A New LC-MS Method for Evaluating the Efficacy of Pesticide Residue Removal from Fruit Surfaces by Washing Agents
Abstract
:1. Introduction
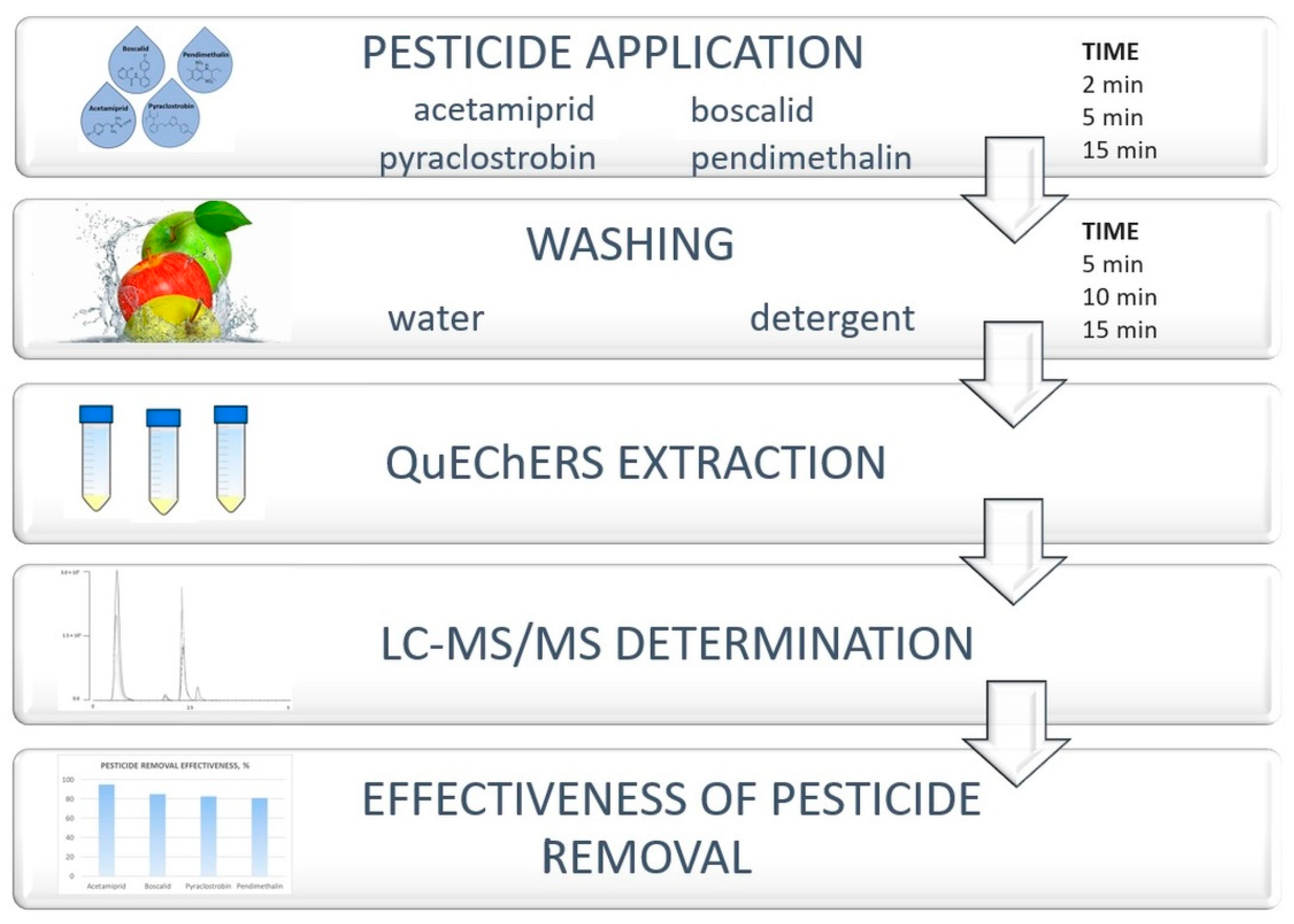
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Sample Preparation
2.3.1. Sample Spiking with Pesticides
2.3.2. Washing Procedure
2.3.3. QuEChERS Extraction
2.4. LC-MS/MS Analysis
2.4.1. MS/MS Parameter Optimization
2.4.2. Chromatographic Condition Optimization
2.4.3. Quantitative Analysis
2.5. Validation of the UHPLC-MS/MS Method
2.5.1. System Suitability Test
2.5.2. Selectivity
2.5.3. Matrix Effect
2.5.4. Accuracy and Precision
2.5.5. Linearity
2.5.6. Limit of Detection and Limit of Quantification
2.5.7. Stability
2.6. Pesticide Removal Efficacy Study
2.7. Statistical Analysis
3. Results and Discussion
3.1. Development of UHPLC-MS/MS Method
3.2. Analytical Method Validation
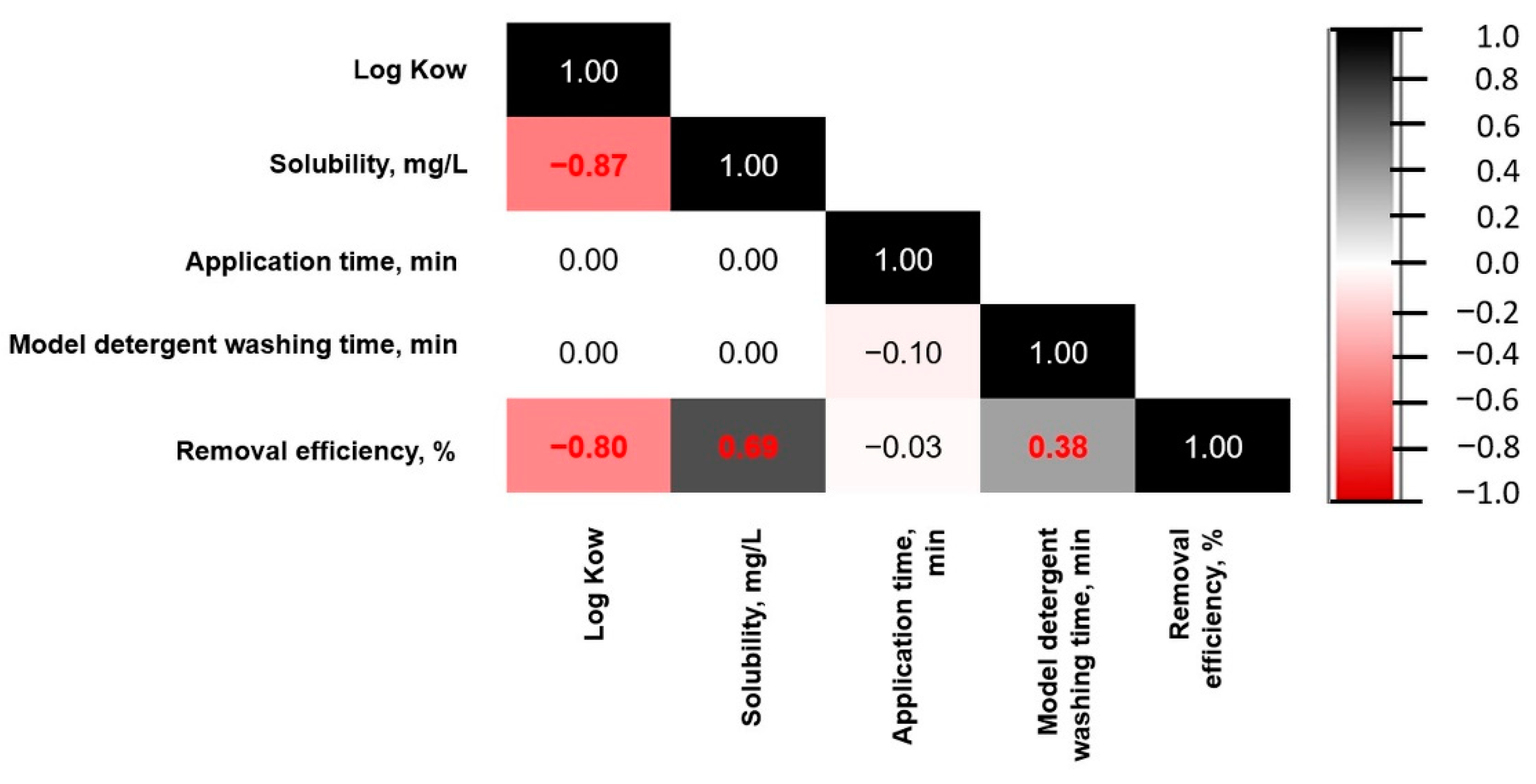
3.3. Pesticide Residuals Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bassil, K.; Vakil, C.; Sanborn, M.; Cole, D.; Kaur, J.; Kerr, K. Cancer Health Effects of Pesticides: Systematic Review. Can. Fam. Phys. 2007, 53, 1704–1711. [Google Scholar]
- Mostafalou, S.; Abdollahi, M. Pesticides and Human Chronic Diseases: Evidences, Mechanisms, and Perspectives. Toxicol. Appl. Pharmacol. 2013, 268, 157–177. [Google Scholar] [CrossRef]
- Bjørling-Poulsen, M.; Andersen, H.R.; Grandjean, P. Potential Developmental Neurotoxicity of Pesticides Used in Europe. Environ. Health 2008, 7, 50. [Google Scholar] [CrossRef] [Green Version]
