Wang, L.; Yang, Y.; Yang, W.; Wang, H.; Lan, T.; Zhang, Q.; Sun, X. Ultrasound-combined sterilization technology: An effective sterilization technique ensuring the microbial safety of grape juice and significantly improving its quality. Foods 2020, 9, 1512. [Google Scholar] [CrossRef]
- Costello, K.M.; Velliou, E.; Gutierrez-Merino, J.; Smet, C.; Kadri, H.E.; Impe, J.F.V.; Bussemaker, M. The effect of ultrasound treatment in combination with nisin on the inactivation of Listeria innocua and Escherichia coli. Ultrason. Sonochem. 2021, 79, 105776. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yuan, Q.; Gao, C.; Wang, X.; Zhu, B.; Wang, J.; Ma, T.; Sun, X. Thermosonication combined with natural antimicrobial nisin: A potential technique ensuring microbiological safety and improving the quality parameters of orange juice. Foods 2021, 10, 1851. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, K.; Kopeć, A.; Dróżdż, T.; Kiełbasa, P.; Ostafin, M.; Bulski, K.; Oziembłowski, M. Effect of pulsed electric field treatment on shelf life and nutritional value of apple juice. J. Food Sci. Technol. 2019, 56, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Gao, Q.Y.; Han, Z.; Zeng, X.A.; Yu, S.J. Structural properties and digestibility of pulsed electric field treated waxy rice starch. Food Chem. 2016, 194, 1313–1319. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; El-Din Bekhit, A.; Liu, Z.W.; Aadil, R.M. Pulsed electric field: A potential alternative towards a sustainable food processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]
- Pataro, G.; Ferrari, G. Limitations of Pulsed Electric Field Utilization in Food Industry; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128164020. [Google Scholar]
- Zhang, Z.; Zhang, B.; Yang, R.; Zhao, W. Recent Developments in the Preservation of Raw Fresh Food by Pulsed Electric Field. Food Rev. Int. 2020, 00, 1–19. [Google Scholar] [CrossRef]
- Calderón-Miranda, M.L.; Barbosa-Cánovas, G.V.; Swanson, B.G. Inactivation of Listeria innocua in skim milk by pulsed electric fields and nisin. Int. J. Food Microbiol. 1999, 51, 19–30. [Google Scholar] [CrossRef]
- Pol, I.E.; Mastwijk, H.C.; Bartels, P.V.; Smid, E.J. Pulsed-electric field treatment enhances the bactericidal action of nisin against Bacillus cereus. Appl. Environ. Microbiol. 2000, 66, 428–430. [Google Scholar] [CrossRef] [PubMed]
- Martínez Viedma, P.; Sobrino López, A.; Ben Omar, N.; Abriouel, H.; Lucas López, R.; Valdivia, E.; Martín Belloso, O.; Gálvez, A. Enhanced bactericidal effect of enterocin AS-48 in combination with high-intensity pulsed-electric field treatment against Salmonella enterica in apple juice. Int. J. Food Microbiol. 2008, 128, 244–249. [Google Scholar] [CrossRef]
- Terebiznik, M.R.; Jagus, R.J.; Cerrutti, P.; de Huergo, M.S.; Pilosof, A.M.R. Combined effect of nisin and pulsed electric fields on the inactivation of Escherichia coli. J. Food Prot. 2000, 63, 741–746. [Google Scholar] [CrossRef]
- Terebiznik, M.; Jagus, R.; Cerrutti, P.; De Huergo, M.S.; Pilosof, A.M.R. Inactivation of Escherichia coli by a combination of nisin, pulsed electric fields, and water activity reduction by sodium chloride. J. Food Prot. 2002, 65, 1253–1258. [Google Scholar] [CrossRef]
- Sobrino-López, A.; Martín-Belloso, O. Enhancing Inactivation of Staphylococcus aureus in Skim Milk. J. Food Prot. 2006, 69, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Aguirre, D.; Dunne, C.P.; Barbosa-Cánovas, G.V. Effect of processing parameters on inactivation of Bacillus cereus spores in milk using pulsed electric fields. Int. Dairy J. 2012, 24, 13–21. [Google Scholar] [CrossRef]
