Successful Manufacturing Protocols of N-Rich Carbon Electrodes Ensuring High ORR Activity: A Review
Abstract
:1. Introduction
2. Metal-Free Cathode Electrocatalysts for Oxygen Reduction Reaction
2.1. Graphene-Based Electrocatalysts
2.1.1. Synthesis of Nitrogen-Doped Graphene-Based Materials via Pyrolysis Method
2.1.2. Synthesis of Nitrogen-Doped Graphene-Based Materials via Hydro(Solvo)Thermal Method
2.1.3. Synthesis of Nitrogen-Doped Graphene-Based Materials via Chemical Vapor Deposition Method
2.2. Carbon Nanotube-Based Electrocatalysts
2.2.1. Synthesis of Nitrogen-Doped Carbon Nanotube-Based Materials via Pyrolysis Method
2.2.2. Synthesis of Nitrogen-Doped Carbon Nanotube-Based Materials via Hydrothermal Method
2.2.3. Synthesis of Nitrogen-Doped Carbon Nanotube-Based Materials via Chemical Vapor Deposition Method
2.3. Porous Carbon Electrocatalysts Based on Natural Precursors
2.4. Carbon Nanofiber-Based Electrocatalysts
2.4.1. Synthesis of Nitrogen-Doped Carbon Nanofiber-Based Materials via Pyrolysis Method
2.4.2. Synthesis of Nitrogen-Doped Carbon Nanofiber-Based Materials via Chemical Vapor Deposition Method
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Frattini, D.; Accardo, G.; Ferone, C.; Cioffi, R. Fabrication and characterization of graphite-cement composites for microbial fuel cells applications. Mater. Res. Bull. 2017, 88, 188–199. [Google Scholar] [CrossRef]
- Tan, P.; Chen, B.; Xu, H.; Zhang, H.; Cai, W.; Ni, M.; Liu, M.; Shao, Z. Flexible Zn-and Li-air batteries: Recent advances, challenges, and future perspectives. Energy Environ. Sci. 2017, 10, 2056–2080. [Google Scholar] [CrossRef]
- Li, L.; Fan, W.; Xuan, J.; Leung, M.K.; Zheng, K.; She, Y. Optimal design of current collectors for microfluidic fuel cell with flow-through porous electrodes: Model and experiment. Appl. Energy 2017, 206, 413–424. [Google Scholar] [CrossRef]
- Ren, S.; Duan, X.; Liang, S.; Zhang, M.; Zheng, H. Bifunctional electrocatalysts for Zn-air batteries: Recent developments and future perspectives. J. Mater. Chem. A 2020, 8, 6144–6182. [Google Scholar] [CrossRef]
- Mulyadi, A.; Zhang, Z.; Dutzer, M.; Liu, W.; Deng, Y. Facile approach for synthesis of doped carbon electrocatalyst from cellulose nanofibrils toward high-performance metal-free oxygen reduction and hydrogen evolution. Nano Energy 2017, 32, 336–346. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Losic, D.; Saint, C.P.; Pant, D. Applications of graphene in microbial fuel cells: The gap between promise and reality. Renew. Sustain. Energy Rev. 2017, 72, 1389–1403. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Qu, K.; Zheng, Y.; Dai, S.; Qiao, S.Z. Graphene oxide-polydopamine derived N,S-codoped carbon nanosheets as superior bifunctional electrocatalysts for oxygen reduction and evolution. Nano Energy 2016, 19, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Pan, F.; Jiang, L.; Fu, X.; Liang, A.; Wei, Z.; Zhang, J.; Sun, G. Catalyst-free synthesis of crumpled boron and nitrogen co-doped graphite layers with tunable bond structure for oxygen reduction reaction. ACS Nano 2014, 8, 3313–3321. [Google Scholar] [CrossRef]
- Zhang, C.; Mahmood, N.; Yin, H.; Liu, F.; Hou, Y. Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef]
- She, Y.; Chen, J.; Zhang, C.; Lu, Z.; Ni, M.; Sit, P.H.-L.; Leung, M.K. Nitrogen-doped graphene derived from ionic liquid as metal-free catalyst for oxygen reduction reaction and its mechanisms. Appl. Energy 2018, 225, 513–521. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, B.; Zhou, Y. Three-dimensional Polypyrrole Derived N-doped Carbon Nanotube Aerogel as a High-performance Metal-free Catalyst for Oxygen Reduction Reaction. ChemCatChem 2019, 11, 5495–5504. [Google Scholar] [CrossRef]
- Bandosz, T.J. Porous Carbons as Oxygen Reduction Electrocatalysts. In Porous Materials; Springer: Berlin/Heidelberg, Germany, 2021; pp. 41–77. [Google Scholar]
- Wu, J.; Ma, L.; Yadav, R.M.; Yang, Y.; Zhang, X.; Vajtai, R.; Lou, J.; Ajayan, P.M. Nitrogen-doped graphene with pyridinic dominance as a highly active and stable electrocatalyst for oxygen reduction. ACS Appl. Mater. Interfaces 2015, 7, 14763–14769. [Google Scholar] [CrossRef]
- Ratso, S.; Kruusenberg, I.; Vikkisk, M.; Joost, U.; Shulga, E.; Kink, I.; Kallio, T.; Tammeveski, K. Highly active nitrogen-doped few-layer graphene/carbon nanotube composite electrocatalyst for oxygen reduction reaction in alkaline media. Carbon 2014, 73, 361–370. [Google Scholar] [CrossRef]
- Guo, D.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef]
- Liang, H.-W.; Zhuang, X.; Brüller, S.; Feng, X.; Müllen, K. Hierarchically porous carbons with optimized nitrogen doping as highly active electrocatalysts for oxygen reduction. Nat. Commun. 2014, 5, 4973. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Wei, Z.; Chen, S.; Qi, X.; Yang, T.; Hu, J.; Wang, D.; Wan, L.J.; Alvi, S.F.; Li, L. Space-confinement-induced synthesis of pyridinic-and pyrrolic-nitrogen-doped graphene for the catalysis of oxygen reduction. Angew. Chem. 2013, 125, 11971–11975. [Google Scholar] [CrossRef]
- Deng, H.; Li, Q.; Liu, J.; Wang, F. Active sites for oxygen reduction reaction on nitrogen-doped carbon nanotubes derived from polyaniline. Carbon 2017, 112, 219–229. [Google Scholar] [CrossRef]
