Synthesis and Characterization of Bi2WxMo1−xO6 Solid Solutions and Their Application in Photocatalytic Desulfurization under Visible Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bi2WxMo1−xO6 Solid Solutions
2.2. Characterization
2.3. Photocatalytic Oxidative Desulfurization
2.4. Active Species Trapping Experiments
2.5. Stability Test
3. Results and Discussion
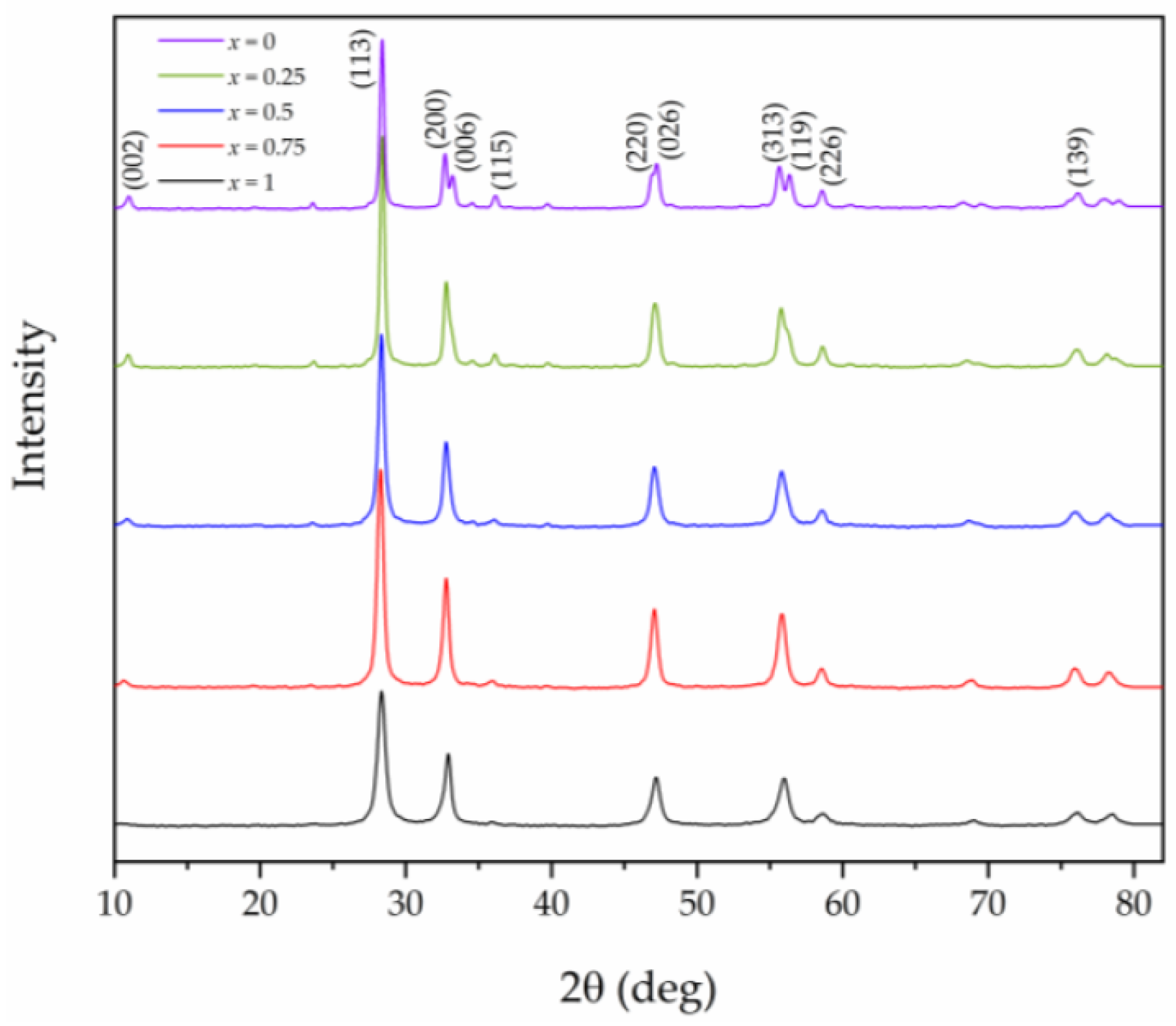
3.1. XRD Analysis
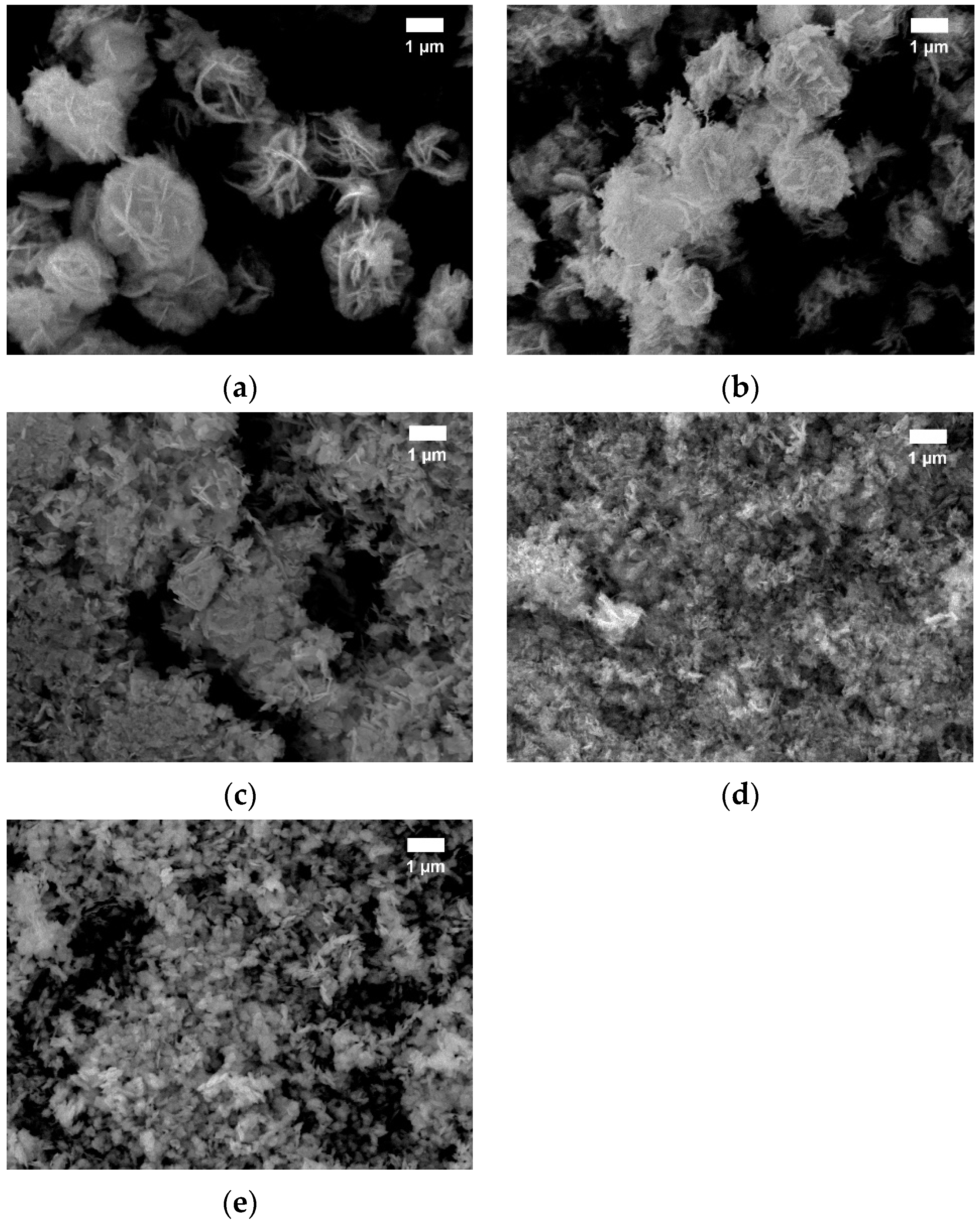
3.2. Morphology
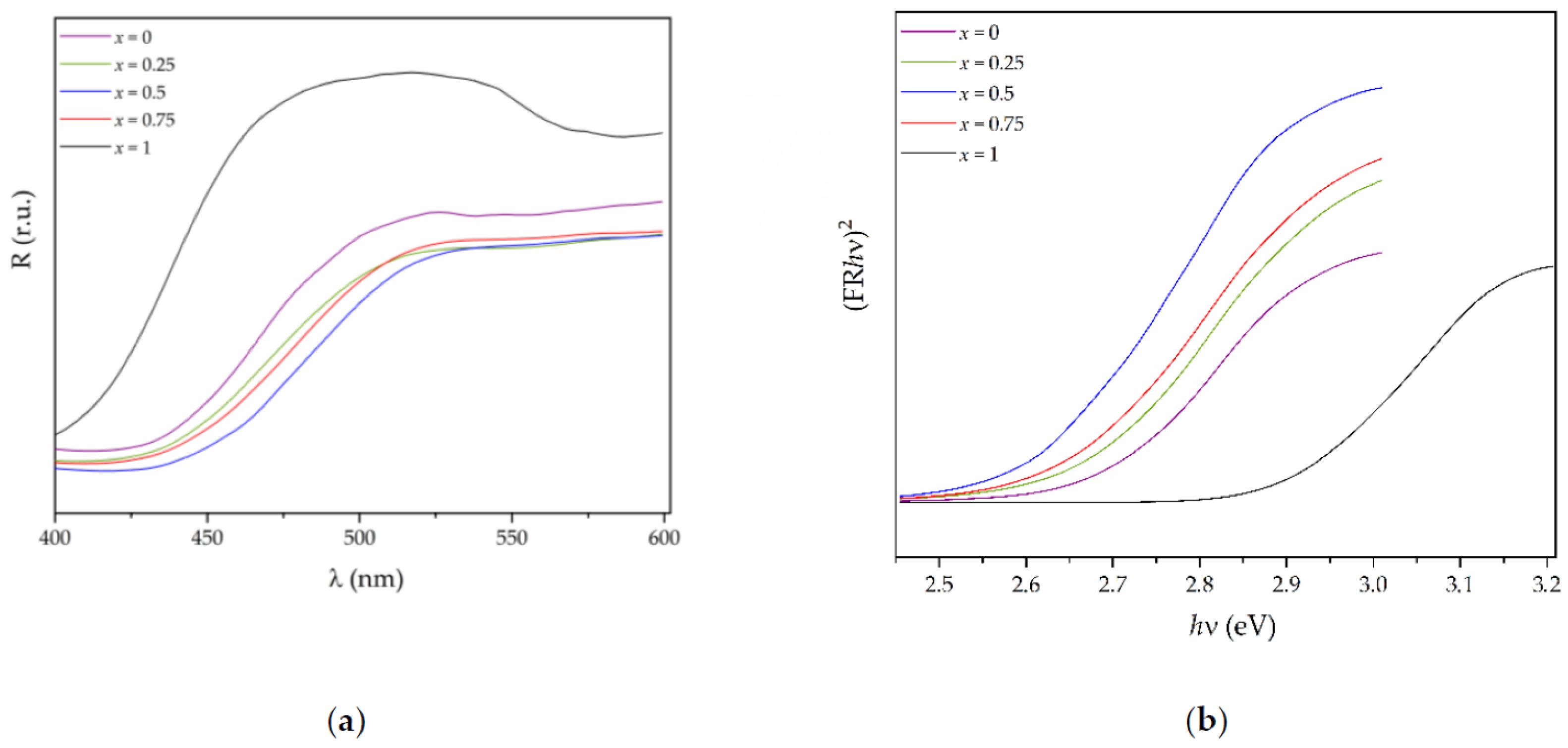
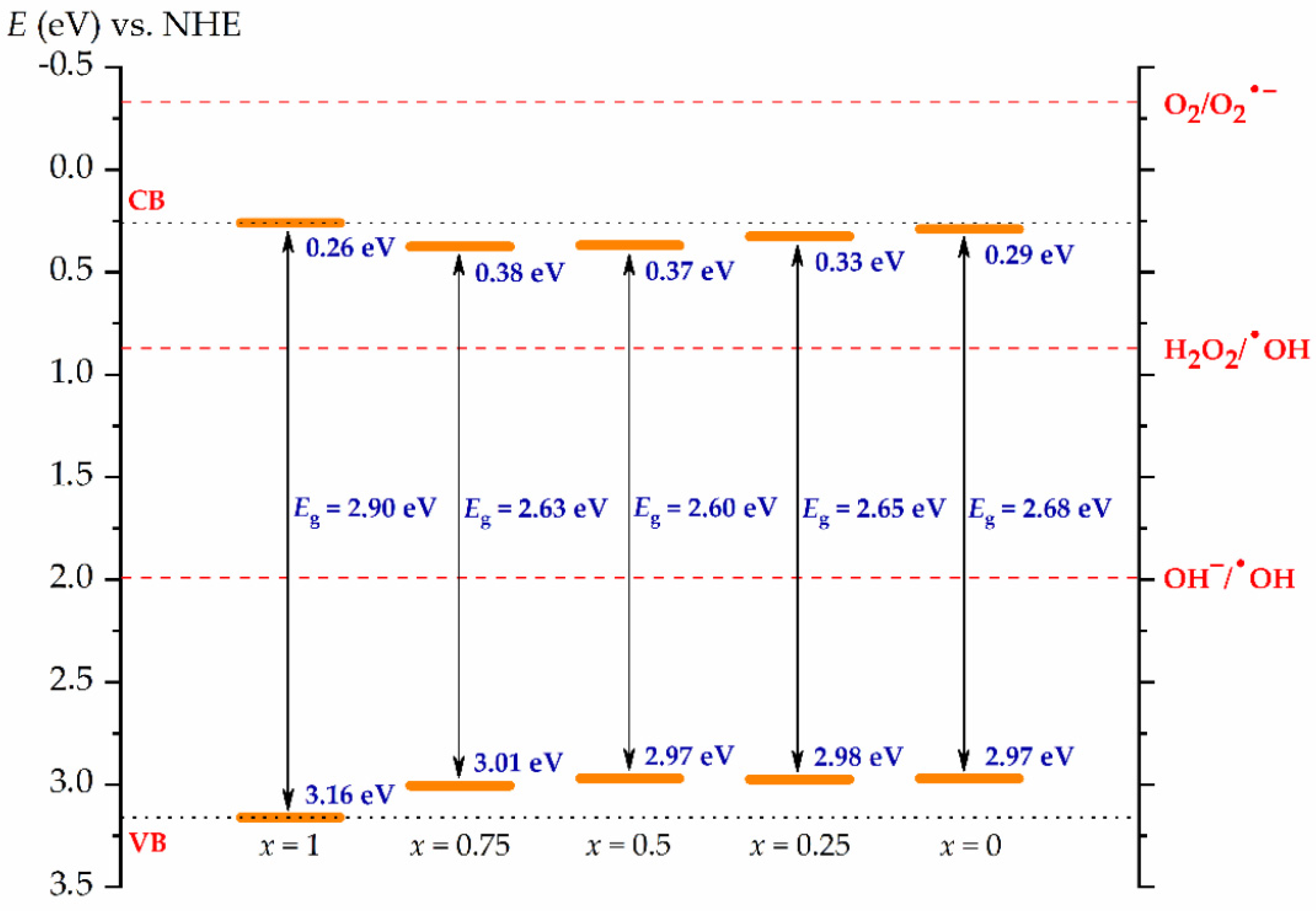
3.3. UV-Vis Analysis and Electronic Structure
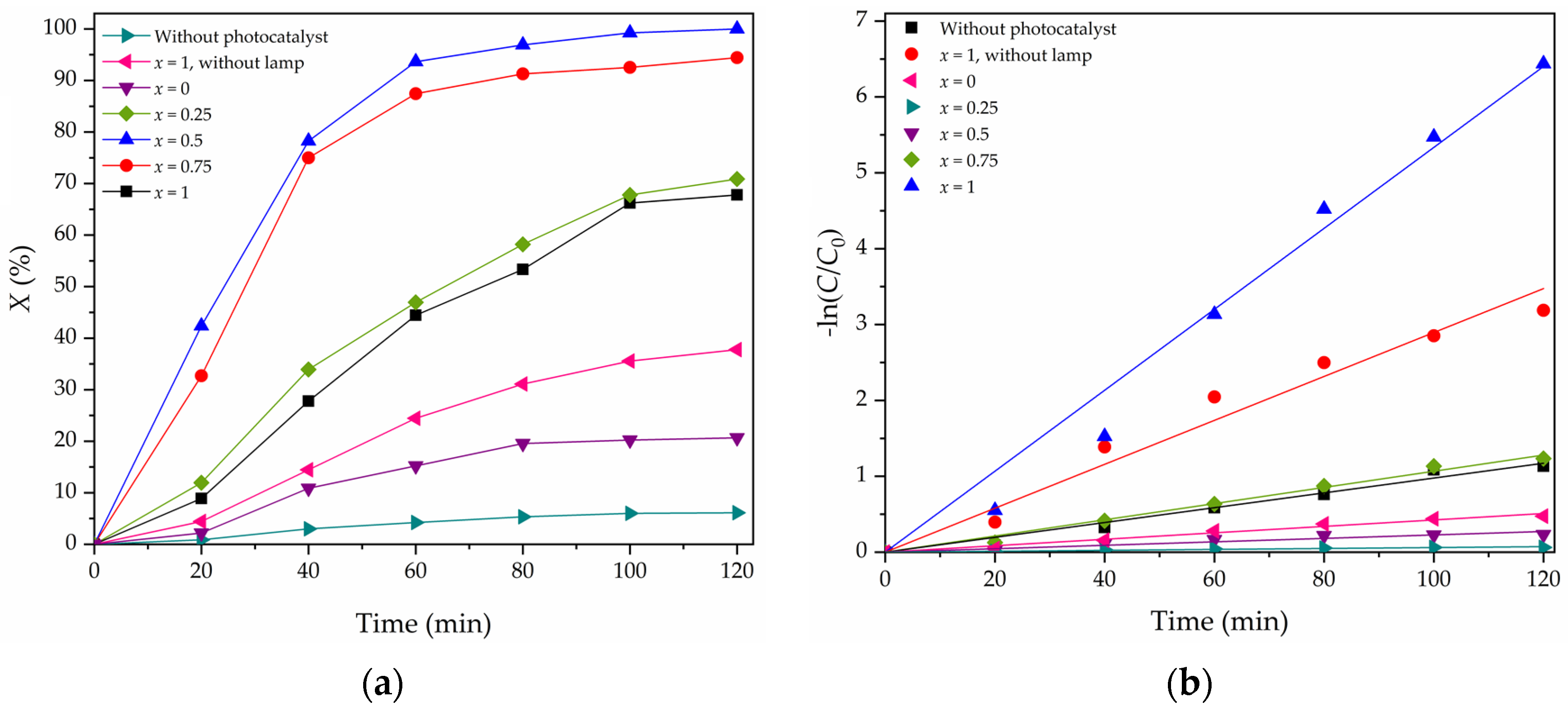
3.4. Photocatalytic Oxidative Desulfurization Performances
| No. 1 | Photocatalyst | C (ppm) 2 | Oxidant | Visible Light Source | Time (min) | X (%) 3 | Main Products 4 | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Nb2O5/Bi2WO6 | 200 | H2O2 | Two 5 W LED lamps | 120 | 99 | DBTO2 | [18] |
| 2 | Fe3O4@SiO2/Bi2WO6/Bi2S3 | 500 | Air | 400 W halogen lamp | 120 | 100 | Not specified | [19] |
| 3 | NiO-Bi2WO6 | 200 | O2 | Not specified | 180 | 95 | BTO2 | [20] |
| 4 | CuO–Fe3O4 | 200 | H2O2 | 350 W Hg lamp | 120 | 80 | Not specified | [38] |
| 5 | Na–g-C3N4 | 200 | O2 | 300 W Xe lamp | 180 | 35 | CO2, H2O | [39] |
| 6 | TiO2@SBA-15 | 50 | H2O2 | 300 W Xe lamp | 90 | 96 | DBTO2 | [40] |
| 7 | N–CeO2–TiO2 | 100 | H2O2 | 300 W Xe lamp | 180 | 94 | DBTO2 | [41] |
| 8 | ZnO/TiO2–SiO2 | 200 | None | 300 W Xe lamp | 240 | 97 | DBTO2 | [42] |
| 9 | CeO2/MIL-101(Fe) | 500 | H2O2 | 500 W Xe lamp | 120 | 90 | DBTO2 | [43] |
| 10 | BiOBr-C3N4/MCM-41 | 200 | H2O2 | 150 W halogen lamp | 120 | 92 | DBTO2 | [44] |
| 11 | Bi2W0.5Mo0.5O6 | 200 | H2O2 | 30 W LED lamp | 120 | 100 | CO2, H2O | This study |
3.5. Kinetics of PODS
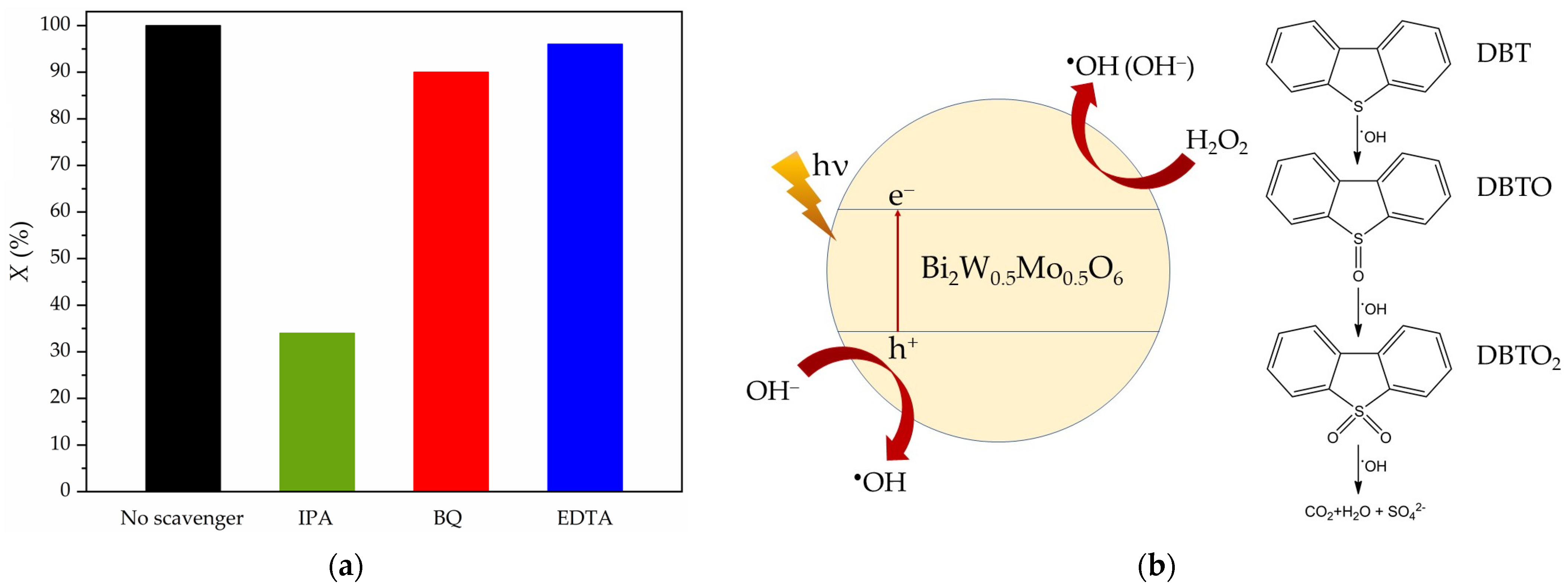
3.6. Active Radical Trapping
3.7. Stability and Recycling Performance of the Photocatalyst
3.8. Future Research Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Wang, D. Photocatalysis: From fundamental principles to materials and applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A.; Abdulrasheed, A.A. A review on recent progression of photocatalytic desulphurization study over decorated photocatalysts. J. Ind. Eng. Chem. 2019, 74, 172–186. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, T.; Liu, H.; Gao, X.; Wang, C.; Wang, G. Desulfurization through photocatalytic oxidation: A critical review. ChemSusChem 2020, 14, 492–511. [Google Scholar] [CrossRef]
