Design and Economic Evaluation of a Hybrid Membrane Separation Process from Multiple Refinery Gases Using a Graphic Synthesis Method
Abstract
:1. Introduction
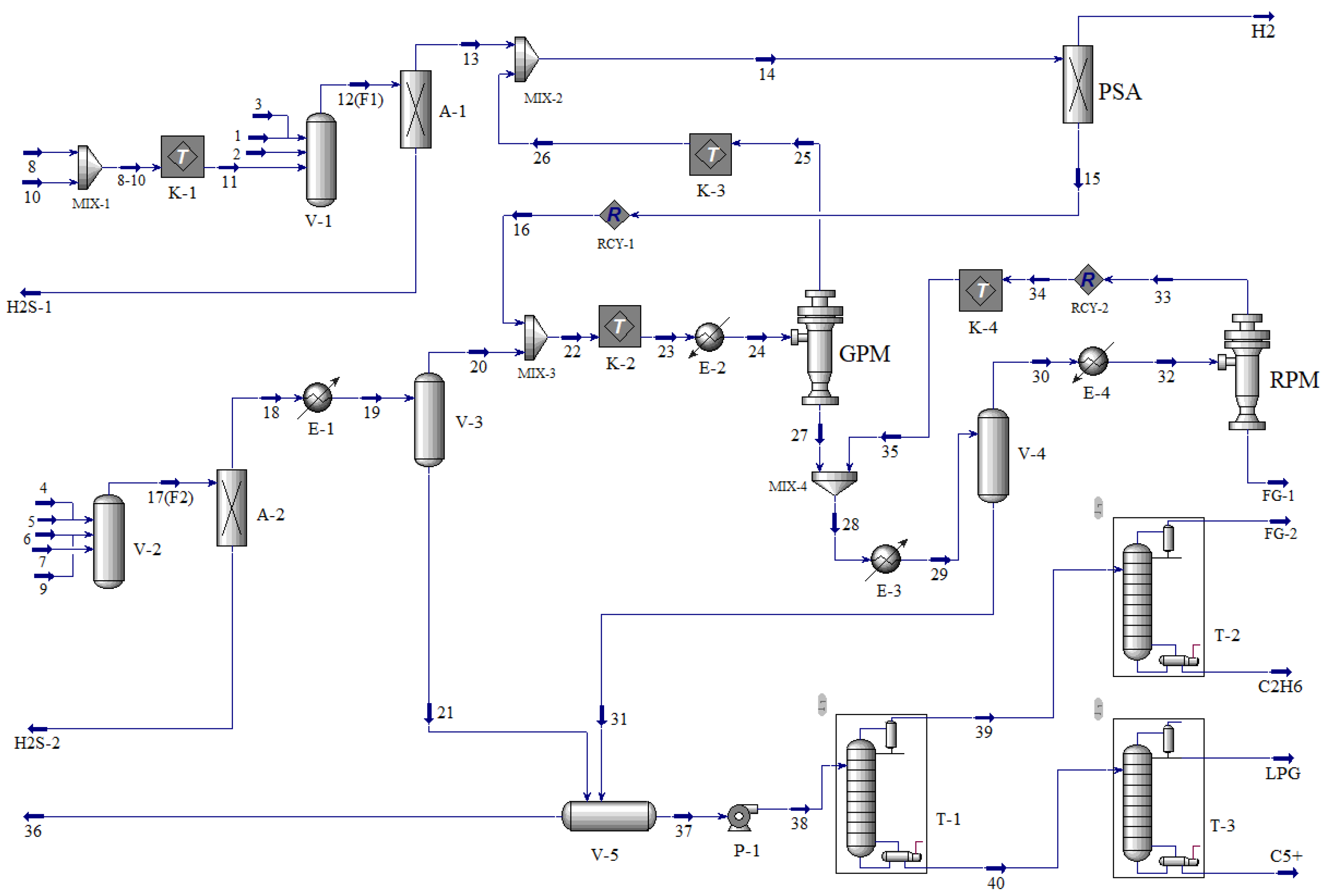
2. Process Design
2.1. Problem Description
2.2. Process Design Based on a Graphic Synthesis Method
2.2.1. Representation and Merging of Feed Gas Streams Based on a Triangular Coordinate System
2.2.2. Separation Sequence Determination and Process Design
3. Sensitivity Analysis
3.1. Hydrogen and Light Hydrocarbon Production
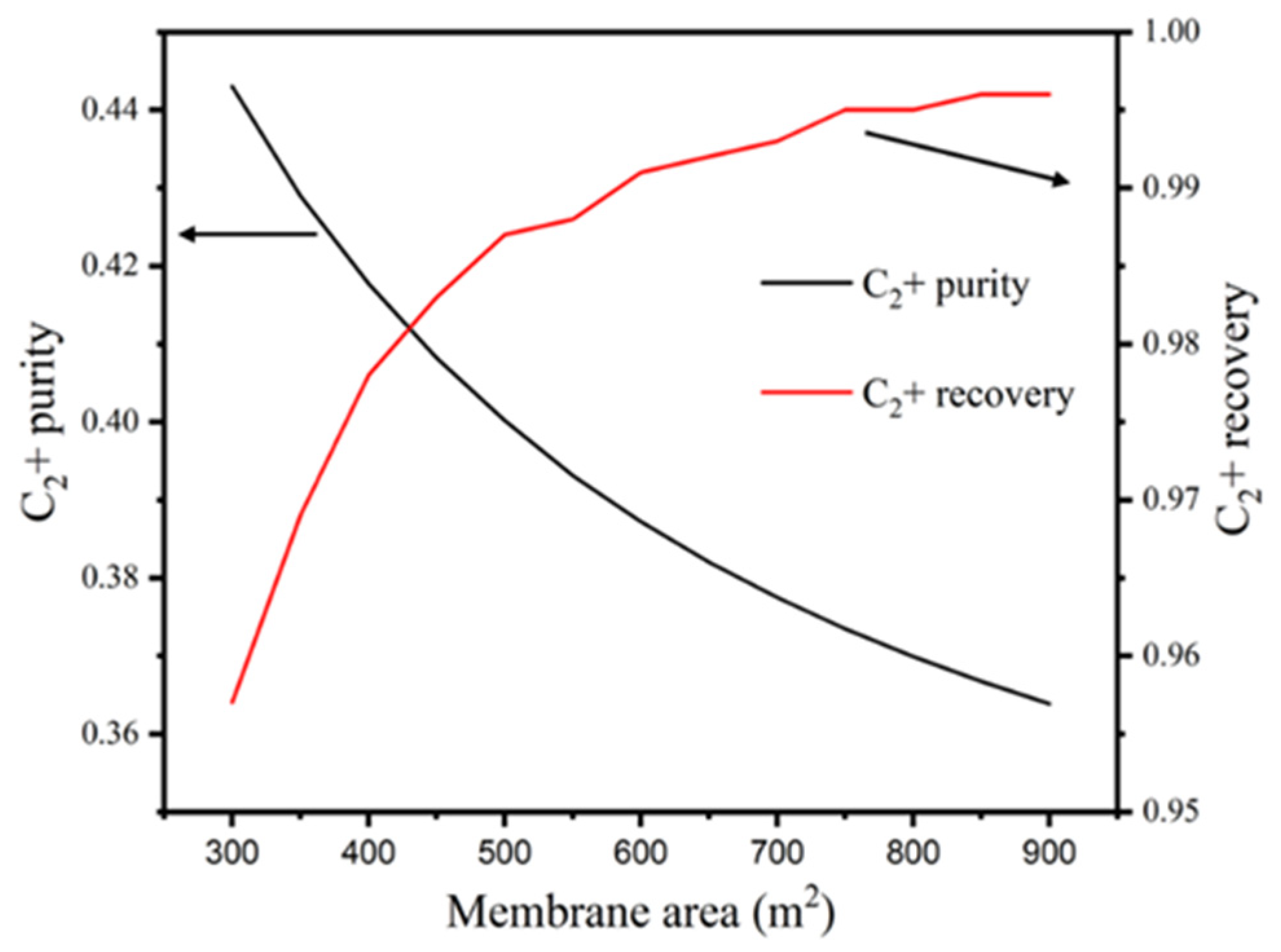
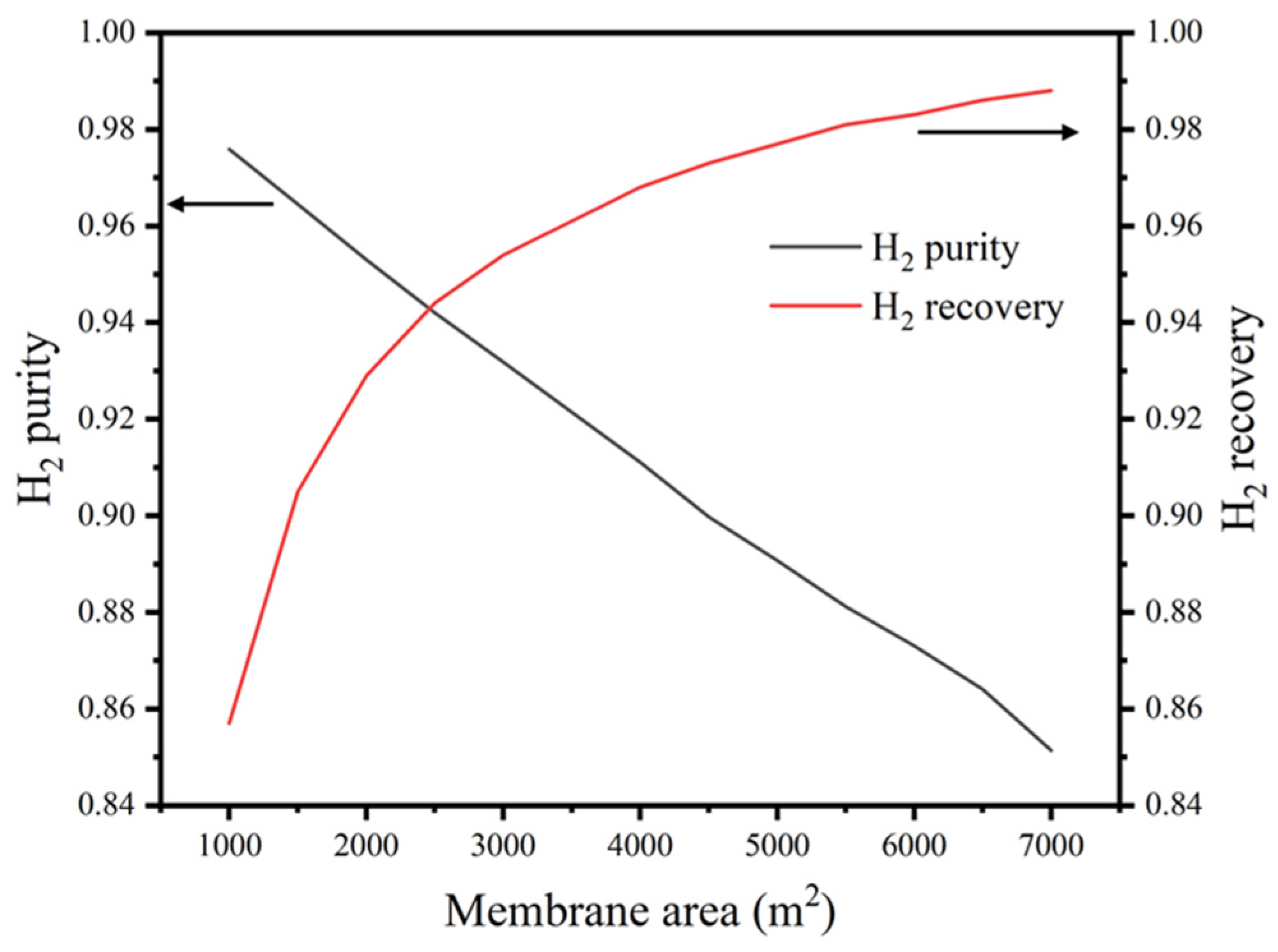
3.2. Membrane Area
3.2.1. Impacts of RPM Area on the Purity and Recovery of C2+
3.2.2. Impacts of GPM Area on the Purity and Recovery of Hydrogen
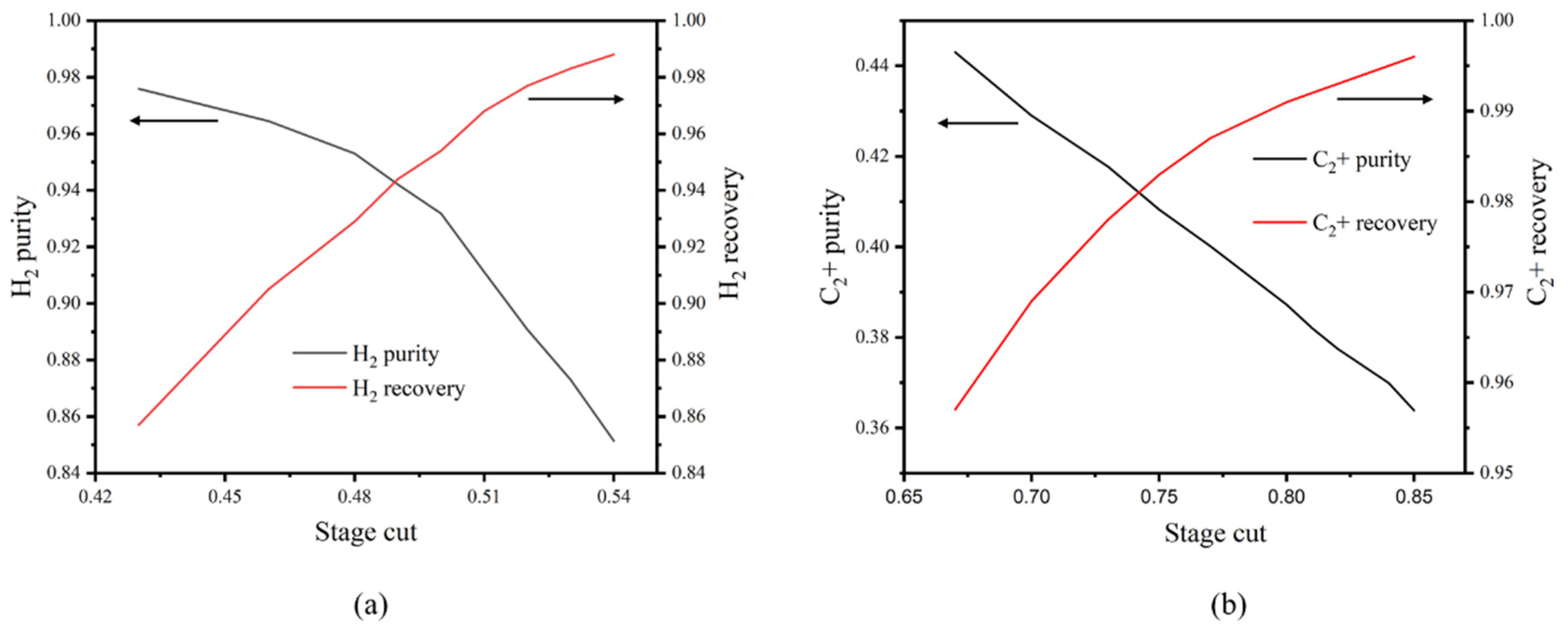
3.3. Impacts of Stage Cut on the Purity and Recovery of C2+ and H2
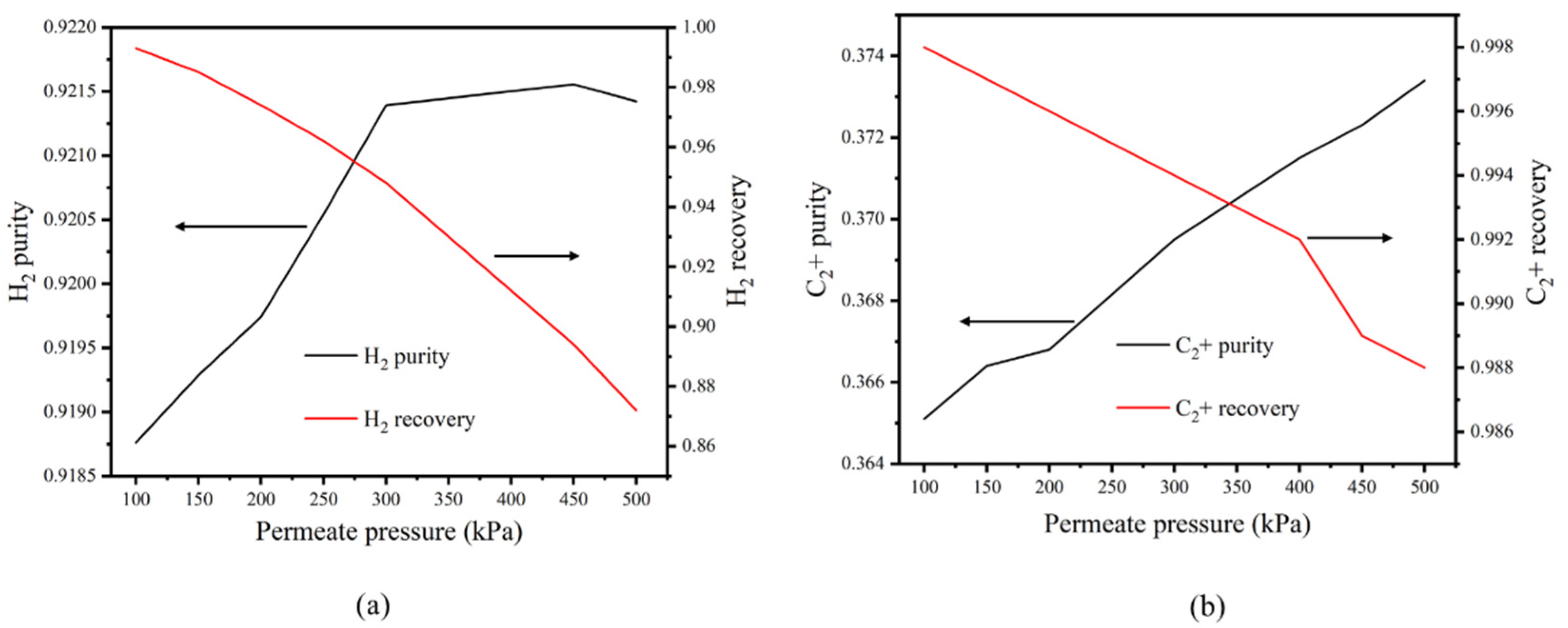
3.4. Impacts of Permeate Pressure on the Purity and Recovery of C2+ and H2
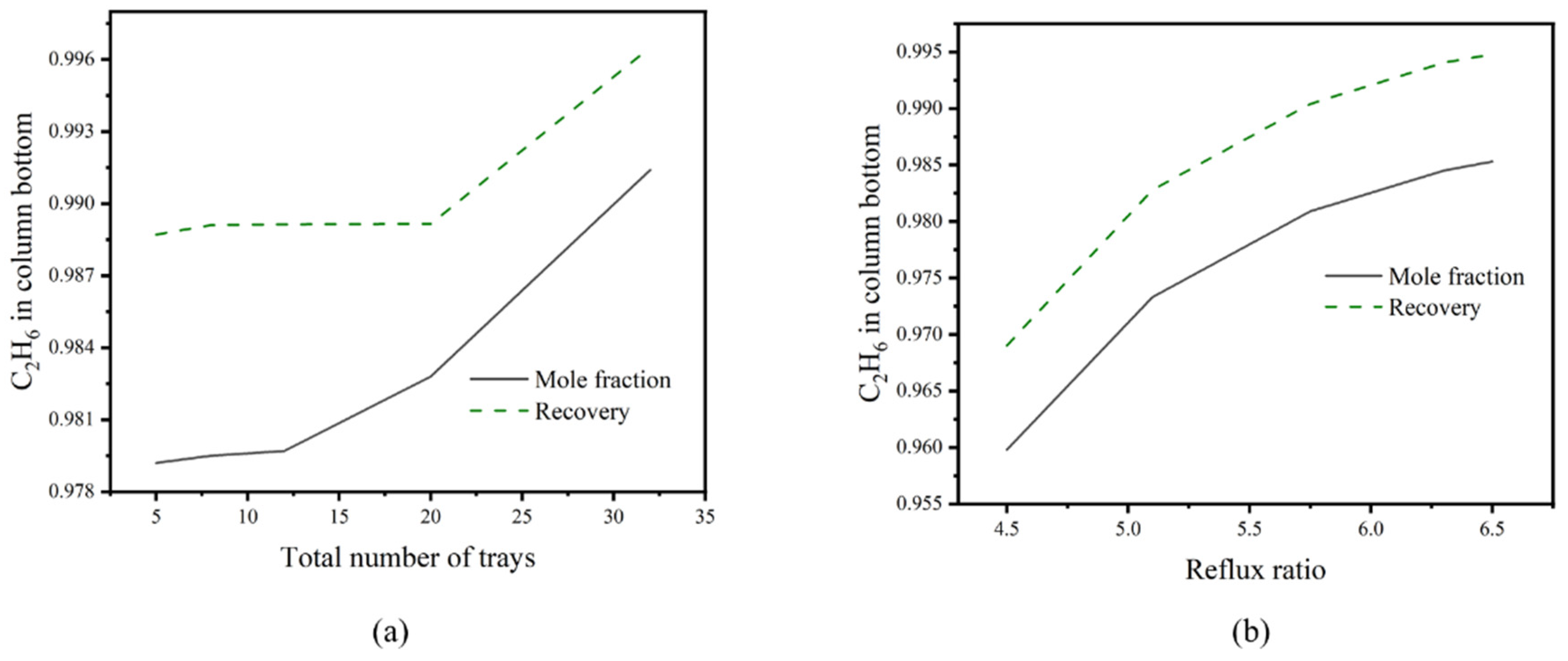
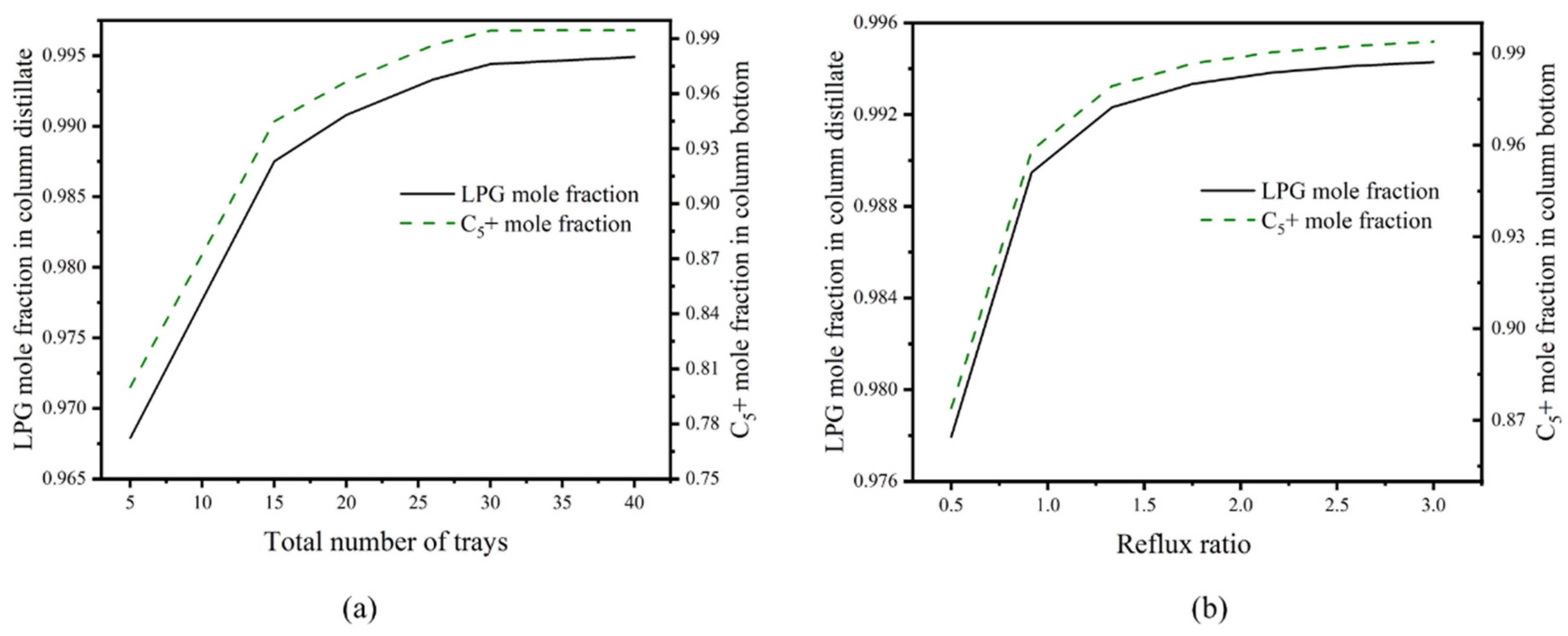
3.5. Design and Optimization of Distillation Columns
4. Economic Assessment
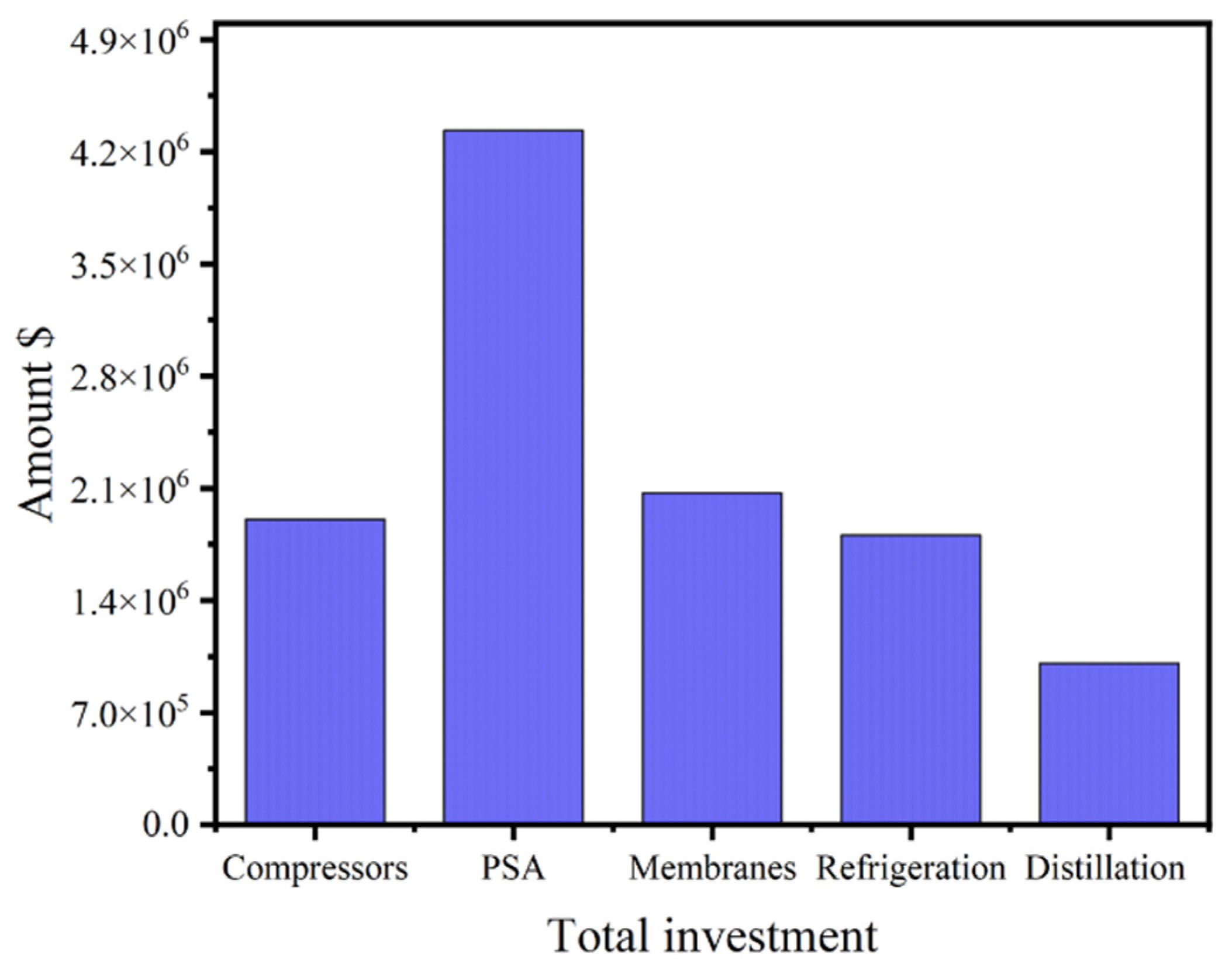
4.1. Impact of Total Investment and OPEX on Process Design
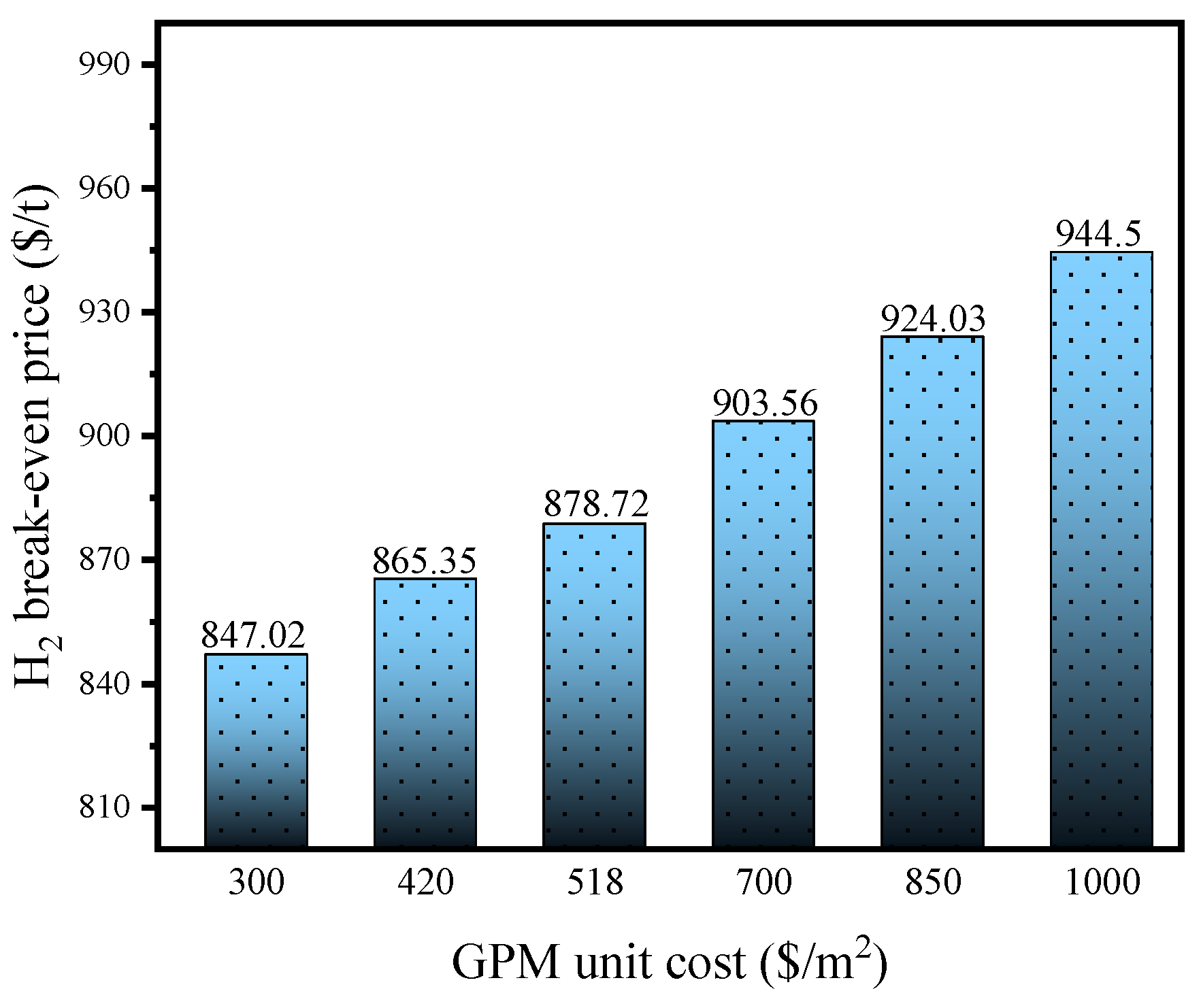
4.2. Break-Even Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Condenser Investment Cost
Appendix A.2. Reboiler Investment Cost
Appendix A.3. Column Vessel Investment Cost
Appendix B
Appendix C
Appendix D
Appendix D.1. Membrane Separation
| Component | GPM | RPM |
|---|---|---|
| H2 | 277.8 | 57.3 |
| CH4 | 2.67 | 73.1 |
| C2H6 | 0.67 | 402 |
| C3H8 | 1.19 | 621 |
| i-C4H10 | 1 | 1500 |
| n-C4H10 | 1 | 1500 |
| n-C5H12 | 1 | 1500 |
Appendix D.2. Pressure Swing Adsorption (PSA)
| Feed Gas | Product | Tail Gas | |
|---|---|---|---|
| Flow (kmol.h−1) | 494.4 | 317.5 | 176.9 |
| Pressure (kPa) | 2500 | 2000 | 650 |
| Temperature (°C) | 39.78 | 39 | 38.65 |
| Mole fraction % | |||
| H2 | 80.2 | 99.91 | 44.83 |
| CH4 | 7.74 | 0.09 | 21.48 |
| C2H6 | 7.13 | 0 | 19.93 |
| C3H8 | 2.33 | 0 | 6.5 |
