Abstract
Urban forests are key to mitigating the Urban Heat Island Effect, which contributes to temperature increases in urban areas. However, the trees in these forests are usually under stress because urban soil is typically degraded. Biochar/compost amendments help with soil management by improving the physiochemical properties and bacterial communities of soil. Here, we compared the physiochemical properties and bacterial communities before and after (1) biochar-only and (2) biochar-based compost amendments. Our results suggested that biochar-only application did not improve soil properties after 1 year of treatment, whereas in the biochar-based compost treatment, the soil properties and bacterial communities changed after just four months. The increase in potassium and decrease in organic material, calcium, and available phosphorus in the soil of the former treatment indicated that the nutrient uptake of its trees had improved. Although there was no significant variation in the soil’s total nitrogen, the higher abundance of potential nitrogen-fixing bacteria in the biochar-based treatment suggested that the soil contained a supplement to nitrogen. Our results show that biochar-based compost amendment improves soil quality and associated bacterial communities in urban forest management.
1. Introduction
Urban forests have environmental and socio-cultural functions. These greenspaces adjust the microclimate and cool rising temperatures [1,2,3]. Urban areas are major contributors to air pollution and climate change. The high proportion of impervious surfaces in urban settings results in higher air temperatures than surrounding green fields, known as the urban heat island (UHI) effect [4]. Well-designed urban greenspaces can mitigate this undesirable condition [5]; specifically, plantations of various tree species in an urban forest can enhance the mitigation [6]. However, urban trees are usually planted in soil that is degraded and compacted due to abandoned architectural materials, thus hindering tree growth and reducing the ecological services from these forests.
Soil organic matter and nutrient levels are critical to maintaining urban trees [7,8,9]. Soil microorganisms play an essential role for mediating soil organic matter decomposition and nutrient cycling [10,11]. Numerous studies have shown that soil microbes are sensitive to plant species composition, biomass, diversity, and soil structure [12,13,14]. Recent studies also found that tree species diversity in an urban forest affect the soil bacterial and fungal communities, indicating that the design of urban greenspaces influences microbial communities of urban soil [15,16].
Biochar and organic compost amendment have been promoted to improve soil physical, chemical, and biological properties in agriculture for years [17,18,19,20]. Biochar as a carbon-rich material produced by pyrolysis can increase soil water-holding capacity [21] and nutrient availability in different types of soil [22,23], and pH in acidic soils [24]. For soil microbes, biochar provides metabolically available carbon sources, which alters the soil microbial activity and community structure [11,25,26]. In addition, the porous structure of biochar provides additional surfaces for microbial growth [17,27]. On the other side, applying organic composted amendment was shown to increase soil organic matter content, stimulating microbial activities and nutrient supply to plants [28,29]. Combined, biochar-based compost amendment improved soil more efficiently than the biochar alone in agriculture [30].
In urban forestry, biochar and organic compost experiments were conducted to evaluate their influences on soil properties and tree growth performance. One result was that a combination of the two had the strongest effects on urban tree soils and growth [31]. Although previous studies have researched the physical and chemical properties of soil after biochar/compost treatments on urban trees [19,31], no study has evaluated the impact of these treatments on the soil microbial community of urban forests.
This study investigated the effects of biochar and biochar-based compost amendments on soil physicochemical properties and bacterial communities associated with nine urban trees collected from two city centers. We added (1) biochar and (2) biochar-based compost to four Diospyros blancoi rhizosphere soils in the Kaohsiung Botanical Garden and on the other four urban tree species, including two Ficus microcarpa, one Melia azedarach, one Swietenia macrophylla, and one Podocarpus nakaii in Tainan’s city center.
2. Materials and Methods
2.1. Preparation of Biochar and Organic Compost
Two biochars, Wood biochar 1 and Bamboo biochar 2, were produced at the pyrolyzing temperature of 500–600 °C and 650 °C, respectively. The two biochars were analyzed and found to have C:N ratios of 138 and 188 (Table 1), respectively. Organic compost was freshly produced from ingredients including mushroom sawdust waste, chicken manure, molasses, and fish powder (Fortune Life Enterprise Co., Ltd., Kaohsiung City, Taiwan); it had 36% organic carbon (Corg), 2% total nitrogen (Ntot), and 0.02% available phosphorus (Table 1). The biochars were mixed with self-produced organic compost for a final C/N of ca. 25; the ratio of biochar 1 and biochar 2 to organic compost was 1:14 and 1:18, respectively (Table 1). Biochar and organic compost were combined at least two weeks before use.

