Effects of Modified Biochar on the Mobility and Speciation Distribution of Cadmium in Contaminated Soil
Abstract
:1. Introduction
2. Material and Methods
2.1. Preparation of Cd-Contaminated Soil
2.2. Preparation of Chitosan–EDTA-Modified Biochar
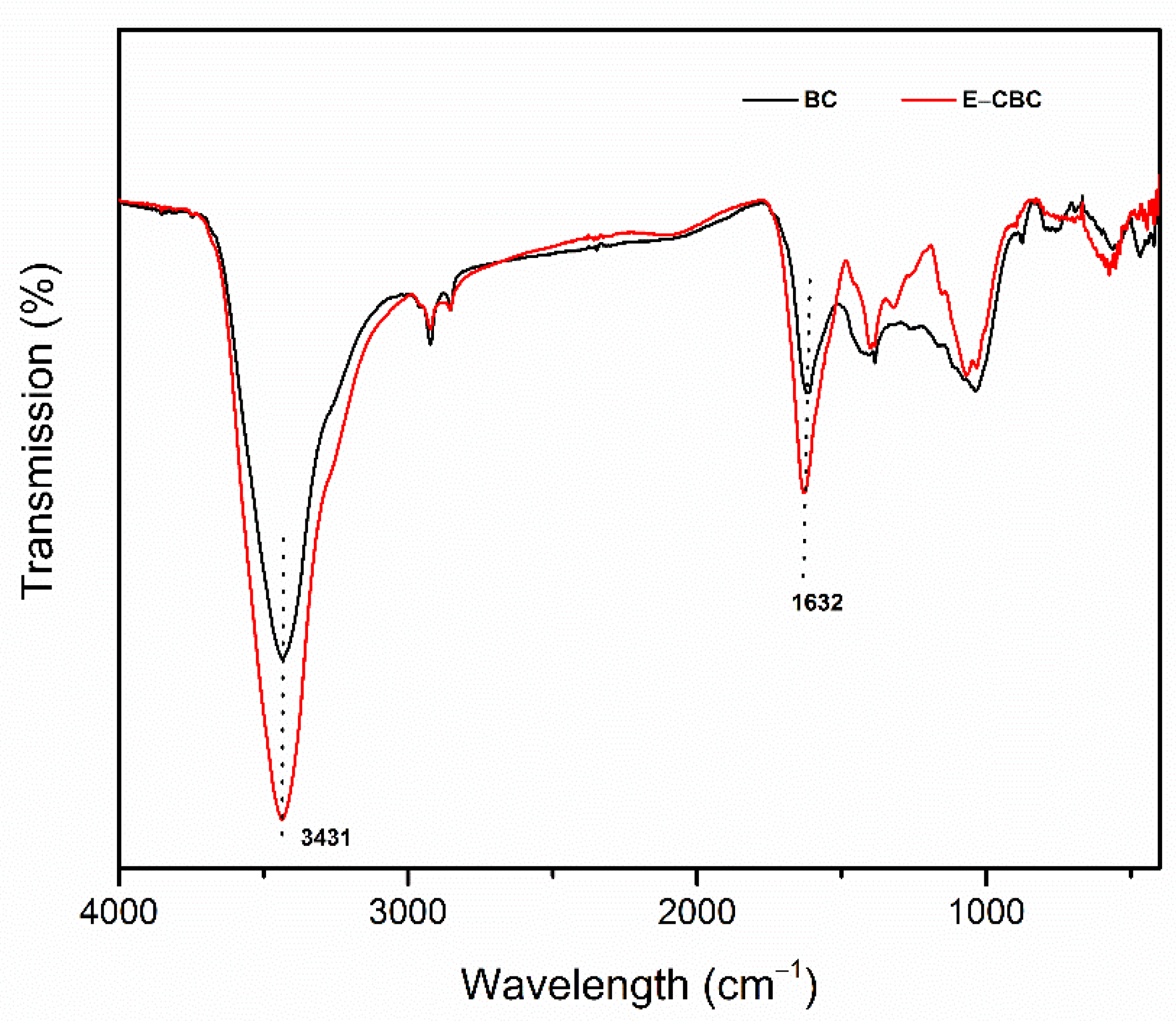
2.3. Characterization
2.4. Soil Incubation Experiment
2.5. Analytical Methods
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Biochars
3.2. Effects of Modified Biochar on Soil pH and SOM
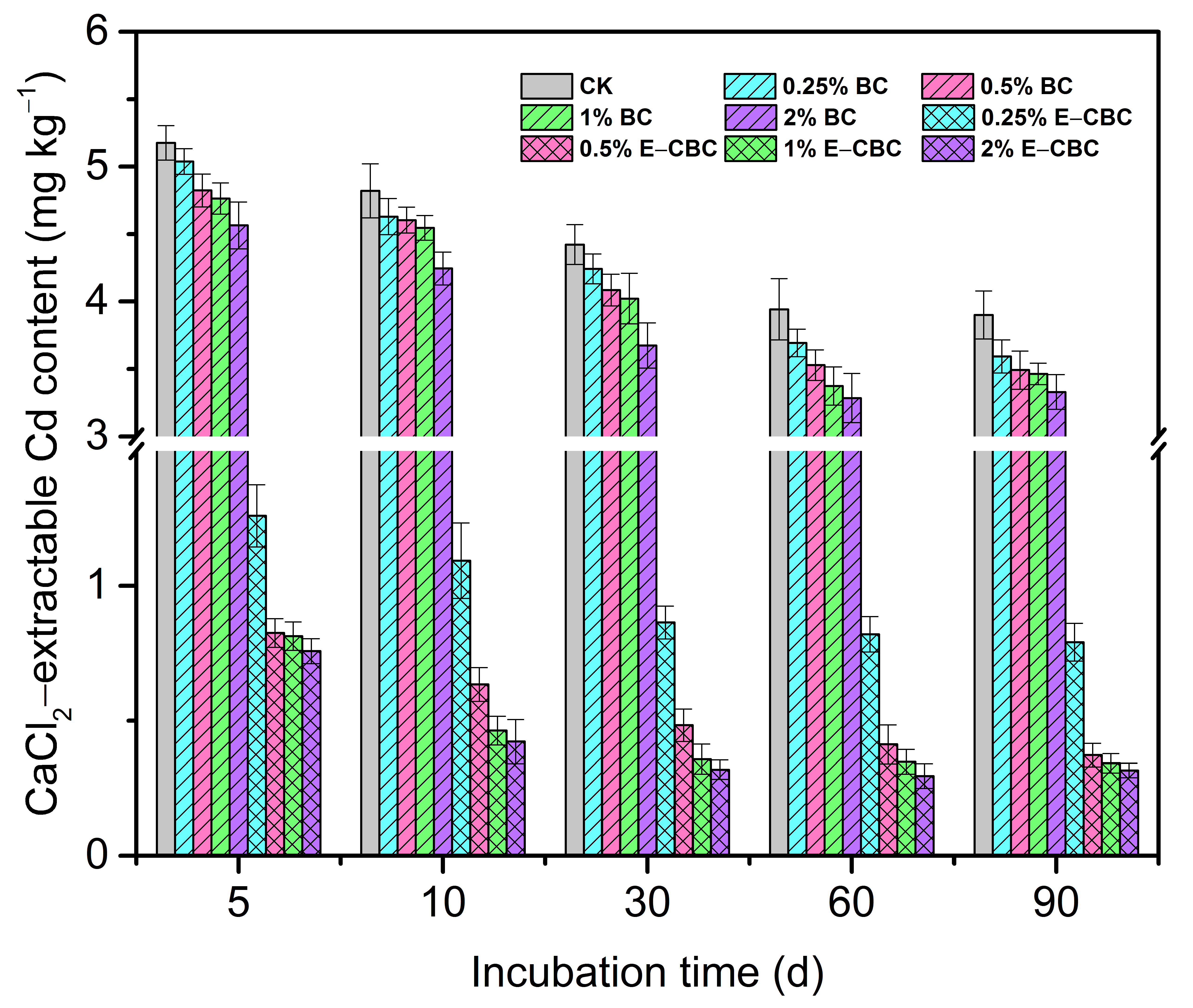
3.3. Effects of Modified Biochar on the Bioavailability of Cd
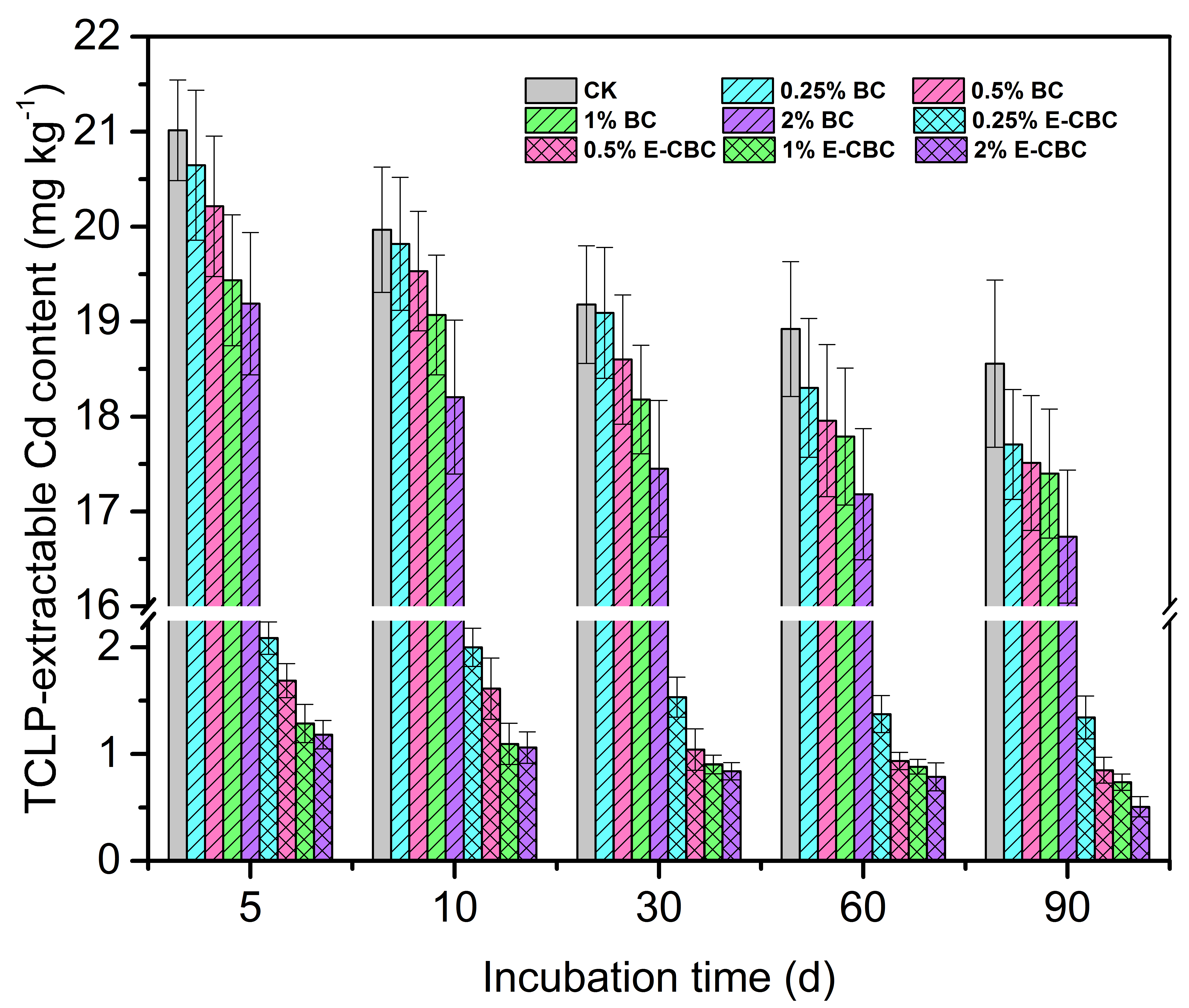
3.4. Effects of Modified Biochar on the Leachability of Cd
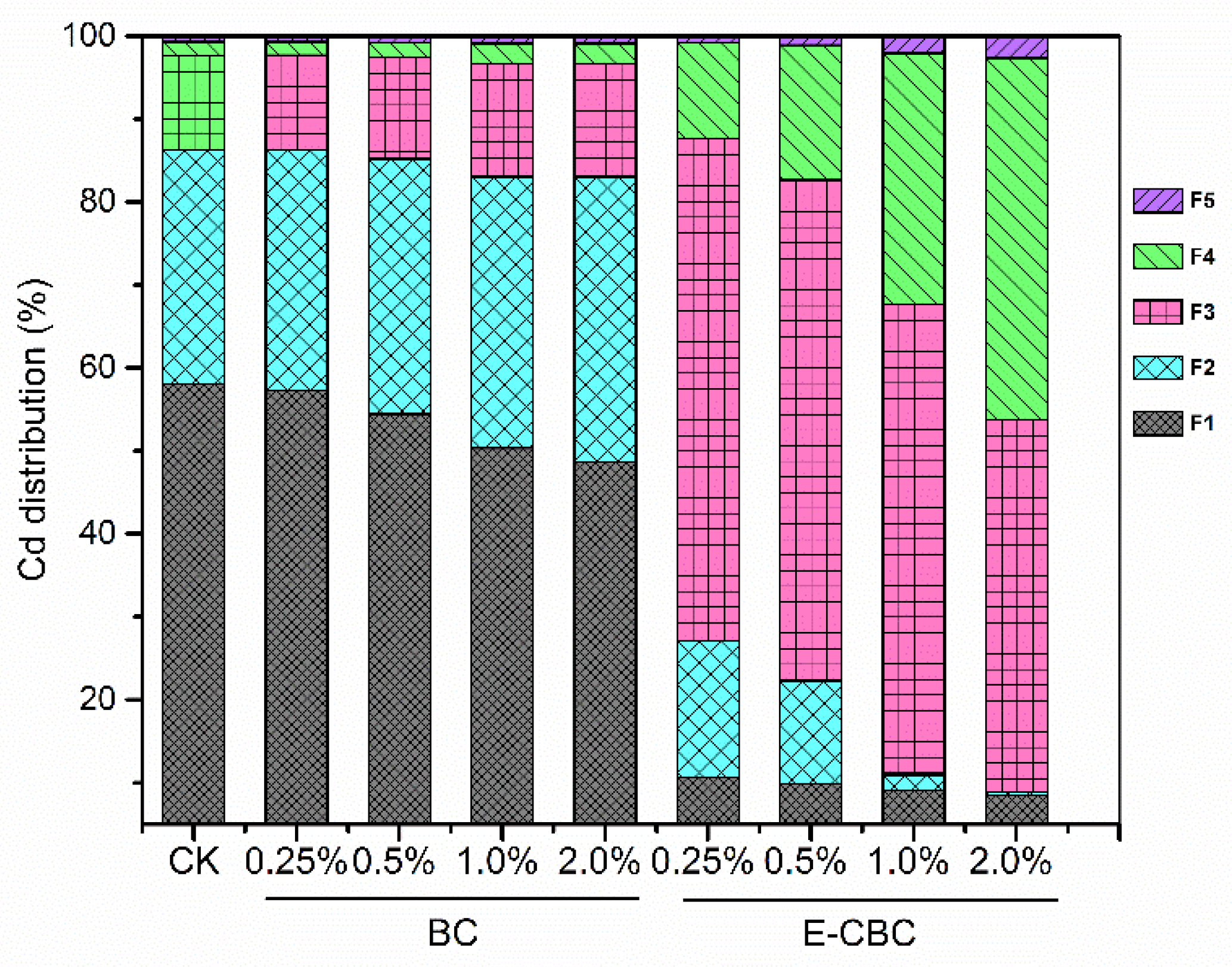
3.5. Effects of Modified Biochar on Speciation of Cd
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Wang, J.; Zhang, C.; Chen, R. Stabilization of heavy metal-contaminated soils by biochar: Challenges and recommendations. Sci. Total Environ. 2020, 729, 139060. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Chakraborty, R.; Myakala, H.; Koti, R.; Famurewa, A.C.; Madhyastha, H.; Vellingiri, B.; George, A.; Gopalakrishnan, A.V. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium)-induced hepatotoxicity—A review. Chemosphere 2021, 271, 132369. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.A.; Ng, H.-S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2022, 287, 129735. [Google Scholar] [CrossRef]
- Kumar, A.; Subrahmanyam, G.; Mondal, R.; Cabral-Pinto, M.M.S.; Shabnam, A.A.; Jigyasu, D.K.; Malyan, S.K.; Fagodiya, R.K.; Khan, S.A.; Kumar, A.; et al. Bio-remediation approaches for alleviation of cadmium contamination in natural resources. Chemosphere 2021, 268, 128855. [Google Scholar] [CrossRef]