| C4H10 | 1.09 | 0 | 3.07 |
| C5+ | 1.38 | 0 | 3.85 |
Appendix D.3. Distillation
Appendix D.4. Shallow Condensation
References
- Liu, H.; Mu, L.; Wang, B.; Liu, B.; Wang, J.; Zhang, X.; Sun, C.; Chen, J.; Jia, M.; Chen, G. Separation of ethylene from refinery dry gas via forming hydrate in w/o dispersion system. Sep. Purif. Technol. 2013, 116, 342–350. [Google Scholar] [CrossRef]
- Sardashti Birjandi, M.R.; Shahraki, F. Off-Gases Optimization in a Hydrogen Network Refinery. Chem. Eng. Technol. 2011, 34, 1974–1982. [Google Scholar] [CrossRef]
- Sardashti Birjandi, M.R.; Shahraki, F.; Birjandi, M.S.; Nobandegani, M.S. Application of global optimization strategies to refinery hydrogen network. Int. J. Hydrogen Energy 2014, 39, 14503–14511. [Google Scholar] [CrossRef]
- Deng, C.; Pan, H.; Li, Y.; Zhou, Y.; Feng, X. Comparative analysis of different scenarios for the synthesis of refinery hydrogen network. Appl. Therm. Eng. 2014, 70, 1162–1179. [Google Scholar] [CrossRef]
- Ruan, X.; Wang, L.; Dai, Y.; Zhang, N.; Yan, X.; He, G. Effective reclamation of vent gas in ethylbenzene dehydrogenation by coupling multi-stage circle absorption and membrane units. Sep. Purif. Technol. 2016, 168, 265–274. [Google Scholar] [CrossRef]
- Li, Y.; Luo, H. Integration of light hydrocarbons cryogenic separation process in refinery based on LNG cold energy utilization. Chem. Eng. Res. Des. 2015, 93, 632–639. [Google Scholar] [CrossRef]
- Zhang, D. Recovery of trace hydrogen by cryogenic adsorption. Sep. Purif. Technol. 2004, 35, 105–112. [Google Scholar] [CrossRef]
- Huang, W.; Jiang, X.; He, G.; Ruan, X.; Chen, B.; Nizamani, A.K.; Li, X.; Wu, X.; Xiao, W. A Novel Process of H2/CO2 Membrane Separation of Shifted Syngas Coupled with Gasoil Hydrogenation. Processes 2020, 8, 590. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Liguori, S.; Kian, K.; Buggy, N.; Anzelmo, B.H.; Wilcox, J. Opportunities and challenges of low-carbon hydrogen via metallic membranes. Prog. Energy Combust. Sci. 2020, 80, 100851. [Google Scholar] [CrossRef]