Table 1.
The chemical properties of the biochar and compost in this study.
2.2. Study Site, Amendment Process, and Sampling Procedures
Nine urban trees distributed in four sites in Tainan or Kaohsiung city of Taiwan were selected for the study: four Diospyros blancoi in the Zuoying Indigenous Botanical Garden (22°40′51.3″ N 120°18′10.6″ E), two Ficus microcarpa in Tainan Municipal Jhongyi Elementary School (22°59′23.0″ N 120°12′11.1″ E), one Melia azedarach and one Swietenia macrophylla Tainan Municipal Jhongshan Junior High School (22°59′06.1″ N 120°12′11.8″ E), and one Podocarpus nakaii in Tainan Park (23°00′02.4″ N 120°12′38.3″ E). Bamboo biochar (biochar 2) was only added to the soil of two Ficus macrocarpa trees and wood biochar was applied in other urban trees.
We aimed to separately investigate the effects of biochar only and biochar with organic compost in combination. Urban forest soil was amended in two parts: first, four Diospyros blancoi were amended with only biochar; then, biochar with compost was added after 1 year. Soil samples were collected for the first time 1 year after biochar amendment and for the second time 4 months after biochar/compost was added. Soil from the other five urban trees was collected directly before biochar/compost was added, and then 4 months later. Five soil cores (0–10 cm depth) were collected as five subsamples around each individual urban tree and then bulked into one composite sample of the spot. Homogenized samples were immediately hand-sieved in the field (≤2 mm) to remove stones, roots, macrofauna, and litter material, and then a partial soil for the microbial analysis was collected in 50 mL Falcon tubes and immediately stored in liquid nitrogen until arriving at the lab. Samples were stored at −20 °C until molecular analysis. The remaining soil samples were air-dried for physicochemical analyses.
2.3. Soil and Biochar Physicochemical Parameters
Total carbon (C) and nitrogen (N) concentrations in biochar were measured by dry combustion at 1000 °C with a CHNS-Elemental Analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). The pH and electric conductivity of rhizosphere soil samples were determined using a WTW Multi 3510 IDS portable meter (Weilheim, Germany). In addition, soil was analyzed for total nitrogen using the Semimacro Kjeldahl method [32]; organic matter using the Walkley–Black procedure [33]; and soil texture [34], inorganic phosphorus, CEC [35], and exchangeable cations (K+, Mg2+, Ca2+, and Na+) [36] by atomic absorption spectrophotometry using a Z 5300 instrument following recommendations of the manufacturer (Hitachi—Science & Technology, Tokyo, Japan). The porosity of soil samples was determined by the method described in [37].
2.4. DNA Extraction, Bacterial Amplicon Libraries, and DNA Sequencing
Soil microbial genomic DNA was extracted from approximately 1 g of soil subsample using a MoBio PowerSoil DNA Isolation Kit (MoBio Laboratories Inc., Carsbad, CA, USA) following the manufacturer’s instructions. The presence and quantity of genomic DNA was checked using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Dreieich, Germany); then, the extracts were stored at −20 °C.
The bacterial communities were detected based on the 16S rRNA V6-V8 region. The amplicon libraries were generated using the universal primer pair, 968F/1391R. The PCR reaction was carried out in a total volume of 50 μL containing 1× Taq Master Mix (Genechain Industrial Co., Ltd., Kaohsiung City, Taiwan), 1 mg/mL of BSA, 0.2 μM of each primer, and 2–5 μg of diluted template DNA (final concentration, 100 ng). PCR was conducted using the following program: initial denaturation at 95 °C for 5 min; 30 cycles of 95 °C for 20 s, 52 °C for 20 s, and 72 °C for 20 s; and a final step of 72 °C for 5 min, then cooling at 4 °C. The PCR products were checked with 1% agarose gel electrophoresis with 1× TAE buffer and SYBR® Green I. The band of expected size (about 424 bp) was cut and purified using a GeneKlean Gel Recovery Kit (Cyrusbioscience, Inc., Taipei City, Taiwan). The purified DNAs were quantified using a NanoDrop spectrophotometer (Thermo Scientific, Vantaa, Finland). In the second round of PCR, individual tags were added to the 5′ends of primers 968F/1391R. The PCR mixture contained 1× Taq Master Mix (Genechain Industrial Co., Ltd., Kaohsiung City, Taiwan), 0.4 μM of each tagged primer, and 100 ng of V6-V8 amplicon for a final volume of 50 μL. The PCR program for tag addition involved an initial denaturation at 94 for 3 min; 20 cycles of 94 °C for 20 s, 52 °C for 10 s, and 72 °C for 30 s; and a final step of 72 °C for 2 min, then cooling at 4 °C. The PCR products were purified and a 200 ng mixture of tagged products was subject to the Illumina HiSeq 2000 sequencing system (San Diego, CA, USA) at Genomics BioSci. & Tech. Co., Ltd. (Taipei, Taiwan).