- Zhao, A.; Masa, J.; Schuhmann, W.; Xia, W. Activation and stabilization of nitrogen-doped carbon nanotubes as electrocatalysts in the oxygen reduction reaction at strongly alkaline conditions. J. Phys. Chem. C 2013, 117, 24283–24291. [Google Scholar] [CrossRef]
- Kruusenberg, I.; Ratso, S.; Vikkisk, M.; Kanninen, P.; Kallio, T.; Kannan, A.M.; Tammeveski, K. Highly active nitrogen-doped nanocarbon electrocatalysts for alkaline direct methanol fuel cell. J. Power Source 2015, 281, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Vikkisk, M.; Kruusenberg, I.; Ratso, S.; Joost, U.; Shulga, E.; Kink, I.; Rauwel, P.; Tammeveski, K. Enhanced electrocatalytic activity of nitrogen-doped multi-walled carbon nanotubes towards the oxygen reduction reaction in alkaline media. RSC Adv. 2015, 5, 59495–59505. [Google Scholar] [CrossRef]
- Ma, R.; Ma, Y.; Dong, Y.; Lee, J.-M. Recent advances in heteroatom-doped graphene materials as efficient electrocatalysts towards the oxygen reduction reaction. Nano Adv. 2016, 1, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.-L.; Xu, D.; Xu, J.-J.; Zhang, X.-B. Oxygen electrocatalysts in metal-air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. npj Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Lee, J.S.; Liu, M.; Cho, J. Recent progress in non-precious catalysts for metal-air batteries. Adv. Energy Mater. 2012, 2, 816–829. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Quan, X.; Chen, S.; Zhao, H.; Yu, H. Nitrogen and sulfur co-doped graphene/carbon nanotube as metal-free electrocatalyst for oxygen evolution reaction: The enhanced performance by sulfur doping. Electrochim. Acta 2016, 204, 169–175. [Google Scholar] [CrossRef]
- Zhu, C.; Li, H.; Fu, S.; Du, D.; Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef]
- Paul, R.; Du, F.; Dai, L.; Ding, Y.; Wang, Z.L.; Wei, F.; Roy, A. 3D heteroatom-doped carbon nanomaterials as multifunctional metal-free catalysts for integrated energy devices. Adv. Mater. 2019, 31, 1805598. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, L.; Yan, X.; Yao, X. Recent progress in oxygen electrocatalysts for zinc-air batteries. Small Methods 2017, 1, 1700209. [Google Scholar] [CrossRef]
- Cong, H.-P.; Chen, J.-F.; Yu, S.-H. Graphene-based macroscopic assemblies and architectures: An emerging material system. Chem. Soc. Rev. 2014, 43, 7295–7325. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-E.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Giovanni, M.; Poh, H.L.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Šaněk, F.; Khezri, B.; Webster, R.D.; Pumera, M. Noble metal (Pd, Ru, Rh, Pt, Au, Ag) doped graphene hybrids for electrocatalysis. Nanoscale 2012, 4, 5002–5008. [Google Scholar] [CrossRef]
- Xiao, J.; Bian, X.; Liao, L.; Zhang, S.; Ji, C.; Liu, B. Nitrogen-doped mesoporous graphene as a synergistic electrocatalyst matrix for high-performance oxygen reduction reaction. ACS Appl. Mater. Interfaces 2014, 6, 17654–17660. [Google Scholar] [CrossRef]
- Cong, H.-P.; Wang, P.; Gong, M.; Yu, S.-H. Facile synthesis of mesoporous nitrogen-doped graphene: An efficient methanol-tolerant cathodic catalyst for oxygen reduction reaction. Nano Energy 2014, 3, 55–63. [Google Scholar] [CrossRef]
- Yan, Y.; Gong, J.; Chen, J.; Zeng, Z.; Huang, W.; Pu, K.; Liu, J.; Chen, P. Recent advances on graphene quantum dots: From chemistry and physics to applications. Adv. Mater. 2019, 31, 1808283. [Google Scholar] [CrossRef]
- Fei, H.; Ye, R.; Ye, G.; Gong, Y.; Peng, Z.; Fan, X.; Samuel, E.L.; Ajayan, P.M.; Tour, J.M. Boron-and nitrogen-doped graphene quantum dots/graphene hybrid nanoplatelets as efficient electrocatalysts for oxygen reduction. ACS Nano 2014, 8, 10837–10843. [Google Scholar] [CrossRef]
- Brownson, D.A.; Kampouris, D.K.; Banks, C.E. Graphene electrochemistry: Fundamental concepts through to prominent applications. Chem. Soc. Rev. 2012, 41, 6944–6976. [Google Scholar] [CrossRef]
- Yuan, W.; Zhou, Y.; Li, Y.; Li, C.; Peng, H.; Zhang, J.; Liu, Z.; Dai, L.; Shi, G. The edge-and basal-plane-specific electrochemistry of a single-layer graphene sheet. Sci. Rep. 2013, 3, 2248. [Google Scholar] [CrossRef] [Green Version]
- Tabish, T.; Zhang, S. Graphene Quantum Dots: Syntheses, Properties, and Biological Applications. In Comprehensive Nanoscience and Nanotechnology, 2nd ed.; Elsevier: New York, NY, USA, 2016. [Google Scholar]
- Jin, H.; Huang, H.; He, Y.; Feng, X.; Wang, S.; Dai, L.; Wang, J. Graphene quantum dots supported by graphene nanoribbons with ultrahigh electrocatalytic performance for oxygen reduction. J. Am. Chem. Soc. 2015, 137, 7588–7591. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Duan, X.; Sun, H.; Kang, J.; Zhang, H.; Tade, M.O.; Wang, S. Facile synthesis of nitrogen-doped graphene via low-temperature pyrolysis: The effects of precursors and annealing ambience on metal-free catalytic oxidation. Carbon 2017, 115, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Niu, Y.; Hu, W. Nitrogen/sulfur-doping of graphene with cysteine as a heteroatom source for oxygen reduction electrocatalysis. J. Colloid Interface Sci. 2017, 505, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Ilnicka, A.; Skorupska, M.; Romanowski, P.; Kamedulski, P.; Lukaszewicz, J.P. Improving the Performance of Zn-Air Batteries with N-Doped Electroexfoliated Graphene. Materials 2020, 13, 2115. [Google Scholar] [CrossRef]
