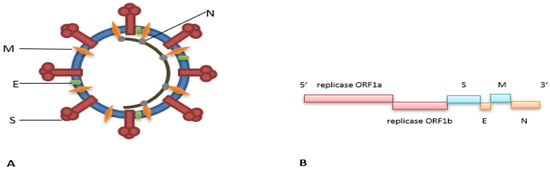
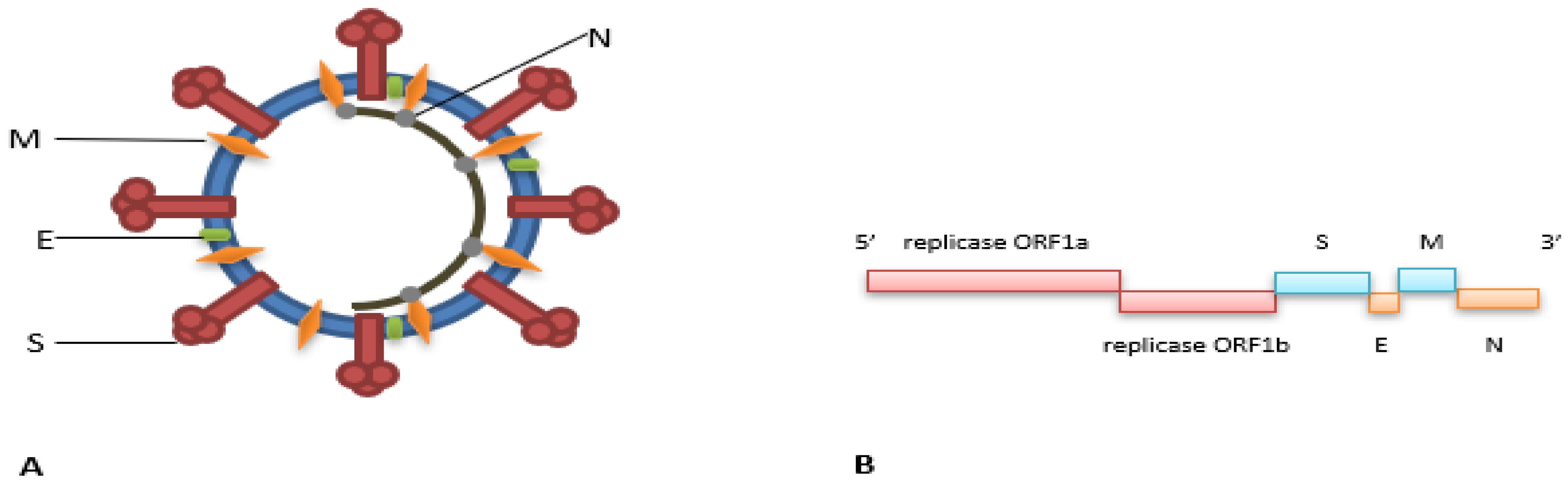
Abstract
Coronaviruses have caused devastation in both human and animal populations, affecting both health and the economy. Amidst the emergence and re-emergence of coronaviruses, humans need to surmount the health and economic threat of coronaviruses through science and evidence-based approaches. One of these approaches is through biotechnology, particularly the heterologous production of biopharmaceutical proteins. This review article briefly describes the genome, general virion morphology, and key structural proteins of different coronaviruses affecting animals and humans. In addition, this review paper also presents the different systems in recombinant protein technology such as bacteria, yeasts, plants, mammalian cells, and insect/insect cells systems used to express key structural proteins in the development of countermeasures such as diagnostics, prophylaxis, and therapeutics in the challenging era of coronaviruses.
1. Introduction
Coronaviruses or CoVs are enveloped, positive-sensed, single-stranded RNA viruses belonging to the order Nidovirales, family Coronaviridae, and subfamily Coronavirinae. Coronaviruses were originally grouped based on serology but are now classified based on phylogenetic relationships. This system of classification divided Coronavirinae into four genera, namely, alpha-, beta-, gamma-, and deltacoronavirus [1]. Generally, alpha- and betacoronaviruses are associated with humans and other mammals, while gamma- and deltacoronaviruses are linked to avians and, to some extent, marine mammals [2]. Their mode of transmission is usually through respiratory droplets, which are particles less than 5–10 μm in size, or alternatively, through fecal–oral routes. However, emerging evidence shows that airborne transmission or inhalation of droplet nuclei (aerosols) that may remain suspended in the air for some time, especially in occluded spaces, is possible [3]. Coronaviruses may not cause apparent infection in their original vertebrate hosts, but once they jump to livestock and humans from wildlife, they may cause mild to severe infection, even reaching epidemic and pandemic proportions if unabated [4,5]. Usually, coronaviruses cause mild to severe respiratory infection, kidney failure, enteric, including hepatic disease, and to some extent, neurological involvement depending on the type of coronavirus and animal host.
The world has seen the devastation of different coronaviruses in agriculture and human populations with grave implications for health systems, the food chain, and the economies of countries affected. The very first known coronavirus that was described and isolated in the 1930s is the infectious bronchitis virus (IBV) [6]. Infectious bronchitis virus belongs to the Genus Gammacoronavirus, which primarily infects the respiratory tract of chickens but also shows tissue tropism for other organs such as the kidney, oviducts, testes, and the alimentary tract [7]. Another avian coronavirus is the turkey coronavirus (TCoV) which also belongs to the Genus Gammacoronavirus and can cause gastrointestinal disease in both young poults and adult turkeys [8]. In turkey poults, TCoV may cause mortality, while it can cause stunting and underperformance in terms of meat and egg yield in adult birds [9]. Both IBV and TCoV are imminent threats to the poultry industry worldwide, which captured the attention of scientists early on due to the economic importance of the poultry industry, as reviewed by Cavanagh [9]. Another coronavirus that has devastated the agriculture sector is the porcine epidemic diarrhea virus (PEDV). The first outbreak of PEDV was reported in England in 1971 among growing and fattening pigs that manifested watery diarrhea [10]. Pensaert and de Bouck (1978) reported that a new coronavirus-like particle was detected in the intestinal contents of pigs during an outbreak of diarrhea in four swine breeding farms in Belgium [11]. Their team replicated Koch’s postulate and designated the novel coronavirus as PEDV CV777, which was unique from the other two coronaviruses, transmissible gastroenteritis virus (TGEV) and porcine hemagglutinating encephalomyelitis virus (PHEV), that also infect hogs [12,13]. Although it does not cause diseases in agricultural and livestock animals, murine hepatitis coronavirus (MHCoV) is of interest to scientists since it can be used as a model to study hepatitis, demyelinating diseases such as multiple sclerosis in humans, and possibly other infections caused by other coronaviruses [14].