- Lončarić, A.; Matanović, K.; Ferrer, P.; Kovač, T.; Šarkanj, B.; Skendrović Babojelić, M.; Lores, M. Peel of Traditional Apple Varieties as a Great Source of Bioactive Compounds: Extraction by Micro-Matrix Solid-Phase Dispersion. Foods 2020, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.-Y.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, Vegetables, and Health: A Comprehensive Narrative, Umbrella Review of the Science and Recommendations for Enhanced Public Policy to Improve Intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef] [Green Version]
- Żurawicz, E.; Kubik, J.; Lewandowski, M.; Rutkowski, K.P.; Zmarlicki, K. The apple industry in Poland. Acta Hortic. 2019, 1261, 13–20. [Google Scholar] [CrossRef]
- Łozowicka, B.; Kaczyński, P.; Rutkowska, E.; Jankowska, M.; Hrynko, I. Evaluation of Pesticide Residues in Fruit from Poland and Health Risk Assessment. Agric. Sci. 2013, 4, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Kowalska, G.; Kowalski, R. Pestycydy–Zakres i Ryzyko Stosowania, Korzyści i Zagrożenia-Praca Przeglądowa. Pesticides–Scope and Risk of Use, Benefits and Hazards. A Review. Ann. Hortic. 2020, 29, 5–25. [Google Scholar] [CrossRef]
- Jankowska, M.; Łozowicka, B.; Kaczyński, P. Comprehensive Toxicological Study over 160 Processing Factors of Pesticides in Selected Fruit and Vegetables after Water, Mechanical and Thermal Processing Treatments and Their Application to Human Health Risk Assessment. Sci. Total Environ. 2019, 652, 1156–1167. [Google Scholar] [CrossRef]
- Szpyrka, E.; Slowik-Borowiec, M.; Matyaszek, A.; Podbielska, M.; Rupar, J. Pesticide residues in raw agricultural products from the south-eastern region of Poland and the acute risk assessment. Rocz. Panstw. Zakl. Hig. 2016, 67, 237–245. [Google Scholar] [PubMed]
- Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels of Pesticides in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC (Text with EEA Relevance). Available online: https://www.legislation.gov.uk/eur/2005/396/contents (accessed on 12 April 2022).