- Iulianelli, A.; Drioli, E. Membrane engineering: Latest advancements in gas separation and pre-treatment processes, petrochemical industry and refinery, and future perspectives in emerging applications. Fuel Processing Technol. 2020, 206, 106464. [Google Scholar] [CrossRef]
- Li, B.; He, G.; Jiang, X.; Dai, Y.; Ruan, X. Pressure swing adsorption/membrane hybrid processes for hydrogen purification with a high recovery. Front. Chem. Sci. Eng. 2016, 10, 255–264. [Google Scholar] [CrossRef]
- Liemberger, W.; Groß, M.; Miltner, M.; Harasek, M. Experimental analysis of membrane and pressure swing adsorption (PSA) for the hydrogen separation from natural gas. J. Clean. Prod. 2017, 167, 896–907. [Google Scholar] [CrossRef]
- Sircar, S.; Waldron, W.E.; Rao, M.B.; Anand, M. Hydrogen production by hybrid SMR–PSA–SSF membrane system. Sep. Purif. Technol. 1999, 17, 11–20. [Google Scholar] [CrossRef]
- Ohs, B.; Falkenberg, M.; Wessling, M. Optimizing hybrid membrane-pressure swing adsorption processes for biogenic hydrogen recovery. Chem. Eng. J. 2019, 364, 452–461. [Google Scholar] [CrossRef]
- Esteves, I.A.; Mota, J.P. Gas separation by a novel hybrid membrane/pressure swing adsorption process. Ind. Eng. Chem. Res. 2007, 46, 5723–5733. [Google Scholar] [CrossRef]
- Mango, F.D. The light hydrocarbons in petroleum: A critical review. Org. Geochem. 1997, 26, 417–440. [Google Scholar] [CrossRef]
- Cui, W.G.; Hu, T.L.; Bu, X.H. Metal-Organic Framework Materials for the Separation and Purification of Light Hydrocarbons. Adv Mater 2020, 32, e1806445. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, P.; Guo, X. Separation of Xylene Isomers: A Review of Recent Advances in Materials. Ind. Eng. Chem. Res. 2017, 56, 14725–14753. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Li, J.; Zhu, T. Adsorption equilibrium and thermodynamics of acetaldehyde/acetone on activated carbon. Sep. Purif. Technol. 2019, 209, 535–541. [Google Scholar] [CrossRef]
- Ma, B.; Deng, C.; Chen, H.; Zhu, M.; Yang, M.; Feng, X. Hybrid Separation Process of Refinery Off-gas toward Near-Zero Hydrogen Emission: Conceptual Design and Techno-economic Analysis. Ind. Eng. Chem. Res. 2020, 59, 8715–8727. [Google Scholar] [CrossRef]