Humans have not been spared from the ravages of coronaviruses. To date, seven coronaviruses have been found to circulate within the human population. Of these, four of the seven human coronaviruses (HCoV), namely 229E, NL63, OC43, and HKU1, cause annual mild to moderate upper respiratory tract illnesses, as reviewed elsewhere by Hulse [15]. However, the other three remaining known HCoV have grievously affected humans in recent history, and one is still occurring as of the writing of this review. These HCoVs are the severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), all of which cause severe and life-threatening respiratory tract infections, which sometimes lead to the death of the infected individuals [16]. The 2002–2003 SARS epidemic originated in Guangzhou, Guangdong Province, People’s Republic of China, and spread to Hong Kong, Singapore, Taiwan, and Canada, wherein the main suspected animal reservoir host was a civet cat (Paguma larvata) [17]. Another coronavirus that caused an epidemic within this decade is the MERS-CoV, which originated in Saudi Arabia [18]. The 2012 MERS-CoV most probably jumped from bats and dromedary camels to humans [19] and affected several countries in the Middle East, Southeast Asia, East Asia, Europe, North Africa, and North America [20]. Lastly, the most damaging of all the infections caused by a coronavirus in human history is Coronavirus Disease-19, or COVID-19, which has already infected around 328 million people and killed more than 5.5 million people worldwide (as of 19 January 2022). Coronavirus Disease-19 is caused by a novel coronavirus, originally named 2019-nCoV but renamed SARS-CoV-2, which originated in the city of Wuhan, in Hubei province, central China [21,22]. The prime suspected animal reservoirs of SARS-CoV-2 are horseshoe bats (Rhinolopus affinis) or Malayan pangolins (Manis javanica) [23]. Table 1 shows the list of coronaviruses, their animal hosts and host receptors, and the diseases they cause in their host.

Table 1.
Veterinary and medically important coronaviruses, their hosts and host receptors, and diseases they cause.
Countries around the world are implementing non-pharmaceutical control measures such as border restrictions and travel bans, community lockdowns, mass testing, contact tracing, isolating and quarantining exposed and suspected individuals, wearing face masks, respiratory etiquette, hand hygiene, and social distancing in their efforts to control the transmission of SARS-CoV-2 [41]. Additionally, pharmacotherapies such as Remdesivir, Hydroxychloroquine, Lopinavir, and Interferon were also tested under the WHO SOLIDARITY clinical trials, but these regimens showed little or no effect on hospitalized COVID-19 patients [42]. Only recently, the US FDA granted Emergency Use Authorization (EUA) to two oral antiviral agents against COVID-19, Molnupiravir and Paxlovid, which were found to reduce the risk of COVID-19-related hospitalization by 50% and 89%, respectively [43,44]. While the non-pharmaceutical strategies are effective in preventing transmission and antiviral drugs may reduce hospitalization and death among infected individuals, the only way to avert the further health and socioeconomic impacts of the COVID-19 pandemic and establish long-term prevention and control measures is to develop a vaccine and a safe and effective vaccination program [45]. As of 10 December 2020, 214 vaccine candidates were under development for COVID-19 [46]. Of these, 52 were in clinical trials, while the remaining 162 were in pre-clinical evaluation. Several vaccine candidates have completed their Phase III clinical trial, and their manufacturers have obtained Emergency Use Authorization (EUA) from regulatory authorities. Vaccine candidates for coronaviruses and other viral pathogens are developed based on different techniques. One of the strategies in vaccine development against coronaviruses is the use of protein subunits, which may be composed of just one type of viral antigen or structural proteins that have the ability to assemble into repeated arrays, also known as virus-like particles (VLPs), produced through recombinant protein technology in various culture systems. Recombinant protein subunit vaccines are produced by the heterologous expression of an epitope-carrying immunogen protein subunit of a target pathogen in a competent host [47], while VLPs are protein subunits that mimic the structure of authentic virus particles that can be presented to the immune system in a more native conformation in the absence of infectious genetic material [48,49]. The use of recombinant protein technology has expanded beyond therapeutic proteins to vaccine engineering and the development of diagnostic platforms for different diseases due to the flexibility of its expression system and proven safety record. This review briefly discusses the key structural proteins of coronaviruses and highlights the different expression systems currently used to produce these recombinant key structural proteins of coronaviruses and their various applications in the rational design of prophylactic vaccines, diagnostics, and therapeutics.
4. Concluding Remarks
We live in an interconnected ecosystem and in a One Health web where humans, animals, and the environment always interact. As long as viruses continue to evolve and adapt to new hosts, humans will be a receptive species to viral pathogens harbored by wildlife, such as coronaviruses. The preservation of the human species through adaptation and survival against viral ravages may be aided by technologies, including biotechnology, such as the production of biopharmaceuticals through recombinant protein expression systems. Today, there are a variety of expression systems to choose from in the development of vaccines, diagnostics, and therapeutics for diseases caused by coronaviruses. Each has its own advantages and disadvantages; thus, the choice of a competent host cell that will serve as a factory for the desired protein will all depend on the protein that ought to be expressed. However, based on the reports presented in this review, the two most successful platforms in delivering recombinant proteins used as protein subunit vaccines, particularly for COVID-19, are the mammalian expression system and the insect cell/insect expression system, which may be attributed to their good secretion capacity, high product yield, and ability to produce complex proteins and high-quality proteins. As more and more proteins are produced and enter clinical trials for diagnostics, prophylaxis, and therapeutic purposes, recombinant protein technology involving different expression systems also becomes better and more sophisticated, especially in the fight against viral pathogens of global public health importance challenging our time and humanity such as coronaviruses.
Author Contributions
Conceptualization, A.B.S.C. and T.-Y.W.; writing—original draft preparation, A.B.S.C.; writing—review and editing, T.-Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology (MOST), Taiwan, grant number MOST-109-2321-B-033-001.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to express our sincere appreciation to Marco Vignuzzi of Viral Population and Pathogenesis Unit, Institute Pasteur, Paris, France, for his invaluable help in ironing out some English grammar errors in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carstens, E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2009). Arch. Virol. 2010, 155, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Milek, J.; Blicharz-Domanska, K. Coronaviruses in Avian Species—Review with Focus on Epidemiology and Diagnosis in Wild Birds. J. Vet. Res. 2018, 62, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.D.; Wang, Z.Y.; Zhang, S.F.; Li, X.; Li, L.; Li, C.; Cui, Y.; Fu, R.B.; Dong, Y.Z.; Chi, X.Y.; et al. Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020, 26, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Muth, D.; Niemeyer, D.; Drosten, C. Hosts and Sources of Endemic Human Coronaviruses. Adv. Virus Res. 2018, 100, 163–188. [Google Scholar] [CrossRef]