2.5. Bioinformatics and Statistical Analyses
Paired-end reads were first merged using USEARCH (v8.0.1623) with default settings and then subjected to MOTHUR (v1.35.1) for quality control to retain sequences (1) 400–450 base pairs (bp) long, (2) with one or more homopolymers no longer than eight bp, (3) without any ambiguous bases, and (4) of an average quality score greater than 20. Chimeric 16S rDNA sequences were detected and removed using UCHIME (implemented in USEARCH) in reference mode and with 3% minimum divergence. Quality-filtered and nonchimeric reads were further analyzed using the UPARSE pipeline [38] to generate Operational Taxonomic Units (OTUs) at the 97% identity level per sample. The OTU representative sequences were searched against the Greengenes 13_5 database using USEARCH global alignment to identify the best hit’s corresponding taxonomy. OTUs with no hits or weak hits (i.e., average of sequence identity and alignment coverage less than 93%) were removed from downstream analysis.
We analyzed the bacterial diversity and community structure with R software [39]. Count data were transformed into relative abundance by dividing by sample size. Soil parameters were log(x + 1) transformed for all analyses. The clustering heat map was generated using a gplots package [40]. Nonmetric Multidimensional Scaling (NMDS) based on Bray–Curtis distances was performed using the Vegan package to visualize the distribution of the bacterial community composition, followed by post hoc regression of individual explanatory variables on the ordination scores. Goodness-of-fit values and their significance were calculated using 999 random permutations. Statistical analysis was carried out by one-way analysis of variance (ANOVA). Differences among treatments were examined using Tukey’s HSD test.
3. Results
3.1. Effects of Biochar and Compost on Soil Physicochemical Properties
The pre-treatment soil from D. blancoi in the botanical garden was a slight alkaline sandy loam and with low total nitrogen (Ntot) (ca. 0.09 ± 0.01%), organic matter (OM) (ca. 1.22 ± 0.19%), and cation exchange capacity (CEC) (ca. 5.12 ± 0.14 cmol(+) kg−1). After the amendment with biochar for 1 year, the soil physicochemical parameters collected from D. blancoi did not change significantly (Table 2a), but soil porosity seemed to have increased. In terms of the addition of biochar and compost in combination, most of the parameters including OM and phosphorus significantly decreased after 4 months, except K+, which remarkably increased from D. blancoi 1 (Table 2b). In the other site, the soil was mainly slightly alkaline loamy sand with low Ntot (0.04–0.1%), OM (0.54–1.84%), and CEC (3.78–5.16 cmol(+) kg−1) in the park (Table 3). After the combination of biochar and compost was added, the soil conductivity of M. azedarach and F. microcarpa 2 significantly increased, while those of other urban trees including S. macrophylla significantly decreased after 4 months. OM generally declined across all trees as phosphorus was only raised in soil of M. azedarach. In terms of cations, only K+ and Mg2+ were significantly increased in soil of M. azedarach, P. nakaii, and F. microcarpa 2.

Table 2.
Physicochemical properties of Diospyros blancoi soil responding to the amendment with (a) biochar and (b) biochar/compost in combination.

Table 3.
Physicochemical properties of the soil of the urban tree species including Swietenia macrophylla, Melia azedarach, Podocarpus nakaii, and Ficus microcarpa responding to the amendment with biochar/compost in combination.
3.2. Response of Soil Microbial Community Composition to Biochar and Compost Addition
A total of 4,860,048 high-quality sequences were obtained across all urban trees; they were classified into 60 phyla, 204 classes, 425 orders, 688 families, 1361 genera, and 20,383 species. The bacterial communities were dominated by Thermoleophilia, followed by Actinobacteria and Alphaproteobacteria at the class level (Supplementary Figure S1). The OTU richness ranged between 4171 and 7845 and the Shannon (H′) diversity ranged between 6.29 and 6.94 (Supplementary Table S1). The OTU richness and Chao1 and Shannon H′ diversity indices of D. blancoi-associated bacteria did not significantly change after the addition of biochar or the combination of biochar/compost (Figure 1a). However, a trend of increasing OTU and Chao1 over the treatment period was observed. For the other four urban tree species, F. microcarpa, M. azedarach, and S. macrophylla showed a trend of increasing H′ diversity, whereas P. nakaii showed decreasing H′ diversity in soil (Figure 1b).