- Skorupska, M.; Ilnicka, A.; Lukaszewicz, J.P. N-doped graphene foam obtained by microwave-assisted exfoliation of graphite. Sci. Rep. 2021, 11, 2044. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Ding, R.; Wang, H.; Wu, J.; Wang, C.; Zhang, C.; Xu, Y.; Wang, L.; Lv, B. Facile synthesis of porous nitrogen-doped holey graphene as an efficient metal-free catalyst for the oxygen reduction reaction. Nano Res. 2017, 10, 305–319. [Google Scholar] [CrossRef]
- Hassani, S.S.; Samiee, L.; Ghasemy, E.; Rashidi, A.; Ganjali, M.R.; Tasharrofi, S. Porous nitrogen-doped graphene prepared through pyrolysis of ammonium acetate as an efficient ORR nanocatalyst. Int. J. Hydrogen Energy 2018, 43, 15941–15951. [Google Scholar] [CrossRef]
- Lin, Z.; Waller, G.; Liu, Y.; Liu, M.; Wong, C.P. Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Kamedulski, P.; Lukaszewicz, J.P.; Witczak, L.; Szroeder, P.; Ziolkowski, P. The Importance of Structural Factors for the Electrochemical Performance of Graphene/Carbon Nanotube/Melamine Powders towards the Catalytic Activity of Oxygen Reduction Reaction. Materials 2021, 14, 2448. [Google Scholar] [CrossRef]
- Zhou, X.; Bai, Z.; Wu, M.; Qiao, J.; Chen, Z. 3-Dimensional porous N-doped graphene foam as a non-precious catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 3343–3350. [Google Scholar] [CrossRef]
- Gu, D.; Zhou, Y.; Ma, R.; Wang, F.; Liu, Q.; Wang, J. Facile synthesis of N-doped graphene-like carbon nanoflakes as efficient and stable electrocatalysts for the oxygen reduction reaction. Nano-Micro Lett. 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Zhi, L.; Tang, K.; Feng, X.; Maier, J.; Müllen, K. Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reactions. Adv. Funct. Mater. 2012, 22, 3634–3640. [Google Scholar] [CrossRef]
- Cong, K.; Radtke, M.; Stumpf, S.; Schröter, B.; McMillan, D.G.; Rettenmayr, M.; Ignaszak, A. Electrochemical stability of the polymer-derived nitrogen-doped carbon: An elusive goal? Mater. Renew. Sustain. Energy 2015, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Wan, K.; Yu, Z.-P.; Liang, Z.-X. Polyaniline-derived ordered mesoporous carbon as an efficient electrocatalyst for oxygen reduction reaction. Catalysts 2015, 5, 1034–1045. [Google Scholar] [CrossRef] [Green Version]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Oxygen-reduction catalysis of N-doped carbons prepared via heat treatment of polyaniline at over 1100 °C. Chem. Commun. 2018, 54, 4441–4444. [Google Scholar] [CrossRef] [Green Version]
- Cheon, J.Y.; Kim, J.H.; Kim, J.H.; Goddeti, K.C.; Park, J.Y.; Joo, S.H. Intrinsic relationship between enhanced oxygen reduction reaction activity and nanoscale work function of doped carbons. J. Am. Chem. Soc. 2014, 136, 8875–8878. [Google Scholar] [CrossRef]
- Florent, M.; Wallace, R.; Bandosz, T.J. Oxygen electroreduction on nanoporous carbons: Textural features vs nitrogen and boron catalytic centers. ChemCatChem 2019, 11, 851–860. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Zhao, S.; Wang, Y.Q.; Lin, X.; Tian, Z.Q.; Shen, P.K. Precursor modulated active sites of nitrogen doped graphene-based carbon catalysts via one-step pyrolysis method for the enhanced oxygen reduction reaction. Electrochim. Acta 2021, 370, 137712. [Google Scholar] [CrossRef]
- Lu, X.; Wang, D.; Ge, L.; Xiao, L.; Zhang, H.; Liu, L.; Zhang, J.; An, M.; Yang, P. Enriched graphitic N in nitrogen-doped graphene as a superior metal-free electrocatalyst for the oxygen reduction reaction. New J. Chem. 2018, 42, 19665–19670. [Google Scholar] [CrossRef]
- Liu, T.; Kou, T.; Bulmahn, D.; Ortuno-Quintana, C.; Liu, G.; Lu, J.Q.; Li, Y. Tuning the electrochemical properties of nitrogen-doped carbon aerogels in a blend of ammonia and nitrogen gases. ACS Appl. Energy Mater. 2018, 1, 5043–5053. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, T.; Zhou, Y.; Li, Y.; Lu, J.; Tian, X.; Wang, D.; Wang, J. Facile synthesis of boron and nitrogen-doped graphene as efficient electrocatalyst for the oxygen reduction reaction in alkaline media. Int. J. Hydrogen Energy 2014, 39, 16043–16052. [Google Scholar] [CrossRef]
- Huang, Z.-H.; Liu, T.-Y.; Song, Y.; Li, Y.; Liu, X.-X. Balancing the electrical double layer capacitance and pseudocapacitance of hetero-atom doped carbon. Nanoscale 2017, 9, 13119–13127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, S.; Artyushkova, K.; Serov, A.; Kiefer, B.; Atanassov, P. Binding energy shifts for nitrogen-containing graphene-based electrocatalysts-experiments and DFT calculations. Surf. Interface Anal. 2016, 48, 293–300. [Google Scholar] [CrossRef]
- Kabir, S.; Artyushkova, K.; Kiefer, B.; Atanassov, P. Computational and experimental evidence for a new TM-N3/C moiety family in non-PGM electrocatalysts. Phys. Chem. Chem. Phys. 2015, 17, 17785–17789. [Google Scholar] [CrossRef]
- Kabir, S.; Artyushkova, K.; Serov, A.; Atanassov, P. Role of nitrogen moieties in N-doped 3D-graphene nanosheets for oxygen electroreduction in acidic and alkaline media. ACS Appl. Mater. Interfaces 2018, 10, 11623–11632. [Google Scholar] [CrossRef]
- Dong, F.; Cai, Y.; Liu, C.; Liu, J.; Qiao, J. Heteroatom (B, N and P) doped porous graphene foams for efficient oxygen reduction reaction electrocatalysis. Int. J. Hydrogen Energy 2018, 43, 12661–12670. [Google Scholar] [CrossRef]
- Skorupska, M.; Ilnicka, A.; Lukaszewicz, J.P. The effect of nitrogen species on the catalytic properties of N-doped graphene. Sci. Rep. 2021, 11, 23970. [Google Scholar] [CrossRef]