- Li, K.; An, X.; Park, K.H.; Khraisheh, M.; Tang, J. A critical review of CO2 photoconversion: Catalysts and reactors. Catal. Today 2014, 224, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Jitan, S.A.; Palmisano, G.; Garlisi, C. Synthesis and surface modification of TiO2-based photocatalysts for the conversion of CO2. Catalysts 2020, 10, 227. [Google Scholar] [CrossRef] [Green Version]
- Belousov, A.S.; Suleimanov, E.V. Application of metal–organic frameworks as an alternative to metal oxide-based photocatalysts for the production of industrially important organic chemicals. Green Chem. 2021, 23, 6172–6204. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, C.; Wang, J.; Xiao, G.; Luo, G. Green synthesis of Ag–TiO2 supported on porous glass with enhanced photocatalytic performance for oxidative desulfurization and removal of dyes under visible light. ACS Sustain. Chem. Eng. 2018, 6, 13276–13286. [Google Scholar] [CrossRef]
- Zhang, G.; Gao, M.; Tian, M.; Zhao, W. In situ hydrothermal preparation and photocatalytic desulfurization performance of graphene wrapped TiO2 composites. J. Solid State Chem. 2019, 279, 120953. [Google Scholar] [CrossRef]
- Man, Z.; Meng, Y.; Lin, X.; Dai, X.; Wang, L.; Liu, D. Assembling UiO-66@TiO2 nanocomposites for efficient photocatalytic degradation of dimethyl sulfide. Chem. Eng. J. 2022, 431, 133952. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Y.; Xie, D.; Zhang, M.; Zuo, N.; Mominou, N.; Jing, C. Ultra-deep desulfurization of model diesel fuel over Pr/Ce–N–TiO2 assisted by visible light. Microporous Mesoporous Mater. 2021, 323, 111258. [Google Scholar] [CrossRef]
- Ortiz-Bustos, J.; Hierro, I.; Pérez, Y. Photocatalytic oxidative desulfurization and degradation of organic pollutants under visible light using TiO2 nanoparticles modified with iron and sulphate ions. Ceram. Int. 2022, 48, 6905–6916. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Ding, Y.; Ma, C.; Liu, X.; Meng, M.; Yan, Y. Fabrication of Bi2WO6/In2O3 photocatalysts with efficient photocatalytic performance for the degradation of organic pollutants: Insight into the role of oxygen vacancy and heterojunction. Adv. Powder Technol. 2020, 31, 2890–2900. [Google Scholar] [CrossRef]