- Góralczyk, K.; Struciński, P.; Korcz, W.; Czaja, K.; Hernik, A.; Snopczyński, T.; Ludwicki, J. The Survey of Pesticide Residues in Food of Plant Origin in Poland, 2004–2007. Rocz. Panstw. Zakładu Zakl. Hig. 2009, 60, 113–119. [Google Scholar]
- Analiza Potencjalnego Zagrożenia Zdrowia Konsumentów Wynikającego z Obecności Pozostałości Pestycydów w Żywności Dostępnej na Polskim Rynku w Roku 2017, Narodowy Instytut Zdrowia Publicznego PZH–Państwowy Instytut Badawczy 31.12.2019. Available online: https://www.pzh.gov.pl/wp-content/uploads/2020/03/Raport-Analiza-potencjalnego-zagro%C5%BCenia-zdrowia-konsument%C3%B3w-...-pozosta%C5%82o%C5%9Bci-pestycyd%C3%B3w-2017-na-stron%C4%99.pdf (accessed on 21 December 2021).
- Łozowicka, B.; Jankowska, M. Comparison of the effects of water and thermal processing on pesticide removal in selected fruit and vegetables. J. Elem. 2016, 21, 99–111. [Google Scholar]
- Wu, Y.; An, Q.; Li, D.; Wu, J.; Pan, C. Comparison of Different Home/Commercial Washing Strategies for Ten Typical Pesticide Residue Removal Effects in Kumquat, Spinach and Cucumber. Int. J. Environ. Res. Public Health 2019, 16, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Huang, J.; Chen, J.; Li, F. Effectiveness of Dishwashing Liquids in Removing Chlorothalonil and Chlorpyrifos Residues from Cherry Tomatoes. Chemosphere 2013, 92, 1022–1028. [Google Scholar] [CrossRef]
- Soltani Nazarloo, A.; Rasooli Sharabiani, V.; Abbaspour Gilandeh, Y.; Taghinezhad, E.; Szymanek, M.; Sprawka, M. Feasibility of Using VIS/NIR Spectroscopy and Multivariate Analysis for Pesticide Residue Detection in Tomatoes. Processes 2021, 9, 196. [Google Scholar] [CrossRef]
- Yang, T.; Doherty, J.; Zhao, B.; Kinchla, A.J.; Clark, J.M.; He, L. Effectiveness of Commercial and Homemade Washing Agents in Removing Pesticide Residues on and in Apples. J. Agric. Food Chem. 2017, 65, 9744–9752. [Google Scholar] [CrossRef]
- Bakırcı, G.T.; Hışıl, Y. Fast and Simple Extraction of Pesticide Residues in Selected Fruits and Vegetables Using Tetrafluoroethane and Toluene Followed by Ultrahigh-Performance Liquid Chromatography/Tandem Mass Spectrometry. Food Chem. 2012, 135, 1901–1913. [Google Scholar] [CrossRef]
- Han, Y.; Zou, N.; Song, L.; Li, Y.; Qin, Y.; Liu, S.; Li, X.; Pan, C. Simultaneous Determination of 70 Pesticide Residues in Leek, Leaf Lettuce and Garland Chrysanthemum Using Modified QuEChERS Method with Multi-Walled Carbon Nanotubes as Reversed-Dispersive Solid-Phase Extraction Materials. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1005, 56–64. [Google Scholar] [CrossRef]
- Botero-Coy, A.M.; Marín, J.M.; Serrano, R.; Sancho, J.V.; Hernández, F. Exploring Matrix Effects in Liquid Chromatography–Tandem Mass Spectrometry Determination of Pesticide Residues in Tropical Fruits. Anal. Bioanal. Chem. 2015, 407, 3667–3681. [Google Scholar] [CrossRef] [Green Version]