- Wang, Z.; Li, P.; Chen, Y.; He, J.; Zhang, W.; Schmidt, O.G.; Li, Y. Pure thiophene-sulfur doped reduced graphene oxide: Synthesis, structure, and electrical properties. Nanoscale 2014, 6, 7281–7287. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Ji, Y.; Lei, Y.; Wang, Y.; Wang, Y.; Li, Y.; Wang, S. Pyridinic-N-dominated doped defective graphene as a superior oxygen electrocatalyst for ultrahigh-energy-density Zn-air batteries. ACS Energy Lett. 2018, 3, 1183–1191. [Google Scholar] [CrossRef]
- Ma, R.; Ren, X.; Xia, B.Y.; Zhou, Y.; Sun, C.; Liu, Q.; Liu, J.; Wang, J. Novel synthesis of N-doped graphene as an efficient electrocatalyst towards oxygen reduction. Nano Res. 2016, 9, 808–819. [Google Scholar] [CrossRef]
- Miao, H.; Li, S.; Wang, Z.; Sun, S.; Kuang, M.; Liu, Z.; Yuan, J. Enhancing the pyridinic N content of Nitrogen-doped graphene and improving its catalytic activity for oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 28298–28308. [Google Scholar] [CrossRef]
- Fernández-Sáez, N.; Villela-Martinez, D.E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Pastrana-Martínez, L.M. Heteroatom-doped graphene aerogels and carbon-magnetite catalysts for the heterogeneous electro-Fenton degradation of acetaminophen in aqueous solution. J. Catal. 2019, 378, 68–79. [Google Scholar] [CrossRef]
- Zhai, C.; Sun, M.; Zhu, M.; Song, S.; Jiang, S. A new method to synthesize sulfur-doped graphene as effective metal-free electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2017, 407, 503–508. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Fang, J.; Villa, C.J.; Xu, Y.; Hao, S.; Li, J.; Liu, Y.; Wolverton, C.; Chen, X. Superior oxygen reduction reaction on phosphorus-doped carbon dot/graphene aerogel for all-solid-state flexible Al-air batteries. Adv. Energy Mater. 2020, 10, 1902736. [Google Scholar] [CrossRef]
- Li, R.; Wei, Z.; Gou, X.; Xu, W. Phosphorus-doped graphene nanosheets as efficient metal-free oxygen reduction electrocatalysts. RSC Adv. 2013, 3, 9978–9984. [Google Scholar] [CrossRef]
- Musico, Y.L.F.; Kakati, N.; Labata, M.F.M.; Ocon, J.D.; Chuang, P.-Y.A. One-pot hydrothermal synthesis of heteroatom co-doped with fluorine on reduced graphene oxide for enhanced ORR activity and stability in alkaline media. Mater. Chem. Phys. 2019, 236, 121804. [Google Scholar] [CrossRef]
- Tam, T.V.; Kang, S.G.; Kim, M.H.; Lee, S.G.; Hur, S.H.; Chung, J.S.; Choi, W.M. Novel graphene hydrogel/B-doped graphene quantum dots composites as trifunctional electrocatalysts for Zn− air batteries and overall water splitting. Adv. Energy Mater. 2019, 9, 1900945. [Google Scholar] [CrossRef]
- Nankya, R.; Lee, J.; Opar, D.O.; Jung, H. Electrochemical behavior of boron-doped mesoporous graphene depending on its boron configuration. Appl. Surf. Sci. 2019, 489, 552–559. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Maegawa, K.; Tan, W.K.; Kawamura, G.; Kar, K.K.; Matsuda, A. Heteroatom doped graphene engineering for energy storage and conversion. Mater. Today 2020, 39, 47–65. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Ge, L.; Jaroniec, M.; Qiao, S.Z. Two-step boron and nitrogen doping in graphene for enhanced synergistic catalysis. Angew. Chem. 2013, 125, 3192–3198. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y.; Zhuang, X.; Li, S.; Wu, D.; Zhang, F.; Feng, X. Low-temperature synthesis of nitrogen/sulfur co-doped three-dimensional graphene frameworks as efficient metal-free electrocatalyst for oxygen reduction reaction. Carbon 2013, 62, 296–301. [Google Scholar] [CrossRef]
- Han, C.; Chen, Z. The mechanism study of oxygen reduction reaction (ORR) on non-equivalent P, N co-doped graphene. Appl. Surf. Sci. 2020, 511, 145382. [Google Scholar] [CrossRef]
- Lin, H.; Chu, L.; Wang, X.; Yao, Z.; Liu, F.; Ai, Y.; Zhuang, X.; Han, S. Boron, nitrogen, and phosphorous ternary doped graphene aerogel with hierarchically porous structures as highly efficient electrocatalysts for oxygen reduction reaction. New J. Chem. 2016, 40, 6022–6029. [Google Scholar] [CrossRef]
- Razmjooei, F.; Singh, K.P.; Song, M.Y.; Yu, J.-S. Enhanced electrocatalytic activity due to additional phosphorous doping in nitrogen and sulfur-doped graphene: A comprehensive study. Carbon 2014, 78, 257–267. [Google Scholar] [CrossRef]
- Zhao, G.; Shi, L.; Xu, J.; Yan, X.; Zhao, T. Role of phosphorus in nitrogen, phosphorus dual-doped ordered mesoporous carbon electrocatalyst for oxygen reduction reaction in alkaline media. Int. J. Hydrogen Energy 2018, 43, 1470–1478. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Müllen, K. 3D nitrogen-doped graphene aerogel-supported Fe3O4 nanoparticles as efficient electrocatalysts for the oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, Q.; Lv, Z.; Dong, H.; Yu, J.; Dong, L. Nitrogen-doped graphene as catalysts and catalyst supports for oxygen reduction in both acidic and alkaline solutions. Int. J. Hydrogen Energy 2013, 38, 1413–1418. [Google Scholar] [CrossRef]
- Kim, I.T.; Song, M.J.; Kim, Y.B.; Shin, M.W. Microwave-hydrothermal synthesis of boron/nitrogen co-doped graphene as an efficient metal-free electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2016, 41, 22026–22033. [Google Scholar] [CrossRef]
- Kim, I.T.; Shin, M.W. Synthesis of nitrogen-doped graphene via simple microwave-hydrothermal process. Mater. Lett. 2013, 108, 33–36. [Google Scholar] [CrossRef]
