The Formation, Stabilization and Separation of Oil–Water Emulsions: A Review
Abstract
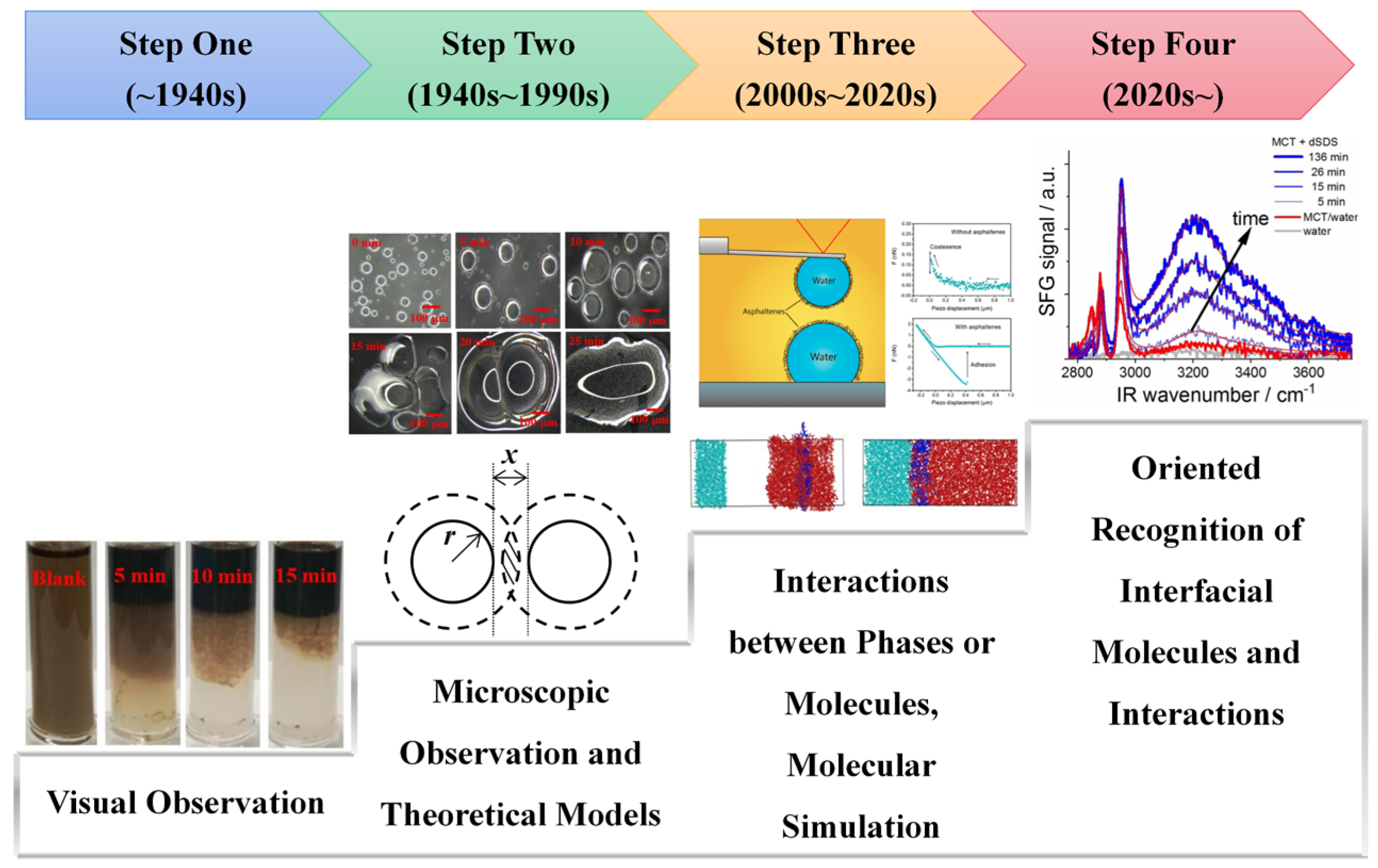
:1. Introduction
2. Generation of Oil–Water Emulsions in Industry
2.1. Classification of Emulsions
2.2. Generation and Impacts of Oil–Water Emulsions
2.2.1. Generation of Oil–Water Emulsions in Industry
2.2.2. The Impact of Oil–Water Emulsions
3. The Stability of Oil–Water Emulsions
3.1. The Roles of Emulsifiers
3.1.1. Small Molecular Emulsifiers
3.1.2. Macro Molecular Emulsifiers
| Large Category | Materials | Example | Systems |
|---|---|---|---|
| Small molecular emulsifier | Simple surfactant | Anionic surfactant: sulfate [83], sulfonate [84], and phosphate [85], carboxylate derivatives [86], etc. Cationic surfactant: mainly ammonium [87,88]. Zwitterionic surfactant: anionic ammonium [89], sulfobetaine-type surfactant [90]. Nonionic surfactant: mainly oxygen-containing surfactant [91]. | Widely existing in various emulsifying systems, including petroleum, organic synthesis, materials, biological medicine, electrochemistry, food industry, etc. |
| Macro-molecular emulsifiers | Heavy petroleum components | Interfacially active asphaltene [98,103]. | Heavy oil, oil sludge. |
| Biological macromolecules | Lipid [107], protein [108], polysaccharide [109,110]. | Biological medicine, food industry. | |
| Polymeric surfactants | Random polymer [111,112]. Block polymer [113]. Branched polymer [114]. | Organic synthesis, materials, biological medicine. | |
| Solid particles as an emulsifier | Inorganic solid particles | Silicon dioxide (SiO2), titanium dioxide (TiO2), ferric oxide (Fe2O3), montmorillonite (MMT), laponite, layered bimetallic hydroxide, etc. | Pharmaceutical industry, oil and gas industry, aerospace industry, etc. |
| Organic solid particles | Poly (N-isopropylacrylamide) micro-gel particles, polyethylene microspheres, block copolymer micelles, etc. | ||
| Surface modified solid particles | Amine-modified lithium saponite particles [115], etc. | ||
| Janus particles | Polymeric Janus particles (PDVB-PNIPAM) [116], P2VN-PAA/PEO polymeric Janus particles [117], etc. |
3.1.3. Solid Particle Emulsifiers
3.2. Interactions between Different Molecules at the Interface: From Macro- to Micro-Scale
3.2.1. Thermodynamics of Colloidal Dispersion Stability: DLVO Theory
3.2.2. Measurements of Interactions between Phases: AFM
- Firstly, AFM can quantify the interactions between phases. In 2004, Gunning et al. [132] attached oil droplets to the end of an AFM cantilever, and they monitored the interactions between droplets as a function of inter-droplet separation. In the same year, Dagastine et al. [133] measured the interaction forces between alkane droplets in an aqueous solution. In 2017, Shi et al. [125] applied this method to W/O systems and explored the role of adsorbed asphaltene in interfacial adhesion. These studies provide quantitative insights into the stability of emulsions.
- Furthermore, AFM improves the theoretical system of colloid science. Liu et al. [134] combined AFM with extended DLVO theory to reveal the stability mechanisms of bitumen droplets. The measured parameters are in excellent agreement with the calculated ones. Wang et al. [135] reviewed the effect of AFM in the theories of deformable droplet interactions, including DLVO forces, non-DLVO forces, and the dynamic film evolution process.
- Additionally, AFM promotes the rational design of functional emulsions. This is mainly reflected in nano-emulsion in the food industry [136,137], and is better absorbed by the digestive system. Food emulsions can be better understood, predicted, and controlled through the bulk phase interactions, and are better absorbed by the digestive system [36].
3.2.3. Visualizing Molecular Interactions: Molecular Dynamics Simulation
- Radial distribution function (RDF, or g (r)). RDF describes how density varies as a function of distance from a reference atom, which may reflect the interactions between reference atoms with statistical atoms. This is calculated by Equation (2) [142]. is the number of particles within a spherical container at distance r from a reference point b. and denote the container thickness and density of atoms in the space, respectively. g (r)~r functional diagrams are applied for analysis [142,144,145]. Usually, sharp peaks exist in the interval of 0.1~1 nm, which are generated by the interactions between emulsifiers and the bulk phase. The strength is reflected by the peak value of g (r), which directly dominates the stability of the emulsion.
- Non-covalent interactions. Non-covalent interactions are general designations of inter-molecular interactions other than covalent bonds, including electrostatic interactions (i.e., hydrogen bonds), van der Waals interactions, steric interactions, etc. [36]. The evolving force fields have fitted appropriate molecular potentials that most correctly express them. In addition, several studies discussed the details of non-covalent interactions. Chen et al. studied the wetting mechanism of amphiphilic collagen fibers by MD simulation. It was found that electrostatic interactions and van der Waals interactions are the driving forces of regional wetting in the hydrophilic and hydrophobic regions, respectively [146]. Lv et al. [145] calculated that the hydrogen bonding networks between the carboxyl group and the water molecules stabilized the petroleum emulsion. Ma et al. [124] illustrated the important role of the reconstruction of non-covalent interactions in demulsification by dissipative particle dynamics simulation.
3.3. Recent Progress on Molecular Oriented Recognition
4. Separation of Oil–Water Emulsions
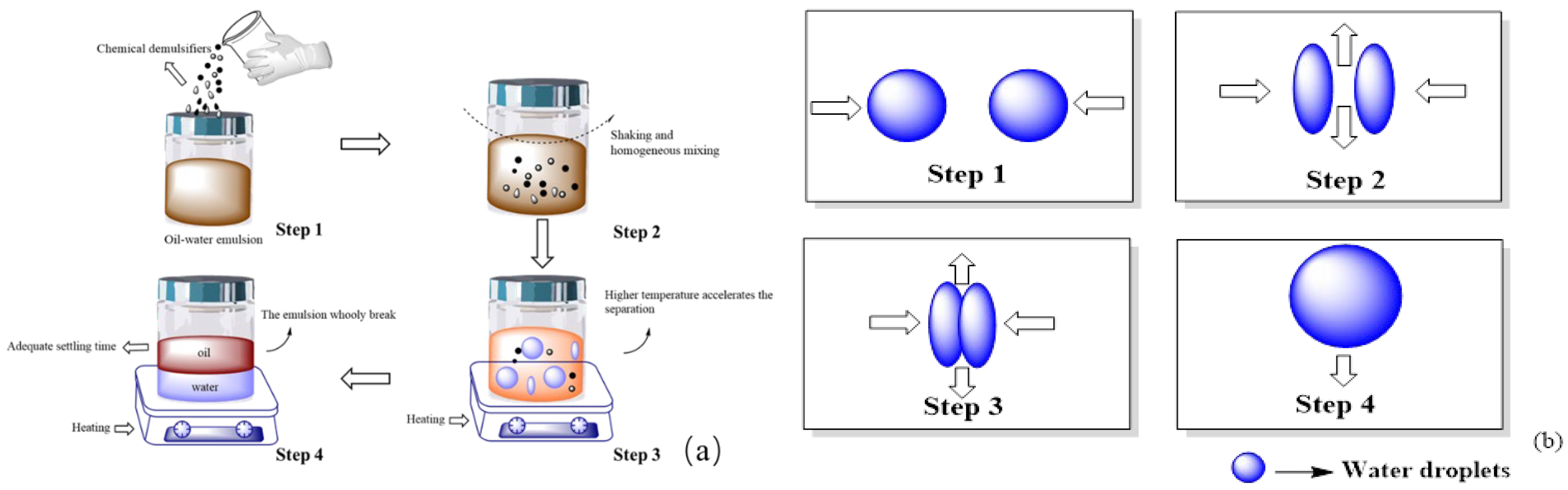
4.1. Common Processes and Mechanisms of Demulsification
4.2. Technologies for Oil–Water Emulsions Separation
4.3. Oil–Water Emulsions Separation Process
4.3.1. Combined Demulsification Process
4.3.2. Demulsification Process in Different Industrial Field
5. Discussions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garti, N. Progress in Stabilization and Transport Phenomena of Double Emulsions in Food Applications. LWT-Food Sci. Technol. 1997, 30, 222–235. [Google Scholar] [CrossRef]
- Okochi, H.; Nakano, M. Preparation and evaluation of w/o/w type emulsions containing vancomycin. Adv. Drug Deliv. Rev. 2000, 45, 5–26. [Google Scholar] [CrossRef]
- Oh, C.; Chung, S.-C.; Shin, S.-I.; Kim, Y.C.; Im, S.-S.; Oh, S.-G. Distribution of Macropores in Silica Particles Prepared by Using Multiple Emulsions. J. Colloid Interface Sci. 2002, 254, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, J.W.; Han, S.H.; Chang, I.S.; Kang, H.H.; Lee, O.S.; Oh, S.G.; Suh, K.D. The stabilization of L-ascorbic acid in aqueous solution and water-in-oil-in-water double emulsion by controlling pH and electrolyte concentration. J. Cosmet. Sci. 2004, 55, 217. [Google Scholar] [CrossRef] [PubMed]
- Schramm, L.L. Emulsions: Fundamentals and Applications in the Petroleum Industry. In Petroleum Emulsion; Schramm, L.L., Ed.; American Chemical Society: Washington, DC, USA, 1992. [Google Scholar]
- Wang, Z.M.; Lun, Q.Y.; Wang, J.; Han, X.; Zhu, W.; Zhang, J.; Song, G.-L. Corrosion mitigation behavior of an alternately wetted steel electrode in oil/water media. Corros. Sci. 2019, 152, 140–152. [Google Scholar] [CrossRef]
- Deng, J. Status and development trend of innocuous treatment and resource utilization of kitchen waste. J. Environ. Eng. Technol. 2019, 9, 637–642. [Google Scholar]