- Orimolade, B.O.; Idris, A.O.; Feleni, U.; Mamba, B. Recent advances in degradation of pharmaceuticals using Bi2WO6 mediated photocatalysis–A comprehensive review. Environ. Pollut. 2021, 289, 117891. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Wei, K.; Li, Y.; Liu, Y.; Zhao, Y.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Carbon dots/Bi2WO6 composite with compensatory photo-electronic effect for overall water photo-splitting at normal pressure. Chin. Chem. Lett. 2021, 32, 2283–2286. [Google Scholar] [CrossRef]
- Chen, P.; Du, T.; Jia, H.; Zhou, L.; Yue, Q.; Wang, H.; Wang, Y. A novel Bi2WO6/Si heterostructure photocatalyst with Fermi level shift in valence band realizes efficient reduction of CO2 under visible light. Appl. Surf. Sci. 2022, 585, 152665. [Google Scholar] [CrossRef]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 photocatalysis: A brief review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Chen, T.; Liu, L.; Hu, C.; Huang, H. Recent advances on Bi2WO6-based photocatalysts for environmental and energy applications. Chin. J. Catal. 2021, 42, 1413–1438. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Liu, J.; Bai, J.; Yang, L. A novel Nb2O5/Bi2WO6 heterojunction photocatalytic oxidative desulfurization catalyst with high visible light-induced photocatalytic activity. RSC Adv. 2017, 7, 51046–51054. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Zhao, Q.; Wang, C.; Shen, H.; Han, X.; Zhang, K.; Wang, D.; Fu, F. Magnetically recyclable Fe3O4@SiO2/Bi2WO6/Bi2S3 with visible-light-driven photocatalytic oxidative desulfurization. Mater. Res. Bull. 2019, 118, 110520. [Google Scholar] [CrossRef]
- Chang, H.; Yi, H.; Zhang, J. Preparation of a NiO-Bi2WO6 catalyst and its photocatalytic oxidative desulfurization performance. Colloid Interface Sci. Commun. 2021, 41, 100381. [Google Scholar] [CrossRef]
- Li, X.; Li, F.; Lu, X.; Zuo, S.; Yao, C.; Ni, C. Development of Bi2W1−xMoxO6/montmorillonite nanocomposite as efficient catalyst for photocatalytic desulfurization. J. Alloys Compd. 2017, 709, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Wang, H.; Song, J.; Zhang, H.; Gao, F.; Zhao, S.; Hu, H. Controlled synthesis of three-dimensional hierarchical Bi2WO6 microspheres with optimum photocatalytic activity. Mater. Res. Bull. 2012, 47, 315–320. [Google Scholar] [CrossRef]