- Towler, G.P.; Mann, R.; Serriere, A.J.; Gabaude, C.M. Refinery hydrogen management: Cost analysis of chemically-integrated facilities. Ind. Eng. Chem. Res. 1996, 35, 2378–2388. [Google Scholar] [CrossRef]
- Alves, J.J.; Towler, G.P. Analysis of refinery hydrogen distribution systems. Ind. Eng. Chem. Res. 2002, 41, 5759–5769. [Google Scholar] [CrossRef]
- Nishida, N.; Stephanopoulos, G.; Westerberg, A.W. A review of process synthesis. AIChE J. 1981, 27, 321–351. [Google Scholar] [CrossRef]
- Hendry, J.E.; Rudd, D.F.; Seader, J.D. Synthesis in the design of chemical processes. AIChE J. 1973, 19, 1–15. [Google Scholar] [CrossRef]
- Bausa, J.; Marquardt, W. Shortcut design methods for hybrid membrane/distillation processes for the separation of nonideal multicomponent mixtures. Ind. Eng. Chem. Res. 2000, 39, 1658–1672. [Google Scholar] [CrossRef]
- Kao, Y.K. Separation vector formulation for the synthesis of multicomponent separation sequences. AIChE J. 1995, 41, 78–96. [Google Scholar] [CrossRef]
- Shenvi, A.A.; Shah, V.H.; Zeller, J.A.; Agrawal, R. A synthesis method for multicomponent distillation sequences with fewer columns. AIChE J. 2012, 58, 2479–2494. [Google Scholar] [CrossRef]
- Ruan, X.; Xiao, H.; Jiang, X.; Yan, X.; Dai, Y.; He, G. Graphic synthesis method for multi-technique integration separation sequences of multi-input refinery gases. Sep. Purif. Technol. 2019, 214, 187–195. [Google Scholar] [CrossRef]
- Mores, P.; Arias, A.; Scenna, N.; Caballero, J.; Mussati, S.; Mussati, M. Membrane-Based Processes: Optimization of Hydrogen Separation by Minimization of Power, Membrane Area, and Cost. Processes 2018, 6, 221. [Google Scholar] [CrossRef] [Green Version]
- Zarca, G.; Urtiaga, A.; Biegler, L.T.; Ortiz, I. An optimization model for assessment of membrane-based post-combustion gas upcycling into hydrogen or syngas. J. Membr. Sci. 2018, 563, 83–92. [Google Scholar] [CrossRef]