- Wang, M.-J.; Li, M.-Y.; Ning, H.; Xue, R.-Y.; Liang, J.-H.; Wang, N.; Luo, X.-S.; Li, G.; Juhasz, A.L.; Ma, L.Q.; et al. Cadmium oral bioavailability is affected by calcium and phytate contents in food: Evidence from leafy vegetables in mice. J. Hazard. Mater. 2022, 424, 127373. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Xia, X.; Wu, S.; Zhou, Z.; Wang, G. Microbial Cd(II) and Cr(VI) resistance mechanisms and application in bioremediation. J. Hazard. Mater. 2021, 401, 123685. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, X.; Luo, L.; Zhou, Y.; Wei, J.; Chen, A.; Tang, L.; Wu, H.; Deng, Y.; Zhang, F.; et al. Remediation of Cu, Pb, Zn and Cd-contaminated agricultural soil using a combined red mud and compost amendment. Int. Biodeterior. Biodegr. 2017, 118, 73–81. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Lian, M.; Zhang, C.; Ruan, X.; Li, T. Long-term stabilization of Cd in agricultural soil using mercapto-functionalized nano-silica (MPTS/nano-silica): A three-year field study. Ecotoxicol. Environ. Saf. 2020, 197, 110600. [Google Scholar] [CrossRef]
- Rizwan, M.S.; Imtiaz, M.; Zhu, J.; Yousaf, B.; Hussain, M.; Ali, L.; Ditta, A.; Ihsan, M.Z.; Huang, G.; Ashraf, M.; et al. Immobilization of Pb and Cu by organic and inorganic amendments in contaminated soil. Geoderma 2021, 385, 114803. [Google Scholar] [CrossRef]
- Ruttens, A.; Adriaensen, K.; Meers, E.; Vocht, A.D.; Geebelen, W.; Carleer, R.; Mench, M.; Vangronsveld, J. Long-term sustainability of metal immobilization by soil amendments: Cyclonic ashes versus lime addition. Environ. Pollut. 2010, 158, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, D.; Sun, X.; Wang, Y.; Wang, Q. In situ removal of cadmium by short-distance migration under the action of a low-voltage electric field and granular activated carbon. Chemosphere 2022, 287, 132208. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, J.; Yin, H.; Zhang, Y.; Pan, H.; Wang, L. Sustainable stabilization/solidification of the Pb, Zn, and Cd contaminated soil by red mud-derived binders. Environ. Pollut. 2021, 284, 117178. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, L.; Yang, W.; Shi, W.; Tong, Y.; Chai, L.; Gao, S.; Liao, Q. Simultaneous immobilization of cadmium and lead in contaminated soils by hybrid bio-nanocomposites of fungal hyphae and nano-hydroxyapatites. Environ. Sci. Pollut. Res. 2018, 25, 11970–11980. [Google Scholar] [CrossRef]
- Shen, Z.; McMillan, O.; Jin, F.; Al-Tabbaa, A. Salisbury biochar did not affect the mobility or speciation of lead in kaolin in a short-term laboratory study. J. Hazard. Mater. 2016, 316, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Gou, J.; Chen, X.; Xaio, S.; Ali, I.; Shang, R.; Wang, D.; Wu, Y.; Han, M.; Luo, X. Application of mixed bacteria-loaded biochar to enhance uranium and cadmium immobilization in a co-contaminated soil. J. Hazard. Mater. 2021, 401, 123823. [Google Scholar] [CrossRef]
- Zhang, R.-H.; Li, Z.-G.; Liu, X.-D.; Wang, B.-C.; Zhou, G.-L.; Huang, X.-X.; Lin, C.-F.; Wang, A.-H.; Brooks, M. Immobilization and bioavailability of heavy metals in greenhouse soils amended with rice straw-derived biochar. Ecol. Eng. 2017, 98, 183–188. [Google Scholar] [CrossRef]
- Zhang, C.; Shan, B.; Zhu, Y.; Tang, W. Remediation effectiveness of Phyllostachys pubescens biochar in reducing the bioavailability and bioaccumulation of metals in sediments. Environ. Pollut. 2018, 242, 1768–1776. [Google Scholar] [CrossRef]
- Dai, S.; Li, H.; Yang, Z.; Dai, M.; Dong, X.; Ge, X.; Sun, M.; Shi, L. Effects of biochar amendments on speciation and bioavailability of heavy metals in coal-mine-contaminated soil. Hum. Ecol. Risk Assess. 2018, 24, 1887–1900. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, K.; Zhan, W.; Huang, L.; Liu, Y.; Li, T.; Yang, Z.; Liao, Q.; Chen, R.; Zhang, C.; et al. Highly effective stabilization of Cd and Cu in two different soils and improvement of soil properties by multiple-modified biochar. Ecotoxicol. Environ. Saf. 2021, 207, 111294. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Tao, M.; Wang, L.; Liu, X.; Xu, J. Changes in heavy metal bioavailability and speciation from a Pb-Zn mining soil amended with biochars from co-pyrolysis of rice straw and swine manure. Sci. Total Environ. 2018, 633, 300–307. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Xu, H.; Song, F.; Ge, H.; Chen, L.; Yue, S.; Yang, W. Effect of biochar on immobilization remediation of Cd-contaminated soil and environmental quality. Environ. Res. 2022, 204, 111840. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Gao, Y.; Du, J.; Zhang, W.; Huang, Y.; Wang, L.; Zhao, Q.; Pan, X. A novel, recyclable magnetic biochar modified by chitosan-EDTA for the effective removal of Pb(II) from aqueous solution. RSC Adv. 2020, 10, 40196. [Google Scholar] [CrossRef]
- Hou, R.; Ouyang, Z.; Li, Y.; Tyler, D.D.; Li, F.; Wilson, G.V. Effects of Tillage and Residue Management on Soil Organic Carbon and Total Nitrogen in the North China Plain. Soil Sci. Soc. Am. J. 2012, 76, 230–240. [Google Scholar] [CrossRef]
- Liang, J.; Yang, Z.; Tang, L.; Zang, G.; Yu, M.; Li, X.; Wu, H.; Qian, Y.; Li, X.; Luo, Y. Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 2017, 181, 281–288. [Google Scholar] [CrossRef]
- Xiao, F.; Cheng, J.; Cao, W.; Yang, C.; Chen, J.; Luo, Z. Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 2019, 540, 579–584. [Google Scholar] [CrossRef]