- Golge, O.; Kabak, B. Evaluation of QuEChERS Sample Preparation and Liquid Chromatography-Triple-Quadrupole Mass Spectrometry Method for the Determination of 109 Pesticide Residues in Tomatoes. Food Chem. 2015, 176, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Mol, H.G.J.; Zomer, P.; García López, M.; Fussell, R.J.; Scholten, J.; de Kok, A.; Wolheim, A.; Anastassiades, M.; Lozano, A.; Fernandez Alba, A. Identification in Residue Analysis Based on Liquid Chromatography with Tandem Mass Spectrometry: Experimental Evidence to Update Performance Criteria. Anal. Chim. Acta 2015, 873, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Núñez, O.; Gallart-Ayala, H.; Ferrer, I.; Moyano, E.; Galceran, M.T. Strategies for the Multi-Residue Analysis of 100 Pesticides by Liquid Chromatography–Triple Quadrupole Mass Spectrometry. J. Chromatogr. A 2012, 1249, 164–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Morgan, M.K.; Graham, S.E.; Starr, J.M. Measurement of Pyrethroids and Their Environmental Degradation Products in Fresh Fruits and Vegetables Using a Modification of the Quick Easy Cheap Effective Rugged Safe (QuEChERS) Method. Talanta 2016, 151, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Prodhan, M.; Papadakis, E.-N.; Papadopoulou-Mourkidou, E. Analysis of Pesticide Residues and Their Variability in Cabbage Using QuEChERS Extraction in Combination with LC-MS/MS. Food Anal. Methods 2016, 9, 3470–3478. [Google Scholar] [CrossRef]
- Stachniuk, A.; Szmagara, A.; Czeczko, R.; Fornal, E. LC-MS/MS Determination of Pesticide Residues in Fruits and Vegetables. J. Environ. Sci. Health Part B 2017, 52, 446–457. [Google Scholar] [CrossRef]
- Dong, F.; Chen, X.; Liu, X.; Xu, J.; Li, Y.; Shan, W.; Zheng, Y. Simultaneous Determination of Five Pyrazole Fungicides in Cereals, Vegetables and Fruits Using Liquid Chromatography/Tandem Mass Spectrometry. J Chromatogr. A 2012, 1262, 98–106. [Google Scholar] [CrossRef]
- Tian, F.; Qiao, C.; Luo, J.; Guo, L.; Pang, T.; Pang, R.; Li, J.; Wang, C.; Wang, R.; Xie, H. Development of a Fast Multi-Residue Method for the Determination of Succinate Dehydrogenase Inhibitor Fungicides in Cereals, Vegetables and Fruits by Modified QuEChERS and UHPLC-MS/M.MS. J. Chromatogr. B 2020, 1152, 122261. [Google Scholar] [CrossRef]
- Concha-Meyer, A.; Grandon, S.; Sepúlveda, G.; Diaz, R.; Yuri, J.A.; Torres, C. Pesticide Residues Quantification in Frozen Fruit and Vegetables in Chilean Domestic Market Using QuEChERS Extraction with Ultra-High-Performance Liquid Chromatography Electrospray Ionization Orbitrap Mass Spectrometry. Food Chem. 2019, 295, 64–71. [Google Scholar] [CrossRef]
- Stringhini, F.; Ribeiro, L.; Rocha, G.; Kuntz, J.; Zanella, R.; Prestes, O.; Adaime, M. Dilution of QuEChERS Extracts Without Cleanup Improves Results in the UHPLC-MS/MS Multiresidue Analysis of Pesticides in Tomato. Food Methods 2021, 14, 1511–1523. [Google Scholar] [CrossRef]