- Ahmad, F.; Lau, K.K.; Shariff, A.M.; Murshid, G. Process simulation and optimal design of membrane separation system for CO2 capture from natural gas. Comput. Chem. Eng. 2012, 36, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Murad Chowdhury, M.H.; Feng, X.; Douglas, P.; Croiset, E. A New Numerical Approach for a Detailed Multicomponent Gas Separation Membrane Model and AspenPlus Simulation. Chem. Eng. Technol. 2005, 28, 773–782. [Google Scholar] [CrossRef]
- Arias, A.; Mores, P.; Scenna, N.; Caballero, J.; Mussati, S.; Mussati, M. Optimal Design of a Two-Stage Membrane System for Hydrogen Separation in Refining Processes. Processes 2018, 6, 284. [Google Scholar] [CrossRef] [Green Version]
- Hoorfar, M.; Alcheikhhamdon, Y.; Chen, B. A novel tool for the modeling, simulation and costing of membrane based gas separation processes using Aspen HYSYS: Optimization of the CO2/CH4 separation process. Comput. Chem. Eng. 2018, 117, 11–24. [Google Scholar] [CrossRef]
- De Guido, G.; Fogli, M.R.; Pellegrini, L.A. Effect of Heavy Hydrocarbons on CO2 Removal from Natural Gas by Low-Temperature Distillation. Ind. Eng. Chem. Res. 2018, 57, 7245–7256. [Google Scholar] [CrossRef]
- Langé, S.; Pellegrini, L.A. Energy Analysis of the New Dual-Pressure Low-Temperature Distillation Process for Natural Gas Purification Integrated with Natural Gas Liquids Recovery. Ind. Eng. Chem. Res. 2016, 55, 7742–7767. [Google Scholar] [CrossRef]
- Lin, H.; He, Z.; Sun, Z.; Kniep, J.; Ng, A.; Baker, R.W.; Merkel, T.C. CO2-selective membranes for hydrogen production and CO2 capture—Part II: Techno-economic analysis. J. Membr. Sci. 2015, 493, 794–806. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Jiang, X.; Xiao, W.; Dong, Y.; El Hamouti, I.; He, G. Dual-membrane natural gas pretreatment process as CO2 source for enhanced gas recovery with synergy hydrocarbon recovery. J. Nat. Gas Sci. Eng. 2016, 34, 563–574. [Google Scholar] [CrossRef]
- Chen, B.; Yang, T.; Xiao, W.; Nizamani, A.k. Conceptual Design of Pyrolytic Oil Upgrading Process Enhanced by Membrane-Integrated Hydrogen Production System. Processes 2019, 7, 284. [Google Scholar] [CrossRef] [Green Version]