- Tulayakul, P.; Boonsoongnern, A.; Kasemsuwan, S.; Wiriyarampa, S.; Pankumnoed, J.; Tippayaluck, S.; Hananantachai, H.; Mingkhwan, R.; Netvichian, R.; Khaodhiar, S. Comparative study of heavy metal and pathogenic bacterial contamination in sludge and manure in biogas and non-biogas swine farms. J. Environ. Sci. 2011, 23, 991–997. [Google Scholar] [CrossRef]
- Van Gerpen, J. Biodiesel processing and production. Fuel Process. Technol. 2015, 86, 1097–1107. [Google Scholar] [CrossRef]
- Zheng, W.; Jin, J.P.; Liu, S.L.; Qu, W.G.; Min, H.H.; Chen, G.Y. Discussion on suitable resource-based methods based on the nature of Chinese kitchen waste. Environ. Sanit. Eng. 2015, 23, 75–83. [Google Scholar]
- Xie, J.; Xin, L.; Hu, X.; Cheng, W.; Liu, W.; Wang, Z. Technical application of safety and cleaner production technology by underground coal gasification in China. J. Clean. Prod. 2020, 250, 119487. [Google Scholar] [CrossRef]
- Shi, J.; Xu, C.; Han, Y.; Han, H. Enhanced anaerobic biodegradation efficiency and mechanism of quinoline, pyridine, and indole in coal gasification wastewater. Chem. Eng. J. 2019, 361, 1019–1029. [Google Scholar] [CrossRef]
- Cui, P.; Mai, Z.; Yang, S.; Qian, Y. Integrated treatment processes for coal-gasification wastewater with high concentration of phenol and ammonia. J. Clean. Prod. 2017, 142, 2218–2226. [Google Scholar] [CrossRef]
- Lee, M.; Jung, J.Y. Pollution risk assessment of oil spill accidents in Garorim Bay of Korea. Mar. Pollut. Bull. 2015, 100, 297–303. [Google Scholar] [CrossRef]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, D.; Datta, D.; Sen, D.; Bhattacharjee, C. Simulation of continuous stirred rotating disk-membrane module: An approach based on surface renewal theory. Chem. Eng. Sci. 2011, 66, 2554–2567. [Google Scholar] [CrossRef]
- Lan, D.; Liang, B.; Bao, C.; Ma, M.; Xu, Y.; Yu, C. Marine oil spill risk mapping for accidental pollution and its application in a coastal city. Mar. Pollut. Bull. 2015, 96, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhang, Q.; Yang, N.; Xu, J.; Liu, J.; Yang, R.; Kunkelmann, C.; Schreiner, E.; Holtze, C.; Mülheims, K.; et al. Molecular dynamics simulations of surfactant adsorption at oil/water interface under shear flow. Particuology 2019, 44, 36–43. [Google Scholar] [CrossRef]
- Abbasi, A.; Malayeri, M.R. Stability of acid in crude oil emulsion based on interaction energies during well stimulation using HCl acid. J. Pet. Sci. Eng. 2022, 212, 110317. [Google Scholar] [CrossRef]
- Jia, H.; Lian, P.; Yan, H.; Yuan, J.; Tang, H.; Wei, X.; Song, J.; He, J.; Lv, K.; Liu, D. Novel molecular insight into the discrepant distributions for ionic surfactants in light oil/water and heavy oil/water systems. Fuel 2021, 304, 121460. [Google Scholar] [CrossRef]
- Jamaly, S.; Giwa, A.; Hasan, S.W. Recent improvements in oily wastewater treatment: Progress, challenges, and future opportunities. J. Environ. Sci. 2015, 37, 15–30. [Google Scholar] [CrossRef]
- Ma, J.; Yao, M.; Yang, Y.; Zhang, X. Comprehensive review on stability and demulsification of unconventional heavy oil-water emulsions. J. Mol. Liq. 2022, 350, 118510. [Google Scholar] [CrossRef]
- Yonguep, E.; Fabrice, K.K.; Katende, J.K.; Chowdhury, M. Formation, stabilization and chemical demulsification of crude oil-in-water emulsions: A review. Pet. Res. 2022, in press. [Google Scholar] [CrossRef]
- Adeyemi, I.; Meribout, M.; Khezzar, L. Recent developments, challenges, and prospects of ultrasound-assisted oil technologies. Ultrason. Sonochemistry 2021, 82, 105902. [Google Scholar] [CrossRef]
- Lim, J.; Wong, S.; Law, M.; Samyudia, Y.; Dol, S. A Review on the Effects of Emulsions on Flow Behaviours and Common Factors Affecting the Stability of Emulsions. J. Appl. Sci. 2015, 15, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Kokal, S.L. Crude-Oil Emulsions: A State-of-the-Art Review. SPE Prod. Facil. 2005, 20, 5–13. [Google Scholar] [CrossRef]
- He, L.; Lin, F.; Li, X.; Sui, H.; Xu, Z. Interfacial sciences in unconventional petroleum production: From fundamentals to applications. Chem. Soc. Rev. 2015, 44, 5446–5494. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Wasan, D. Particle—fluid interactions with application to solid-stabilized emulsions part II. The effect of adsorbed water. Colloids Surf. 1986, 19, 107–122. [Google Scholar] [CrossRef]
- Winsor, P.A. Binary and multicomponent solutions of amphiphilic compounds. Solubilization and the formation, structure, and theoretical significance of liquid crystalline solutions. Chem. Rev. 1968, 68, 1–40. [Google Scholar] [CrossRef]
- McClements, D.J. Lipid-based Emulsions and emulsifiers. Food Lipid Chem. Nutr. Biotechnol. 2008, 3, 63–98. [Google Scholar]
- Fingas, M.; Fieldhouse, B. Studies of the formation process of water-in-oil emulsions. Mar. Pollut. Bull. 2003, 47, 369–396. [Google Scholar] [CrossRef]
- Umar, A.A.; Saaid, I.B.M.; Sulaimon, A.A.; Pilus, R.B.M. A review of petroleum emulsions and recent progress on water-in-crude oil emulsions stabilized by natural surfactants and solids. J. Pet. Sci. Eng. 2018, 165, 673–690. [Google Scholar] [CrossRef]
- Gillberg, G.; Lehtinen, H.; Friberg, S. NMR and IR investigation of the conditions determining the stability of microemulsions. J. Colloid Interface Sci. 1970, 33, 40–53. [Google Scholar] [CrossRef]
- Friberg, S.; Mandell, L.; Fontell, K.; Lindblad, C.-G.; Lindberg, A.A.; Jansen, G.; Lamm, B.; Samuelsson, B. Mesomorphous Phases in Systems of Water-Nonionic Emulsifier-Hydrocarbon. Acta Chem. Scand. 1969, 23, 1055–1057. [Google Scholar] [CrossRef]
- Winsor, P.A. Hydrotropy, solubilisation and related emulsification processes. Trans. Faraday Soc. 1948, 44, 451–471. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practices, and Techniques; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Guzey, D.; McClements, D.J. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interface Sci. 2006, 128–130, 227–248. [Google Scholar] [CrossRef]
- Yan, X.; Ma, C.; Cui, F.; McClements, D.J.; Liu, X.; Liu, F. Protein-stabilized Pickering emulsions: Formation, stability, properties, and applications in foods. Trends Food Sci. Technol. 2020, 103, 293–303. [Google Scholar] [CrossRef]
- Hayase, M. Cosmetic Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 10; pp. 149–154. [Google Scholar]
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and applications of particle stabilized emulsions in cosmetic formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kushnick, A.P. Effect of Demulsifiers on Interfacial Properties Governing Crude Oil Demulsification; ACS Publications: Washington, DC, USA, 1989; pp. 364–374. [Google Scholar]
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Thring, R.W.; Hu, X.; Song, X. Oil recovery from refinery oily sludge via ultrasound and freeze/thaw. J. Hazard. Mater. 2012, 203–204, 195–203. [Google Scholar] [CrossRef]
- Ren, G.; Zhou, M.; Zhang, Q.; Xu, X.; Li, Y.; Su, P.; Paidar, M.; Bouzek, K. Cost-efficient improvement of coking wastewater biodegradability by multi-stages flow through peroxi-coagulation under low current load. Water Res. 2019, 154, 336–348. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, C.; Yan, B. Emission characteristics and associated health risk assessment of volatile organic compounds from a typical coking wastewater treatment plant. Sci. Total Environ. 2019, 693, 133417. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.Q.; Lee, B.C.Y.; Ong, S.L.; Hu, J.Y. Fluidized-bed Fenton technologies for recalcitrant industrial wastewater treatment: Recent advances, challenges and perspective. Water Res. 2021, 190, 116692. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, B.R.; Reedy, R.C.; Male, F.; Walsh, M. Water Issues Related to Transitioning from Conventional to Unconventional Oil Production in the Permian Basin. Environ. Sci. Technol. 2017, 51, 10903–10912. [Google Scholar] [CrossRef] [PubMed]