- Guo, J.; Tong, M.; Tang, J.; Bian, H.; Wan, X.; He, L.; Hou, R. Analysis of Multiple Pesticide Residues in Polyphenol-Rich Agricultural Products by UPLC-MS/MS Using a Modified QuEChERS Extraction and Dilution Method. Food 2019, 274, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Hajslová, J.; Zrostlíková, J. Matrix Effects in (Ultra)Trace Analysis of Pesticide Residues in Food and Biotic Matrices. J. Chromatogr. A 2003, 1000, 181–197. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.; štajnbaher, D.; Schenck, F. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehotay, S.; Kok, A.; Hiemstra, M.; Bodegraven, P. Validation of a Fast and Easy Method for the Determination of Residues from 229 Pesticides in Fruits and Vegetables Using Gas and Liquid Chromatography and Mass Spectrometric Detection. J. AOAC Int. 2005, 88, 595–614. [Google Scholar] [CrossRef] [Green Version]
- Lehotay, S.; Mastovska, K.; Lightfield, A. Use of Buffering Other Means to Improve Results of Problematic Pesticides in a Fast Easy Method for Residue Analysis of Fruits Vegetables. J. AOAC Int. 2005, 88, 615–629. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.D.; Lee, B.S.; Lee, B.R.; Lee, D.M.; Lee, G.-H. A Multiresidue Method for the Determination of 109 Pesticides in Rice Using the Quick Easy Cheap Effective Rugged and Safe (QuEChERS) Sample Preparation Method and Gas Chromatography/Mass Spectrometry with Temperature Control and Vacuum Concentration. Rapid Commun. Mass Spectrom. 2007, 21, 3115–3122. [Google Scholar] [CrossRef]
- EN 15662:2008—Foods of Plant Origin-Determination of Pesticide Residues Using GC-MS and/or LC-MS/MS Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE-QuEChERS-Method. Available online: https://standards.iteh.ai/catalog/standards/cen/9f9e56e8-ac1c-4f3e-9f91-23d42703dd8a/en-15662-2008 (accessed on 6 March 2022).
- Lequang, H. AOAC Official Method 2007.01 Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate Gas Chromatography/Mass Spectrometry and Liquid Chromatography/Tandem Mass Spectrometry First Action 2007. Available online: https://www.academia.edu/33471645/AOAC_Official_Method_2007_01_Pesticide_Residues_in_Foods_by_Acetonitrile_Extraction_and_Partitioning_with_Magnesium_Sulfate_Gas_Chromatography_Mass_Spectrometry_and_Liquid_Chromatography_Tandem_Mass_Spectrometry_First_Action_2007 (accessed on 6 February 2022).
- Kaushik, G.; Satya, S.; Naik, S.N. Food Processing a Tool to Pesticide Residue Dissipation—A Review. Food Res. 2009, 42, 26–40. [Google Scholar] [CrossRef]
- Hao, J.; Liu, H.; Chen, T.; Zhou, Y.; Su, Y.-C.; Li, L. Reduction of Pesticide Residues on Fresh Vegetables with Electrolyzed Water Treatment. J. Food Sci. 2011, 76, C520–C524. [Google Scholar] [CrossRef]
- Qi, H.; Huang, Q.; Hung, Y.-C. Effectiveness of Electrolyzed Oxidizing Water Treatment in Removing Pesticide Residues and Its Effect on Produce Quality. Food Chem. 2018, 239, 561–568. [Google Scholar] [CrossRef]
- European Commission. Technical Material and Preparations: Guidance for Generating and Reporting Methods of Analysis in Support of Pre- and Post-Registration Data Requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99-Rev.4; European Commission: Brussels, Belgium, 2000. [Google Scholar]