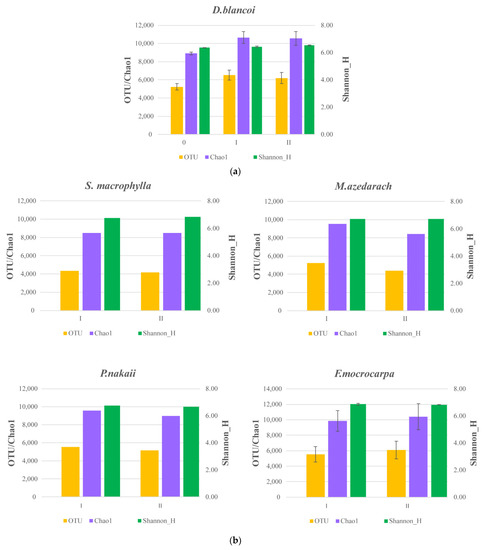
Figure 1.
The change in the OTU richness, Chao1, and Shannon H′ of the bacterial community composition associated with (a) D. blancoi (n = 4) and (b) S. macrophylla (n = 1), M. azedarach (n = 1), P. nakaii (n = 1), and F. microcarpa (n = 2) across treatments at both sites. 0 denotes the original soil; I and II denote soil after the addition of biochar and biochar/compost in combination, respectively. Different lower-case letters denote significant difference.
The NMDS ordination plot indicated that the urban tree-associated soil bacterial community composition changed when biochar only or a combination of biochar/compost was added (Figure 2) (Stress = 0.09). Specifically, the amendment with biochar/compost yielded a remarkable shift in the D. blancoi (Diospyros) soil. In addition, different biochar substances, i.e., bamboo and wood biochars, also influenced soil bacterial assemblages as F. microcarpa (Ficus) treated with bamboo biochar were discrete from other urban trees treated with wood biochar on the ordination plot. Furthermore, the heat map was applied to demonstrate the relative abundance of the 60 dominant bacteria species associated with individual urban trees across treatments (Figure 3). Specifically, the relative abundance of the following increased after the addition of biochar and compost in combination: Bradyrhizobium elkanii associated with F. microcarpa 1 (Ficus_1) while Nitrospira associated with P. nakaii (Podocarpus) and Bacillus with M. azedarach (Melia).
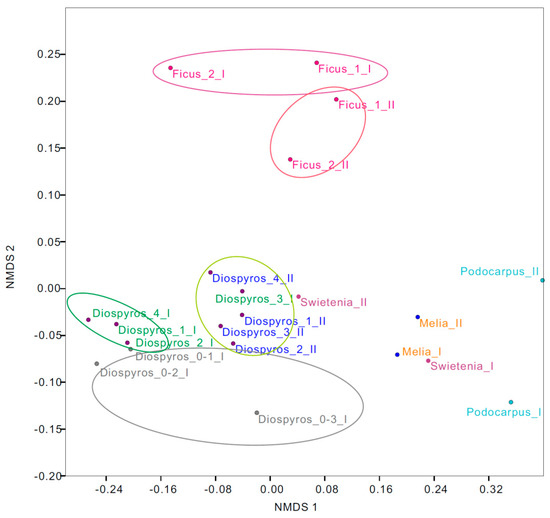
Figure 2.
Nonmetric multidimensional scaling (NMDS) ordination of the nine urban trees at two study sites across the treatments based on bacterial communities. Diospyros = D. blancoi, Swietenia = S. macrophylla, Melia = M. azedarach, Podocarpus = P. nakaii, and Ficus = F. microcarpa across treatments at both sites. 0–1~0–3 denote the original soil; I and II denote soil after addition of biochar and of biochar/compost in combination, respectively. Stress = 0.09.
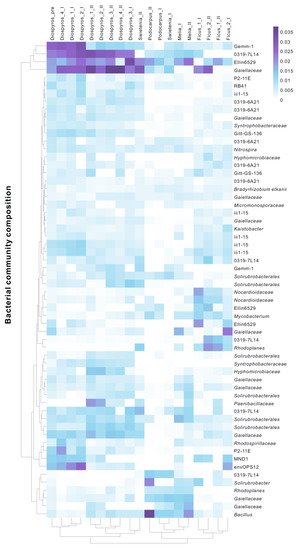
Figure 3.