- Altunina, L.K.; Kuvshinov, V.A. Enhance Oil Recovery by Surfactant Compositional Systems; Nauka: Novosibirsk, Russia, 1995; p. 198. [Google Scholar]
- Umar, A.A.; Saaid, I.M.; Sulaimon, A.A. The roles of polar compounds in the stability and flow behavior of water-in-oil emulsions. In ICIPEG; Springer: Berlin/Heidelberg, Germany, 2016; pp. 643–653. [Google Scholar]
- Fakhru’L-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, W.; Gryta, M. Application of ultrafiltration ceramic membrane for separation of oily wastewater generated by maritime transportation. Sep. Purif. Technol. 2021, 261, 118259. [Google Scholar] [CrossRef]
- Qingxin, L.; Congbao, K.; Hao, W.; Changkai, Z. Application of oil degrading bacterium to treat oil field wastewater. Ind. Water Treat. 2003, 23, 13–16. [Google Scholar]
- Bengani-Lutz, P.; Zaf, R.D.; Emecen, P.; Çulfaz, Z.; Asatekin, A. Extremely fouling resistant zwitterionic copolymer membranes with ~1 nm pore size for treating municipal, oily and textile wastewater streams. J. Membr. Sci. 2017, 543, 184–194. [Google Scholar] [CrossRef]
- Bosi Data Research Center. 2014–2019 China Lubricating Oil Market Status Analysis and Investment Prospects Research Report; Bosidata: Hangzhou, China, 2014. [Google Scholar]
- Makki, H.F.; Abdulameer, I.R. Aluminum Rubbish as a Coagulant for Oily Wastewater Treatment. J. Eng. 2016, 22, 55–71. [Google Scholar]
- El Naggar, A.M.A.; El-Din, M.R.N.; Mishrif, M.R.; Nassar, I.M.; El-Din, M.R.N. Highly Efficient Nano-Structured Polymer-Based Membrane/Sorbent for Oil Adsorption from O/W Emulsion Conducted of Petroleum Wastewater. J. Dispers. Sci. Technol. 2014, 36, 118–128. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Nanofibrous metal–organic framework composite membrane for selective efficient oil/water emulsion separation. J. Membr. Sci. 2017, 543, 10–17. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, M.; Zhuang, D. Wastewater treatment and emerging contaminants: Bibliometric analysis. Chemosphere 2022, 297, 133932. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Bin Emran, T.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud Univ.-Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Karbowska, B. Presence of thallium in the environment: Sources of contaminations, distribution and monitoring methods. Environ. Monit. Assess. 2016, 188, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coetzee, J.J.; Bansal, N.; Chirwa, E.M.N. Chromium in Environment, Its Toxic Effect from Chromite-Mining and Ferrochrome Industries, and Its Possible Bioremediation. Expo. Health 2020, 12, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Sundar, J.S. Chakravarty Antimony toxicity. Int. J. Environ. Res. Public Health 2010, 7, 4267–4277. [Google Scholar] [CrossRef]
- Hou, S.; Yuan, L.; Jin, P.; Ding, B.; Qin, N.; Li, L.; Liu, X.; Wu, Z.; Zhao, G.; Deng, Y. A clinical study of the effects of lead poisoning on the intelligence and neurobehavioral abilities of children. Theor. Biol. Med. Model. 2013, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef]
- Hayat, M.T.; Nauman, M.; Nazir, N.; Ali, S.; Bangash, N. Environmental Hazards of Cadmium: Past, Present, and Future. In Cadmium Toxicity and Tolerance in Plants; Academic Press: Cambridge, MA, USA, 2018; pp. 163–183. [Google Scholar]
- Zhang, X.; Yang, L.; Li, Y.; Li, H.; Wang, W.; Ye, B. Impacts of lead/zinc mining and smelting on the environment and human health in China. Environ. Monit. Assess. 2012, 184, 2261–2273. [Google Scholar] [CrossRef]
- Samuel, O.; Othman, M.H.D.; Kamaludin, R.; Sinsamphanh, O.; Abdullah, H.; Puteh, M.H.; Kurniawan, T.A.; Li, T.; Ismail, A.F.; Rahman, M.A.; et al. Oilfield-produced water treatment using conventional and membrane-based technologies for beneficial reuse: A critical review. J. Environ. Manag. 2022, 308, 114556. [Google Scholar] [CrossRef]
- Konkel, L. Salting the Earth: The environmental impact of oil and gas wastewater spills Environ. Health Perspect. 1984, 124, 230–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varjani, S.; Joshi, R.; Srivastava, V.K.; Ngo, H.H.; Guo, W. Treatment of wastewater from petroleum industry: Current practices and perspectives. Environ. Sci. Pollut. Res. 2020, 27, 27172–27180. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A. Bioprocess Evaluation of Petroleum Wastewater Treatment with Zinc Oxide Nanoparticle for the Production of Methane Gas: Process Assessment and Modelling. Appl. Biochem. Biotechnol. 2019, 190, 851–866. [Google Scholar] [CrossRef] [PubMed]
- Eklund, R.L.; Knapp, L.C.; Sandifer, P.A.; Colwell, R.C. Oil Spills and Human Health: Contributions of the Gulf of Mexico Research Initiative. GeoHealth 2019, 3, 391–406. [Google Scholar] [CrossRef]
- Nowak, P.; Kucharska, K.; Kamiński, M. Ecological and health effects of lubricant oils emitted into the environment. Int. J. Environ. Res. Public Health 2019, 16, 3002. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhang, M.; Zou, H.; Li, X.; Xing, M.; Fang, X.; He, J. Genetic damage and lipid peroxidation in workers occupationally exposed to organic bentonite particles. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 751, 40–44. [Google Scholar] [CrossRef]
- Singh, P.; Ojha, A.; Borthakur, A.; Singh, R.; Lahiry, D.; Tiwary, D.; Mishra, P.K. Emerging trends in photodegradation of petrochemical wastes: A review. Environ. Sci. Pollut. Res. 2016, 23, 22340–22364. [Google Scholar] [CrossRef]
- Ishak, S.; Malakahmad, A.; Isa, M.H. Refinery wastewater biological treatment: A short review. J. Sci. Ind. Res. 2012, 71, 251–256. [Google Scholar]
- Ramirez, M.I.; Arevalo, A.P.; Sotomayor, S.; Bailon-Moscoso, N. Contamination by oil crude extraction-refinement and their effects on human health. Environ. Pollut. 2017, 231, 415–425. [Google Scholar] [CrossRef]
- Sima, N.A.K.; Ebadi, A.; Reiahisamani, N.; Rasekh, B. Bio-based remediation of petroleum-contaminated saline soils: Challenges, the current state-of-the-art and future prospects. J. Environ. Manag. 2019, 250, 109476. [Google Scholar] [CrossRef]
- Almeda, R.; Cosgrove, S.; Buskey, E.J. Oil Spills and Dispersants Can Cause the Initiation of Potentially Harmful Dinoflagellate Blooms (“Red Tides”). Environ. Sci. Technol. 2018, 52, 5718–5724. [Google Scholar] [CrossRef] [PubMed]
- Asatekin, A.; Mayes, A.M. Oil Industry Wastewater Treatment with Fouling Resistant Membranes Containing Amphiphilic Comb Copolymers. Environ. Sci. Technol. 2009, 43, 4487–4492. [Google Scholar] [CrossRef] [PubMed]
- Karthick, A.; Roy, B.; Chattopadhyay, P. A review on the application of chemical surfactant and surfactant foam for remediation of petroleum oil contaminated soil. J. Environ. Manag. 2019, 243, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, D.; Huang, C.; Huang, Y.; Yang, D.; Zhang, H.; Liu, Q.; Tang, T.; El-Din, M.G.; Kemppi, T.; et al. Stabilization mechanism and chemical demulsification of water-in-oil and oil-in-water emulsions in petroleum industry: A review. Fuel 2021, 286, 119390–119419. [Google Scholar] [CrossRef]
- Azad, A.R.M.; Ugelstad, J.; Fitch, R.M.; Hansen, F.K. Emulsification and Emulsion Polymerization of Styrene Using Mixtures of Cationic Surfactant and Long Chain Fatty Alcohols or Alkanes as Emulsifiers. In Emulsion Polymerization; ACS Publications: Washington, DC, USA, 1976; pp. 1–23. [Google Scholar]
- Asselah, A.; Pinazo, A.; Mezei, A.; Pérez, L.; Tazerouti, A. Self-Aggregation and Emulsifying Properties of Methyl Ester Sulfonate Surfactants. J. Surfactants Deterg. 2017, 20, 1453–1465. [Google Scholar] [CrossRef]