- Li, G. Electrospinning fabrication and photocatalytic activity of Bi2WO6 nanofibers. J. Mater. Sci. Mater. Electron. 2017, 28, 12320–12325. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, W.; Sun, S.; Zhang, L. A general synthesis strategy for one-dimensional Bi2MO6 (M = Mo, W) photocatalysts using an electrospinning method. CrystEngComm 2013, 15, 7959–7964. [Google Scholar] [CrossRef]
- Voronkova, V.I.; Kharitonova, E.P.; Rudnitskaya, O.G. Refinement of Bi2WO6 and Bi2MoO6 polymorphism. J. Alloys Compd. 2009, 487, 274–279. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, P.; Deng, M.; Shen, H.; Feng, Z.; Li, H. Hybrid density functional theory study of native defects and nonmetal (C, N, S, and P) doping in a Bi2WO6 photocatalyst. ACS Omega 2020, 5, 29081–29091. [Google Scholar] [CrossRef]
- Mulliken, R.S. A new electroaffinity scale; together with data on valence states and on valence ionization potentials and electron affinities. J. Chem. Phys. 1934, 2, 782–793. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic structures of molecules XI. Electroaffinity, molecular orbitals and dipole moments. J. Chem. Phys. 1935, 3, 573–585. [Google Scholar] [CrossRef]
- Halouani, F.E.; Deschanvres, A. Interfaces semi-conducteur-electrolyte: Correlations entre le potentiel de bande plate et les echelles d’electronegativite. Mater. Res. Bull. 1982, 17, 1045–1052. [Google Scholar] [CrossRef]
- Gao, J.; Zeng, W.; Tang, B.; Zhong, M.; Liu, Q.-J. Density functional characterization of Bi-based photocatalysts: BiTaO4, Bi4Ta2O11 and Bi7Ta3O18. Mater. Sci. Semicond. Process. 2021, 121, 105447. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and absolute hardness of Lewis acids and bases. J. Am. Chem. Soc. 1985, 107, 6801–6806. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, M.; Yang, J.; Wang, Y.; Yu, C. Nanosheet-based Bi2MoxW1−xO6 solid solutions with adjustable band gaps and enhanced visible-light-driven photocatalytic activities. J. Phys. Chem. C 2010, 114, 18812–18818. [Google Scholar] [CrossRef]