- Ye, Z.W.; Yuan, S.; Yuen, K.S.; Fung, S.Y.; Chan, C.P.; Jin, D.Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020, 16, 1686–1697. [Google Scholar] [CrossRef]
- Cavanagh, D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007, 38, 281–297. [Google Scholar] [CrossRef]
- Raj, G.D.; Jones, R.C. Infectious bronchitis virus: Immunopathogenesis of infection in the chicken. Avian Pathol. 1997, 26, 677–706. [Google Scholar] [CrossRef]
- Ismail, M.; Tang, Y.; Saif, Y. Pathogenicity of turkey coronavirus in turkeys and chickens. Avian Dis. 2003, 47, 515–522. [Google Scholar] [CrossRef][Green Version]
- Cavanagh, D. Coronaviruses in poultry and other birds. Avian Pathol. 2005, 34, 439–448. [Google Scholar] [CrossRef]
- Lee, C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 2015, 12, 193. [Google Scholar] [CrossRef]
- Pensaert, M.; De Bouck, P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef]
- Liu, Q.; Gerdts, V. Transmissible Gastroenteritis Virus of Pigs and Porcine Epidemic Diarrhea Virus (Coronaviridae). Encycl. Virol. 2021, 2, 850–853. [Google Scholar] [CrossRef]
- Mora-Diaz, J.C.; Pineyro, P.E.; Houston, E.; Zimmerman, J.; Gimenez-Lirola, L.G. Porcine Hemagglutinating Encephalomyelitis Virus: A Review. Front. Vet. Sci. 2019, 6, 53. [Google Scholar] [CrossRef]
- Korner, R.W.; Majjouti, M.; Alcazar, M.A.A.; Mahabir, E. Of Mice and Men: The Coronavirus MHV and Mouse Models as a Translational Approach to Understand SARS-CoV-2. Viruses 2020, 12, 880. [Google Scholar] [CrossRef]
- Hulse, J.D. Human coronaviruses: The deadly seven. ACTA Sci. Microbiol. 2020, 3, 86–89. [Google Scholar] [CrossRef]
- Chen, B.; Tian, E.K.; He, B.; Tian, L.; Han, R.; Wang, S.; Xiang, Q.; Zhang, S.; El Arnaout, T.; Cheng, W. Overview of lethal human coronaviruses. Signal Transduct. Target Ther. 2020, 5, 89. [Google Scholar] [CrossRef]
- Anderson, R.M.; Fraser, C.; Ghani, A.C.; Donnelly, C.A.; Riley, S.; Ferguson, N.M.; Leung, G.M.; Lam, T.H.; Hedley, A.J. Epidemiology, transmission dynamics and control of SARS: The 2002–2003 epidemic. Philos. Trans. R Soc. Lond B Biol. Sci. 2004, 359, 1091–1105. [Google Scholar] [CrossRef]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Mohd, H.A.; Al-Tawfiq, J.A.; Memish, Z.A. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) origin and animal reservoir. Virol. J. 2016, 13, 87. [Google Scholar] [CrossRef]
- Al Mutair, A.; Ambani, Z. Narrative review of Middle East respiratory syndrome coronavirus (MERS-CoV) infection: Updates and implications for practice. J. Int. Med. Res. 2020, 48, 300060519858030. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef]
- Liu, C.; Tang, J.; Ma, Y.; Liang, X.; Yang, Y.; Peng, G.; Qi, Q.; Jiang, S.; Li, J.; Du, L.; et al. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J. Virol 2015, 89, 6121–6125. [Google Scholar] [CrossRef] [PubMed]
- Schultze, B.; Krempl, C.; Ballesteros, M.L.; Shaw, L.; Schauer, R.; Enjuanes, L.; Herrler, G. Transmissible gastroenteritis coronavirus, but not the related porcine respiratory coronavirus, has a sialic acid (N-glycolylneuraminic acid) binding activity. J. Virol. 1996, 70, 5634–5637. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Gao, W.; Lu, H.; Zhao, K.; Ding, N.; Liu, W.; Zhao, J.; Lan, Y.; Tang, B.; Jin, Z.; et al. A small region of porcine hemagglutinating encephalomyelitis virus spike protein interacts with the neural cell adhesion molecule. Intervirology 2015, 58, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Dveksler, G.; Pensiero, M.; Cardellichio, C.; Williams, R.; Jiang, G.; Holmes, K.; Dieffenbach, C. Cloning of the mouse hepatitis virus (MHV) receptor: Expression in human and hamster cell lines confers susceptibility to MHV. J. Virol. 1991, 65, 6881–6891. [Google Scholar] [CrossRef] [PubMed]
- Ambepitiya Wickramasinghe, I.N.; de Vries, R.P.; Weerts, E.A.; van Beurden, S.J.; Peng, W.; McBride, R.; Ducatez, M.; Guy, J.; Brown, P.; Eterradossi, N.; et al. Novel Receptor Specificity of Avian Gammacoronaviruses That Cause Enteritis. J. Virol 2015, 89, 8783–8792. [Google Scholar] [CrossRef]
- Promkuntod, N.; van Eijndhoven, R.E.; de Vrieze, G.; Grone, A.; Verheije, M.H. Mapping of the receptor-binding domain and amino acids critical for attachment in the spike protein of avian coronavirus infectious bronchitis virus. Virology 2014, 448, 26–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Buckles, E.; Whittaker, G.R. Expression of the C-type lectins DC-SIGN or L-SIGN alters host cell susceptibility for the avian coronavirus, infectious bronchitis virus. Vet. Microbiol. 2012, 157, 285–293. [Google Scholar] [CrossRef]
- Ji, W.; Peng, Q.; Fang, X.; Li, Z.; Li, Y.; Xu, C.; Zhao, S.; Li, J.; Chen, R.; Mo, G.; et al. Structures of a deltacoronavirus spike protein bound to porcine and human receptors. Nat. Commun. 2022, 13, 1467. [Google Scholar] [CrossRef]
- Li, W.; Hulswit, R.J.G.; Kenney, S.P.; Widjaja, I.; Jung, K.; Alhamo, M.A.; van Dieren, B.; van Kuppeveld, F.J.M.; Saif, L.J.; Bosch, B.J. Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross-species transmissibility. Proc. Natl. Acad. Sci. USA 2018, 115, E5135–E5143. [Google Scholar] [CrossRef]
- Li, Z.; Tomlinson, A.C.; Wong, A.H.; Zhou, D.; Desforges, M.; Talbot, P.J.; Benlekbir, S.; Rubinstein, J.L.; Rini, J.M. The human coronavirus HCoV-229E S-protein structure and receptor binding. eLife 2019, 8, e51230. [Google Scholar] [CrossRef]
- Hofmann, H.; Pyrc, K.; Van Der Hoek, L.; Geier, M.; Berkhout, B.; Pöhlmann, S. Human coronavirus NL63 employs the severe acute respiratory syndrome coronavirus receptor for cellular entry. Proc. Natl. Acad. Sci. USA 2005, 102, 7988–7993. [Google Scholar] [CrossRef]
- Hulswit, R.J.G.; Lang, Y.; Bakkers, M.J.G.; Li, W.; Li, Z.; Schouten, A.; Ophorst, B.; van Kuppeveld, F.J.M.; Boons, G.J.; Bosch, B.J.; et al. Human coronaviruses OC43 and HKU1 bind to 9-O-acetylated sialic acids via a conserved receptor-binding site in spike protein domain A. Proc. Natl. Acad. Sci. USA 2019, 116, 2681–2690. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Li, W.; Hulswit, R.J.G.; Widjaja, I.; Raj, V.S.; McBride, R.; Peng, W.; Widagdo, W.; Tortorici, M.A.; van Dieren, B.; Lang, Y.; et al. Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc. Natl. Acad. Sci. USA 2017, 114, E8508–E8517. [Google Scholar] [CrossRef]
- Raj, V.S.; Mou, H.; Smits, S.L.; Dekkers, D.H.; Muller, M.A.; Dijkman, R.; Muth, D.; Demmers, J.A.; Zaki, A.; Fouchier, R.A.; et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 2013, 495, 251–254. [Google Scholar] [CrossRef]
- Baker, A.N.; Richards, S.J.; Guy, C.S.; Congdon, T.R.; Hasan, M.; Zwetsloot, A.J.; Gallo, A.; Lewandowski, J.R.; Stansfeld, P.J.; Straube, A.; et al. The SARS-COV-2 Spike Protein Binds Sialic Acids and Enables Rapid Detection in a Lateral Flow Point of Care Diagnostic Device. ACS Cent. Sci. 2020, 6, 2046–2052. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Flaxman, S.; Mishra, S.; Gandy, A.; Unwin, H.J.T.; Mellan, T.A.; Coupland, H.; Whittaker, C.; Zhu, H.; Berah, T.; Eaton, J.W.; et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature 2020, 584, 257–261. [Google Scholar] [CrossRef]
- Consortium, W.H.O.S.T.; Pan, H.; Peto, R.; Henao-Restrepo, A.M.; Preziosi, M.P.; Sathiyamoorthy, V.; Abdool Karim, Q.; Alejandria, M.M.; Hernandez Garcia, C.; Kieny, M.P.; et al. Repurposed Antiviral Drugs for Covid-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martin-Quiros, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Pfizer’s Novel. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate#.YnIDOcZfvMY.link<EUA (accessed on 30 December 2021).