- Martínez Viedma, P.; Abriouel, H.; Sobrino López, A.; Ben Omar, N.; Lucas López, R.; Valdivia, E.; Martín Belloso, O.; Gálvez, A. Effect of enterocin AS-48 in combination with high-intensity pulsed-electric field treatment against the spoilage bacterium Lactobacillus diolivorans in apple juice. Food Microbiol. 2009, 26, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Sobrino-Lopez, A.; Viedma-Martínez, P.; Abriouel, H.; Valdivia, E.; Gálvez, A.; Martin-Belloso, O. The effect of adding antimicrobial peptides to milk inoculated with Staphylococcus aureus and processed by high-intensity pulsed-electric field. J. Dairy Sci. 2009, 92, 2514–2523. [Google Scholar] [CrossRef]
- Viedma, P.M.; López, A.S.; Nabil Omar, B.E.N.; Abriouel, H.; López, R.L.; Belloso, O.M.; Gálvez, A. Increased inactivation of exopolysaccharide-producing pediococcus parvulus in apple Juice by combined treatment with enterocin AS-48 and high-intensity pulsed electric field. J. Food Prot. 2010, 73, 39–43. [Google Scholar] [CrossRef]
- Mok, J.H.; Pyatkovskyy, T.; Yousef, A.; Sastry, S.K. Combined effect of shear stress and moderate electric field on the inactivation of Escherichia coli K12 in apple juice. J. Food Eng. 2019, 262, 121–130. [Google Scholar] [CrossRef]
- Mok, J.H.; Pyatkovskyy, T.; Yousef, A.; Sastry, S.K. Synergistic effects of shear stress, moderate electric field, and nisin for the inactivation of Escherichia coli K12 and Listeria innocua in clear apple juice. Food Control 2020, 113, 107209. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hsu, C.-P.; Wang, C.-Y. Healthy expectations of high hydrostatic pressure treatment in food processing industry. J. Food Drug Anal. 2020, 28, 1–13. [Google Scholar] [CrossRef]
- Sehrawat, R.; Kaur, B.P.; Nema, P.K.; Tewari, S.; Kumar, L. Microbial inactivation by high pressure processing: Principle, mechanism and factors responsible. Food Sci. Biotechnol. 2021, 30, 19–35. [Google Scholar] [CrossRef]
- Aaliya, B.; Valiyapeediyekkal Sunooj, K.; Navaf, M.; Parambil Akhila, P.; Sudheesh, C.; Ahmed Mir, S.; Sabu, S.; Sasidharan, A.; Theingi Hlaing, M.; George, J. Recent trends in bacterial decontamination of food products by hurdle technology: A synergistic approach using thermal and non-thermal processing techniques. Food Res. Int. 2021, 147, 110514. [Google Scholar] [CrossRef]
- Daher, D.; Le Gourrierec, S.; Pérez-Lamela, C. Effect of high pressure processing on the microbial inactivation in fruit preparations and other vegetable based beverages. Agriculture 2017, 7, 72. [Google Scholar] [CrossRef]
- Guillou, S.; Membré, J.-M. Inactivation of Listeria monocytogenes, Staphylococcus aureus, and Salmonella enterica under High Hydrostatic Pressure: A Quantitative Analysis of Existing Literature Data. J. Food Prot. 2019, 82, 1802–1814. [Google Scholar] [CrossRef] [PubMed]
- Dallagnol, A.M.; Barrio, Y.; Cap, M.; Szerman, N.; Castellano, P.; Vaudagna, S.R.; Vignolo, G. Listeria Inactivation by the Combination of High Hydrostatic Pressure and Lactocin AL705 on Cured-Cooked Pork Loin Slices. Food Bioprocess Technol. 2017, 10, 1824–1833. [Google Scholar] [CrossRef]
- Black, E.P.; Linton, M.; McCall, R.D.; Curran, W.; Fitzgerald, G.F.; Kelly, A.L.; Patterson, M.F. The combined effects of high pressure and nisin on germination and inactivation of Bacillus spores in milk. J. Appl. Microbiol. 2008, 105, 78–87. [Google Scholar] [CrossRef]
- Gao, Y.L.; Ju, X.R. Exploiting the combined effects of high pressure and moderate heat with nisin on inactivation of Clostridium botulinum spores. J. Microbiol. Methods 2008, 72, 20–28. [Google Scholar] [CrossRef]
- Hereu, A.; Bover-Cid, S.; Garriga, M.; Aymerich, T. High hydrostatic pressure and biopreservation of dry-cured ham to meet the Food Safety Objectives for Listeria monocytogenes. Int. J. Food Microbiol. 2012, 154, 107–112. [Google Scholar] [CrossRef]
- Pokhrel, P.R.; Toniazzo, T.; Boulet, C.; Oner, M.E.; Sablani, S.S.; Tang, J.; Barbosa-Cánovas, G.V. Inactivation of Listeria innocua and Escherichia coli in carrot juice by combining high pressure processing, nisin, and mild thermal treatments. Innov. Food Sci. Emerg. Technol. 2019, 54, 93–102. [Google Scholar] [CrossRef]