- Schwertmann, L.; Grünert, A.; Pougin, A.; Sun, C.; Wark, M.; Marschall, R. Understanding the influence of lattice composition on the photocatalytic activity of defect-pyrochlore-structured semiconductor mixed oxides. Adv. Funct. Mater. 2015, 25, 905–912. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, D.; Wang, B.; Li, D.; Yan, Z.; Chen, Y.; Shen, J.; Zhang, Z.; Wang, X. Monolayer Bi2W1–xMoxO6 solid solutions for structural polarity to boost photocatalytic reduction of nitrobenzene under visible light. ACS Sustain. Chem. Eng. 2021, 9, 2465–2474. [Google Scholar] [CrossRef]
- Hu, Y.; Mao, L.; Guan, X.; Tucker, K.A.; Xie, H.; Wu, X.; Shi, J. Layered perovskite oxides and their derivative nanosheets adopting different modification strategies towards better photocatalytic performance of water splitting. Renew. Sustain. Energy Rev. 2020, 119, 109527. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, L.; Yao, W.; Zhu, Y. Photocatalytic properties of nanosized Bi2WO6 catalysts synthesized via a hydrothermal process. Appl. Catal. B 2006, 66, 100–110. [Google Scholar] [CrossRef]
- Ammar, S.H.; Kareem, Y.S.; Ali, A.D. Photocatalytic oxidative desulfurization of liquid petroleum fuels using magnetic CuO–Fe3O4 nanocomposites. J. Environ. Chem. Eng. 2018, 6, 6780–6787. [Google Scholar] [CrossRef]
- Zhang, X.; Song, H.; Sun, C.; Chen, C.; Han, F.; Li, X. Photocatalytic oxidative desulfurization and denitrogenation of fuels over sodium doped graphitic carbon nitride nanosheets under visible light irradiation. Mater. Chem. Phys. 2019, 226, 34–43. [Google Scholar] [CrossRef]
- Guo, G.; Guo, H.; Wang, F.; France, L.J.; Yang, W.; Mei, Z.; Yu, Y. Dye-sensitized TiO2@SBA-15 composites: Preparation and their application in photocatalytic desulfurization. Green Energy Environ. 2020, 5, 114–120. [Google Scholar] [CrossRef]
- Lu, X.; Li, X.; Chen, F.; Chen, Z.; Qian, J.; Zhang, Q. Biotemplating synthesis of N-doped two-dimensional CeO2–TiO2 nanosheets with enhanced visible light photocatalytic desulfurization performance. J. Alloys Compd. 2020, 815, 152326. [Google Scholar] [CrossRef]
- Zhou, K.; Ding, Y.; Zhang, L.; Wu, H.; Guo, J. Synthesis of mesoporous ZnO/TiO2–SiO2 composite material and its application in photocatalytic adsorption desulfurization without the addition of an extra oxidant. Dalton Trans. 2020, 49, 1600–1612. [Google Scholar] [CrossRef] [PubMed]