The heat map of the 60 dominant bacteria species associated with individual urban trees across treatments. Diospyros = D. blancoi, Swietenia = S. macrophylla, Melia = M. azedarach, Podocarpus = P. nakaii, and Ficus = F. microcarpa. Pre denotes the original soil; I and II denote soil after the addition of biochar and of biochar/compost in combination, respectively.
3.3. Soil Physicochemical Parameters Shaped Urban Tree-Associated Bacterial Community Composition
NMDS ordination based on bacterial community composition associated with the post hoc correlation among soil physicochemical parameters indicated that soil parameters—including pH, sand percentage, available phosphorus (P), total nitrogen (Ntot), organic matter (OM), and electrical conductivity (EC)—significantly triggered the bacterial community composition across treatments (Figure 4). Specifically, soil pH was the most significant variable explaining the shifts in bacterial assemblages across treatments, followed by P and silt percentage. In addition, biochar type also seemed to explain the variance in bacterial assemblages, as F. microcarpa (Ficus) was distributed separately from others on the ordination plot.
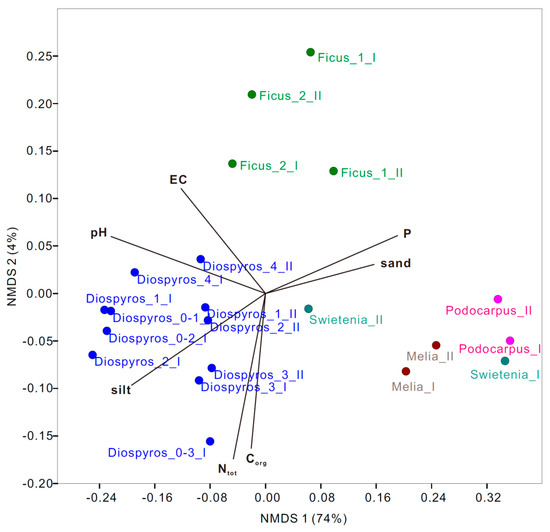
Figure 4.
NMDS ordination of the nine urban trees at two study sites across the treatments based on bacterial community compositions at the species level (>1% relative abundance). Soil physicochemical parameters with a significant goodness of fit based on post hoc correlations (p ≤ 0.05) are represented as vectors.
Furthermore, we found that the change in bacterial composition might have included the emergence of several potential nitrogen-fixing bacterial groups (Figure 5). In urban tree D. blancoi soil samples, Paenibacillus, Rhizobiales, and Frankiaceae had clearly increased after the treatment, whereas Frankia, Hyphomicrobiaceae, and Azospirillum were increased in the soil of the urban tree F. microcarpa. Similarly, the bacteria Azospirillum increased in the soil samples of M. azedarach and P. nakaii, but not in S. macrophylla, while the relative abundances of Frankia and Hyphomicrobiaceae increased dramatically in S. macrophylla after treatment.
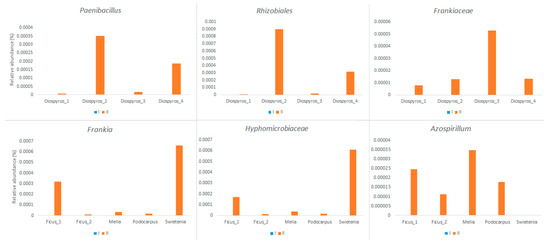
Figure 5.
Potential nitrogen-fixing bacterial groups were detected in the rhizosphere soil after the amendment of biochar-based compost. Diospyros = D. blancoi, Swietenia = S. macrophylla, Melia = M. azedarach, Podocarpus = P. nakaii, and Ficus = F. microcarpa.
4. Discussion
4.1. Newly Developed Biochar-Based Compost Improved the Physicochemical Properties of Soil
Our result showed that biochar-based compost changed the soil properties more efficiently than biochar-only treatment. Although there was no significant increase in soil properties after 1 year of the biochar-only treatment, we suggest that the soil porosity would be significantly higher after repeating or extending the biochar amendments due to the porous internal structure of biochar [20]. This indicates that the water holding capacity would increase.