- Akkuş-Dağdeviren, Z.B.; Wolf, J.D.; Kurpiers, M.; Shahzadi, I.; Steinbring, C.; Bernkop-Schnürch, A. Charge reversal self-emulsifying drug delivery systems: A comparative study among various phosphorylated surfactants. J. Colloid Interface Sci. 2021, 589, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Y.; Li, J.; Wang, M.; Wang, Z. Study on the properties and self-assembly of fatty alcohol ether carboxylic ester anionic surfactant and cationic surfactant in a mixed system. New J. Chem. 2019, 43, 12494–12502. [Google Scholar] [CrossRef]
- Li, H.-P.; Zhao, H.; Liao, K. The Preparation of Asphalt Emulsions with Dissymmetric Gemini Quaternary Ammonium Salts Cationic Surfactants. Energy Sources Part A Recover. Util. Environ. Eff. 2013, 35, 2285–2293. [Google Scholar] [CrossRef]
- Mahmoud, S.A.; Dardir, M.M. Synthesis and Evaluation of a New Cationic Surfactant for Oil-Well Drilling Fluid. J. Surfactants Deterg. 2010, 14, 123–130. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Yuan, T.; Wang, C.; Gao, R.; Hu, G.; Xu, J.; Zhao, J. Synthesis and properties of zwitterionic gemini surfactants for enhancing oil recovery. J. Mol. Liq. 2020, 311, 113179. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, L.; Guo, X. Synthesis of Sulfobetaine-Type Zwitterionic Gemini Surfactants (EAPMAC) and Their Oilfield Application Properties. J. Surfactants Deterg. 2019, 22, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Bowers, R.R.; Temkin, A.M.; Guillette, L.J.; Baatz, J.E.; Spyropoulos, D.D. The commonly used nonionic surfactant Span 80 has RXRα transactivation activity, which likely increases the obesogenic potential of oil dispersants and food emulsifiers. Gen. Comp. Endocrinol. 2016, 238, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Opawale, F.O.; Burgess, D.J. Influence of Interfacial Properties of Lipophilic Surfactants on Water-in-Oil Emulsion Stability. J. Colloid Interface Sci. 1998, 197, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Ghaicha, L.; Leblanc, R.M.; Villamagna, F.; Chattopadhyay, A.K. Monolayers of Mixed Surfactants at the Oil-Water Interface, Hydrophobic Interactions, and Stability of Water-in-Oil Emulsions. Langmuir 1995, 11, 585–590. [Google Scholar] [CrossRef]
- Wollenweber, C.; Makievski, A.; Miller, R.; Daniels, R. Adsorption of hydroxypropyl methylcellulose at the liquid/liquid interface and the effect on emulsion stability. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 172, 91–101. [Google Scholar] [CrossRef]
- Morais, W.J.S.; Franceschi, E.; Dariva, C.; Borges, G.R.; Santos, A.F.; Santana, C.C. Dilatational Rheological Properties of Asphaltenes in Oil–Water Interfaces: Langmuir Isotherm and Influence of Time, Concentration, and Heptol Ratios. Energy Fuels 2017, 31, 10233–10244. [Google Scholar] [CrossRef]
- Tchoukov, P.; Yang, F.; Xu, Z.; Dabros, T.; Czarnecki, J.; Sjöblom, J. Role of Asphaltenes in Stabilizing Thin Liquid Emulsion Films. Langmuir 2014, 30, 3024–3033. [Google Scholar] [CrossRef]
- Langevin, D.; Argillier, J.-F. Interfacial behavior of asphaltenes. Adv. Colloid Interface Sci. 2016, 233, 83–93. [Google Scholar] [CrossRef]
- Yang, F.; Tchoukov, P.; Dettman, H.; Teklebrhan, R.B.; Liu, L.; Dabros, T.; Czarnecki, J.; Masliyah, J.; Xu, Z. Asphaltene Subfractions Responsible for Stabilizing Water-in-Crude Oil Emulsions. Part 2: Molecular Representations and Molecular Dynamics Simulations. Energy Fuels 2015, 29, 4783–4794. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Zhang, X.; Sui, H.; He, L.; Wang, S. A novel oxygen-containing demulsifier for efficient breaking of water-in-oil emulsions. Chem. Eng. J. 2020, 385, 123826. [Google Scholar] [CrossRef]
- Jian, C.; Poopari, M.R.; Liu, Q.; Zerpa, N.; Zeng, H.; Tang, T. Reduction of Water/Oil Interfacial Tension by Model Asphaltenes: The Governing Role of Surface Concentration. J. Phys. Chem. B 2016, 120, 5646–5654. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Greenfield, M.L. Chemical compositions of improved model asphalt systems for molecular simulations. Fuel 2014, 115, 347–356. [Google Scholar] [CrossRef]
- Deniz, C.U.; Yasar, M.; Klein, M.T. Stochastic Reconstruction of Complex Heavy Oil Molecules Using an Artificial Neural Network. Energy Fuels 2017, 31, 11932–11938. [Google Scholar] [CrossRef]
- Rocha, J.A.; Baydak, E.; Yarranton, H.W. What Fraction of the Asphaltenes Stabilizes Water-in-Bitumen Emulsions? Energy Fuels 2018, 32, 1440–1450. [Google Scholar] [CrossRef]
- Gray, M.R.; Tykwinski, R.R.; Stryker, J.M.; Tan, X. Supramolecular Assembly Model for Aggregation of Petroleum Asphaltenes. Energy Fuels 2011, 25, 3125–3134. [Google Scholar] [CrossRef]
- Kim, Y.H.; Wasan, D.T. Effect of Demulsifier Partitioning on the Destabilization of Water-in-Oil Emulsions. Ind. Eng. Chem. Res. 1996, 35, 1141–1149. [Google Scholar] [CrossRef]
- Grenoble, Z.; Trabelsi, S. Mechanisms, performance optimization and new developments in demulsification processes for oil and gas applications. Adv. Colloid Interface Sci. 2018, 260, 32–45. [Google Scholar] [CrossRef]
- Osborn, H.T.; Akoh, C.C. Effect of emulsifier type, droplet size, and oil concentration on lipid oxidation in structured lipid-based oil-in-water emulsions. Food Chem. 2004, 84, 451–456. [Google Scholar] [CrossRef]
- McClements, D.J. Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]
- Nakamura, A.; Takahashi, T.; Yoshida, R.; Maeda, H.; Corredig, M. Emulsifying properties of soybean soluble polysaccharide. Food Hydrocoll. 2004, 18, 795–803. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Lee, D.; Kim, J.H. Emulsion polymerization of styrene using an alkali-soluble random copolymer as polymeric emulsifier. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 2865–2872. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Kim, J.-H.; Min, T.-I. Role of alkali-soluble random copolymer in emulsion polymerization. Asp. E 1999, 153, 89–97. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Ma, J. Recent Research Progress in the Synthesis and Properties of Amphiphilic Block Co-polymers and Their Applications in Emulsion Polymerization. Des. Monomers Polym. 2009, 12, 19–41. [Google Scholar] [CrossRef]
- Edwards, S.E.; Flynn, S.; Hobson, J.J.; Chambon, P.; Cauldbeck, H.; Rannard, S.P. Mucus-responsive functionalized emulsions: Design, synthesis and study of novel branched polymers as functional emulsifiers. RSC Adv. 2020, 10, 30463–30475. [Google Scholar] [CrossRef]
- Ding, P.; Liu, W.; Zhao, Z. Roles of short amine in preparation and sizing performance of partly hydrolyzed ASA emulsion stabilized by Laponite particles. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 384, 150–156. [Google Scholar] [CrossRef]
- Nie, L.; Liu, S.; Shen, W.; Chen, D.; Jiang, M. One-pot synthesis of amphiphilic polymeric Janus particles and their self-assembly into supermicelles with a narrow size distribution. Angew. Chem. Int. Ed. 2007, 119, 6437–6440. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, G.; Zhu, L.; Chen, D.; Jiang, M. Nanoscale Tubular and Sheetlike Superstructures from Hierarchical Self-Assembly of Polymeric Janus Particles. Angew. Chem. Int. Ed. 2008, 47, 10171–10174. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Cui, D.; Jiang, W.; Han, L.; Niu, N. Preparation and application of Janus nanoparticles: Recent development and prospects. Coord. Chem. Rev. 2021, 454, 214318. [Google Scholar] [CrossRef]
- Whitby, C.P.; Wanless, E.J.J.M. Controlling Pickering emulsion destabilization: A route to fabricating new materials by phase inversion. Materials 2016, 9, 626. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Song, H.; Li, J.; Feng, Y.; Shi, Z.; Wang, X.; Lin, Q. Synthesis of bacterial cellulose and bacterial cellulose nanocrystals for their applications in the stabilization of olive oil pickering emulsion. Food Hydrocoll. 2017, 72, 127–135. [Google Scholar] [CrossRef]