- Taraqqi-A-Kamal, A.; Atkinson, C.J.; Khan, A.; Zhang, K.; Sun, P.; Akther, S.; Zhang, Y. Biochar remediation of soil: Linking biochar production with function in heavy metal contaminated soils. Plant Soil Environ. 2021, 67, 183–201. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Tan, X.; Zeng, G.; Zhou, Y.; Liu, S.; Yin, Z.; Jiang, L.; Li, M.; Wen, J. The effect of several activated biochars on Cd immobilization and microbial community composition during in-situ remediation of heavy metal contaminated sediment. Sci. Total Environ. 2018, 208, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wen, J.; Wang, Q.; Xing, L.; Hu, X. Mobilization or immobilization? The effect of HDTMA-modified biochar on As mobility and bioavailability in soil. Ecotoxicol. Environ. Saf. 2021, 207, 111565. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Cui, P.-X.; Fang, G.-D.; Wang, Y.; Wang, S.-Q.; Zhou, D.-M.; Zhang, W.; Wang, Y.-J. Biochar decreased the bioavailability of Zn to rice and wheat grains: Insights from microscopic to macroscopic scales. Sci. Total Environ. 2018, 621, 160–167. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Manevski, K.; Andersen, M.N.; Li, Y.; Wei, Z.; Liu, F. Biochar and alternate wetting-drying cycles improving rhizosphere soil nutrients availability and tobacco growth by altering root growth strategy in Ferralsol and Anthrosol. Sci. Total Environ. 2022, 806, 150513. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Q.; Li, M.; Yuan, X.; Uchimiya, M.; Wang, S.; Zhang, Z.; Ji, T.; Wang, Y.; Zhao, Y. Rhizospheric pore-water content predicts the biochar-attenuated accumulation, translocation, and toxicity of cadmium to lettuce. Ecotoxicol. Environ. Saf. 2021, 208, 111675. [Google Scholar] [CrossRef] [PubMed]