- Sun, X.; Lin, L.; Sun, L.; Zhang, J.; Rui, D.; Li, J.; Wang, M.; Tan, C.; Kang, N.; Wei, D. Low-temperature and rapid growth of large single-crystalline graphene with ethane. Small 2018, 14, 1702916. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.J.; Du, P.; Hu, K.; Gao, J.; Li, H.; Liu, P.; Ina, T.; Ohara, K.; Ito, Y.; Chen, M. Metal and nonmetal codoped 3D nanoporous graphene for efficient bifunctional electrocatalysis and rechargeable Zn-air batteries. Adv. Mater. 2019, 31, 1900843. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Li, L.; Zhao, Y.; Shen, Y.; Wang, S.; Shao, G. Nitrogen-doped vertical graphene nanosheets by high-flux plasma enhanced chemical vapor deposition as efficient oxygen reduction catalysts for Zn-air batteries. J. Mater. Chem. A 2020, 8, 23248–23256. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Y.; Wang, Y.; Zhang, H.; Huang, L.; Yu, G. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 2009, 9, 1752–1758. [Google Scholar] [CrossRef]
- Yazici, M.S.; Azder, M.A.; Salihoglu, O. CVD grown graphene as catalyst for acid electrolytes. Int. J. Hydrogen Energy 2018, 43, 10710–10716. [Google Scholar] [CrossRef]
- Yang, J.; Hu, P.; Yu, G. Design of carbon sources: Starting point for chemical vapor deposition of graphene. 2D Mater. 2019, 6, 042003. [Google Scholar] [CrossRef]
- Shinde, D.B.; Chaturvedi, P.; Vlassiouk, I.V.; Smirnov, S.N. Unique role of dimeric carbon precursors in graphene growth by chemical vapor deposition. Carbon Trends 2021, 5, 100093. [Google Scholar] [CrossRef]
- Granzier-Nakajima, T.; Fujisawa, K.; Anil, V.; Terrones, M.; Yeh, Y.-T. Controlling nitrogen doping in graphene with atomic precision: Synthesis and characterization. Nanomaterials 2019, 9, 425. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef]
- Wu, X.; Xie, Z.; Sun, M.; Lei, T.; Zuo, Z.; Xie, X.; Liang, Y.; Huang, Q. Edge-rich and (N,S)-doped 3D porous graphene as an efficient metal-free electrocatalyst for the oxygen reduction reaction. RSC Adv. 2016, 6, 90384–90387. [Google Scholar] [CrossRef]
- Xu, J.; Dong, G.; Jin, C.; Huang, M.; Guan, L. Sulfur and nitrogen co-doped, few-layered graphene oxide as a highly efficient electrocatalyst for the oxygen-reduction reaction. ChemSusChem 2013, 6, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Nunna, B.B.; Mandal, D.; Lee, E.S. A review of nitrogen-doped graphene catalysts for proton exchange membrane fuel cells-synthesis, characterization, and improvement. Nano-Struct. Nano-Objects 2018, 15, 140–152. [Google Scholar] [CrossRef]
- Wang, H.-F.; Tang, C.; Zhang, Q. Template growth of nitrogen-doped mesoporous graphene on metal oxides and its use as a metal-free bifunctional electrocatalyst for oxygen reduction and evolution reactions. Catal. Today 2018, 301, 25–31. [Google Scholar] [CrossRef]
- Shi, J.-L.; Tang, C.; Huang, J.-Q.; Zhu, W.; Zhang, Q. Effective exposure of nitrogen heteroatoms in 3D porous graphene framework for oxygen reduction reaction and lithium-sulfur batteries. J. Energy Chem. 2018, 27, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Minakshi Sundaram, M.; Watcharatharapong, T.; Laird, D.; Euchner, H.; Ahuja, R. Zn Metal Atom Doping on the Surface Plane of One-Dimesional NiMoO4 Nanorods with Improved Redox Chemistry. ACS Appl. Mater. Interfaces 2020, 12, 44815–44829. [Google Scholar] [CrossRef]
- Sharma, P.; Minakshi Sundaram, M.; Watcharatharapong, T.; Jungthawan, S.; Ahuja, R. Tuning the Nanoparticle Interfacial Properties and Stability of the Core-Shell Structure in Zn-Doped NiMoO4@AWO4. ACS Appl. Mater. Interfaces 2021, 13, 56116–56130. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Qiu, H.J.; Fujita, T.; Tanabe, Y.; Tanigaki, K.; Chen, M. Bicontinuous nanoporous N-doped graphene for the oxygen reduction reaction. Adv. Mater. 2014, 26, 4145–4150. [Google Scholar] [CrossRef]
- Neupane, S.; Li, W. Carbon nanotube arrays: Synthesis, properties, and applications. In Three-Dimensional Nanoarchitectures; Springer: Berlin/Heidelberg, Germany, 2011; pp. 261–285. [Google Scholar]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube-A review on Synthesis, Properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Wu, Z.; Song, M.; Wang, J.; Liu, X. Recent progress in nitrogen-doped metal-free electrocatalysts for oxygen reduction reaction. Catalysts 2018, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Peng, L.; Yang, F.; Liu, Y.; Xie, Z. Improving ORR activity of carbon nanotubes by hydrothermal carbon deposition method. J. Energy Chem. 2017, 26, 712–718. [Google Scholar] [CrossRef] [Green Version]
- Sa, Y.J.; Park, C.; Jeong, H.Y.; Park, S.H.; Lee, Z.; Kim, K.T.; Park, G.G.; Joo, S.H. Carbon nanotubes/heteroatom-doped carbon core-sheath nanostructures as highly active, metal-free oxygen reduction electrocatalysts for alkaline fuel cells. Angew. Chem. 2014, 126, 4186–4190. [Google Scholar] [CrossRef]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon nanotubes: Applications in pharmacy and medicine. BioMed Res. Int. 2013, 2013, 578250. [Google Scholar] [CrossRef] [Green Version]
- Poudel, Y.R.; Li, W. Synthesis, properties, and applications of carbon nanotubes filled with foreign materials: A review. Mater. Today Phys. 2018, 7, 7–34. [Google Scholar] [CrossRef]
- Trogadas, P.; Fuller, T.F.; Strasser, P. Carbon as catalyst and support for electrochemical energy conversion. Carbon 2014, 75, 5–42. [Google Scholar] [CrossRef]