- Schaffer DeRoo, S.; Pudalov, N.J.; Fu, L.Y. Planning for a COVID-19 Vaccination Program. JAMA 2020, 323, 2458–2459. [Google Scholar] [CrossRef]
- Novel Coronavirus Landscape COVID-19. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 8 January 2021).
- Hansson, M.; Nygren, P.A.k.; Ståhl, S. Design and production of recombinant subunit vaccines. Biotechnol. Appl. Biochem. 2000, 32, 95–107. [Google Scholar] [CrossRef]
- Noad, R.; Roy, P. Virus-like particles as immunogens. Trends Microbiol. 2003, 11, 438–444. [Google Scholar] [CrossRef]
- Grgacic, E.V.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.; Netland, J. Coronaviruses post-SARS: Update on replication and pathogenesis. Nat. Rev. Microbiol. 2009, 7, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Enjuanes, L.; Ziebuhr, J.; Snijder, E.J. Nidovirales: Evolving the largest RNA virus genome. Virus Res. 2006, 117, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Tok, T.T.; Tatar, G. Structures and functions of coronavirus proteins: Molecular modeling of viral nucleoprotein. Int. J. Virol. Infect. Dis. 2017, 2, 001–007. [Google Scholar]
- Woo, P.C.; Huang, Y.; Lau, S.K.; Yuen, K.Y. Coronavirus genomics and bioinformatics analysis. Viruses 2010, 2, 1804–1820. [Google Scholar] [CrossRef]
- Ziebuhr, J. The coronavirus replicase. Curr. Top. Microbiol. Immunol. 2005, 287, 57–94. [Google Scholar]
- Bárcena, M.; Oostergetel, G.T.; Bartelink, W.; Faas, F.G.; Verkleij, A.; Rottier, P.J.; Koster, A.J.; Bosch, B.J. Cryo-electron tomography of mouse hepatitis virus: Insights into the structure of the coronavirion. Proc. Natl. Acad. Sci. USA 2009, 106, 582–587. [Google Scholar] [CrossRef]
- Song, H.C.; Seo, M.Y.; Stadler, K.; Yoo, B.J.; Choo, Q.L.; Coates, S.R.; Uematsu, Y.; Harada, T.; Greer, C.E.; Polo, J.M.; et al. Synthesis and characterization of a native, oligomeric form of recombinant severe acute respiratory syndrome coronavirus spike glycoprotein. J. Virol. 2004, 78, 10328–10335. [Google Scholar] [CrossRef]
- Beniac, D.R.; Andonov, A.; Grudeski, E.; Booth, T.F. Architecture of the SARS coronavirus prefusion spike. Nat. Struct. Mol. Biol. 2006, 13, 751–752. [Google Scholar] [CrossRef]
- Millet, J.K.; Jaimes, J.A.; Whittaker, G.R. Molecular diversity of coronavirus host cell entry receptors. FEMS Microbiol. Rev. 2021, 45, fuaa057. [Google Scholar] [CrossRef]
- Li, F. Receptor recognition mechanisms of coronaviruses: A decade of structural studies. J. Virol. 2015, 89, 1954–1964. [Google Scholar] [CrossRef]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, M.L.; Chien, C.S.; Yarmishyn, A.A.; Yang, Y.P.; Lai, W.Y.; Luo, Y.H.; Lin, Y.T.; Chen, Y.J.; Chang, P.C.; et al. Highlight of Immune Pathogenic Response and Hematopathologic Effect in SARS-CoV, MERS-CoV, and SARS-Cov-2 Infection. Front. Immunol. 2020, 11, 1022. [Google Scholar] [CrossRef]
- Lu, G.; Wang, Q.; Gao, G.F. Bat-to-human: Spike features determining ‘host jump’ of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015, 23, 468–478. [Google Scholar] [CrossRef]
- De Haan, C.A.; Kuo, L.; Masters, P.S.; Vennema, H.; Rottier, P.J. Coronavirus particle assembly: Primary structure requirements of the membrane protein. J. Virol. 1998, 72, 6838–6850. [Google Scholar] [CrossRef]
- Neuman, B.W.; Kiss, G.; Kunding, A.H.; Bhella, D.; Baksh, M.F.; Connelly, S.; Droese, B.; Klaus, J.P.; Makino, S.; Sawicki, S.G.; et al. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011, 174, 11–22. [Google Scholar] [CrossRef]
- Niemann, H.; Geyer, R.; Klenk, H.; Linder, D.; Stirm, S.; Wirth, M. The carbohydrates of mouse hepatitis virus (MHV) A59: Structures of the O-glycosidically linked oligosaccharides of glycoprotein E1. EMBO J. 1984, 3, 665–670. [Google Scholar] [CrossRef]
- Holmes, K.V.; Doller, E.W.; Sturman, L.S. Tunicamycin resistant glycosylation of a coronavirus glycoprotein: Demonstration of a novel type of viral glycoprotein. Virology 1981, 115, 334–344. [Google Scholar] [CrossRef]
- Wissink, E.H.J.; Kroese, M.V.; Maneschijn-Bonsing, J.G.; Meulenberg, J.J.M.; van Rijn, P.A.; Rijsewijk, F.A.M.; Rottier, P.J.M. Significance of the oligosaccharides of the porcine reproductive and respiratory syndrome virus glycoproteins GP2a and GP5 for infectious virus production. J. Gen. Virol. 2004, 85, 3715–3723. [Google Scholar] [CrossRef] [PubMed]
- de Haan, C.A.M.; de Wit, M.; Kuo, L.; Montalto-Morrison, C.; Haagmans, B.L.; Weiss, S.R.; Masters, P.S.; Rottier, P.J.M. The glycosylation status of the murine hepatitis coronavirus M protein affects the interferogenic capacity of the virus in vitro and its ability to replicate in the liver but not the brain. Virology 2003, 312, 395–406. [Google Scholar] [CrossRef]
- Narayanan, K.; Makino, S. Characterization of nucleocapsid-M protein interaction in murine coronavirus. Adv. Exp. Med. Biol. 2001, 494, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Cagliani, R.; Forni, D.; Clerici, M.; Sironi, M. Computational Inference of Selection Underlying the Evolution of the Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2. J. Virol. 2020, 94, e00411-20. [Google Scholar] [CrossRef]
- Hu, Y.; Wen, J.; Tang, L.; Zhang, H.; Zhang, X.; Li, Y.; Wang, J.; Han, Y.; Li, G.; Shi, J.; et al. The M Protein of SARS-CoV: Basic Structural and Immunological Properties. Genom. Proteom. Bioinform. 2003, 1, 118–130. [Google Scholar] [CrossRef]
- Raamsman, M.J.; Locker, J.K.; De Hooge, A.; De Vries, A.A.; Griffiths, G.; Vennema, H.; Rottier, P.J. Characterization of the coronavirus mouse hepatitis virus strain A59 small membrane protein E. J. Virol. 2000, 74, 2333–2342. [Google Scholar] [CrossRef]
- Thiel, V.; Siddell, S.G. Internal ribosome entry in the coding region of murine hepatitis virus mRNA 5. J. Gen. Virol. 1994, 75, 3041–3046. [Google Scholar] [CrossRef]
- Ruch, T.R.; Machamer, C.E. The coronavirus E protein: Assembly and beyond. Viruses 2012, 4, 363–382. [Google Scholar] [CrossRef]