In general, the biochar-based compost amendment changed the following soil chemical properties: conductivity, the amount of organic matter, available phosphorus, calcium, and potassium (Table 2b and Table 3). The treatment led to a decrease in organic matter, indicating a positive priming effect on SOC mineralization in the short term. This finding agrees with a hypothesis from a previous study: the presence of organic matter with biochar increases C mineralization in soil in the short term [41]. The mechanism may involve the biochar-promoting soil aggregation [17], which protects SOC from leaching [20] and highly stimulates bacteria to degrade organic carbon from the compost [28,29].
Moreover, the increased soil potassium after amendments may not only increase anti-stress and antipathogen responses, but also improve the osmotic uptake of other nutrients in water by roots, such as calcium and phosphorus. Consequently, we saw the calcium, available phosphorus, and conductivity decrease in the soil (Table 2b and Table 3).
4.2. Microbial Community Structure Is Driven by Biochar/Compost Amendment
The bacterial alpha diversities in the soil of urban trees were high, based on the OTU richness and Shannon H’ index (Figure 1). Previous studies [42,43] also found that the alpha diversities increase after biochar/compost treatment. Although there was no significant variation in the alpha diversities of soil bacterial communities, the bacterial community compositions changed after treatment (Figure 2, Figure 3 and Figure 4).
Comparing the bacterial communities among all D. blancoi soil samples, the samples after biochar-based compost treatment were isolated from biochar-treated samples and control samples (samples marked in blue in Figure 2). This suggests that combined compost and biochar had stronger effects than the effects of biochar-only on the bacterial communities. This result is consistent with that of a previous study [31], which examined the tree growth and soil properties in a tropical urban environment. The other soil samples from different tree species had similar patterns as D. blancoi, and the samples after biochar-based compost treatment had different bacterial communities from the samples before treatment (Figure 2 and Figure 3). Different biochar substances and tree species influenced soil bacterial assemblages as well. The F. microcarpa (Ficus) soil samples were isolated on the y-axis in the nMDS analysis (Figure 2 and Figure 4), which was treated with different kinds of biochar from the other samples. In addition, Nocardioidaceae, Gaiellaceae, and Chloroflexi Ellin 6529 were more dominant in the F. microcarpa soil samples than other samples (Figure 3).
Furthermore, in the nMDS and heat map analyses (Figure 2 and Figure 3), the samples were grouped by three of the four tree species (S. macrophylla was excluded). These findings agree with previous studies that concluded that the bacterial community structure in soil is related to urban tree species [16]. Interestingly, applying biochar-based compost increased the relative abundance of potential nitrogen-fixing bacterial groups (Figure 5), and the tree species affected the species of the nitrogen-fixing bacteria in the soil as well.
4.3. The Interaction between Bacterial Community Structure and Physicochemical Properties
The physicochemical properties—pH, phosphorus, and silt percentage in soil—were highly associated with the bacterial communities. They were potential key parameters shaping the bacterial communities. As mentioned in the previous paragraph, the aggregation of soil after biochar/compost amendment further enhances the activity of some bacteria in the soil. This consequently changes the pH value in soil, though there was no significant difference after 4 months of amendments. The activated bacteria may have come from the compost, as they help decompose organic carbon and mineralize other nutrients. Lastly, the mineralized nutrients, such as nitrogen and phosphate, were absorbed by the roots of urban trees, resulting in a decrease in those concentrations in soil.
5. Conclusions
This study measured changes in soil physical and chemical properties and the variation in bacterial communities before and after applying biochar-based compost. We developed a new organic compost and applied biochar to it, which successfully improved the soil properties and fertility and increased the relative abundance of nitrogen-fixing bacteria in the soil. Finally, our results demonstrated that the biochar-based compost we used more efficiently remediated soil fertilities than biochar-only treatment in the urban forests.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr10040682/s1, Table S1: The OTU richness, diversity indices, evenness, and estimated OTU richness of urban trees across treatments at both sites; Figure S1: The dominant bacterial community composition (>0.01) at class level across treatments of urban trees at both sites.
Author Contributions
Conceptualization, Y.-T.W.; sample collection, Y.-C.H.; sample analysis, Z.-T.L. and J.-H.S.; writing—original draft preparation, J.-H.S. and Y.-T.W.; writing—review and editing, S.-H.J. and M.-L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Taiwan Forestry Research Institute (Grant numbers 108AS-17.1.3-F1-G1 and 109AS-15.1.3-F1-G4), and by Ministry of Science and Technology (Grant number MOST 108-2313-B-020-011-MY3).
Data Availability Statement
Accession numbers will be provided during review.