- Huo, Q.; Liu, G.; Sun, H.; Fu, Y.; Ning, Y.; Zhang, B.; Zhang, X.; Gao, J.; Miao, J.; Zhang, X.; et al. CeO2-modified MIL-101(Fe) for photocatalysis extraction oxidation desulfurization of model oil under visible light irradiation. Chem. Eng. J. 2021, 422, 130036. [Google Scholar] [CrossRef]
- Abedini, F.; Allahyari, S.; Rahemi, N. Oxidative desulfurization of dibenzothiophene and simultaneous adsorption of products on BiOBr-C3N4/MCM-41 visible-light-driven core–shell nano photocatalyst. Appl. Surf. Sci. 2021, 569, 151086. [Google Scholar] [CrossRef]
- Majid, M.F.; Zaid, H.F.M.; Kait, C.F.; Jumbri, K.; Yuan, L.C.; Rajasuriyan, S. Futuristic advance and perspective of deep eutectic solvent for extractive desulfurization of fuel oil: A review. J. Mol. Liq. 2020, 306, 112870. [Google Scholar] [CrossRef]
- Tahir, S.; Qazi, U.Y.; Naseem, Z.; Tahir, N.; Zahid, M.; Javaid, R.; Shahid, I. Deep eutectic solvents as alternative green solvents for the efficient desulfurization of liquid fuel: A comprehensive review. Fuel 2021, 305, 121502. [Google Scholar] [CrossRef]
- Mandizadeh, S.; Salavati-Niasari, M.; Sadri, M. Hydrothermal synthesis, characterization and magnetic properties of BaFe2O4 nanostructure as a photocatalytic oxidative desulfurization of dibenzothiophene. Sep. Purif. Technol. 2017, 175, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Pham, X.N.; Nguyen, M.B.; Ngo, H.S.; Doan, H.V. Highly efficient photocatalytic oxidative desulfurization of dibenzothiophene with sunlight irradiation using green catalyst of Ag@AgBr/Al-SBA-15 derived from natural halloysite. J. Ind. Eng. Chem. 2020, 90, 358–370. [Google Scholar] [CrossRef]
- Lu, X.; Chen, F.; Qian, J.; Fu, M.; Jiang, Q.; Zhang, Q. Facile fabrication of CeF3/g-C3N4 heterojunction photocatalysts with upconversion properties for enhanced photocatalytic desulfurization performance. J. Rare Earths 2021, 39, 1204–1210. [Google Scholar] [CrossRef]
- Deng, F.; Lu, X.; Pei, X.; Luo, X.; Luo, S.; Dionysiou, D.D. Fabrication of ternary reduced graphene oxide/SnS2/ZnFe2O4 composite for high visible-light photocatalytic activity and stability. J. Hazard. Mater. 2017, 332, 149–161. [Google Scholar] [CrossRef]