- European Commission. Guidance Document on Residue Analytical Methods. SANCO/825/00-Rev. 8.1; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Melnyk, A.; Namieśnik, J.; Wolska, L. Theory and Recent Applications of Coacervate-Based Extraction Techniques. Trends Anal. Chem. 2015, 71, 282–292. [Google Scholar] [CrossRef]
- Arya, S.S.; Kaimal, A.M.; Chib, M.; Sonawane, S.K.; Show, P.L. Novel, Energy Efficient and Green Cloud Point Extraction: Technology and Applications in Food Processing. J. Food Sci. Technol. 2019, 56, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Trufelli, H.; Palma, P.; Famiglini, G.; Cappiello, A. An Overview of Matrix Effects in Liquid Chromatography-Mass Spectrometry. Mass Spectrom. Rev. 2011, 30, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.A.Z.; de Queiroz, M.E.L.R.; Neves, A.A.; de Oliveira, A.F.; Prates, L.H.F.; de Freitas, J.F.; Heleno, F.F.; Faroni, L.R.D. Use of Ozone and Detergent for Removal of Pesticides and Improving Storage Quality of Tomato. Food Res. Int. 2019, 125, 108626. [Google Scholar] [CrossRef] [PubMed]
- Christia, C.; Bizani, E.; Christophoridis, C.; Fytianos, K. Pesticide Residues in Fruit Samples: Comparison of Different QuEChERS Methods Using Liquid Chromatography–Tandem Mass Spectrometry. Environ. Sci. Pollut. Res. 2015, 22, 13167–13178. [Google Scholar] [CrossRef]
- Mandal, S.; Poi, R.; Ansary, I.; Hazra, D.K.; Bhattacharyya, S.; Karmakar, R. Validation of a Modified QuEChERS Method to Determine Multiclass Multipesticide Residues in Apple, Banana and Guava Using GC–MS and LC–MS/MS and Its Application in Real Sample Analysis. SN Appl. Sci. 2020, 2, 188. [Google Scholar] [CrossRef] [Green Version]
- Masiá, A.; Blasco, C.; Picó, Y. Last Trends in Pesticide Residue Determination by Liquid Chromatography–Mass Spectrometry. Trends Environ. Anal. Chem. 2014, 2, 11–24. [Google Scholar] [CrossRef]
- Nguyen, D.T.-T.; Guillarme, D.; Rudaz, S.; Veuthey, J.-L. Fast Analysis in Liquid Chromatography Using Small Particle Size and High Pressure. J. Sep. Sci. 2006, 29, 1836–1848. [Google Scholar] [CrossRef]
- Walter, T.H.; Andrews, R.W. Recent Innovations in UHPLC Columns and Instrumentation. Trends Anal. Chem. 2014, 63, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Chow, W.; Chang, J.; Wong, J.W. Ultrahigh-Performance Liquid Chromatography Electrospray Ionization Q-Orbitrap Mass Spectrometry for the Analysis of 451 Pesticide Residues in Fruits and Vegetables: Method Development and Validation. J. Agric. Food Chem. 2014, 62, 10375–10391. [Google Scholar] [CrossRef]
- Stachniuk, A.; Fornal, E. Liquid Chromatography-Mass Spectrometry in the Analysis of Pesticide Residues in Food. Food Anal. Methods 2016, 9, 1654–1665. [Google Scholar] [CrossRef] [Green Version]
- Botitsi, H.V.; Garbis, S.D.; Economou, A.; Tsipi, D.F. Current Mass Spectrometry Strategies for the Analysis of Pesticides and Their Metabolites in Food and Water Matrices. Mass Spectrom. Rev. 2010, 30, 907–939. [Google Scholar] [CrossRef] [PubMed]
- Kittlaus, S.; Schimanke, J.; Kempe, G.; Speer, K. Assessment of Sample Cleanup and Matrix Effects in the Pesticide Residue Analysis of Foods Using Postcolumn Infusion in Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 8399–8410. [Google Scholar] [CrossRef] [PubMed]