- DeDiego, M.L.; Nieto-Torres, J.L.; Jimenez-Guardeno, J.M.; Regla-Nava, J.A.; Alvarez, E.; Oliveros, J.C.; Zhao, J.; Fett, C.; Perlman, S.; Enjuanes, L. Severe acute respiratory syndrome coronavirus envelope protein regulates cell stress response and apoptosis. PLoS Pathog. 2011, 7, e1002315. [Google Scholar] [CrossRef]
- Grunewald, M.E.; Fehr, A.R.; Athmer, J.; Perlman, S. The coronavirus nucleocapsid protein is ADP-ribosylated. Virology 2018, 517, 62–68. [Google Scholar] [CrossRef]
- Zuwala, K.; Golda, A.; Kabala, W.; Burmistrz, M.; Zdzalik, M.; Nowak, P.; Kedracka-Krok, S.; Zarebski, M.; Dobrucki, J.; Florek, D.; et al. The nucleocapsid protein of human coronavirus NL63. PLoS ONE 2015, 10, e0117833. [Google Scholar] [CrossRef]
- Stertz, S.; Reichelt, M.; Spiegel, M.; Kuri, T.; Martinez-Sobrido, L.; Garcia-Sastre, A.; Weber, F.; Kochs, G. The intracellular sites of early replication and budding of SARS-coronavirus. Virology 2007, 361, 304–315. [Google Scholar] [CrossRef]
- McBride, R.; van Zyl, M.; Fielding, B.C. The coronavirus nucleocapsid is a multifunctional protein. Viruses 2014, 6, 2991–3018. [Google Scholar] [CrossRef]
- Chen, Z.; Pei, D.; Jiang, L.; Song, Y.; Wang, J.; Wang, H.; Zhou, D.; Zhai, J.; Du, Z.; Li, B.; et al. Antigenicity analysis of different regions of the severe acute respiratory syndrome coronavirus nucleocapsid protein. Clin. Chem. 2004, 50, 988–995. [Google Scholar] [CrossRef]
- Hu, H.; Tao, L.; Wang, Y.; Chen, L.; Yang, J.; Wang, H. Enhancing immune responses against SARS-CoV nucleocapsid DNA vaccine by co-inoculating interleukin-2 expressing vector in mice. Biotechnol. Lett. 2009, 31, 1685–1693. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Tsoi, H.W.; Chen, Z.W.; Wong, B.H.; Zhang, L.; Chan, J.K.; Wong, L.P.; He, W.; Ma, C.; et al. SARS coronavirus spike polypeptide DNA vaccine priming with recombinant spike polypeptide from Escherichia coli as booster induces high titer of neutralizing antibody against SARS coronavirus. Vaccine 2005, 23, 4959–4968. [Google Scholar] [CrossRef]
- Wang, M.; Fu, T.; Hao, J.; Li, L.; Tian, M.; Jin, N.; Ren, L.; Li, C. A recombinant Lactobacillus plantarum strain expressing the spike protein of SARS-CoV-2. Int. J. Biol. Macromol. 2020, 160, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.M.; Al-Amri, S.S.; Al-Subhi, T.L.; Siddiq, L.A.; Hassan, A.M.; Alawi, M.M.; Alhabbab, R.Y.; Hindawi, S.I.; Mohammed, O.B.; Amor, N.S.; et al. Development and validation of different indirect ELISAs for MERS-CoV serological testing. J. Immunol. Methods 2019, 466, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, E.G.; Miao, C.; Haupt, T.E.; Anderson, L.J.; Haynes, L.M. Development of a recombinant truncated nucleocapsid protein based immunoassay for detection of antibodies against human coronavirus OC43. J. Virol. Methods 2011, 177, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Lee, B.H.; Seok, S.H.; Baek, M.W.; Lee, H.Y.; Kim, D.J.; Na, Y.R.; Noh, K.J.; Park, S.H.; Kumar, D.N.; et al. Production of specific antibodies against SARS-coronavirus nucleocapsid protein without cross reactivity with human coronaviruses 229E and OC43. J. Vet. Sci. 2010, 11, 165–167. [Google Scholar] [CrossRef]
- Zou, N.; Wang, F.; Duan, Z.; Xia, J.; Wen, X.; Yan, Q.; Liu, P.; Cao, S.; Huang, Y. Development and characterization of neutralizing monoclonal antibodies against the S1 subunit protein of QX-like avian infectious bronchitis virus strain Sczy3. Monoclon. Antib. Immunodiagn. Immunother. 2015, 34, 17–24. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Wei, F.; Dong, Y.; Zhu, L.; Han, W.; Wang, L.; Shen, Z. Prokaryotic Expression of Truncated S1 Protein of Porcine Epidemic Diarrhea Virus and Production of Monoclonal Antibodies to Recombinant Protein. Monoclon. Antib. Immunodiagn. Immunother. 2015, 34, 327–333. [Google Scholar] [CrossRef]
- Luo, S.X.; Fan, J.H.; Opriessnig, T.; Di, J.M.; Liu, B.J.; Zuo, Y.Z. Development and application of a recombinant M protein-based indirect ELISA for the detection of porcine deltacoronavirus IgG antibodies. J. Virol. Methods 2017, 249, 76–78. [Google Scholar] [CrossRef]
- Elia, G.; Fiermonte, G.; Pratelli, A.; Martella, V.; Camero, M.; Cirone, F.; Buonavoglia, C. Recombinant M protein-based ELISA test for detection of antibodies to canine coronavirus. J. Virol. Methods 2003, 109, 139–142. [Google Scholar] [CrossRef]
- Su, M.; Li, C.; Guo, D.; Wei, S.; Wang, X.; Geng, Y.; Yao, S.; Gao, J.; Wang, E.; Zhao, X.; et al. A recombinant nucleocapsid protein-based indirect enzyme-linked immunosorbent assay to detect antibodies against porcine deltacoronavirus. J. Vet. Med. Sci. 2016, 78, 601–606. [Google Scholar] [CrossRef]
- Finger, P.F.; Pepe, M.S.; Dummer, L.A.; Magalhaes, C.G.; de Castro, C.C.; de Oliveira Hubner, S.; Leite, F.P.L.; Ritterbusch, G.A.; Esteves, P.A.; Conceicao, F.R. Combined use of ELISA and Western blot with recombinant N protein is a powerful tool for the immunodiagnosis of avian infectious bronchitis. Virol. J. 2018, 15, 189. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Kamble, N.M.; Pillai, A.S.; Gaikwad, S.S.; Khulape, S.A.; Reddy, M.R.; Mohan, C.M.; Kataria, J.M.; Dey, S. Recombinant nucleocapsid protein based single serum dilution ELISA for the detection of antibodies to infectious bronchitis virus in poultry. J. Virol. Methods 2014, 209, 1–6. [Google Scholar] [CrossRef]
- Abdelwahab, M.; Loa, C.C.; Wu, C.C.; Lin, T.L. Recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay for detection of antibody to turkey coronavirus. J. Virol. Methods 2015, 217, 36–41. [Google Scholar] [CrossRef]
- Chen, W.H.; Du, L.; Chag, S.M.; Ma, C.; Tricoche, N.; Tao, X.; Seid, C.A.; Hudspeth, E.M.; Lustigman, S.; Tseng, C.T.; et al. Yeast-expressed recombinant protein of the receptor-binding domain in SARS-CoV spike protein with deglycosylated forms as a SARS vaccine candidate. Hum. Vaccin. Immunother. 2014, 10, 648–658. [Google Scholar] [CrossRef]