- William, L. Luyben, Distillation Design and Control Using Aspen Simulation; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Gallucci, F.; Fernandez, E.; Corengia, P.; van Sint Annaland, M. Recent advances on membranes and membrane reactors for hydrogen production. Chem. Eng. Sci. 2013, 92, 40–66. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Marcoberardino, G.; Vitali, D.; Spinelli, F.; Binotti, M.; Manzolini, G. Green Hydrogen Production from Raw Biogas: A Techno-Economic Investigation of Conventional Processes Using Pressure Swing Adsorption Unit. Processes 2018, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Voldsund, M.; Jordal, K.; Anantharaman, R. Hydrogen production with CO2 capture. Int. J. Hydrogen Energy 2016, 41, 4969–4992. [Google Scholar] [CrossRef]
- Skarstrom, C.W. Method and Apparatus for Fractionating Gaseous Mixtures by Adsorption. U.S. Patent No. 2,944,627, 12 July 1960. [Google Scholar]
- Adhikari, S.; Fernando, S. Hydrogen membrane separation techniques. Ind. Eng. Chem. Res. 2006, 45, 875–881. [Google Scholar] [CrossRef]
- Hinchliffe, A.B.; Porter, K.E. A Comparison of Membrane Separation and Distillation. Chem. Eng. Res. Des. 2000, 78, 255–268. [Google Scholar] [CrossRef]
- Lu, H.; Ma, G.; Cao, L.; Azimi, M. Optimization of light hydrocarbon recovery system in condensate gas field. Energy Rep. 2019, 5, 1209–1221. [Google Scholar] [CrossRef]




| Streams | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Pressure (MPag) | 2.50 | 2.40 | 2.50 | 0.75 | 0.67 | 0.86 | 1.20 | 1.15 | 0.58 | 0.7 |
| Flowrate (Nm3·h−1) | 600 | 2788 | 2415 | 1326 | 2053 | 1229 | 1034 | 600 | 900 | 1200 |
| Mole fraction % | ||||||||||
| H2 | 98.43 | 63.34 | 79.95 | 31.23 | 34.42 | 13.45 | 35.01 | 75.78 | 3.49 | 69.45 |
| N2 | 0.00 | 0.00 | 0.00 | 2.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.41 |
| O2 | 0.00 | 0.00 | 0.00 | 0.23 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.50 |
| CH4 | 0.88 | 14.25 | 8.92 | 12.17 | 10.19 | 2.60 | 5.64 | 4.27 | 1.19 | 3.35 |