- Kalchayanand, N.; Sikes, T.; Dunne, C.P.; Ray, B. Hydrostatic pressure and electroporation have increased bactericidal efficiency in combination with bacteriocins. Appl. Environ. Microbiol. 1994, 60, 4174–4177. [Google Scholar] [CrossRef]
- Masschalck, B.; García-Graells, C.; Van Haver, E.; Michiels, C.W. Inactivation of high pressure resistant Escherichia coli by lysozyme and nisin under high pressure. Innov. Food Sci. Emerg. Technol. 2000, 1, 39–47. [Google Scholar] [CrossRef]
- Morgan, S.M.; Ross, R.P.; Beresford, T.; Hill, C. Combination of hydrostatic pressure and lacticin 3147 causes increased killing of Staphylococcus and Listeria. J. Appl. Microbiol. 2000, 88, 414–420. [Google Scholar] [CrossRef]
- Garriga, M.; Aymerich, M.T.; Costa, S.; Monfort, J.M.; Hugas, M. Bactericidal synergism through bacteriocins and high pressure in a meat model system during storage. Food Microbiol. 2002, 19, 509–518. [Google Scholar] [CrossRef]
- López-Pedemonte, T.J.; Roig-Sagués, A.X.; Trujillo, A.J.; Capellas, M.; Guamis, B. Inactivation of spores of Bacillus cereus in cheese by high hydrostatic pressure with the addition of nisin or lysozyme. J. Dairy Sci. 2003, 86, 3075–3081. [Google Scholar] [CrossRef]
- Lee, J.; Kaletunç, G. Inactivation of Salmonella Enteritidis strains by combination of high hydrostatic pressure and nisin. Int. J. Food Microbiol. 2010, 140, 49–56. [Google Scholar] [CrossRef]
- Pérez Pulido, R.; Toledo del Árbol, J.; Grande Burgos, M.J.; Gálvez, A. Bactericidal effects of high hydrostatic pressure treatment singly or in combination with natural antimicrobials on Staphylococcus aureus in rice pudding. Food Control 2012, 28, 19–24. [Google Scholar] [CrossRef]
- de Alba, M.; Bravo, D.; Medina, M. Inactivation of Escherichia coli O157: H7 in dry-cured ham by high-pressure treatments combined with biopreservatives. Food Control 2013, 31, 508–513. [Google Scholar] [CrossRef]
- Pérez Pulido, R.; Toledo, J.; Grande, M.J.; Gálvez, A.; Lucas, R. Analysis of the effect of high hydrostatic pressure treatment and enterocin AS-48 addition on the bacterial communities of cherimoya pulp. Int. J. Food Microbiol. 2015, 196, 62–69. [Google Scholar] [CrossRef]
- Toledo del Árbol, J.; Pérez Pulido, R.; Grande Burgos, M.J.; Gálvez, A.; Lucas López, R. Inactivation of leuconostocs in cherimoya pulp by high hydrostatic pressure treatments applied singly or in combination with enterocin AS-48. LWT-Food Sci. Technol. 2016, 65, 1054–1058. [Google Scholar] [CrossRef]
- Komora, N.; Maciel, C.; Pinto, C.A.; Ferreira, V.; Brandão, T.R.S.; Saraiva, J.M.A.; Castro, S.M.; Teixeira, P. Non-thermal approach to Listeria monocytogenes inactivation in milk: The combined effect of high pressure, pediocin PA-1 and bacteriophage P100. Food Microbiol. 2020, 86, 103315. [Google Scholar] [CrossRef]
- Costello, K.M.; Smet, C.; Gutierrez-Merino, J.; Bussemaker, M.; Van Impe, J.F.; Velliou, E.G. The impact of food model system structure on the inactivation of Listeria innocua by cold atmospheric plasma and nisin combined treatments. Int. J. Food Microbiol. 2021, 337, 108948. [Google Scholar] [CrossRef]
- Ferreira, T.V.; Mizuta, A.G.; Menezes, J.L.D.; Dutra, T.V.; Bonin, E.; Castro, J.C.; Szczerepa, M.M.D.A.; Pilau, E.J.; Nakamura, C.V.; Mikcha, J.M.G.; et al. Effect of ultraviolet treatment (UV–C) combined with nisin on industrialized orange juice in Alicyclobacillus acidoterrestris spores. LWT 2020, 133, 109911. [Google Scholar] [CrossRef]
- Pérez-Lamela, C.; Franco, I.; Falqué, E. Impact of high-pressure processing on antioxidant activity during storage of fruits and fruit products: A review. Molecules 2021, 26, 5265. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).