- Chen, W.H.; Chag, S.M.; Poongavanam, M.V.; Biter, A.B.; Ewere, E.A.; Rezende, W.; Seid, C.A.; Hudspeth, E.M.; Pollet, J.; McAtee, C.P.; et al. Optimization of the Production Process and Characterization of the Yeast-Expressed SARS-CoV Recombinant Receptor-Binding Domain (RBD219-N1), a SARS Vaccine Candidate. J. Pharm. Sci. 2017, 106, 1961–1970. [Google Scholar] [CrossRef]
- Chuck, C.P.; Wong, C.H.; Chow, L.M.; Fung, K.P.; Waye, M.M.; Tsui, S.K. Expression of SARS-coronavirus spike glycoprotein in Pichia pastoris. Virus Genes 2009, 38, 1–9. [Google Scholar] [CrossRef]
- Liu, R.-S.; Yang, K.-Y.; Lin, J.; Lin, Y.-W.; Zhang, Z.-H.; Zhang, J.; Xia, N.-S. High-yield expression of recombinant SARS coronavirus nucleocapsid protein in methylotrophic yeast Pichia pastoris. World J. Gastroenterol. WJG 2004, 10, 3602. [Google Scholar] [CrossRef]
- Pogrebnyak, N.; Golovkin, M.; Andrianov, V.; Spitsin, S.; Smirnov, Y.; Egolf, R.; Koprowski, H. Severe acute respiratory syndrome (SARS) S protein production in plants: Development of recombinant vaccine. Proc. Natl. Acad. Sci. USA 2005, 102, 9062–9067. [Google Scholar] [CrossRef]
- Li, H.-Y.; Ramalingam, S.; Chye, M.-L. Accumulation of recombinant SARS-CoV spike protein in plant cytosol and chloroplasts indicate potential for development of plant-derived oral vaccines. Exp. Biol. Med. 2006, 231, 1346–1352. [Google Scholar] [CrossRef]
- Zheng, N.; Xia, R.; Yang, C.; Yin, B.; Li, Y.; Duan, C.; Liang, L.; Guo, H.; Xie, Q. Boosted expression of the SARS-CoV nucleocapsid protein in tobacco and its immunogenicity in mice. Vaccine 2009, 27, 5001–5007. [Google Scholar] [CrossRef]
- Gomez, N.; Wigdorovitz, A.; Castanon, S.; Gil, F.; Ordá, R.; Borca, M.; Escribano, J. Oral immunogenicity of the plant derived spike protein from swine-transmissible gastroenteritis coronavirus. Arch. Virol. 2000, 145, 1725–1732. [Google Scholar] [CrossRef]
- Tien, N.Q.; Huy, N.X.; Kim, M.Y. Improved expression of porcine epidemic diarrhea antigen by fusion with cholera toxin B subunit and chloroplast transformation in Nicotiana tabacum. Plant Cell Tissue Organ Cult. 2019, 137, 213–223. [Google Scholar] [CrossRef]
- Demurtas, O.C.; Massa, S.; Illiano, E.; De Martinis, D.; Chan, P.K.; Di Bonito, P.; Franconi, R. Antigen Production in Plant to Tackle Infectious Diseases Flare Up: The Case of SARS. Front. Plant Sci. 2016, 7, 54. [Google Scholar] [CrossRef]
- Tai, W.; He, L.; Zhang, X.; Pu, J.; Voronin, D.; Jiang, S.; Zhou, Y.; Du, L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: Implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020, 17, 613–620. [Google Scholar] [CrossRef]
- Tai, W.; Wang, Y.; Fett, C.A.; Zhao, G.; Li, F.; Perlman, S.; Jiang, S.; Zhou, Y.; Du, L. Recombinant Receptor-Binding Domains of Multiple Middle East Respiratory Syndrome Coronaviruses (MERS-CoVs) Induce Cross-Neutralizing Antibodies against Divergent Human and Camel MERS-CoVs and Antibody Escape Mutants. J. Virol. 2017, 91, e01651-16. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Erdos, G.; Huang, S.; Kenniston, T.W.; Balmert, S.C.; Carey, C.D.; Raj, V.S.; Epperly, M.W.; Klimstra, W.B.; Haagmans, B.L.; et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine 2020, 55, 102743. [Google Scholar] [CrossRef] [PubMed]
- Elshabrawy, H.A.; Coughlin, M.M.; Baker, S.C.; Prabhakar, B.S. Human monoclonal antibodies against highly conserved HR1 and HR2 domains of the SARS-CoV spike protein are more broadly neutralizing. PLoS ONE 2012, 7, e50366. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Shi, M.; Li, J.; Song, P.; Li, N. Construction of SARS-CoV-2 Virus-Like Particles by Mammalian Expression System. Front. Bioeng. Biotechnol. 2020, 8, 862. [Google Scholar] [CrossRef]
- Kuo, T.Y.; Lin, M.Y.; Coffman, R.L.; Campbell, J.D.; Traquina, P.; Lin, Y.J.; Liu, L.T.; Cheng, J.; Wu, Y.C.; Wu, C.C.; et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020, 10, 20085. [Google Scholar] [CrossRef]
- Hsieh, S.M.; Liu, W.D.; Huang, Y.S.; Lin, Y.J.; Hsieh, E.F.; Lian, W.C.; Chen, C.; Janssen, R.; Shih, S.R.; Huang, C.G.; et al. Safety and immunogenicity of a Recombinant Stabilized Prefusion SARS-CoV-2 Spike Protein Vaccine (MVC-COV1901) Adjuvanted with CpG 1018 and Aluminum Hydroxide in healthy adults: A Phase 1, dose-escalation study. EClinicalMedicine 2021, 38, 100989. [Google Scholar] [CrossRef]
- Thachil, A.; Gerber, P.F.; Xiao, C.T.; Huang, Y.W.; Opriessnig, T. Development and application of an ELISA for the detection of porcine deltacoronavirus IgG antibodies. PLoS ONE 2015, 10, e0124363. [Google Scholar] [CrossRef]
- Esposito, D.; Mehalko, J.; Drew, M.; Snead, K.; Wall, V.; Taylor, T.; Frank, P.; Denson, J.P.; Hong, M.; Gulten, G.; et al. Optimizing high-yield production of SARS-CoV-2 soluble spike trimers for serology assays. Protein Expr. Purif. 2020, 174, 105686. [Google Scholar] [CrossRef]
- Mehalko, J.; Drew, M.; Snead, K.; Denson, J.P.; Wall, V.; Taylor, T.; Sadtler, K.; Messing, S.; Gillette, W.; Esposito, D. Improved production of SARS-CoV-2 spike receptor-binding domain (RBD) for serology assays. Protein Expr. Purif. 2021, 179, 105802. [Google Scholar] [CrossRef]
- Siu, Y.L.; Teoh, K.T.; Lo, J.; Chan, C.M.; Kien, F.; Escriou, N.; Tsao, S.W.; Nicholls, J.M.; Altmeyer, R.; Peiris, J.S.; et al. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008, 82, 11318–11330. [Google Scholar] [CrossRef]
- Li, J.; Ulitzky, L.; Silberstein, E.; Taylor, D.R.; Viscidi, R. Immunogenicity and protection efficacy of monomeric and trimeric recombinant SARS coronavirus spike protein subunit vaccine candidates. Viral. Immunol. 2013, 26, 126–132. [Google Scholar] [CrossRef]
- Coleman, C.M.; Liu, Y.V.; Mu, H.; Taylor, J.K.; Massare, M.; Flyer, D.C.; Smith, G.E.; Frieman, M.B. Purified coronavirus spike protein nanoparticles induce coronavirus neutralizing antibodies in mice. Vaccine 2014, 32, 3169–3174. [Google Scholar] [CrossRef]