- Chirwa, E.M.N.; Mampholo, T.; Fayemiwo, O. Biosurfactants as demulsifying agents for oil recovery from oily sludge-performance evaluation. Water Sci. Technol. 2013, 67, 2875–2881. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Yang, Y.; Jiang, J.; Liu, X.; Jiang, M. Research and application of particle emulsifiers. Prog. Chem. 2011, 23, 65. [Google Scholar]
- Lagaly, G.; Reese, M.; Abend, S. Smectites as colloidal stabilizers of emulsions: I. Preparation and properties of emulsions with smectites and nonionic surfactants. Appl. Clay Sci. 1999, 14, 83–103. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.; Li, X.; Sui, H.; He, L. Mechanisms on the stability and instability of water-in-oil emulsion stabilized by interfacially active asphaltenes: Role of hydrogen bonding reconstructing. Fuel 2021, 297, 120763. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, L.; Xie, L.; Lu, X.; Liu, Q.; He, J.; Mantilla, C.A.; Berg, F.G.A.V.D.; Zeng, H. Surface Interaction of Water-in-Oil Emulsion Droplets with Interfacially Active Asphaltenes. Langmuir 2017, 33, 1265–1274. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Götz, V.; Peukert, W. Effect of Surfactants on the Molecular Structure of the Buried Oil/Water Interface. Angew. Chem. Int. Ed. 2021, 60, 25143–25150. [Google Scholar] [CrossRef]
- Dimitrova, T.D.; Leal-Calderon, F.J. Forces between emulsion droplets stabilized with Tween 20 and proteins. Langmuir 1999, 15, 8813–8821. [Google Scholar] [CrossRef]
- Salou, M.; Siffert, B.; Jada, A. Study of the stability of bitumen emulsions by application of DLVO theory. Colloids Surfaces A Physicochem. Eng. Asp. 1998, 142, 9–16. [Google Scholar] [CrossRef]
- De Vleeschauwer, D.; Van der Meeren, P.J. Colloid chemical stability and interfacial properties of mixed phospholipid–non-ionic surfactant stabilized oil-in-water emulsions. Colloids Surfaces. A Physicochem. Eng. Asp. 1999, 152, 59–66. [Google Scholar] [CrossRef]
- Bizmark, N.; Ioannidis, M.A. Ethyl Cellulose Nanoparticles at the Alkane–Water Interface and the Making of Pickering Emulsions. Langmuir 2017, 33, 10568–10576. [Google Scholar] [CrossRef] [PubMed]
- Petkov, J.; Sénéchal, J.; Guimberteau, F.; Leal-Calderon, F. Indirect Evidence for Non-DLVO Forces in Emulsions. Langmuir 1998, 14, 4011–4016. [Google Scholar] [CrossRef]
- Gunning, A.P.; Mackie, A.R.; Wilde, A.P.J.; Morris, V.J. Atomic Force Microscopy of Emulsion Droplets: Probing Droplet−Droplet Interactions. Langmuir 2003, 20, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Dagastine, R.; Stevens, G.; Chan, D.; Grieser, F. Forces between two oil drops in aqueous solution measured by AFM. J. Colloid Interface Sci. 2004, 273, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, Z.; Masliyah, J. Colloidal forces between bitumen surfaces in aqueous solutions measured with atomic force microscope. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 260, 217–228. [Google Scholar] [CrossRef]
- Wang, W.; Li, K.; Ma, M.; Jin, H.; Angeli, P.; Gong, J. Review and perspectives of AFM application on the study of deformable drop/bubble interactions. Adv. Colloid Interface Sci. 2015, 225, 88–97. [Google Scholar] [CrossRef]
- Morris, V.J.; Woodward, N.C.; Gunning, A.P. Atomic force microscopy as a nanoscience tool in rational food design. J. Sci. Food Agric. 2011, 91, 2117–2125. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, Y.; Liu, Y.; Liu, X.; Rui, Z. Molecular Dynamics-Based Simulation on Chemical Flooding Produced Emulsion Formation and Stabilization: A Critical Review. Arab. J. Sci. Eng. 2020, 45, 7161–7173. [Google Scholar] [CrossRef]
- Li, B.; Zhang, L.; Liu, S.; Fan, M. Effects of Surfactant Headgroups on Oil-in-Water Emulsion Droplet Formation: An Experimental and Simulation Study. J. Surfactants Deterg. 2019, 22, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; Zhao, J.; Zhang, L.; Liang, Z.; Wang, J. Design, synthesis and characterization of bitumen emulsifiers based on molecular simulation. Kem. Ind. 2019, 68, 1–6. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Ren, S. Molecular Dynamics Simulation of Self-Aggregation of Asphaltenes at an Oil/Water Interface: Formation and Destruction of the Asphaltene Protective Film. Energy Fuels 2015, 29, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Dehaghani, A.H.S.; Taleghani, M.S.; Badizad, M.H.; Daneshfar, R. Simulation study of the Gachsaran asphaltene behavior within the interface of oil/water emulsion: A case study. Colloids Interface Sci. Commun. 2019, 33, 100202. [Google Scholar] [CrossRef]
- Niu, Z.; Ma, X.; Manica, R.; Yue, T. Molecular Destabilization Mechanism of Asphaltene Model Compound C5Pe Interfacial Film by EO-PO Copolymer: Experiments and MD Simulation. J. Phys. Chem. C 2019, 123, 10501–10508. [Google Scholar] [CrossRef]
- Duan, M.; Song, X.; Zhao, S.; Fang, S.; Wang, F.; Zhong, C.; Luo, Z. Layer-by-Layer Assembled Film of Asphaltenes/Polyacrylamide and Its Stability of Water-in-Oil Emulsions: A Combined Experimental and Simulation Study. J. Phys. Chem. C 2017, 121, 4332–4342. [Google Scholar] [CrossRef]
- Lv, G.; Gao, F.; Liu, G.; Yuan, S. The properties of asphaltene at the oil-water interface: A molecular dynamics simulation. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 515, 34–40. [Google Scholar] [CrossRef]
- Chen, G.; Hao, B.; Wang, Y.; Wang, Y.; Xiao, H.; Li, H.; Huang, X.; Shi, B. Insights into Regional Wetting Behaviors of Amphiphilic Collagen for Dual Separation of Emulsions. ACS Appl. Mater. Interfaces 2021, 13, 18209–18217. [Google Scholar] [CrossRef] [PubMed]
- Baiz, C.R.; Błasiak, B.; Bredenbeck, J.; Cho, M.; Choi, J.-H.; Corcelli, S.A.; Dijkstra, A.G.; Feng, C.-J.; Garrett-Roe, S.; Ge, N.-H.; et al. Vibrational Spectroscopic Map, Vibrational Spectroscopy, and Intermolecular Interaction. Chem. Rev. 2020, 120, 7152–7218. [Google Scholar] [CrossRef]
- Wang, H.; Gao, T.; Xiong, W. Self-Phase-Stabilized Heterodyne Vibrational Sum Frequency Generation Microscopy. ACS Photon. 2017, 4, 1839–1845. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Gruenbaum, S.M.; Skinner, J.L. Theoretical vibrational sum-frequency generation spectroscopy of water near lipid and surfactant monolayer interfaces. II. Two-dimensional spectra. J. Chem. Phys. 2014, 141, 22D505. [Google Scholar] [CrossRef]
- Singh, P.C.; Nihonyanagi, S.; Yamaguchi, S.; Tahara, T. Ultrafast vibrational dynamics of water at a charged interface revealed by two-dimensional heterodyne-detected vibrational sum frequency generation. J. Chem. Phys. 2012, 137, 094706. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Gruenbaum, S.M.; Skinner, J.L. Theoretical vibrational sum-frequency generation spectroscopy of water near lipid and surfactant monolayer interfaces. J. Chem. Phys. 2014, 141, 18C502. [Google Scholar] [CrossRef] [PubMed]
- Pullanchery, S.; Kulik, S.; Rehl, B.; Hassanali, A.; Roke, S.J.S. Charge transfer across C–HO hydrogen bonds stabilizes oil droplets in water. Science 2021, 374, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Kilpatrick, P.K. Water-in-Crude Oil Emulsion Stabilization: Review and Unanswered Questions. Energy Fuels 2012, 26, 4017–4026. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [Green Version]
- Marrucci, G. A theory of coalescence. Chem. Eng. Sci. 1969, 24, 975–985. [Google Scholar] [CrossRef]