- Dozwolone Substancje Dodatkowe. Dz.U.2010.232.1525-OpenLEX. Available online: https://sip.lex.pl/akty-prawne/dzu-dziennik-ustaw/dozwolone-substancje-dodatkowe-17665215 (accessed on 8 March 2022).
- Valverde, A.; Aguilera, A.; Rodríguez, M.; Boulaid, M.; Soussi-El Begrani, M. Pesticide Residue Levels in Peppers Grown in a Greenhouse after Multiple Applications of Pyridaben and Tralomethrin. J. Agric. Food Chem. 2002, 50, 7303–7307. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W. How Effective Are Common Household Preparations on Removing Pesticide Residues from Fruit and Vegetables? A Review: Removal of Pesticide Residues. J. Sci. Food Agric. 2018, 98, 2857–2870. [Google Scholar] [CrossRef]
- Boulaid, M.; Aguilera, A.; Camacho, F.; Soussi, M.; Valverde, A. Effect of Household Processing and Unit-to-Unit Variability of Pyrifenox, Pyridaben, and Tralomethrin Residues in Tomatoes. J. Agric. Food Chem. 2005, 53, 4054–4058. [Google Scholar] [CrossRef]
- Finizio, A.; Vighi, M.; Sandroni, D. Determination of N-Octanol/Water Partition Coefficient (Kow) of Pesticide Critical Review and Comparison of Methods. Chemosphere 1997, 34, 131–161. [Google Scholar] [CrossRef]
- Rao, C.S.; Bhushan, V.S.; Reddy, H.; Darsi, R.; Aruna, M.; Ramesh, B. Risk Mitigation Methods for Removal of Pesticide Residues in Brinjal for Food Safety. Univers. J. Agric. Agric. Res. 2014, 2, 279–283. [Google Scholar] [CrossRef]
- Tomer, V.; Sangha, J.; Singh, B.; Takkar, R. Efficacy of Processing Treatments on Cypermethrin Residues in Okra (Abelmoschus Esculentus). Nutr. Food Sci. 2014, 44, 545–553. [Google Scholar] [CrossRef]
- Yu-shan, Z.; Xiao-peng, L.I.; Hong-mei, L.I.U.; Yao-kun, Z.; Fan-fei, Z.; Qin-jie, Y.U.; Hao, L.I.; Jian-wen, C. Study on Universal Cleaning Solution in Removing Blended Pesticide Residues in Chinese Cabbage. J. Environ. Chem. Ecotoxicol. 2013, 5, 202–207. [Google Scholar] [CrossRef]
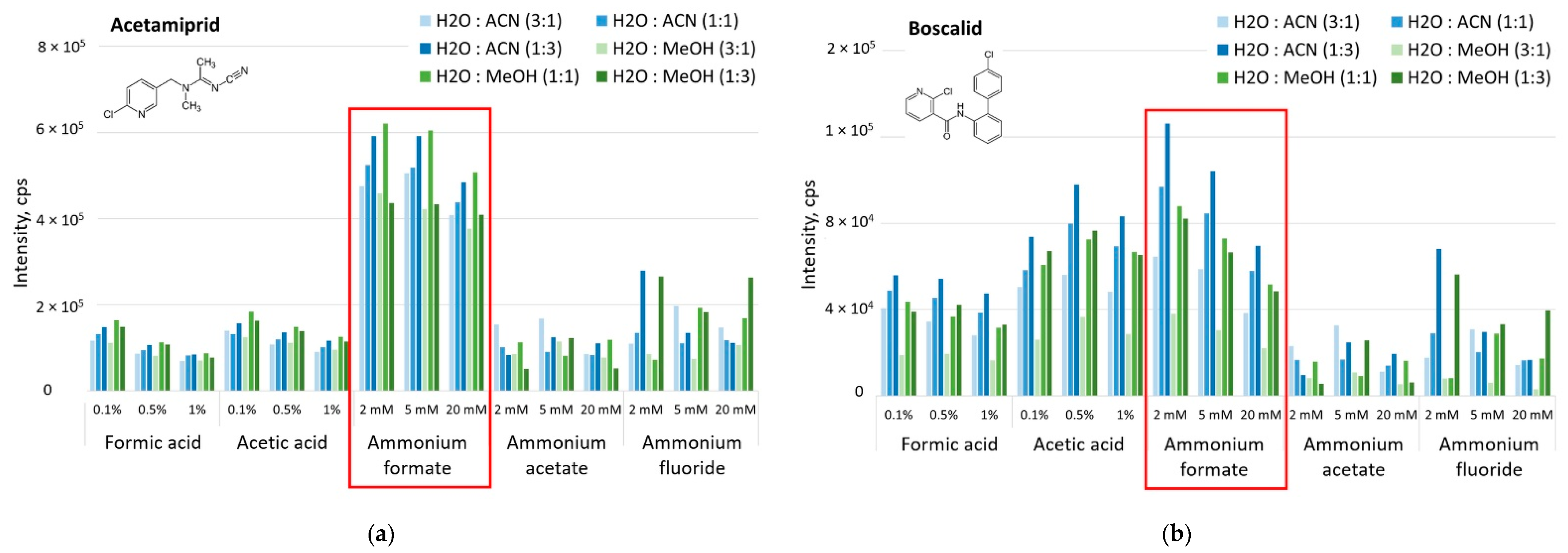

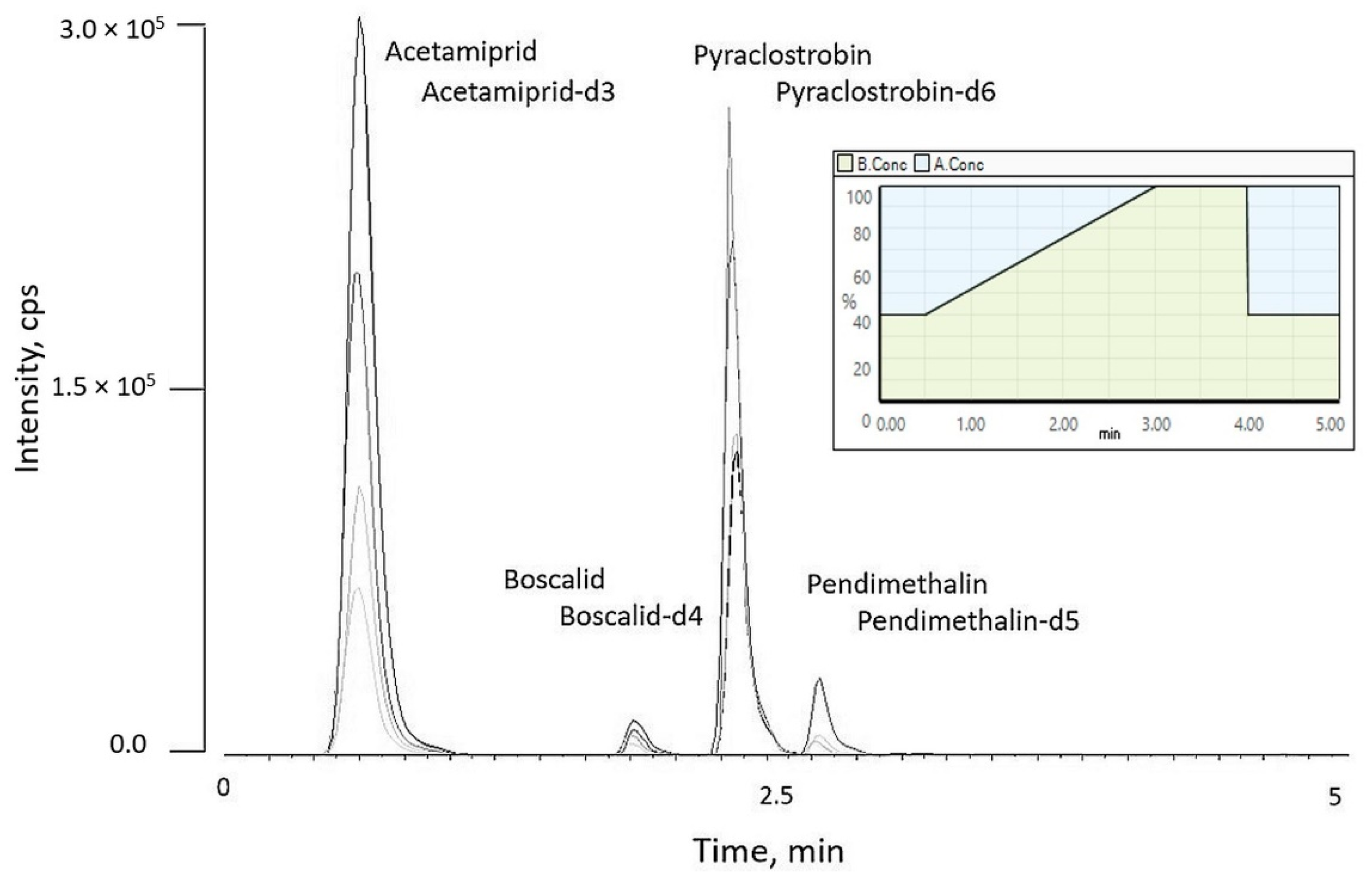
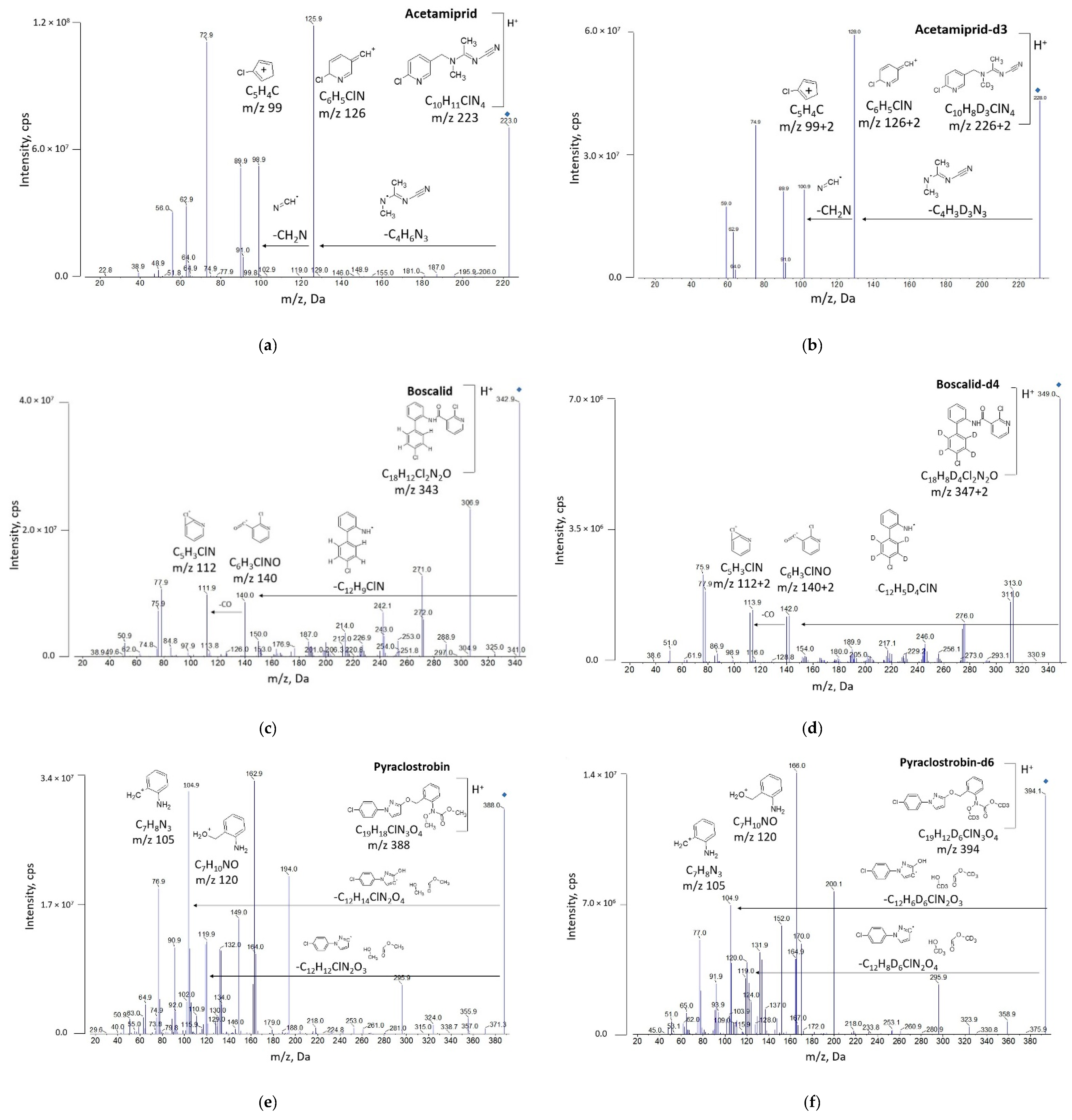
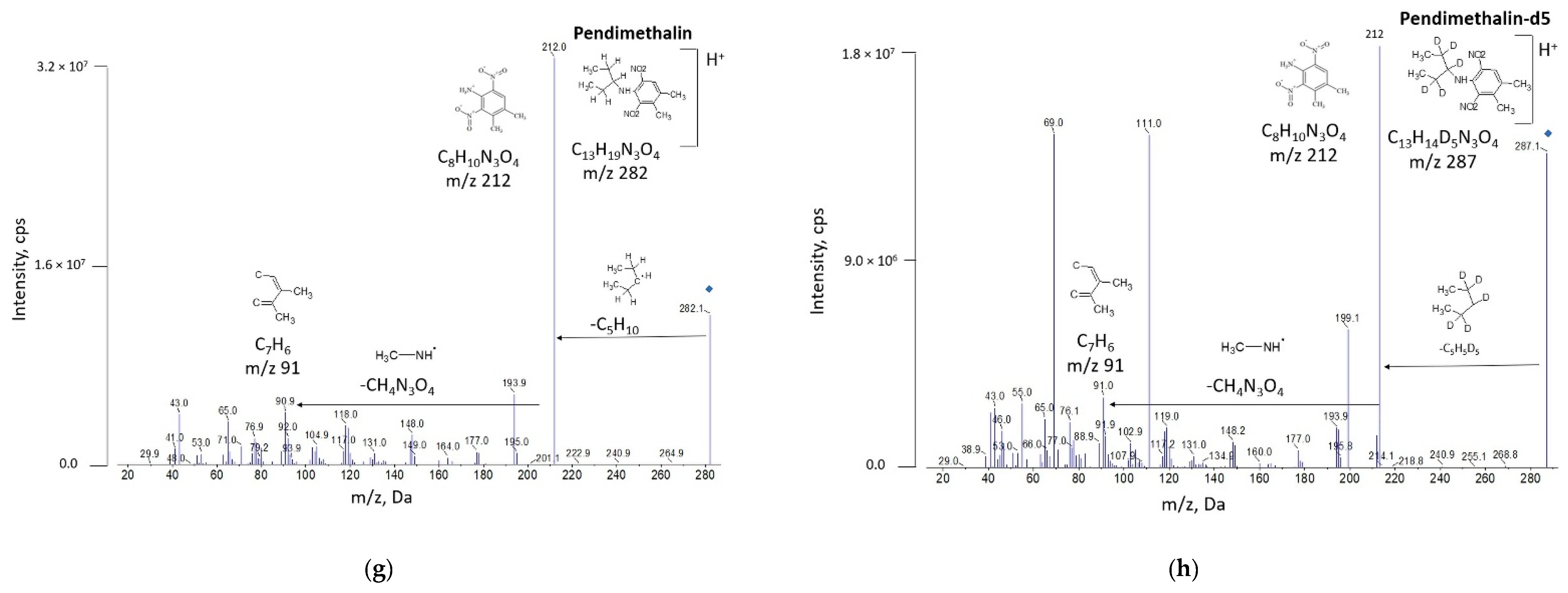


| Pesticide | Acetamiprid | Boscalid | Pyraclostrobin | Pendimethalin |
|---|---|---|---|---|
| Chemical structure | 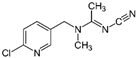 |  | 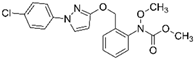 |  |
| Chemical formula | C10H11ClN4 | C18H12Cl2N2O | C19H18ClN3O4 | C13H19N3O4 |
| Molecular mass, Da | 222 | 342 | 387 | 281 |
| Category | Insecticide | Fungicide | Fungicide | Herbicide |
| Chemical group | Neonicotinoid | Carboxamide | Strobilurin | Dinitroaniline |
| Mode of action | Systemic | Systemic | Systemic | Non-systemic |
| Molar mass, g/mol | 222.67 | 343.21 | 387.82 | 281.31 |
| Threshold of toxicological concern * | High (Class III) | |||
| MRL for apple, mg/kg | 0.4 | 3 | 0.5 | 0.05 |
| Water solubility 20 °C, mg/L | 2950 | 4.6 | 1.9 | 0.33 |
| Log Kow in 20 °C pH 7 | 0.8 | 2.96 | 3.99 | 5.2 |
| pKa | 0.7 | Not applicable | −0.23 | 2.8 |