- Fujita, R.; Hino, M.; Ebihara, T.; Nagasato, T.; Masuda, A.; Lee, J.M.; Fujii, T.; Mon, H.; Kakino, K.; Nagai, R.; et al. Efficient production of recombinant SARS-CoV-2 spike protein using the baculovirus-silkworm system. Biochem. Biophys. Res. Commun. 2020, 529, 257–262. [Google Scholar] [CrossRef]
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef]
- Keech, C.; Glenn, G.M.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; et al. First-in-Human Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef]
- Hsu, C.W.; Chang, M.H.; Chang, H.W.; Wu, T.Y.; Chang, Y.C. Parenterally Administered Porcine Epidemic Diarrhea Virus-Like Particle-Based Vaccine Formulated with CCL25/28 Chemokines Induces Systemic and Mucosal Immune Protectivity in Pigs. Viruses 2020, 12, 1122. [Google Scholar] [CrossRef]
- Yilmaz, H.; Faburay, B.; Turan, N.; Cotton-Caballero, M.; Cetinkaya, B.; Gurel, A.; Yilmaz, A.; Cizmecigil, U.Y.; Aydin, O.; Tarakci, E.A.; et al. Production of Recombinant N Protein of Infectious Bronchitis Virus Using the Baculovirus Expression System and Its Assessment as a Diagnostic Antigen. Appl. Biochem. Biotechnol. 2019, 187, 506–517. [Google Scholar] [CrossRef]
- Severance, E.G.; Bossis, I.; Dickerson, F.B.; Stallings, C.R.; Origoni, A.E.; Sullens, A.; Yolken, R.H.; Viscidi, R.P. Development of a nucleocapsid-based human coronavirus immunoassay and estimates of individuals exposed to coronavirus in a U.S. metropolitan population. Clin. Vaccine Immunol. 2008, 15, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Takami, Y.; Kumar Deo, V.; Park, E.Y. Preparation of virus-like particle mimetic nanovesicles displaying the S protein of Middle East respiratory syndrome coronavirus using insect cells. J. Biotechnol. 2019, 306, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ko, H.L.; Lee, E.Y.; Park, H.J.; Kim, Y.S.; Kim, Y.S.; Cho, N.H.; Park, M.S.; Lee, S.M.; Kim, J.; et al. Development of a diagnostic system for detection of specific antibodies and antigens against Middle East respiratory syndrome coronavirus. Microbiol. Immunol. 2018, 62, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wan, Y.; Liu, C.; Yount, B.; Gully, K.; Yang, Y.; Auerbach, A.; Peng, G.; Baric, R.; Li, F. Structure of mouse coronavirus spike protein complexed with receptor reveals mechanism for viral entry. PLoS Pathog. 2020, 16, e1008392. [Google Scholar] [CrossRef]
- Rosano, G.L.; Ceccarelli, E.A. Recombinant protein expression in Escherichia coli: Advances and challenges. Front. Microbiol. 2014, 5, 172. [Google Scholar] [CrossRef]
- Cohen, S.N.; Chang, A.C.; Boyer, H.W.; Helling, R.B. Construction of biologically functional bacterial plasmids in vitro. Proc. Natl. Acad. Sci. USA 1973, 70, 3240–3244. [Google Scholar] [CrossRef]
- Ouranidis, A.; Davidopoulou, C.; Tashi, R.-K.; Kachrimanis, K. Pharma 4.0 Continuous MRNA Drug Products Manufacturing. Pharmaceutics 2021, 13, 1371. [Google Scholar] [CrossRef]
- Baeshen, N.A.; Baeshen, M.N.; Sheikh, A.; Bora, R.S.; Ahmed, M.M.M.; Ramadan, H.A.; Saini, K.S.; Redwan, E.M. Cell factories for insulin production. Microb. Cell Factories 2014, 13, 1–9. [Google Scholar] [CrossRef]
- Krzeslak, J.; Braun, P.; Voulhoux, R.; Cool, R.H.; Quax, W.J. Heterologous production of Escherichia coli penicillin G acylase in Pseudomonas aeruginosa. J. Biotechnol. 2009, 142, 250–258. [Google Scholar] [CrossRef]
- Schleicher, L.; Muras, V.; Claussen, B.; Pfannstiel, J.; Blombach, B.; Dibrov, P.; Fritz, G.; Steuber, J. Vibrio natriegens as Host for Expression of Multisubunit Membrane Protein Complexes. Front. Microbiol. 2018, 9, 2537. [Google Scholar] [CrossRef]
- Chen, R. Bacterial expression systems for recombinant protein production: E. coli and beyond. Biotechnol. Adv. 2012, 30, 1102–1107. [Google Scholar] [CrossRef]
- Cai, D.; Rao, Y.; Zhan, Y.; Wang, Q.; Chen, S. Engineering Bacillus for efficient production of heterologous protein: Current progress, challenge and prospect. J. Appl. Microbiol. 2019, 126, 1632–1642. [Google Scholar] [CrossRef]
- Cui, W.; Han, L.; Suo, F.; Liu, Z.; Zhou, L.; Zhou, Z. Exploitation of Bacillus subtilis as a robust workhorse for production of heterologous proteins and beyond. World J. Microbiol. Biotechnol. 2018, 34, 145. [Google Scholar] [CrossRef]
- Morello, E.; Bermudez-Humaran, L.G.; Llull, D.; Sole, V.; Miraglio, N.; Langella, P.; Poquet, I. Lactococcus lactis, an efficient cell factory for recombinant protein production and secretion. J. Mol. Microbiol. Biotechnol. 2008, 14, 48–58. [Google Scholar] [CrossRef]
- Yzturk, S.; Yalik, P.; Yzdamar, T.H. Fed-Batch Biomolecule Production by Bacillus subtilis: A State of the Art Review. Trends Biotechnol. 2016, 34, 329–345. [Google Scholar] [CrossRef]
- Kang, Z.; Yang, S.; Du, G.; Chen, J. Molecular engineering of secretory machinery components for high-level secretion of proteins in Bacillus species. J. Ind. Microbiol. Biotechnol. 2014, 41, 1599–1607. [Google Scholar] [CrossRef]
- Goffeau, A.; Barrell, B.G.; Bussey, H.; Davis, R.W.; Dujon, B.; Feldmann, H.; Galibert, F.; Hoheisel, J.D.; Jacq, C.; Johnston, M. Life with 6000 genes. Science 1996, 274, 546–567. [Google Scholar] [CrossRef]
- Nielsen, J. Production of biopharmaceutical proteins by yeast: Advances through metabolic engineering. Bioengineered 2013, 4, 207–211. [Google Scholar] [CrossRef]
- Baghban, R.; Farajnia, S.; Rajabibazl, M.; Ghasemi, Y.; Mafi, A.; Hoseinpoor, R.; Rahbarnia, L.; Aria, M. Yeast Expression Systems: Overview and Recent Advances. Mol. Biotechnol. 2019, 61, 365–384. [Google Scholar] [CrossRef]
- Vieira Gomes, A.M.; Souza Carmo, T.; Silva Carvalho, L.; Mendonca Bahia, F.; Parachin, N.S. Comparison of Yeasts as Hosts for Recombinant Protein Production. Microorganisms 2018, 6, 38. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, S.J.; Kang, H.A. Yeast synthetic biology for the production of recombinant therapeutic proteins. FEMS Yeast Res. 2015, 15, 1–16. [Google Scholar] [CrossRef]