- Ratso, S.; Kruusenberg, I.; Sarapuu, A.; Rauwel, P.; Saar, R.; Joost, U.; Aruväli, J.; Kanninen, P.; Kallio, T.; Tammeveski, K. Enhanced oxygen reduction reaction activity of iron-containing nitrogen-doped carbon nanotubes for alkaline direct methanol fuel cell application. J. Power Source 2016, 332, 129–138. [Google Scholar] [CrossRef]
- Choi, C.H.; Chung, M.W.; Kwon, H.C.; Chung, J.H.; Woo, S.I. Nitrogen-doped graphene/carbon nanotube self-assembly for efficient oxygen reduction reaction in acid media. Appl. Catal. B Environ. 2014, 144, 760–766. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Higgins, D.; Chen, Z. Highly durable graphene nanosheet supported iron catalyst for oxygen reduction reaction in PEM fuel cells. J. Electrochem. Soc. 2011, 159, B86. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.H.; Park, S.H.; Woo, S.I. Phosphorus-nitrogen dual doped carbon as an effective catalyst for oxygen reduction reaction in acidic media: Effects of the amount of P-doping on the physical and electrochemical properties of carbon. J. Mater. Chem. 2012, 22, 12107–12115. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Rinawati, M.; Huang, W.-H.; Cheng, Y.-S.; Lin, P.-H.; Chen, K.-J.; Chang, L.-Y.; Ho, K.-C.; Su, W.-N.; Yeh, M.-H. Surface-engineered N-doped carbon nanotubes with B-doped graphene quantum dots: Strategies to develop highly-efficient noble metal-free electrocatalyst for online-monitoring dissolved oxygen biosensor. Carbon 2022, 186, 406–415. [Google Scholar] [CrossRef]
- Lu, X.; Yim, W.-L.; Suryanto, B.H.; Zhao, C. Electrocatalytic oxygen evolution at surface-oxidized multiwall carbon nanotubes. J. Am. Chem. Soc. 2015, 137, 2901–2907. [Google Scholar] [CrossRef]
- Ren, M.; Chang, F.; Miao, R.; He, X.; Yang, L.; Wang, X.; Bai, Z. Strained lattice platinum-palladium alloy nanowires for efficient electrocatalysis. Inorg. Chem. Front. 2020, 7, 1713–1718. [Google Scholar] [CrossRef]
- Chang, F.; Bai, Z.; Li, M.; Ren, M.; Liu, T.; Yang, L.; Zhong, C.-J.; Lu, J. Strain-Modulated Platinum-Palladium Nanowires for Oxygen Reduction Reaction. Nano Lett. 2020, 20, 2416–2422. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Zhang, D.; Lu, Y.; Yang, C.; Du, S.; Li, W.; Tao, L.; Wang, S. Advanced Cathode Electrocatalysts for Fuel Cells: Understanding, Construction, and Application of Carbon-Based and Platinum-Based Nanomaterials. ACS Mater. Lett. 2021, 3, 1610–1634. [Google Scholar] [CrossRef]
- Hu, X.; Wu, Y.; Li, H.; Zhang, Z. Adsorption and activation of O2 on nitrogen-doped carbon nanotubes. J. Phys. Chem. C 2010, 114, 9603–9607. [Google Scholar] [CrossRef]
- Wang, Y.; Song, W.; Li, M.; Wu, Z. Oxygen reduction reaction mechanisms on heteroatom-doped single-walled carbon nanotube catalysts: Insights from a theoretical study. J. Electrochem. Soc. 2019, 166, F670. [Google Scholar] [CrossRef]
- Zhang, P.; Hou, X.; Mi, J.; He, Y.; Lin, L.; Jiang, Q.; Dong, M. From two-dimension to one-dimension: The curvature effect of silicon-doped graphene and carbon nanotubes for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2014, 16, 17479–17486. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Zhang, R.; Li, Z.; Zhou, L.; Shao, M.; Wei, M. Highly efficient metal-free electrocatalysts toward oxygen reduction derived from carbon nanotubes@ polypyrrole core-shell hybrids. J. Mater. Chem. A 2016, 4, 18008–18014. [Google Scholar] [CrossRef]
- Xie, W.; Li, J.; Song, Y.; Li, S.; Li, J.; Shao, M. Hierarchical carbon Microtube@ Nanotube core-shell structure for high-performance oxygen electrocatalysis and Zn-air battery. Nano-Micro Lett. 2020, 12, 97. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.-J.; Hu, J.-S.; Zhang, X.; Jiang, Y.; Yu, B.-B.; Wei, Z.-D.; Wan, L.-J. In situ nitrogen-doped nanoporous carbon nanocables as an efficient metal-free catalyst for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 10154–10160. [Google Scholar] [CrossRef]
- Xue, L.; Li, Y.; Liu, X.; Liu, Q.; Shang, J.; Duan, H.; Dai, L.; Shui, J. Zigzag carbon as efficient and stable oxygen reduction electrocatalyst for proton exchange membrane fuel cells. Nat. Commun. 2018, 9, 3819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, Y.; Song, H.; Liu, Y.; Cheng, Y.; Li, W.; Chen, Y.; Fan, Y.; Liu, B.; Lu, S. Boron-nitrogen-doped carbon dots on multi-walled carbon nanotubes for efficient electrocatalysis of oxygen reduction reactions. J. Colloid Interface Sci. 2021, 600, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Patil, I.M.; Lokanathan, M.; Ganesan, B.; Swami, A.; Kakade, B. Carbon nanotube/boron nitride nanocomposite as a significant bifunctional electrocatalyst for oxygen reduction and oxygen evolution reactions. Chem. Eur. J. 2017, 23, 676–683. [Google Scholar] [CrossRef]
- Suryanto, B.H.; Chen, S.; Duan, J.; Zhao, C. Hydrothermally driven transformation of oxygen functional groups at multiwall carbon nanotubes for improved electrocatalytic applications. ACS Appl. Mater. Interfaces 2016, 8, 35513–35522. [Google Scholar] [CrossRef] [PubMed]
- Likodimos, V.; Steriotis, T.A.; Papageorgiou, S.K.; Romanos, G.E.; Marques, R.R.; Rocha, R.P.; Faria, J.L.; Pereira, M.F.; Figueiredo, J.L.; Silva, A.M. Controlled surface functionalization of multiwall carbon nanotubes by HNO3 hydrothermal oxidation. Carbon 2014, 69, 311–326. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, W. Hierarchical carbon material of N-doped carbon quantum dots in-situ formed on N-doped carbon nanotube for efficient oxygen reduction. Appl. Surf. Sci. 2019, 495, 143597. [Google Scholar] [CrossRef]