- Shentu, J.; Li, X.; Han, R.; Chen, Q.; Shen, D.; Qi, S. Effect of site hydrological conditions and soil aggregate sizes on the stabilization of heavy metals (Cu, Ni, Pb, Zn) by biochar. Sci. Total Environ. 2022, 802, 149949. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Wang, X.; Chen, C.; Peng, B.; Tan, C.; Li, H. Varying effect of biochar on Cd, Pb and As mobility in a multi-metal contaminated paddy soil. Chemosphere 2016, 152, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Gao, Y.; Du, J.; Zhang, W.; Huang, Y.; Zhao, Q.; Duan, L.; Liu, Y.; Naidu, R.; Pan, X. Single and binary adsorption behaviour and mechanisms of Cd2+, Cu2+ and Ni2+ onto modified biochar in aqueous solutions. Processes 2021, 9, 1829. [Google Scholar] [CrossRef]
- Kiran, Y.K.; Barkat, A.; Cui, X.; Feng, Y.; Pan, F.; Tang, L.; Yang, X. Cow manure and cow manure-derived biochar application as a soil amendment for reducing cadmium availability and accumulation by Brassica chinensis L. in acidic red soil. J. Integr. Agric. 2017, 16, 725–734. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, J.; Chen, F.; Yu, R.; Hu, G.; Zhang, S. Remediation of soil polluted with Cd in a postmining area using thiourea-modified biochar. Int. J. Environ. Res. Public Health 2020, 17, 7654. [Google Scholar] [CrossRef]
- Li, H.; Ye, X.; Geng, Z.; Zhou, H.; Guo, X.; Zhang, Y.; Zhao, H.; Wang, G. The influence of biochar type on long-term stabilization for Cd and Cu in contaminated paddy soils. J. Hazard. Mater. 2016, 304, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Hu, H.; Fu, Q.; Li, Z.; Xing, Z.; Ali, U.; Zhu, J.; Liu, Y. Remediation of Pb, Cd, and Cu contaminated soil by co-pyrolysis biochar derived from rape straw and orthophosphate: Speciation transformation, risk evaluation and mechanism inquiry. Sci. Total Environ. 2020, 730, 139119. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Sohrabi, T. Distribution and contamination pattern of heavy metals from surface sediments in the southern part of Caspian Sea, Iran. Chem. Spec. Bioavailab. 