Acknowledgments
Hong-Chang Xie helped with the biochar and compost treatments and helped analyze the physiochemical properties of the soil. Jun Li and Zhi-Jun Zhuang helped analyze the physiochemical properties of the soil.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wong, N.H.; Yu, C. Study of green areas and urban heat island in a tropical city. Habitat Int. 2005, 29, 547–558. [Google Scholar] [CrossRef]
- Aram, F.; Higueras Garcia, E.; Solgi, E.; Mansournia, S. Urban green space cooling effect in cities. Heliyon 2019, 5, e01339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, D.; Liu, W.; Gan, T.; Liu, K.; Chen, Q. A review of mitigating strategies to improve the thermal environment and thermal comfort in urban outdoor spaces. Sci. Total Environ. 2019, 661, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Solecki, W.D.; Rosenzweig, C.; Parshall, L.; Pope, G.; Clark, M.; Cox, J.; Wiencke, M. Mitigation of the heat island effect in urban New Jersey. Glob. Environ. Chang. Part B Environ. Hazards 2005, 6, 39–49. [Google Scholar] [CrossRef]
- Tan, Z.; Lau, K.K.-L.; Ng, E. Urban tree design approaches for mitigating daytime urban heat island effects in a high-density urban environment. Energy Build. 2016, 114, 265–274. [Google Scholar] [CrossRef]
- Wang, X.; Dallimer, M.; Scott, C.E.; Shi, W.; Gao, J. Tree species richness and diversity predicts the magnitude of urban heat island mitigation effects of greenspaces. Sci. Total Environ. 2021, 770, 145211. [Google Scholar] [CrossRef]
- Tiessen, H.; Cuevas, E.; Chacon, P. The Role of Soil Organic-Matter in Sustaining Soil Fertility. Nature 1994, 371, 783–785. [Google Scholar] [CrossRef]
- Bationo, A.; Kihara, J.; Vanlauwe, B.; Waswa, B.; Kimetu, J. Soil organic carbon dynamics, functions and management in West African agro-ecosystems. Agric. Syst. 2007, 94, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Vanlauwe, B.; Bationo, A.; Chianu, J.; Giller, K.E.; Merckx, R.; Mokwunye, U.; Ohiokpehai, O.; Pypers, P.; Tabo, R.; Shepherd, K.D.; et al. Integrated soil fertility management Operational definition and consequences for implementation and dissemination. Outlook Agric. 2010, 39, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Igalavithana, A.D.; Kim, K.H.; Jung, J.M.; Heo, H.S.; Kwon, E.E.; Tack, F.M.G.; Tsang, D.C.W.; Jeon, Y.J.; Ok, Y.S. Effect of biochars pyrolyzed in N2 and CO2, and feedstock on microbial community in metal(loid)s contaminated soils. Environ. Int. 2019, 126, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chao, L.; Li, J.; Ding, Z.; Wang, S.; Li, F.; Bao, Y. The Distribution and Turnover of Bacterial Communities in the Root Zone of Seven Stipa Species Across an Arid and Semi-arid Steppe. Front. Microbiol. 2021, 12, 782621. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, K.; Wurzburger, N.; Zhang, J. Relationships between plant diversity and soil microbial diversity vary across taxonomic groups and spatial scales. Ecosphere 2020, 11, e02999. [Google Scholar] [CrossRef] [Green Version]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition-Current Knowledge and Future Directions. Front. Plant. Sci. 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyner, J.L.; Kerwin, J.; Deeb, M.; Lozefski, G.; Prithiviraj, B.; Paltseva, A.; McLaughlin, J.; Groffman, P.; Cheng, Z.; Muth, T.R. Green Infrastructure Design Influences Communities of Urban Soil Bacteria. Front. Microbiol. 2019, 10, 982. [Google Scholar] [CrossRef] [Green Version]
- Ao, L.; Zhao, M.; Li, X.; Sun, G. Different Urban Forest Tree Species Affect the Assembly of the Soil Bacterial and Fungal Community. Microb. Ecol. 2021, 83, 447–458. [Google Scholar] [CrossRef]
- Jien, S.-H.; Wang, C.-S. Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena 2013, 110, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Hseu, Z.Y.; Jien, S.H.; Chien, W.H.; Liou, R.C. Impacts of biochar on physical properties and erosion potential of a mudstone slopeland soil. Sci. World J. 2014, 2014, 602197. [Google Scholar] [CrossRef]
- Somerville, P.D.; Farrell, C.; May, P.B.; Livesley, S.J. Biochar and compost equally improve urban soil physical and biological properties and tree growth, with no added benefit in combination. Sci. Total Environ. 2020, 706, 135736. [Google Scholar] [CrossRef]