- Chen, L.; Cui, X.; Wang, Y.; Wang, M.; Cui, F.; Wei, C.; Huang, W.; Hua, Z.; Zhang, L.; Shi, J. One-Step Hydrothermal Synthesis of Nitrogen-Doped Carbon Nanotubes as an Efficient Electrocatalyst for Oxygen Reduction Reactions. Chem. Asian J. 2014, 9, 2915–2920. [Google Scholar] [CrossRef]
- El-Sawy, A.M.; Mosa, I.M.; Su, D.; Guild, C.J.; Khalid, S.; Joesten, R.; Rusling, J.F.; Suib, S.L. Controlling the active sites of sulfur-doped carbon nanotube-graphene nanolobes for highly efficient oxygen evolution and reduction catalysis. Adv. Energy Mater. 2016, 6, 1501966. [Google Scholar] [CrossRef]
- Alexeyeva, N.; Shulga, E.; Kisand, V.; Kink, I.; Tammeveski, K. Electroreduction of oxygen on nitrogen-doped carbon nanotube modified glassy carbon electrodes in acid and alkaline solutions. J. Electroanal. Chem. 2010, 648, 169–175. [Google Scholar] [CrossRef]
- Alexeyeva, N.; Tammeveski, K.; Lopez-Cudero, A.; Solla-Gullón, J.; Feliu, J. Electroreduction of oxygen on Pt nanoparticle/carbon nanotube nanocomposites in acid and alkaline solutions. Electrochim. Acta 2010, 55, 794–803. [Google Scholar] [CrossRef]
- Wong, W.; Daud, W.; Mohamad, A.; Kadhum, A.; Loh, K.; Majlan, E. Influence of nitrogen doping on carbon nanotubes towards the structure, composition and oxygen reduction reaction. Int. J. Hydrogen Energy 2013, 38, 9421–9430. [Google Scholar] [CrossRef]
- Gonzalez, I.Z.; Valenzuela-Muñiz, A.; Alonso-Nuñez, G.; Farías, M.; Gomez, Y.V. Influence of the synthesis parameters in carbon nanotubes doped with nitrogen for oxygen electroreduction. ECS J. Solid State Sci. Technol. 2017, 6, M3135. [Google Scholar] [CrossRef] [Green Version]
- Alexander, C.T.; Abakumov, A.M.; Forslund, R.P.; Johnston, K.P.; Stevenson, K.J. Role of the carbon support on the oxygen reduction and evolution activities in LaNiO3 composite electrodes in alkaline solution. ACS Appl. Energy Mater. 2018, 1, 1549–1558. [Google Scholar] [CrossRef]
- Yang, G.; Choi, W.; Pu, X.; Yu, C. Scalable synthesis of bi-functional high-performance carbon nanotube sponge catalysts and electrodes with optimum C-N-Fe coordination for oxygen reduction reaction. Energy Environ. Sci. 2015, 8, 1799–1807. [Google Scholar] [CrossRef]
- Yasuda, S.; Furuya, A.; Uchibori, Y.; Kim, J.; Murakoshi, K. Iron-nitrogen-doped vertically aligned carbon nanotube electrocatalyst for the oxygen reduction reaction. Adv. Funct. Mater. 2016, 26, 738–744. [Google Scholar] [CrossRef]
- Mi, J.-L.; Liang, J.-H.; Yang, L.-P.; Wu, B.; Liu, L. Effect of Zn on size control and oxygen reduction reaction activity of Co nanoparticles supported on N-doped carbon nanotubes. Chem. Mater. 2019, 31, 8864–8874. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, L.; Chen, S.; Wang, X.; Ma, Y.; Wu, Q.; Jiang, Y.; Qian, W.; Hu, Z. Can boron and nitrogen co-doping improve oxygen reduction reaction activity of carbon nanotubes? J. Am. Chem. Soc. 2013, 135, 1201–1204. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, S.; Li, H.; Zan, Y.; Li, X.; Zhu, Y.; Dou, M.; Wang, F. Sustainable carbonaceous materials derived from biomass as metal-free electrocatalysts. Adv. Mater. 2019, 31, 1805718. [Google Scholar] [CrossRef]
- Song, M.Y.; Park, H.Y.; Yang, D.S.; Bhattacharjya, D.; Yu, J.S. Seaweed-derived heteroatom-doped highly porous carbon as an electrocatalyst for the oxygen reduction reaction. ChemSusChem 2014, 7, 1755–1763. [Google Scholar] [CrossRef]
- Liu, F.; Liu, L.; Li, X.; Zeng, J.; Du, L.; Liao, S. Nitrogen self-doped carbon nanoparticles derived from spiral seaweeds for oxygen reduction reaction. RSC Adv. 2016, 6, 27535–27541. [Google Scholar] [CrossRef]
- Escobar, B.; Pérez-Salcedo, K.; Alonso-Lemus, I.; Pacheco, D.; Barbosa, R. N-doped porous carbon from Sargassum spp. as metal-free electrocatalysts for oxygen reduction reaction in alkaline media. Int. J. Hydrogen Energy 2017, 42, 30274–30283. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, R.; Zang, Y.; Liu, G.; Wang, G.; Zhang, Y.; Zhang, H.; Zhao, H. Co/CoO nanoparticles immobilized on Co-N-doped carbon as trifunctional electrocatalysts for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Chem. Commun. 2016, 52, 5946–5949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Zhang, H.; Liu, S.; Zhang, X.; Wu, T.; Ge, X.; Zang, Y.; Zhao, H.; Wang, G. Shrimp-shell derived carbon nanodots as carbon and nitrogen sources to fabricate three-dimensional N-doped porous carbon electrocatalysts for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2016, 18, 4095–4101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Wang, K.; Song, H.; Li, H.; Ji, S.; Wang, Z.; Li, S.; Wang, R. N-doped porous carbon material made from fish-bones and its highly electrocatalytic performance in the oxygen reduction reaction. RSC Adv. 2015, 5, 48965–48970. [Google Scholar] [CrossRef]
- Liu, F.; Peng, H.; You, C.; Fu, Z.; Huang, P.; Song, H.; Liao, S. High-performance doped carbon catalyst derived from nori biomass with melamine promoter. Electrochim. Acta 2014, 138, 353–359. [Google Scholar] [CrossRef]
- Ilnicka, A.; Skorupska, M.; Tyc, M.; Kowalska, K.; Kamedulski, P.; Zielinski, W.; Lukaszewicz, J.P. Green algae and gelatine derived nitrogen rich carbon as an outstanding competitor to Pt loaded carbon catalysts. Sci. Rep. 2021, 11, 7084. [Google Scholar] [CrossRef]