- Cao, Y.; Ren, Y.; Zhang, J.; Xie, T.; Lin, Y. Activation of H2O2 by photo-generated electrons for enhanced visible light driven methylene blue degradation with ZnFe2O4/BiVO4 heterojunction. Opt. Mater. 2021, 121, 111637. [Google Scholar] [CrossRef]
- Belousov, A.S.; Suleimanov, E.V.; Fukina, D.G. Pyrochlore oxides as visible light-responsive photocatalysts. New J. Chem. 2021, 45, 22531–22558. [Google Scholar] [CrossRef]
- Li, C.; Chen, G.; Sun, J.; Feng, Y.; Liu, J.; Dong, H. Ultrathin nanoflakes constructed erythrocyte-like Bi2WO6 hierarchical architecture via anionic self-regulation strategy for improving photocatalytic activity and gas-sensing property. Appl. Catal. B 2015, 163, 415–423. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Yuan, Q.; He, J.; Au, C.-T.; Yin, S.-F. A green and efficient photocatalytic route for the highly-selective oxidation of saturated alpha-carbon C–H bonds in aromatic alkanes over flower-like Bi2WO6. Chem. Commun. 2016, 52, 1274–1277. [Google Scholar] [CrossRef]
- Li, C.; Chen, G.; Sun, J.; Rao, J.; Han, Z.; Hu, Y.; Zhou, Y. A novel mesoporous single-crystal-like Bi2WO6 with enhanced photocatalytic activity for pollutants degradation and oxygen production. ACS Appl. Mater. Interfaces 2015, 7, 25716–25724. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Y.; Lee, S.C. Salt-assisted synthesis of hollow Bi2WO6 microspheres with superior photocatalytic activity for NO removal. Chin. J. Catal. 2017, 38, 348–356. [Google Scholar] [CrossRef]
- Nagyné-Kovács, T.; Shahnazarova, G.; Lukács, I.E.; Szabó, A.; Hernadi, K.; Igricz, T.; László, K.; Szilágyi, I.M.; Pokol, G. Effect of pH in the hydrothermal preparation of Bi2WO6 nanostructures. Materials 2019, 12, 1728. [Google Scholar] [CrossRef] [Green Version]

| x | Perovskite | Molar Ratio of Starting Materials | Temperature and Holding Time |
|---|---|---|---|
| 1 | Bi2WO6 | Na2WO4:Bi(NO3)3 = 1:2 | 160 °C, 12 h |
| 0.75 | Bi2W0.75Mo0.25O6 | Na2WO4:Na2MoO4:Bi(NO3)3 = 1.5:0.5:4 | 160 °C, 12 h |
| 0.5 | Bi2W0.5Mo0.5O6 | Na2WO4:Na2MoO4:Bi(NO3)3 = 1:1:4 | 160 °C, 12 h |
| 0.25 | Bi2W0.25Mo0.75O6 | Na2WO4:Na2MoO4:Bi(NO3)3 = 0.5:1.5:4 | 160 °C, 12 h |
| 0 | Bi2MoO6 | Na2MoO4:Bi(NO3)3 = 1:2 | 160 °C, 12 h |