- Lee, M.-H.; Chang, E.-H.; Lee, C.-H.; Chen, J.-Y.; Jien, S.-H. Effects of Biochar on Soil Aggregation and Distribution of Organic Carbon Fractions in Aggregates. Processes 2021, 9, 1431. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 202–203, 183–191. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Luo, X.; Wang, Z.; Xing, B. Biochar-induced negative carbon mineralization priming effects in a coastal wetland soil: Roles of soil aggregation and microbial modulation. Sci. Total Environ. 2018, 610–611, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Hu, A.; Zhao, Z.; Liu, X.; Jiang, C.; Zhang, Z. Biochar with large specific surface area recruits N2O-reducing microbes and mitigate N2O emission. Soil Biol. Biochem. 2021, 156, 108212. [Google Scholar] [CrossRef]
- Whitman, T.; Pepe-Ranney, C.; Enders, A.; Koechli, C.; Campbell, A.; Buckley, D.H.; Lehmann, J. Dynamics of microbial community composition and soil organic carbon mineralization in soil following addition of pyrogenic and fresh organic matter. ISME J. 2016, 10, 2918–2930. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Lee, S.E.; Lee, Y.H.; Tsang, D.C.W.; Rinklebe, J.; Kwon, E.E.; Ok, Y.S. Heavy metal immobilization and microbial community abundance by vegetable waste and pine cone biochar of agricultural soils. Chemosphere 2017, 174, 593–603. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Zhang, H.; Selim, H.M. Competitive Sorption-Desorption Kinetics of Arsenate and Phosphate in Soils. Soil Sci. 2008, 173, 3–12. [Google Scholar] [CrossRef]
- Tandy, S.; Healey, J.R.; Nason, M.A.; Williamson, J.C.; Jones, D.L. Remediation of metal polluted mine soil with compost: Co-composting versus incorporation. Environ. Pollut. 2009, 157, 690–697. [Google Scholar] [CrossRef]
- Naeem, M.A.; Khalid, M.; Aon, M.; Abbas, G.; Amjad, M.; Murtaza, B.; Khan, W.-U.-D.; Ahmad, N. Combined application of biochar with compost and fertilizer improves soil properties and grain yield of maize. J. Plant. Nutr. 2018, 41, 112–122. [Google Scholar] [CrossRef]
- Ghosh, S.; Ow, L.F.; Wilson, B. Influence of biochar and compost on soil properties and tree growth in a tropical urban environment. Int. J. Environ. Sci. Technol. 2014, 12, 1303–1310. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, C.C. Methods of Soil and Tissue Analysis Used in the Analytical Laboratory; Information Report; Canadian Forestry Service, Maritimes Forest Research Centre: Fredericton, NB, Canada, 1977; M-X-78.
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis; Part 3. Chemical Methods, SSSA Book Series No. 5; SSSA and ASA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size Analysis. In Methods of Soil Analysis; Wiley Online Library: Hoboken, NJ, USA, 1986; pp. 383–411. [Google Scholar]
- Rhoades, J.D. Cation Exchange Capacity. In Methods of Soil Analysis; Wiley Online Library: Hoboken, NJ, USA, 1986; pp. 149–157. [Google Scholar]
- Thomas, G.W. Exchangeable Cations. In Methods of Soil Analysis; Wiley Online Library: Hoboken, NJ, USA, 1986; pp. 159–165. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; Lewis Publishers: Boca Raton, FL, USA, 1993. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A language and environment for statistical computing. MSOR Connect. 2014, 1, 2673. [Google Scholar]
- Warnes, G.R.; Bolker, B.M.; Bonebakker, L.; Gentleman, R.; Liaw, W.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; Schwartz, M.L.; et al. Gplots: Various R Programming Tools for Plotting Data. R Package Version 3.1.1. 2009. Available online: https://cran.r-project.org/web/packages/gplots/gplots.pdf (accessed on 5 March 2022).
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Dai, Z.; Xiong, X.; Zhu, H.; Xu, H.; Leng, P.; Li, J.; Tang, C.; Xu, J. Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 2021, 3, 239–254. [Google Scholar] [CrossRef]
- Jien, S.H.; Kuo, Y.L.; Liao, C.S.; Wu, Y.T.; Igalavithana, A.D.; Tsang, D.C.W.; Ok, Y.S. Effects of field scale in situ biochar incorporation on soil environment in a tropical highly weathered soil. Environ. Pollut. 2021, 272, 116009. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).