2015, 27, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, H.; Wei, T.; Cao, G.; Yu, S.; Ren, X.; Jia, H.; Saleem, A.; Hua, L.; Guo, J.; Li, Y. Study of soil microorganisms modified wheat straw and biochar for reducing cadmium leaching potential and bioavailability. Chemosphere 2021, 273, 129644. [Google Scholar] [CrossRef] [PubMed]


| pH | SOM (g kg−1) | Total Cd Concentration (mg kg−1) | CaCl2-Extractable Cd Concentration (mg kg−1) | TCLP-Extractable Cd Concentration (mg kg−1) |
|---|---|---|---|---|
| 7.57 | 13.3 | 0.18 | 0.05 | 0.07 |
| pH | Pore Diameter (nm) | Pore Volume (cc g−1) | Surface Area (m2 g−1) | Element Content | |||
|---|---|---|---|---|---|---|---|
| C (%) | N (%) | O (%) | |||||
| BC | 9.44 | 5.35 | 0.044 | 49.26 | 83.9 | 2.72 | 13.38 |
| E–CBC | 8.17 | 12.38 | 0.017 | 6.06 | 65.33 | 6.77 | 27.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.; Ji, H.; Gao, Y.; Yang, Z.; Ji, L.; Zhao, Q.; Liu, Y.; Pan, X. Effects of Modified Biochar on the Mobility and Speciation Distribution of Cadmium in Contaminated Soil. Processes 2022, 10, 818. https://doi.org/10.3390/pr10050818
Zheng L, Ji H, Gao Y, Yang Z, Ji L, Zhao Q, Liu Y, Pan X. Effects of Modified Biochar on the Mobility and Speciation Distribution of Cadmium in Contaminated Soil. Processes. 2022; 10(5):818. https://doi.org/10.3390/pr10050818
Chicago/Turabian StyleZheng, Liwen, Hongying Ji, Yongchao Gao, Zhongfeng Yang, Lei Ji, Qingqing Zhao, Yanju Liu, and Xiangliang Pan. 2022. "Effects of Modified Biochar on the Mobility and Speciation Distribution of Cadmium in Contaminated Soil" Processes 10, no. 5: 818. https://doi.org/10.3390/pr10050818
APA StyleZheng, L., Ji, H., Gao, Y., Yang, Z., Ji, L., Zhao, Q., Liu, Y., & Pan, X. (2022). Effects of Modified Biochar on the Mobility and Speciation Distribution of Cadmium in Contaminated Soil. Processes, 10(5), 818. https://doi.org/10.3390/pr10050818