| C2H6 | 0.23 | 11.15 | 4.98 | 20.21 | 15.97 | 39.90 | 24.04 | 2.44 | 5.31 | 23.94 |
| C3H8 | 0.08 | 4.73 | 2.51 | 14.45 | 14.61 | 38.79 | 20.23 | 0.69 | 30.92 | 2.38 |
| i-C4H10 | 0.02 | 1.09 | 0.55 | 3.91 | 4.03 | 2.39 | 6.86 | 0.19 | 16.66 | 0.12 |
| n-C4H10 | 0.02 | 1.11 | 0.99 | 3.71 | 7.78 | 2.31 | 4.45 | 1.31 | 40.36 | 0.20 |
| C5+ | 0.07 | 1.02 | 1.11 | 3.29 | 8.16 | 0.53 | 3.56 | 14.48 | 0.00 | 0.12 |
| H2S | 0.10 | 3.31 | 1.00 | 8.78 | 4.78 | 0.00 | 0.04 | 0.85 | 1.95 | 1 μL/l |
| Stream | 13 | 18 | H2 | C2H6 | LPG | C5+ | FG |
|---|---|---|---|---|---|---|---|
| Pressure/kPa | 2500 | 680 | 2000 | 2150 | 1400 | 1450 | 2100 |
| Temperature/°C | 40 | 31 | 39 | −7.6 | 56.68 | 142.3 | −3 |
| Rate/kmol·h−1 | 333.5 | 281.7 | 317.4 | 94.77 | 126.4 | 17.23 | 59.17 |
| Mole fraction % | |||||||
| H2 | 74.48 | 26.63 | 99.91 | 0 | 0 | 0 | 10.27 |
| N2 | 0.06 | 0.43 | 0 | 0 | 0 | 0 | 2.42 |
| O2 | 0.08 | 0.05 | 0 | 0 | 0 | 0 | 0.68 |
| CH4 | 9.15 | 7.48 | 0.09 | 0.89 | 0 | 0 | 84.58 |
| C2H6 | 9.77 | 21.93 | 0 | 98.28 | 0 | 0 | 2.04 |
| C3H8 | 3.02 | 23.09 | 0 | 0.83 | 58.94 | 0.03 | 0.01 |
| i-C4H10 | 0.63 | 6.10 | 0 | 0 | 15.26 | 0.44 | 0 |
| n-C4H10 | 0.87 | 10.26 | 0 | 0 | 24.85 | 2.82 | 0 |
| n-C5H12 | 1.93 | 4.04 | 0 | 0 | 0.94 | 96.69 | 0 |
| H2S | 0.00 | 0.00 | 0 | 0 | 0 | 0 | 0 |
| Streams | 39 | 40 | FG-2 | C2H6 | LPG | C5+ |
|---|---|---|---|---|---|---|
| Key component | C2H6 | C3+ | CH4 | C2H6 | LPG | C5+ |
| Mole fraction of key component | 0.8273 | 0.9962 | 0.923 | 0.9828 | 0.991 | 0.967 |
| Pressure (kPa) | 2300 | 2350 | 2100 | 2150 | 1400 | 1450 |
| Temperature (°C) | −9.1 | 90.67 | −84.57 | −7.6 | 56.68 | 142.3 |
| Item | Unit | Price | Reference |
|---|---|---|---|
| Membrane | $/m2 | 420/1000 1 | [5] |
| Compressor | $/kW0.95 | 1620 | [5] |
| Refrigeration | $/kW | 1000 | [39] |
| PSA | $ | 4.33 × 106 | Equation (A7) |
| Heat exchangers | $/m2 | 1500 × A0.6 | [5] |
| Pump | $/(m3h−1) | 1.67 × 103 | [5] |
| Electricity | $/kWh | 0.13 | [5] |
| Steam | $/t | 14.50 | [40] |
| Refrigeration cost | $/GJ | 56.17 | [22] |