- Ogata, K.; Nishikawa, H.; Ohsugi, M. A Yeast Capable of Utilizing Methanol. Agric. Biol. Chem. 2014, 33, 1519–1520. [Google Scholar] [CrossRef]
- Cereghino, J.L.; Cregg, J.M. Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol. Rev. 2000, 24, 45–66. [Google Scholar] [CrossRef]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia pastoris: A highly successful expression system for optimal synthesis of heterologous proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef]
- Gasser, B.; Mattanovich, D. A yeast for all seasons—Is Pichia pastoris a suitable chassis organism for future bioproduction? FEMS Microbiol. Lett. 2018, 365, fny181. [Google Scholar] [CrossRef]
- Tran, A.M.; Nguyen, T.T.; Nguyen, C.T.; Huynh-Thi, X.M.; Nguyen, C.T.; Trinh, M.T.; Tran, L.T.; Cartwright, S.P.; Bill, R.M.; Tran-Van, H. Pichia pastoris versus Saccharomyces cerevisiae: A case study on the recombinant production of human granulocyte-macrophage colony-stimulating factor. BMC Res. Notes 2017, 10, 148. [Google Scholar] [CrossRef]
- Chen, W.H.; Hotez, P.J.; Bottazzi, M.E. Potential for developing a SARS-CoV receptor-binding domain (RBD) recombinant protein as a heterologous human vaccine against coronavirus infectious disease (COVID)-19. Hum. Vaccin Immunother. 2020, 16, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Barta, A.; Sommergruber, K.; Thompson, D.; Hartmuth, K.; Matzke, M.A.; Matzke, A.J. The expression of a nopaline synthase—human growth hormone chimaeric gene in transformed tobacco and sunflower callus tissue. Plant Mol. Biol. 1986, 6, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E.P. Plant-based vaccines against viruses. Virol. J. 2014, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sijmons, P.C.; Dekker, B.M.; Schrammeijer, B.; Verwoerd, T.C.; Van Den Elzen, P.J.; Hoekema, A. Production of correctly processed human serum albumin in transgenic plants. Bio/Technology 1990, 8, 217–221. [Google Scholar] [CrossRef]
- Mason, H.S.; Lam, D.; Arntzen, C.J. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. USA 1992, 89, 11745–11749. [Google Scholar] [CrossRef]
- Usha, R.; Rohll, J.B.; Spall, V.E.; Shanks, M.; Maule, A.J.; Johnson, J.E.; Lomonossoff, G.P. Expression of an animal virus antigenic site on the surface of a plant virus particle. Virology 1993, 197, 366–374. [Google Scholar] [CrossRef]
- Shanmugaraj, B.; Malla, A.; Phoolcharoen, W. Emergence of Novel Coronavirus 2019-nCoV: Need for Rapid Vaccine and Biologics Development. Pathogens 2020, 9, 148. [Google Scholar] [CrossRef]
- Moon, K.B.; Park, J.S.; Park, Y.I.; Song, I.J.; Lee, H.J.; Cho, H.S.; Jeon, J.H.; Kim, H.S. Development of Systems for the Production of Plant-Derived Biopharmaceuticals. Plants 2019, 9, 30. [Google Scholar] [CrossRef]
- Scherer, W.F.; Syverton, J.T.; Gey, G.O. Studies on the propagation in vitro of poliomyelitis viruses: IV. Viral. multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J. Exp. Med. 1953, 97, 695–710. [Google Scholar] [CrossRef]
- Dumont, J.; Euwart, D.; Mei, B.; Estes, S.; Kshirsagar, R. Human cell lines for biopharmaceutical manufacturing: History, status, and future perspectives. Crit. Rev. Biotechnol. 2016, 36, 1110–1122. [Google Scholar] [CrossRef]
- Pellicer, A.; Wigler, M.; Axel, R.; Silverstein, S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell 1978, 14, 133–141. [Google Scholar] [CrossRef]
- Hacker, D.L.; Wurn, F.M. Protein Production in Mammalian Cells. eLS 2007, 1–5. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef]
- Owczarek, B.; Gerszberg, A.; Hnatuszko-Konka, K. A Brief Reminder of Systems of Production and Chromatography-Based Recovery of Recombinant Protein Biopharmaceuticals. Biomed. Res. Int. 2019, 2019, 4216060. [Google Scholar] [CrossRef]
- Khan, K.H. Gene expression in Mammalian cells and its applications. Adv. Pharm. Bull. 2013, 3, 257–263. [Google Scholar] [CrossRef]
- Harrison, R.L.; Herniou, E.A.; Jehle, J.A.; Theilmann, D.A.; Burand, J.P.; Becnel, J.J.; Krell, P.J.; van Oers, M.M.; Mowery, J.D.; Bauchan, G.R.; et al. ICTV Virus Taxonomy Profile: Baculoviridae. J. Gen. Virol. 2018, 99, 1185–1186. [Google Scholar] [CrossRef]
- Ikeda, M.; Hamajima, R.; Kobayashi, M. Baculoviruses: Diversity, evolution and manipulation of insects. Entomol. Sci. 2015, 18, 1–20. [Google Scholar] [CrossRef]
- Kost, T.A.; Condreay, J.P.; Jarvis, D.L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 2005, 23, 567–575. [Google Scholar] [CrossRef]
- Summers, M.D. Milestones Leading to the Genetic Engineering of Baculoviruses as Expression Vector Systems and Viral. Pesticides. Insect Viruses Biotechnol. Appl. 2006, 68, 3–73. [Google Scholar]
- Smith, G.E.; Summers, M.D.; Fraser, M. Production of human beta interferon in insect cells infected with a baculovirus expression vector. Mol. Cell. Biol. 1983, 3, 2156–2165. [Google Scholar] [PubMed]
- van Oers, M.M.; Pijlman, G.P.; Vlak, J.M. Thirty years of baculovirus-insect cell protein expression: From dark horse to mainstream technology. J. Gen. Virol. 2015, 96, 6–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-J.; Jinn, T.R.; Chen, Y.-J.; Deng, M.-C.; Hwang, C.-S.; Hsieh, F.-C.; Kao, S.-S.; Chen, Y.-J.; Tung, K.-L.; Wu, T.Y.; et al. Vaccination with hemagglutinin produced in Trichoplusia ni larvae protects chickens against lethal H5N1 challenge. J. Taiwan Inst. Chem. Eng. 2011, 42, 223–227. [Google Scholar] [CrossRef]
- Ahrens, U.; Kaden, V.; Drexler, C.; Visser, N. Efficacy of the classical swine fever (CSF) marker vaccine Porcilis® Pesti in pregnant sows. Vet. Microbiol. 2000, 77, 83–97. [Google Scholar] [CrossRef]
- Harper, D.M. Impact of vaccination with Cervarix (trade mark) on subsequent HPV-16/18 infection and cervical disease in women 15-25 years of age. Gynecol. Oncol. 2008, 110, S11–S17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).