- Ravera, F.; Dziza, K.; Santini, E.; Cristofolini, L.; Liggieri, L. Emulsification and emulsion stability: The role of the interfacial properties. Advances in Colloid and Interface. Science 2021, 288, 102344. [Google Scholar]
- Zhang, Z.; Xu, G.Y.; Wang, F.; Dong, S.L.; Li, Y.M. Characterization and demulsification of poly (ethylene oxide)–block–poly (propylene oxide)–block–poly (ethylene oxide) copolymers. J. Colloid Interface Sci. 2014, 277, 464–470. [Google Scholar] [CrossRef]
- Delgado-Linares, J.G.; Pereira, J.C.; Rondón, M.; Bullon, J.; Salager, J.L. Breaking of water-in-crude oil emulsions. 6. Estimating the demulsifier performance at optimum formulation from both the required dose and the attained instability. Energy Fuels 2016, 30, 5483–5491. [Google Scholar] [CrossRef]
- Mohammed, R.; Bailey, A.; Luckham, P.; Taylor, S. Dewatering of crude oil emulsions 2. Interfacial properties of the asphaltic constituents of crude oil. Colloids Surfaces A Physicochem. Eng. Asp. 1993, 80, 237–242. [Google Scholar] [CrossRef]
- Bourrel, M.; Salager, J.; Schechter, R.; Wade, W. A correlation for phase behavior of nonionic surfactants. J. Colloid Interface Sci. 1980, 75, 451–461. [Google Scholar] [CrossRef]
- Hirasaki, G.J.; Miller, C.A.; Raney, O.G.; Poindexter, M.K.; Nguyen, D.T.; Hera, J. Separation of Produced Emulsions from Surfactant Enhanced Oil Recovery Processes. Energy Fuels 2010, 25, 555–561. [Google Scholar] [CrossRef]
- Goldszal, A.; Bourrel, M. Demulsification of Crude Oil Emulsions: Correlation to Microemulsion Phase Behavior. Ind. Eng. Chem. Res. 2000, 39, 2746–2751. [Google Scholar] [CrossRef]
- Nguyen, D.; Sadeghi, N.; Houston, C. Chemical Interactions and Demulsifier Characteristics for Enhanced Oil Recovery Applications. Energy Fuels 2012, 26, 2742–2750. [Google Scholar] [CrossRef]
- Bera, A.; Mandal, A.; Guha, B.B. Synergistic Effect of Surfactant and Salt Mixture on Interfacial Tension Reduction between Crude Oil and Water in Enhanced Oil Recovery. J. Chem. Eng. Data 2014, 59, 89–96. [Google Scholar] [CrossRef]
- Gaonkar, A.G. Effects of salt, temperature, and surfactants on the interfacial tension behavior of a vegetable oil/water system. J. Colloid Interface Sci. 1992, 149, 256–260. [Google Scholar] [CrossRef]
- Al-Sabagh, A.M.; Kandile, N.G.; El-Ghazawy, R.A.; El-Din, M.R.N. Synthesis and evaluation of some new demulsifiers based on bisphenols for treating waterin-crude oil emulsions. Egypt. J. Pet 2011, 20, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.G.; Taylor, A.S.; Graham, D.E. Emulsification and demulsification related to crude oil production. Colloids Surf. 1985, 15, 175–189. [Google Scholar] [CrossRef]
- Menon, V.B.; Wasan, D.T. Demulsification. In Encyclopedia of Emulsion Technology, Applications; Becher, P., Ed.; Marcel Dekker: New York, NY, USA, 1985; Volume 2, pp. 1–76. [Google Scholar]
- Hu, C.; Liu, S.; Fang, S.; Xiang, W.; Duan, M. Dissipative particle dynamics investigation of demulsification process and mechanism of comb-like block polyether. Polym. Adv. Technol. 2018, 29, 3171–3180. [Google Scholar] [CrossRef]
- Chen, T.; Mohammed, R.; Bailey, A.; Luckham, P.; Taylor, S. Dewatering of crude oil emulsions 4. Emulsion resolution by the application of an electric field. Colloids Surf. A Physicochem. Eng. Asp. 1994, 83, 273–284. [Google Scholar] [CrossRef]
- Kotsaridou-Nagel, M.; Kragert, B. Demulsifying water-in-oil emulsions through chemical addition. Erdoel Erdgas Kohle 1996, 112, 72–79. [Google Scholar]
- Urdahl, O.; Sjöblom, J. Water-in-crude oil emulsions from the Norwegian Continental Shelf. A stabilization and destabilization study. J. Dispers. Sci. Technol. 1995, 16, 557–574. [Google Scholar] [CrossRef]
- Hartands, S.; Jeelani, S.A.K. A study of demulsifying mechanism on crude oil demulsifier. Colloids Surf. 1994, 88, 289–302. [Google Scholar]
- Bhardwaj, A.; Hartland, S.J. Kinetics of coalescence of water droplets in water-in-crude. Disper. Sci. Technol. 1994, 15, 133–136. [Google Scholar] [CrossRef]
- Sjöblom, J. Emulsions—A Fundamental and Practical Approach; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992; pp. 103–106. [Google Scholar]
- Bhardwaj, A.; Hartland, S. Dynamics of Emulsification and Demulsification of Water in Crude Oil Emulsions. Ind. Eng. Chem. Res. 1994, 33, 1271–1279. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Acosta, E.J.; Scamehorn, J.F.; Sabatini, D.A. Microemulsion phase behavior of anionic-cationic surfactant mixtures: Effect of tail branching. J. Surfactants Deterg. 2006, 9, 169–179. [Google Scholar] [CrossRef]
- Kaler, E.W.; Herrington, K.L.; Murthy, A.K.; Zasadzinski, J.A.N. Phase behavior and structures of mixtures of anionic and cationic surfactants. J. Phys. Chem. 1992, 96, 6698–6707. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Fakhru’L-Razi, A.; Abdullah, L.C.; Elnashaie, S.S.E.H.; Pendashteh, A. Demulsification techniques of water-in-oil and oil-in-water emulsions in petroleum industry. Sep. Purif. Technol. 2016, 170, 377–407. [Google Scholar] [CrossRef]
- Detloff, T.; Lerche, D. Centrifugal separation in tube and disc geometries: Experiments and theoretical models. Acta Mech. 2008, 201, 83–94. [Google Scholar] [CrossRef]
- Chiesa, M.; Ingebrigtsen, S.; Melheim, J.; Hemmingsen, P.; Hansen, E.; Hestad, Ø. Investigation of the role of viscosity on electrocoalescence of water droplets in oil. Sep. Purif. Technol. 2006, 50, 267–277. [Google Scholar] [CrossRef]
- Guo Kun, Q. Analysis on factors influencing crude oil demulsification effectiveness by ultrasonic wave. Pet. Geol. Recovery Effic. 2005, 12, 76–78. [Google Scholar]
- Tan, W.; Yang, X.; Tan, X. Study on Demulsification of Crude Oil Emulsions by Microwave Chemical Method. Sep. Sci. Technol. 2007, 42, 1367–1377. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W.; Knozowska, K. Hydrophobic Ceramic Membranes for Water Desalination. Appl. Sci. 2017, 7, 402. [Google Scholar] [CrossRef] [Green Version]
- Tawalbeh, M.; Al Mojjly, A.; Al-Othman, A.; Hilal, N. Membrane separation as a pre-treatment process for oily saline water. Desalination 2018, 447, 182–202. [Google Scholar] [CrossRef] [Green Version]
- Munirasu, S.; Abu Haija, M.; Banat, F. Use of membrane technology for oil field and refinery produced water treatment—A review. Process Saf. Environ. Prot. 2016, 100, 183–202. [Google Scholar] [CrossRef]
- Nadarajah, N.; Singh, A.; Ward, O.P. Evaluation of a mixed bacterial culture for demulsification of water-in-petroleum oil emulsions. World J. Microbiol. Biotechnol. 2002, 18, 435–440. [Google Scholar] [CrossRef]
- Choudhary, P.; Srivastava, R.K.; Mahendra, S.N.; Motahhir, S. Sustainable Solution for Crude Oil and Natural Gas Separation using Concentrated Solar Power Technology. IOP Conf. Ser. Mater. Sci. Eng. 2017, 225, 12134. [Google Scholar] [CrossRef] [Green Version]
- Sousa, A.M.; Pereira, M.J.; Matos, H.A. Oil-in-water and water-in-oil emulsions formation and demulsification. J. Pet. Sci. Eng. 2021, 210, 110041. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, B.; Zhang, X.; Li, W.; Gong, H. Heat strengthening of double-field coupling demulsification of industrial waste oil emulsion. Appl. Petrochem. Res. 2018, 9, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Zhang, Z.; Yu, J.; Ye, X. Performance characteristics of a hybrid membrane pilot-scale plant for oilfield-produced wastewater. Desalination 2008, 225, 113–122. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, S.; Ye, C.; Wang, M.; Cheng, H.; Wen, H.; Meng, X. Treatment of waste filature oil/water emulsion by combined demulsification and reverse osmosis. Sep. Purif. Technol. 2008, 63, 264–268. [Google Scholar] [CrossRef]