| x | a (Å) | b (Å) | c (Å) | V (Å3) | D113 (nm) | D002 (nm) | SBET (m2·g−1) | Eg (eV) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.446 (2) | 5.458 (9) | 16.36 (1) | 486.7 (7) | 13.02 | - | 25.8 | 2.90 |
| 0.75 | 5.468 (4) | 5.446 (2) | 16.39 (5) | 487.9 (7) | 17.18 | - | 19.3 | 2.63 |
| 0.5 | 5.455 (3) | 5.461 (1) | 16.37 (6) | 487.6 (3) | 17.96 | 13.28 | 16.4 | 2.60 |
| 0.25 | 5.449 (6) | 5.468 (7) | 16.34 (1) | 486.8 (5) | 24.28 | 20.70 | 14.8 | 2.65 |
| 0 | 5.471 (3) | 5.480 (6) | 16.25 (1) | 487.3 (3) | 28.38 | 21.81 | 15.3 | 2.68 |
| x | Perovskite | Bi | W | Mo | O |
|---|---|---|---|---|---|
| 1 | Bi2WO6 | 20.96 | 11.94 | - | 67.10 |
| 0.75 | Bi2W0.75Mo0.25O6 | 22.13 | 8.82 | 2.59 | 65.42 |
| 0.5 | Bi2W0.5Mo0.5O6 | 20.93 | 6.11 | 5.55 | 67.22 |
| 0.25 | Bi2W0.25Mo0.75O6 | 21.56 | 2.79 | 7.75 | 65.90 |
| 0 | Bi2MoO6 | 21.15 | - | 11.35 | 67.50 |
| Atom | I | A | χ |
|---|---|---|---|
| Bi | 7.29 | 0.94 | 4.12 |
| W | 7.98 | 0.82 | 4.40 |
| Mo | 7.10 | 0.75 | 3.93 |
| O | 13.61 | 1.46 | 7.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belousov, A.S.; Suleimanov, E.V.; Parkhacheva, A.A.; Fukina, D.G.; Koryagin, A.V.; Titaev, D.N.; Lazarev, M.A. Synthesis and Characterization of Bi2WxMo1−xO6 Solid Solutions and Their Application in Photocatalytic Desulfurization under Visible Light. Processes 2022, 10, 789. https://doi.org/10.3390/pr10040789
Belousov AS, Suleimanov EV, Parkhacheva AA, Fukina DG, Koryagin AV, Titaev DN, Lazarev MA. Synthesis and Characterization of Bi2WxMo1−xO6 Solid Solutions and Their Application in Photocatalytic Desulfurization under Visible Light. Processes. 2022; 10(4):789. https://doi.org/10.3390/pr10040789
Chicago/Turabian StyleBelousov, Artem S., Evgeny V. Suleimanov, Alina A. Parkhacheva, Diana G. Fukina, Andrey V. Koryagin, Dmitry N. Titaev, and Mikhail A. Lazarev. 2022. "Synthesis and Characterization of Bi2WxMo1−xO6 Solid Solutions and Their Application in Photocatalytic Desulfurization under Visible Light" Processes 10, no. 4: 789. https://doi.org/10.3390/pr10040789
APA StyleBelousov, A. S., Suleimanov, E. V., Parkhacheva, A. A., Fukina, D. G., Koryagin, A. V., Titaev, D. N., & Lazarev, M. A. (2022). Synthesis and Characterization of Bi2WxMo1−xO6 Solid Solutions and Their Application in Photocatalytic Desulfurization under Visible Light. Processes, 10(4), 789. https://doi.org/10.3390/pr10040789