| Cold water | $/t | 0.10 | [5] |
| Depreciation time | Year | 5 for membrane, 5 for PSA 2, 15 for others | |
| Item | Capital Cost ($106) | Depreciation Cost ($106/Year) |
|---|---|---|
| Compressors | 1.91 | 0.13 |
| Refrigeration | 1.80 | 0.12 |
| PSA | 4.33 | 0.86 |
| Membranes | 2.07 | 0.41 |
| Distillation | 1.008 | 0.07 |
| Adsorption | 0.40 | 0.03 |
| Heat exchangers | 0.002 | 1.4 × 10−4 |
| Pumps | 0.029 | 1.97 × 10−3 |
| Other equipment cost 1 | 1.73 | 0.12 |
| Item | Cost ($106/Year) |
|---|---|
| Electricity | 1.66 |
| Steam | 0.55 |
| Refrigeration | 2.92 |
| Cold water | 0.30 |
| Component | Flowrate (103t/Year) | Unit Price ($103/t) a | Amount ($106/Year) |
|---|---|---|---|
| H2 | 5.1296 | 3.015 | 15.47 |
| C2H6 | 22.224 | 0.72 | 16.00 |
| LPG | 49.368 | 0.98 | 48.38 |
| C5+ | 11.416 | 1.08 | 12.28 |
| FG | 7.248 | 0.50 | 3.62 |
| Feed gases (F1 + F2) | 99.944 | 0.50 | 49.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, J.A.S.; Cheng, A.; Ruan, X.; Jiang, X.; Wang, H.; He, G.; Xiao, W. Design and Economic Evaluation of a Hybrid Membrane Separation Process from Multiple Refinery Gases Using a Graphic Synthesis Method. Processes 2022, 10, 820. https://doi.org/10.3390/pr10050820
Perez JAS, Cheng A, Ruan X, Jiang X, Wang H, He G, Xiao W. Design and Economic Evaluation of a Hybrid Membrane Separation Process from Multiple Refinery Gases Using a Graphic Synthesis Method. Processes. 2022; 10(5):820. https://doi.org/10.3390/pr10050820
Chicago/Turabian StylePerez, Juan Aron Stron, Andi Cheng, Xuehua Ruan, Xiaobin Jiang, Hanli Wang, Gaohong He, and Wu Xiao. 2022. "Design and Economic Evaluation of a Hybrid Membrane Separation Process from Multiple Refinery Gases Using a Graphic Synthesis Method" Processes 10, no. 5: 820. https://doi.org/10.3390/pr10050820
APA StylePerez, J. A. S., Cheng, A., Ruan, X., Jiang, X., Wang, H., He, G., & Xiao, W. (2022). Design and Economic Evaluation of a Hybrid Membrane Separation Process from Multiple Refinery Gases Using a Graphic Synthesis Method. Processes, 10(5), 820. https://doi.org/10.3390/pr10050820