- Pintarič, Z.N.; Škof, G.P.; Kravanja, Z. MILP synthesis of separation processes for waste oil-in-water emulsions treatment. Front. Chem. Sci. Eng. 2016, 10, 120–130. [Google Scholar] [CrossRef]
- Abidli, A.; Huang, Y.; Cherukupally, P.; Bilton, A.M.; Park, C.B. Novel separator skimmer for oil spill cleanup and oily wastewater treatment: From conceptual system design to the first pilot-scale prototype development. Environ. Technol. Innov. 2020, 18, 100598. [Google Scholar] [CrossRef]
- Ceschia, E.; Harjani, J.R.; Liang, C.; Ghoshouni, Z.; Andrea, T.; Brown, R.S.; Jessop, P.G. Switchable anionic surfactants for the remediation of oil-contaminated sand by soil washing. RSC Adv. 2014, 4, 4638–4645. [Google Scholar] [CrossRef]
- Long, X.; Zhang, G.; Han, L.; Meng, Q. Dewatering of floated oily sludge by treatment with rhamnolipid. Water Res. 2013, 47, 4303–4311. [Google Scholar] [CrossRef]
- Dudek, M.; Vik, E.A.; Aanesen, S.V.; Øye, G. Colloid chemistry and experimental techniques for understanding fundamental behaviour of produced water in oil and gas production. Adv. Colloid Interface Sci. 2020, 276, 102105. [Google Scholar] [CrossRef]
- Salgado, H.; Mariño, L.; Pacheco, R. Overcoming tight emulsion problems. Pet. Technol. Q. 2014, 19, 105–109. [Google Scholar]
- de Souza, T.S.; Dias, F.F.; Koblitz, M.G.B.; Bell, J.M.D.M. Effects of enzymatic extraction of oil and protein from almond cake on the physicochemical and functional properties of protein extracts. Food Bioprod. Process. 2020, 122, 280–290. [Google Scholar] [CrossRef]
- Moura, J.M.L.N.; Maurer, D.; Jung, S.; Johnson, L.A. Pilot-Plant Proof-of-Concept for Integrated, Countercurrent, Two-Stage, Enzyme-Assisted Aqueous Extraction of Soybeans. J. Am. Oil Chem. Soc. 2010, 88, 1649–1658. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Rosentrater, K.A.; Sekhon, J.; Wang, T.; Jung, S.; Johnson, L.A. Economic Feasibility of Soybean Oil Production by Enzyme-Assisted Aqueous Extraction Processing. Food Bioprocess Technol. 2019, 12, 539–550. [Google Scholar] [CrossRef]
- Mukherjee, S.; Das, P.; Sen, R. Towards commercial production of microbial surfactants. Trends Biotechnol. 2006, 24, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Lotfabad, T.B.; Ebadipour, N.; RoostaAzad, R. Evaluation of a recycling bioreactor for biosurfactant production byPseudomonas aeruginosaMR01 using soybean oil waste. J. Chem. Technol. Biotechnol. 2016, 91, 1368–1377. [Google Scholar] [CrossRef]
- Zoubeik, M.; Ismail, M.; Salama, A.; Henni, A. New Developments in Membrane Technologies Used in the Treatment of Produced Water: A Review. Arab. J. Sci. Eng. 2018, 43, 2093–2118. [Google Scholar] [CrossRef]
- Abd Halim, N.S.; Wirzal, M.D.H.; Hizam, S.M.; Bilad, M.R.; Nordin, N.A.H.M.; Sambudi, N.S.; Putra, Z.A.; Yusoff, A.R.M. Recent Development on Electrospun Nanofiber Membrane for Produced Water Treatment: A review. J. Environ. Chem. Eng. 2021, 9, 104613. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Boyraz, E.; Maryska, J.; Kucerova, K. A Review on Membrane Technology and Chemical Surface Modification for the Oily Wastewater Treatment. Materials 2020, 13, 493. [Google Scholar] [CrossRef] [Green Version]
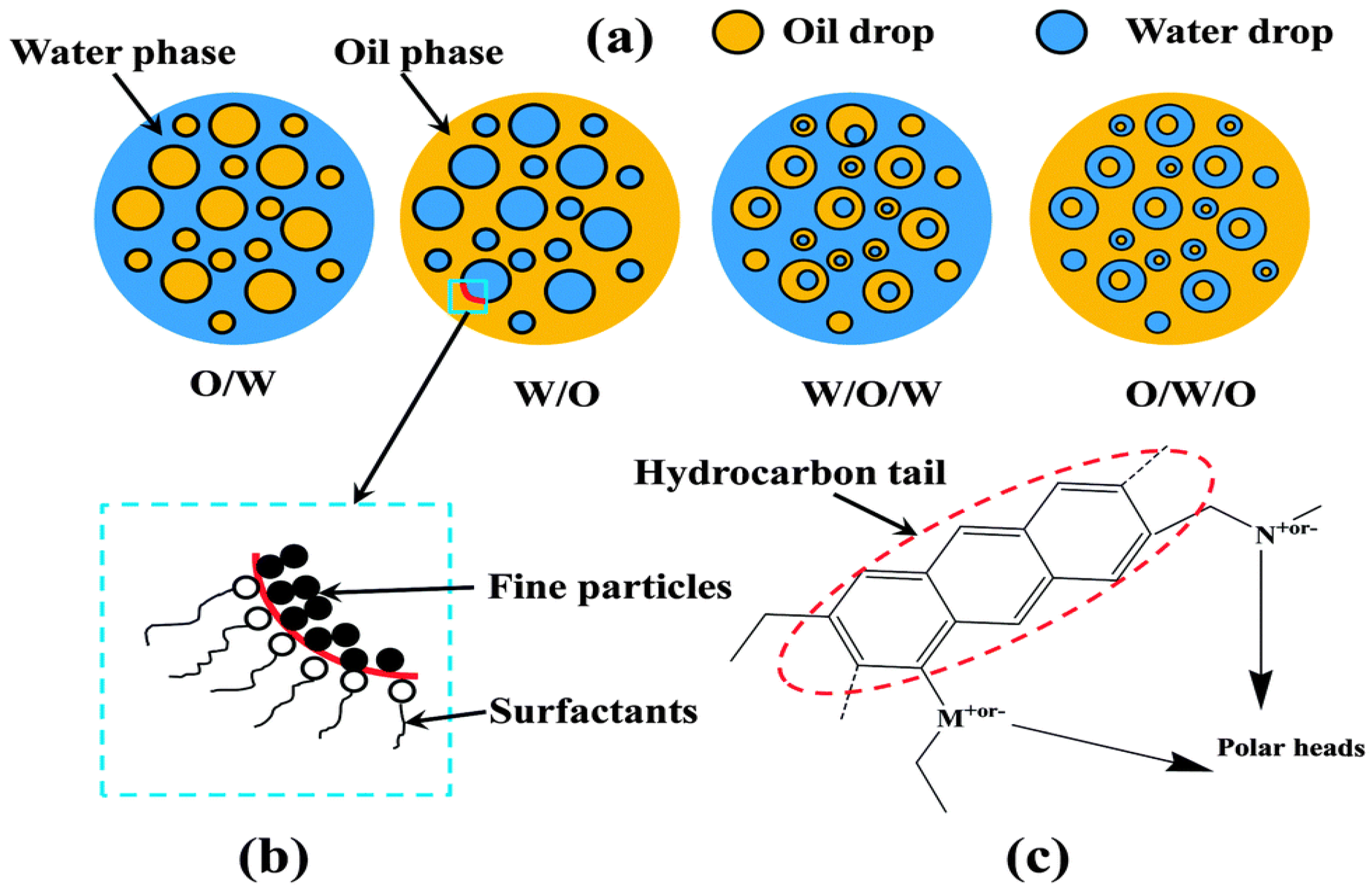

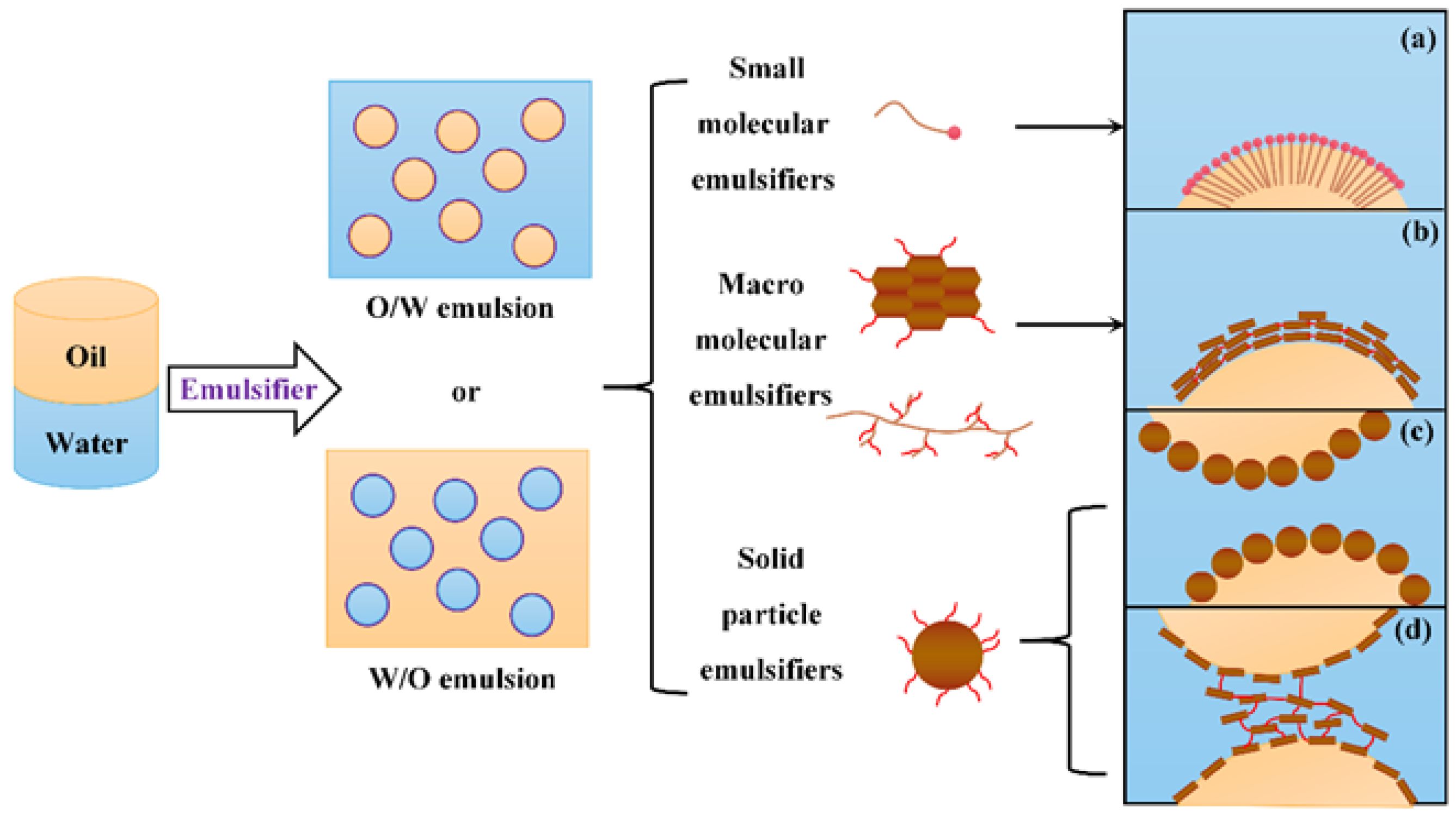
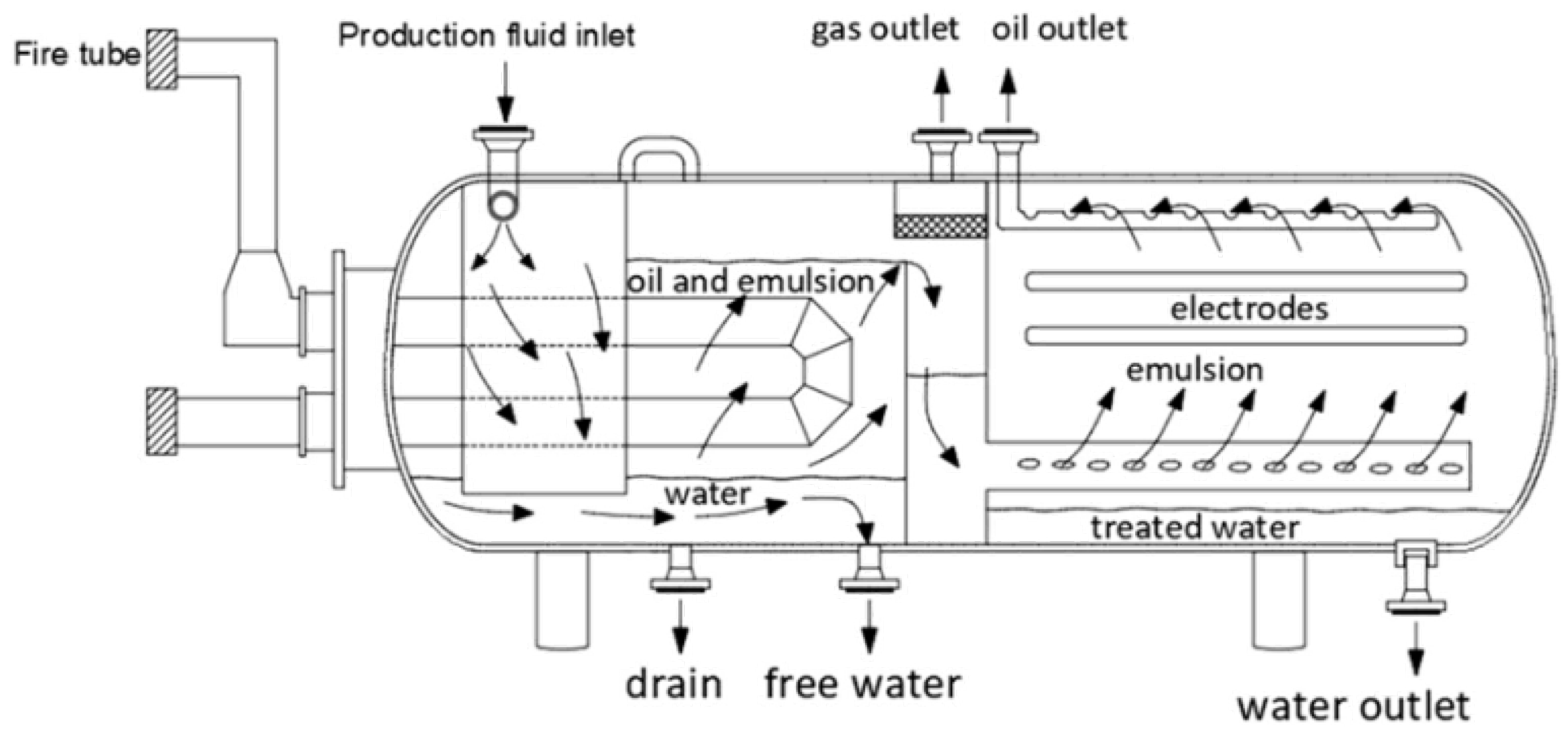
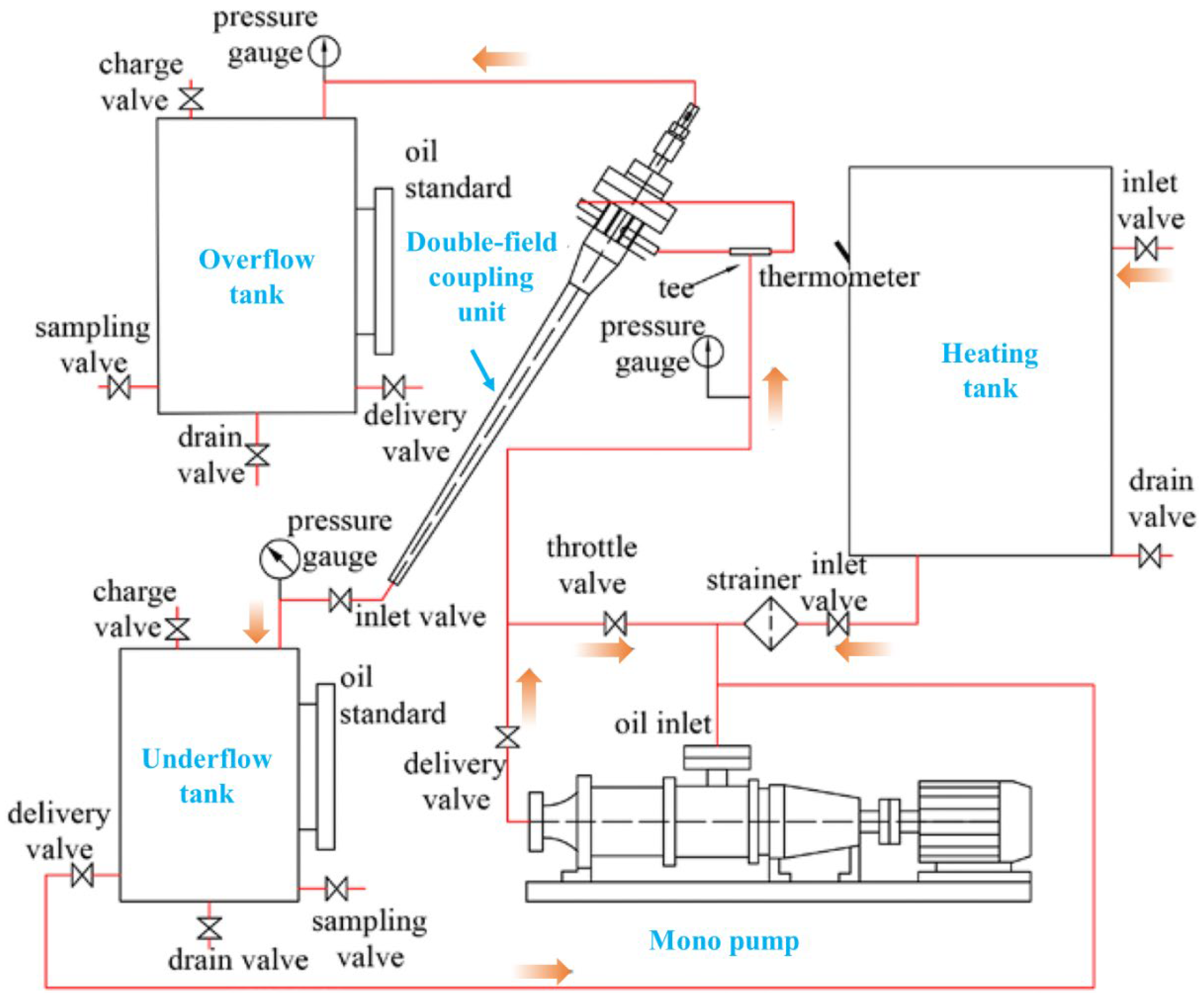

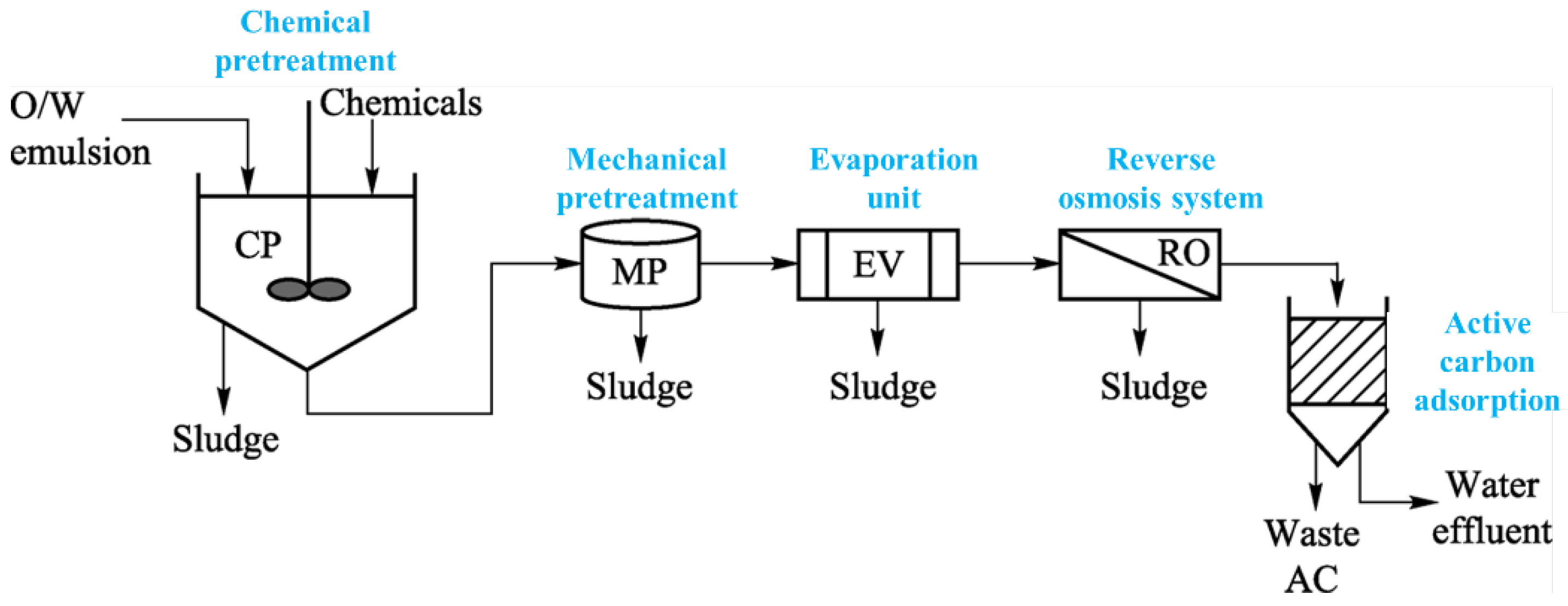

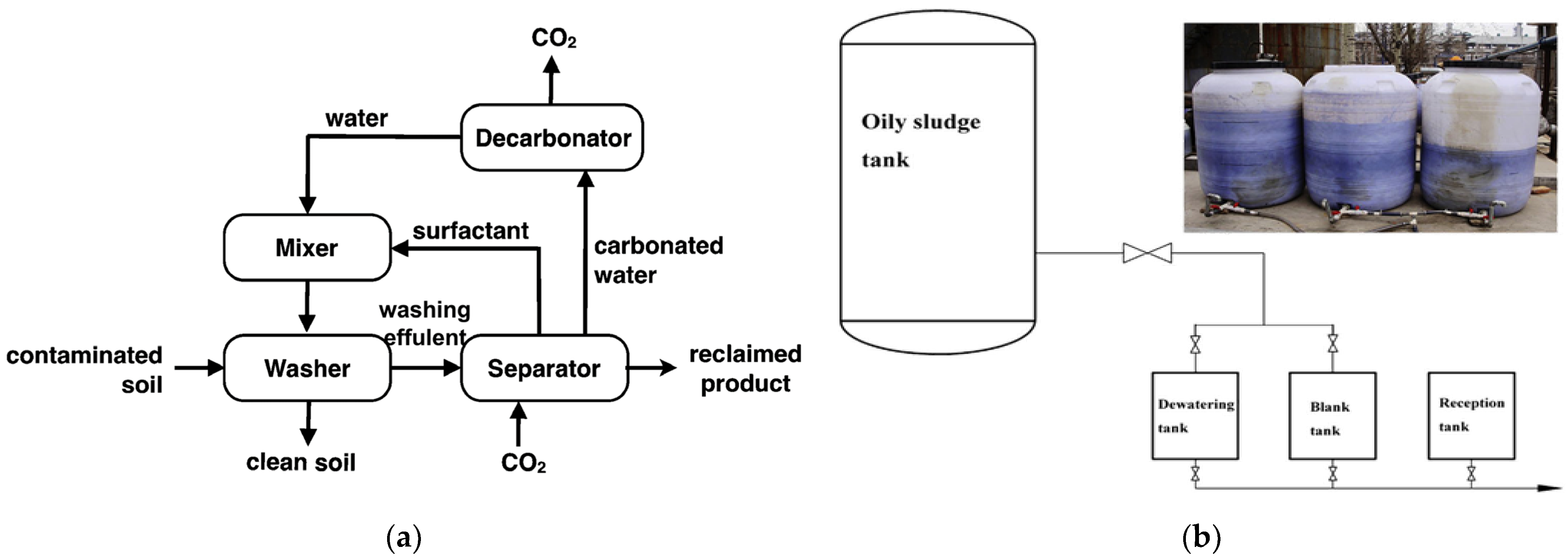
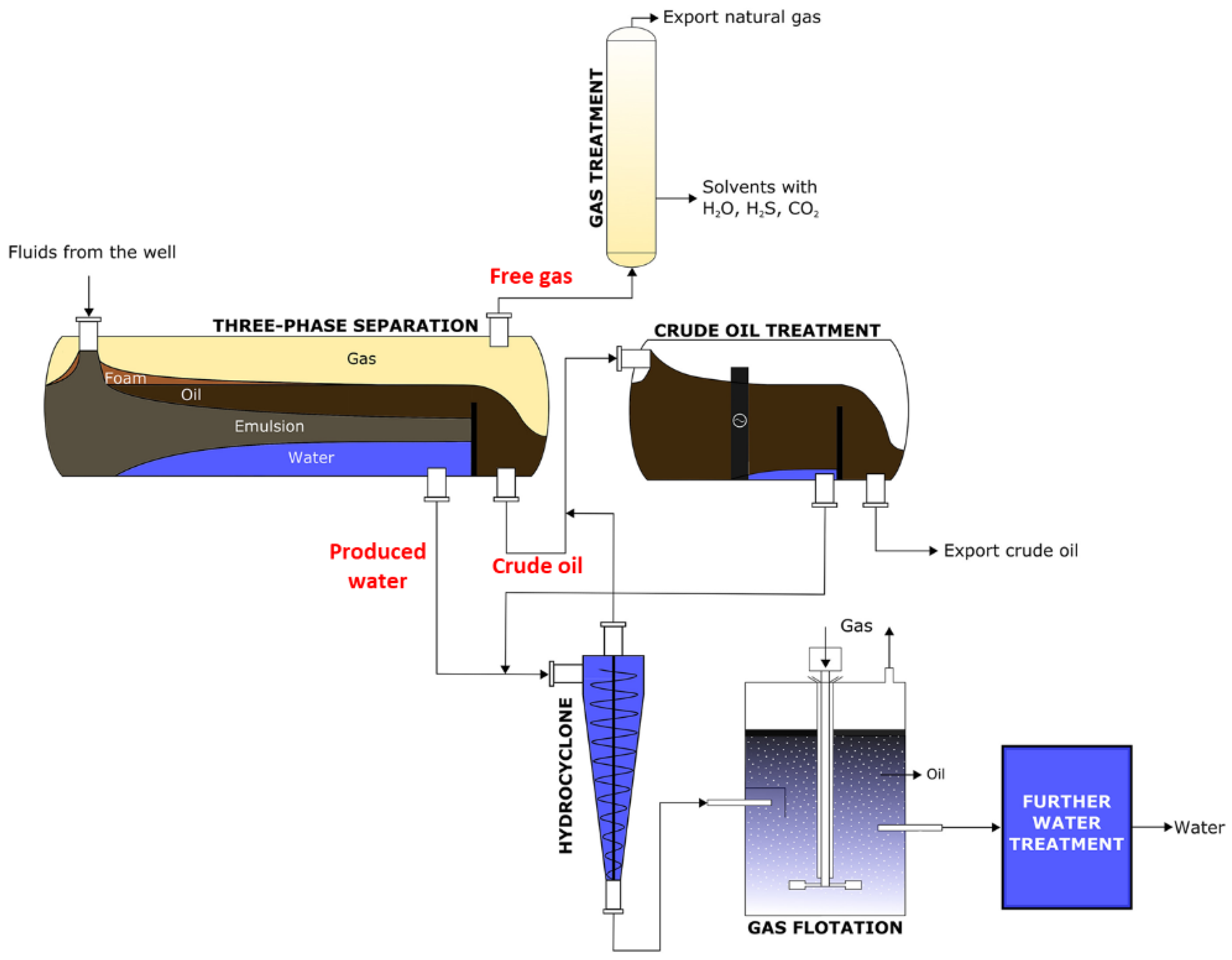
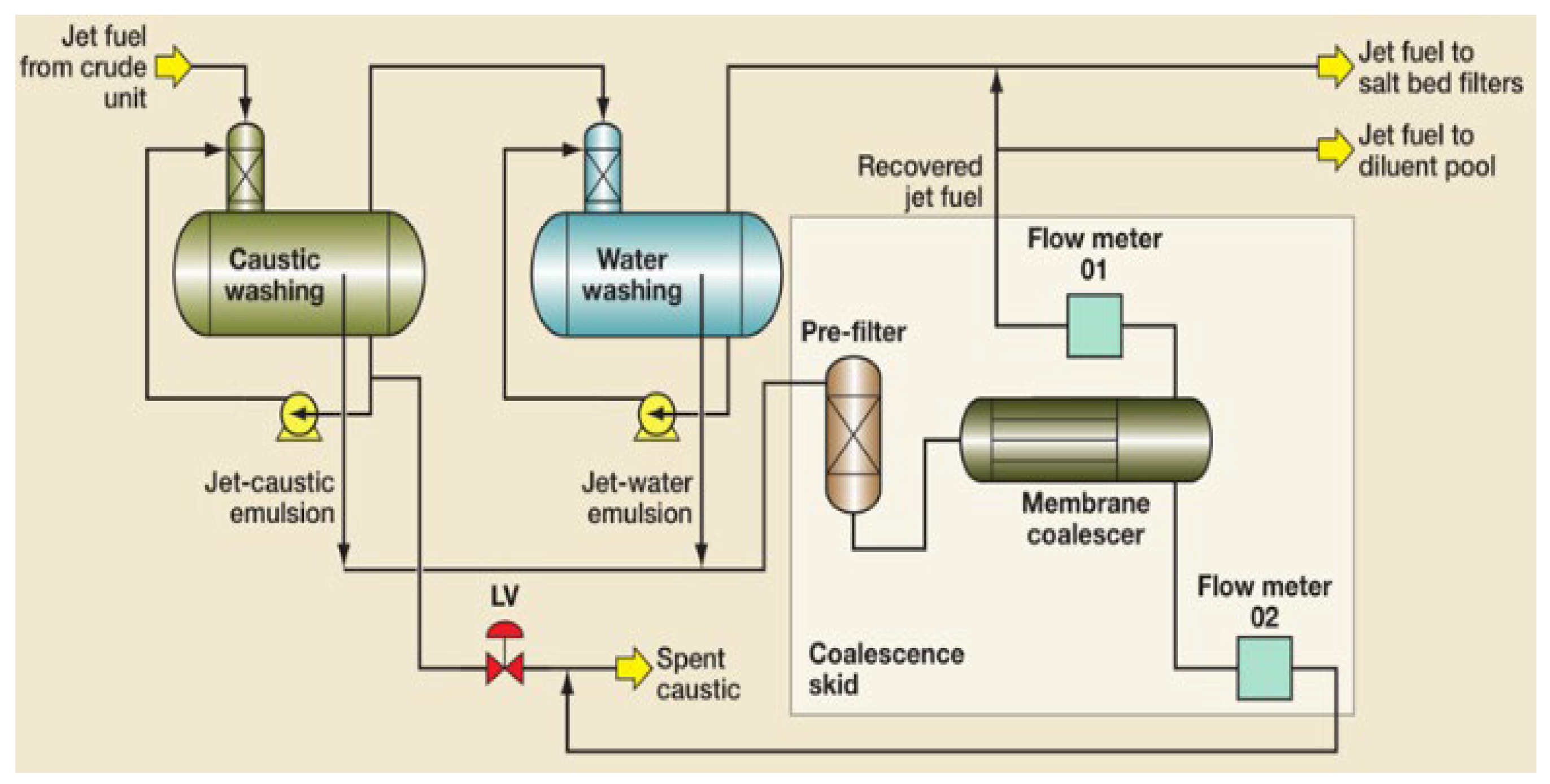

| Author | Basis | Categories | Description |
|---|---|---|---|
| Bansbach [26] | The size of droplets in the dispersed phase | Tight emulsion Loose emulsion | Tight emulsions refer to emulsions that contain very small-sized droplets in the dispersed phase and that do not completely separate within a few hours due to their special structure. Loose emulsions, on the other hand, contain relatively large droplets in the dispersed phase, which allows separation within a few minutes. |
| Fingas et al. [31] | The stability, appearance, and rheological measurements | Stable water-in-oil emulsion Meso-stable water-in-oil emulsion Entrained water Unstable water-in-oil emulsion | Emulsions are considered to be in stable and meso-stable states [32]. Asphaltenes and resins trigger off a tough and stable visco-elastic interfacial film. Meso-stable emulsions are those emulsions between stable and unstable states that are not fully stabilized due to insufficient asphaltene contents, resulting in the possibility of degradation. |
| Friberg et al. [33,34] | The size of droplets in the dispersed phase | Macro-emulsion Micro-emulsion | In general, the majority of emulsions are macro-emulsions. The size of the dispersed droplets in macro-emulsions is generally larger than 0.1 μm. Thermodynamically, they are unstable as the oil and water phases tend to coalesce and finally separate over time due to the decrease in interfacial energy. The droplet size in micro-emulsions is generally less than 10 nm. It is formed due to the severe low interfacial energy of two immiscible liquids. The micro-emulsion is considered a thermodynamically stable mixture. |
| Order | Phase Equilibria | Description |
|---|---|---|
| I | Oil-in-water (O/W) | This type of emulsion contains a water-soluble surfactant, and the surfactant exists in water when forming monomers (Winsor I). |
| II | Water-in-oil (W/O) | This type of emulsion contains an oil-soluble surfactant, and the surfactant-rich oil phase exists at the same time as the water. (Winsor II). |
| III | Three-phase system | It is also called middle-phase micro-emulsion. A middle phase of rich surfactant coexists with superfluous water and oil (Winsor III). |
| IV | Micellar solution | Adding sufficient amounts of surfactant and alcohol can form an isotropic solution of suspended single-phase micelles. |
| Properties | Macro-Emulsion | Micro-Emulsion |
|---|---|---|
| Transparency | Cloudy | Optically transparent |
| Droplet’s size | >0.1 nm | 0.01~0.1 μm |
| Drop shape | Generally, spherical | Spherical |
| Thermodynamic stability | Unstable, stratification after centrifugation | Stable |
| Type | Diagram | Description |
|---|---|---|
| Winsor I | (a) | The single-phase region is occupied by an O/W micro-emulsion phase, and the two-phase region is occupied by an O/W micro-emulsion in equilibrium with the excess oil phase. |
| Winsor II | (c) | The single-phase region in diagram (c) is occupied by a W/O micro-emulsion, and the two-phase region is occupied by a W/O micro-emulsion in equilibrium with the excess water phase. |
| Winsor III | (b) | In the three-phase region, the micro-emulsion is in equilibrium with the excess water phase and the excess oil phase at the same time, and the three-phase composition does not change with the overall composition (system point). |
| Industrial Fields | Common Types of Emulsions | Description |
|---|---|---|
| Petroleum | W/O, O/W | Further exploitation of the oil field causes the produced fluid to gradually change from W/O to O/W emulsions. |
| Coal chemical | O/W, W/O, sludge | The wastewater is generated from the coal liquification and gasification process, in which the components are resistant to degradation. |
| Metalworking | O/W, W/O | Used hydraulic oil, used lubricating oil, metal cutting fluid, and coolant, etc. |
| Food | O/W, W/O, multilayer emulsions, Pickering emulsion | Various food products, both natural and man-made, exist in part or whole as emulsions, or in the emulsified form at certain times during the manufacturing process, including milk, cream, fruit drinks, infant formula, soups, cake batter, salad dressings, mayonnaise, creamy condiments, desserts, salad cream, ice cream, coffee whitening agents, spreads, butter, and margarine [36,37]. Protein-stabilized Pickering emulsions in the food industry have three main applications including formulation of spread-like products, encapsulation of bioactive components, and protection of lipids [38]. |