| Name According to INCI Nomenclature | Concentration, wt.% |
|---|---|
| MD | |
| Laureth-4 | 3.8 |
| Laureth-10 | 6.2 |
| Aqua | to 100 |
| Sodium citrate | 5.0 |
| Benzyl alcohol Benzoic acid Dehydroacetic acid Tocopherol | 0.5 |
| Validation Parameter | Acetamiprid | Boscalid | Pendimethalin | Pyraclostrobin | Acceptance Criterion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 223 > 126 | 223 > 99 | 343 > 140 | 343 > 112 | 282 > 212 | 282 > 91 | 388 > 105 | 388 > 120 | ||||
| SST | 2.2 | 3.4 | 6.0 | 4.0 | 5.2 | 9.6 | 1.5 | 1.9 | <10% | ||
| Selectivity | ΔtR, min | LQC | 0.02 | 0.05 | 0.03 | 0.04 | <±0.1 min | ||||
| HQC | 0.01 | 0.03 | 0.02 | 0.03 | |||||||
| conf. IRD, % | LQC | 4.1 | −0.8 | −8.3 | −8.9 | ±30% | |||||
| HQC | 4.0 | −1.4 | −11 | −6.3 | |||||||
| S/N ≥ 3 | 30.8 | 6.4 | 12.5 | 8.5 | 49 | 37.2 | 18.5 | 9.5 | ≥3 | ||
| Matrix effect, % | LQC | −4 | 1 | 0 | 0 | −1 | 2 | −8 | 1 | negligible < 20% | |
| HQC | 1 | 2 | −2 | 3 | −2 | 3 | −2 | 5 | |||
| Precision, % | LQC | 3.6 | 5.8 | 6.9 | 6.1 | 8.5 | 13.7 | 10.0 | 3.7 | RSD < 20% | |
| HQC | 1.1 | 3.2 | 4.8 | 3.9 | 2.7 | 2.3 | 2.1 | 3.0 | |||
| Accuracy, % | LQC | 98 | 98 | 103 | 90 | 99 | 97 | 89 | 94 | 70–110% | |
| HQC | 101 | 99 | 99 | 105 | 105 | 99 | 103 | 99 | |||
| Linearity | R | 0.9997 | 0.9999 | 0.9990 | 0.9963 | 0.9986 | 0.9979 | 0.9996 | 0.9991 | R > 0.99 | |
| Calibr. curve equation | y = 38.4x + 0.00194 y = 38.3x − 0.00064 | y = 52.4x + 0.00564 y = 48.3x + 0.0141 | y = 147x + 0.00147 y = 38.4x + 0.00085 | y = 15.4x + 0.00672 y = 15.7x + 0.00732 | |||||||
| Calibr. range | 0.003–0.4 mg/kg (0.0003–0.04 μg/mL) | 30% LOQ—30xLOQ | |||||||||
| LOD | 0.003 mg/kg (0.0003 μg/mL) | <30% LOQ | |||||||||
| LOQ | 0.01 mg/kg (0.001 μg/mL) | 0.01 mg/kg | |||||||||
| Stability, % | LQC | 2.9 | 5.7 | −7.2 | −2.3 | 2.2 | −5.3 | −1.8 | −3.2 | <10% | |
| HQC | 2.0 | −2.0 | 7.6 | −2.6 | 3.6 | 0.5 | 3.7 | −1.2 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarębska, M.; Hordyjewicz-Baran, Z.; Wasilewski, T.; Zajszły-Turko, E.; Stanek, N. A New LC-MS Method for Evaluating the Efficacy of Pesticide Residue Removal from Fruit Surfaces by Washing Agents. Processes 2022, 10, 793. https://doi.org/10.3390/pr10040793
Zarębska M, Hordyjewicz-Baran Z, Wasilewski T, Zajszły-Turko E, Stanek N. A New LC-MS Method for Evaluating the Efficacy of Pesticide Residue Removal from Fruit Surfaces by Washing Agents. Processes. 2022; 10(4):793. https://doi.org/10.3390/pr10040793
Chicago/Turabian StyleZarębska, Magdalena, Zofia Hordyjewicz-Baran, Tomasz Wasilewski, Ewa Zajszły-Turko, and Natalia Stanek. 2022. "A New LC-MS Method for Evaluating the Efficacy of Pesticide Residue Removal from Fruit Surfaces by Washing Agents" Processes 10, no. 4: 793. https://doi.org/10.3390/pr10040793
APA StyleZarębska, M., Hordyjewicz-Baran, Z., Wasilewski, T., Zajszły-Turko, E., & Stanek, N. (2022). A New LC-MS Method for Evaluating the Efficacy of Pesticide Residue Removal from Fruit Surfaces by Washing Agents. Processes, 10(4), 793. https://doi.org/10.3390/pr10040793