| Cosmetics | O/W, W/O, multilayer emulsions, Pickering emulsion | Cosmetic emulsions are formulated with hydrophilic materials, hydrophobic materials, surfactants, and often additional materials are also added to the formulation to enhance its performance value, improve the sensory, provide fragrance, etc. [39]. With solid particles, Pickering emulsions are widely used in color cosmetics products [40]. |
| Order | Sources |
|---|---|
| 1 | Lubricating oil is generated by lubrication, cooling, transmission, and other systems in the machining process, emulsified oil for cooling and transmission, etc. |
| 2 | Oily wastewater is produced when cleaning machine parts. |
| 3 | Oily wastewater is produced by oil leakage when conducting tractors and other tests, mainly containing diesel oil and gasoline. |
| 4 | Oily wastewater is discharged by rinsing the floor, tanks, and other equipment in the workshop, which is the main source of oily wastewater from mechanical processing. |
| Order | Type | Emulsion Droplet Size | Description |
|---|---|---|---|
| 1 | Floating oil | >100 μm | Also called “oil slick”. The oil slick is the present form of most oily wastewater, which, once at rest, quickly floats and can float on the water surface as a continuous oil film. |
| 2 | Dispersed oil | 10~100 μm | The dispersed oil is unstable, which aggregates and forms larger oil droplets that float on the surface. |
| 3 | Emulsified oil | 0.1~10 μm | The surface of emulsified oil is usually covered with a negatively charged bilayer that is relatively stable and hardly floats on the water surface. |
| 4 | Dissolved oil | <0.1 μm | The dissolved oil is dispersed in water as molecules. The uniform system formed by oil and water is very stable and difficult to remove with conventional methods. |
| 5 | Oil–solids mixture | / | The oil adheres to the surface of the small solid particles in water to form an oil–solids mixture. |
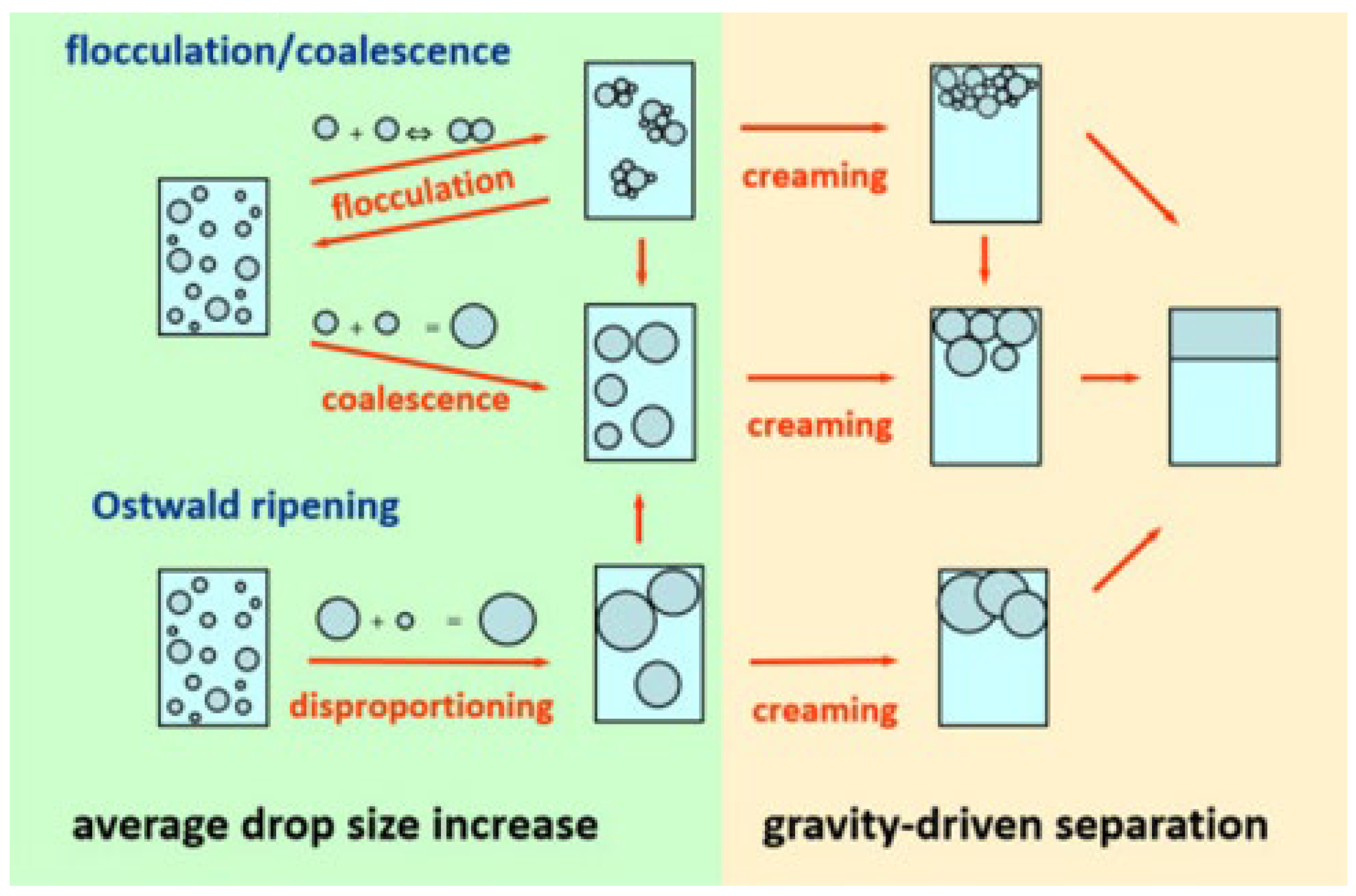
| Demulsification Process | Definition | Details |
|---|---|---|
| Sedimentation | The difference between water and oil density causes the fall of water droplets due to gravity, and the gravity is greater than buoyancy. | It depends on the difference between oil and water density. |
| Creaming | The separation of emulsions into denser parts (cream) and other parts without actually breaking. | |
| Flocculation | It refers to the agglomeration of suspended droplets in an emulsion, or the formation of floccules, which can accelerate the coagulation of droplets and achieve the purpose of separation. |
|
| Coalescence | Two or more separate groups pull each other to reach the slightest contact, and the process acts on miscible particles. | The influencing factors of the interfacial film include viscosity, elasticity, and the dynamics of drainage. |
| Aggregation | It corresponds to accumulating the suspended droplets. | It is the most common process, resulting in the instability of colloidal systems. |
| Ostwald ripening | At the later stage of the precipitation phase precipitated by supersaturated solid solution, the size of precipitated phase particles is different. Due to the dissolution of smaller particles, larger particles continue to grow, thus increasing the average size of particles. | It is generally experienced in water/oil emulsions, and other liquid or solid solutions. |
| Phase separation | Oil and water completely separate into two distinct phases. | It relies on time and types of emulsifier. |
| Order | Theory | Description |
|---|---|---|
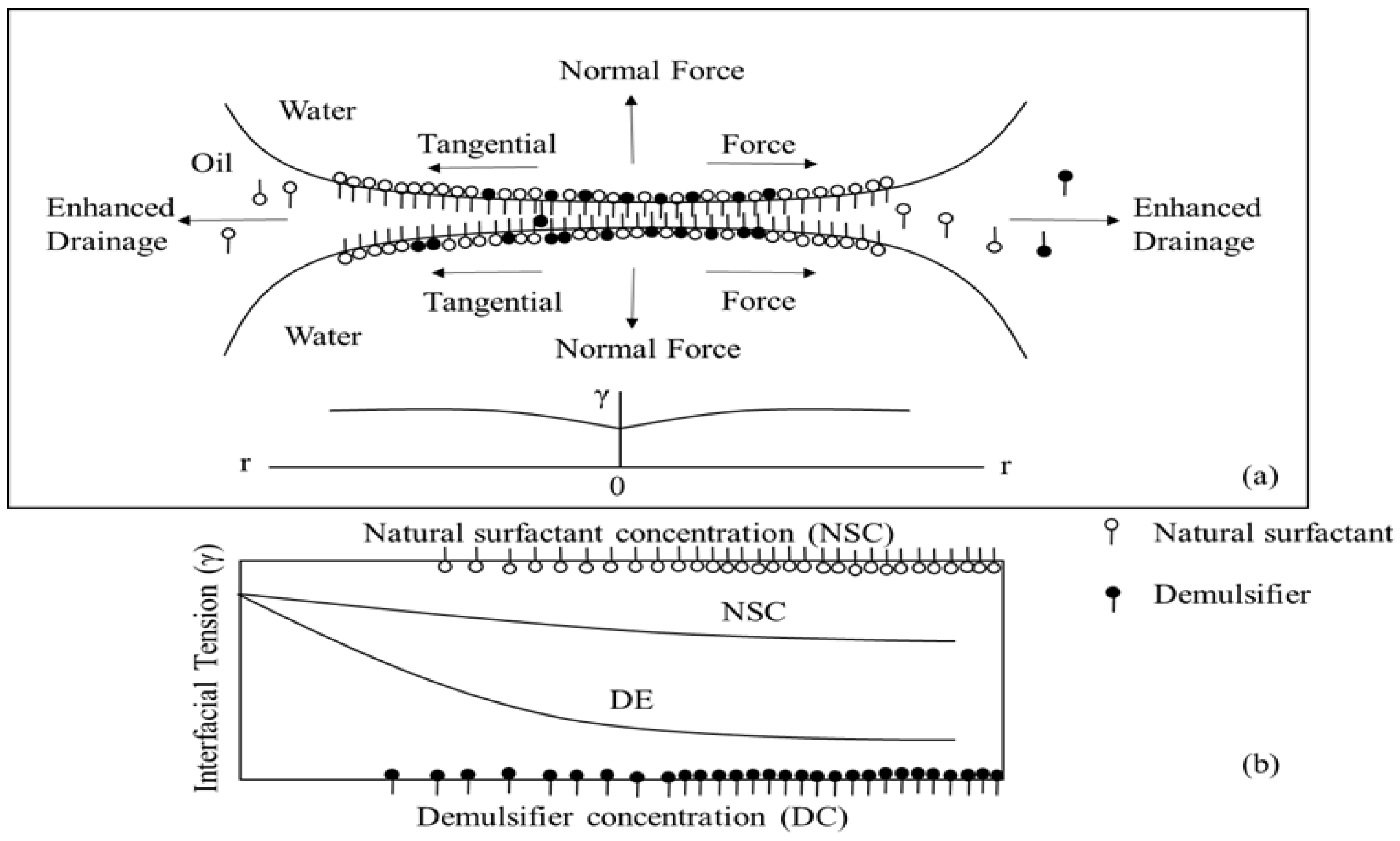
| 1 | Replacement or displacement [169,170] | The surfactivity of the demulsifier is higher than that of the natural surfactants in crude oil, thus demulsifier can replace or displace the surfactants at the oil–water interface to disrupt the stability of the interfacial film. |
| 2 | Reverse acting [171] | The demulsifier can change the type of emulsion. Depending on the properties of the demulsifiers, the O/W emulsion and W/O emulsion convert to each other. The oil droplets and water droplets are separated by gravity. |
| 3 | Electrostatic adsorption [172] | The demulsifier having an opposite charge to the interfacial film of the emulsion neutralizes the repulsive force between the interfacial films to demulsify the emulsion. This mechanism is generally applied to ionic demulsifiers. |
| 4 | Dispersion-Solubilization [173] | Some types of demulsifiers have a solubilizing effect. The demulsifier in the emulsion forms micelles and dissolves the surfactants. |
| 5 | Coalescence-Flocculation [174] | The molecular chain of demulsifiers can be adsorbed on the interfacial film of the droplets and form a loose pellet centered on the demulsifier, which increases the contact area between the droplets and the probability of collision. |
| Treatment | Advantages | Disadvantages |
|---|---|---|
| Centrifugation | High efficiency, lesser operational time. | High cost of maintenance and energy for rotating. |
| Hydrocyclone | High efficiency, compact modules, output for smaller oil particles. | High cost of maintenance and energy, fouling. |
| Bio-demulsification | Better adaptability, strong versatility, non-toxic, eco-friendly, degradable. | High cost. |
| Heating | These techniques are widely used, easy operating and efficient while being used in combination with other methods. | Low efficiency, longer time aimed at O/W emulsion with higher water content, often used in conjunction with other methods. |
| Electric dehydration | The possibility of short circuiting, will consume lots of energy and increase investment costs. | |
| Gravity | Unsatisfactory demulsification effect, huge equipment demand. | |
| Microwave | Fast-speeding, no hysteresis effect. | Low dehydration rate, long settling time. |
| Ultrasonic | No pollution, no emission, low energy consumption, strong universality. | Difficulties in industrial scale-up, high cost of equipment. |
| Magnetic | More suitable for sewage treatment. | Currently in the preliminary stage of research. |
| Membrane | High efficiency, low energy consumption, wide application range. | Low membrane flux, small processing capacity, membrane fouling. |
| In situ extraction | Floating oil can be continuously collected from the water surface. | High cost. |
| Gas flotation | Higher efficiency, fixed parts, robust and durable, easy operation. | Large quantity of skim volume, lateness in separation time, high amount of air generated. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Zhou, J.; He, C.; He, L.; Li, X.; Sui, H. The Formation, Stabilization and Separation of Oil–Water Emulsions: A Review. Processes 2022, 10, 738. https://doi.org/10.3390/pr10040738
Tian Y, Zhou J, He C, He L, Li X, Sui H. The Formation, Stabilization and Separation of Oil–Water Emulsions: A Review. Processes. 2022; 10(4):738. https://doi.org/10.3390/pr10040738
Chicago/Turabian StyleTian, Ying, Jingjing Zhou, Changqing He, Lin He, Xingang Li, and Hong Sui. 2022. "The Formation, Stabilization and Separation of Oil–Water Emulsions: A Review" Processes 10, no. 4: 738. https://doi.org/10.3390/pr10040738
APA StyleTian, Y., Zhou, J., He, C., He, L., Li, X., & Sui, H. (2022). The Formation, Stabilization and Separation of Oil–Water Emulsions: A Review. Processes, 10(4), 738. https://doi.org/10.3390/pr10040738