- Xuan, C.; Wu, Z.; Lei, W.; Wang, J.; Guo, J.; Wang, D. Nitrogen-Doped Hierarchical Porous Carbons Derived from Sodium Alginate as Efficient Oxygen Reduction Reaction Electrocatalysts. ChemCatChem 2017, 9, 809–815. [Google Scholar] [CrossRef]
- Shu, J.; Niu, Q.; Wang, N.; Nie, J.; Ma, G. Alginate derived Co/N doped hierarchical porous carbon microspheres for efficient oxygen reduction reaction. Appl. Surf. Sci. 2019, 485, 520–528. [Google Scholar] [CrossRef]
- Ilnicka, A.; Lukaszewicz, J.P.; Shimanoe, K.; Yuasa, M. Urea treatment of nitrogen-doped carbon leads to enhanced performance for the oxygen reduction reaction. J. Mater. Res. 2018, 33, 1612–1624. [Google Scholar] [CrossRef]
- Guo, D.; Wei, H.; Chen, X.; Liu, M.; Ding, F.; Yang, Z.; Yang, Y.; Wang, S.; Yang, K.; Huang, S. 3D hierarchical nitrogen-doped carbon nanoflower derived from chitosan for efficient electrocatalytic oxygen reduction and high performance lithium-sulfur batteries. J. Mater. Chem. A 2017, 5, 18193–18206. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Gontarek, E.; Lieder, M.; Titirici, M.-M. Salt melt synthesis of curved nitrogen-doped carbon nanostructures: ORR kinetics boost. Appl. Surf. Sci. 2018, 435, 543–551. [Google Scholar] [CrossRef]
- El-Nagar, G.A.; Hassan, M.A.; Fetyan, A.; Kayarkatte, M.K.; Lauermann, I.; Roth, C. A promising N-doped carbon-metal oxide hybrid electrocatalyst derived from crustacean’s shells: Oxygen reduction and oxygen evolution. Appl. Catal. B Environ. 2017, 214, 137–147. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Lieder, M.; Jablonska, M. N-doped mesoporous carbon nanosheets obtained by pyrolysis of a chitosan-melamine mixture for the oxygen reduction reaction in alkaline media. RSC Adv. 2015, 5, 44969–44977. [Google Scholar] [CrossRef]
- Wu, K.H.; Wang, D.W.; Su, D.S.; Gentle, I.R. A discussion on the activity origin in metal-free nitrogen-doped carbons for oxygen reduction reaction and their mechanisms. ChemSusChem 2015, 8, 2772–2788. [Google Scholar] [CrossRef]
- Chen, X.; Liang, Y.; Wan, L.; Xie, Z.; Easton, C.D.; Bourgeois, L.; Wang, Z.; Bao, Q.; Zhu, Y.; Tao, S. Construction of porous N-doped graphene layer for efficient oxygen reduction reaction. Chem. Eng. Sci. 2019, 194, 36–44. [Google Scholar] [CrossRef]
- Tan, A.-D.; Wang, Y.-F.; Fu, Z.-Y.; Tsiakaras, P.; Liang, Z.-X. Highly effective oxygen reduction reaction electrocatalysis: Nitrogen-doped hierarchically mesoporous carbon derived from interpenetrated nonporous metal-organic frameworks. Appl. Catal. B Environ. 2017, 218, 260–266. [Google Scholar] [CrossRef]
- Yang, H.; Kou, S.; Li, Z.; Chang, Z.; Wang, M.; Liu, Z.; Lu, G. 3D interconnected nitrogen-self-doped carbon aerogels as efficient oxygen reduction electrocatalysts derived from biomass gelatin. RSC Adv. 2019, 9, 40301–40308. [Google Scholar] [CrossRef] [Green Version]
- Nam, G.; Park, J.; Kim, S.T.; Shin, D.-B.; Park, N.; Kim, Y.; Lee, J.-S.; Cho, J. Metal-Free Ketjenblack Incorporated Nitrogen-Doped Carbon Sheets Derived from Gelatin as Oxygen Reduction Catalysts. Nano Lett. 2014, 14, 1870–1876. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Xu, D.; Zhong, H.-X.; Wang, J.; Meng, F.-L.; Zhang, X.-B. Gelatin-derived sustainable carbon-based functional materials for energy conversion and storage with controllability of structure and component. Sci. Adv. 2015, 1, e1400035. [Google Scholar] [CrossRef] [Green Version]
- Schnepp, Z.; Zhang, Y.; Hollamby, M.J.; Pauw, B.R.; Tanaka, M.; Matsushita, Y.; Sakka, Y. Doped-carbon electrocatalysts with trimodal porosity from a homogeneous polypeptide gel. J. Mater. Chem. A 2013, 1, 13576–13581. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Z.; Ma, J.; Dong, X.; Ku, W.; Wang, M.; Sun, H.; Liang, S.; Lu, G. Nitrogen and cobalt-doped porous biocarbon materials derived from corn stover as efficient electrocatalysts for aluminum-air batteries. Energy 2018, 162, 453–459. [Google Scholar] [CrossRef]
- Pan, F.; Cao, Z.; Zhao, Q.; Liang, H.; Zhang, J. Nitrogen-doped porous carbon nanosheets made from biomass as highly active electrocatalyst for oxygen reduction reaction. J. Power Source 2014, 272, 8–15. [Google Scholar] [CrossRef]
- Borghei, M.; Laocharoen, N.; Kibena-Põldsepp, E.; Johansson, L.-S.; Campbell, J.; Kauppinen, E.; Tammeveski, K.; Rojas, O.J. Porous N,P-doped carbon from coconut shells with high electrocatalytic activity for oxygen reduction: Alternative to Pt-C for alkaline fuel cells. Appl. Catal. B Environ. 2017, 204, 394–402. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Chen, S.; Wang, Z.; Hou, H.; Zhao, F. Cellulose-derived nitrogen and phosphorus dual-doped carbon as high performance oxygen reduction catalyst in microbial fuel cell. J. Power Source 2015, 273, 1189–1193. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Calo, J.M.; Cazorla-Amorós, D.; Linares-Solano, A. Carbon activation with KOH as explored by temperature programmed techniques, and the effects of hydrogen. Carbon 2007, 45, 2529–2536. [Google Scholar] [CrossRef]
- Qiao, W.; Yoon, S.-H.; Mochida, I. KOH Activation of Needle Coke to Develop Activated Carbons for High-Performance EDLC. Energy Fuels 2006, 20, 1680–1684. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Q.; Hu, J. High Hydrogen Storage Capacity of Porous Carbons Prepared by Using Activated Carbon. J. Am. Chem. Soc. 2009, 131, 7016–7022. [Google Scholar] [CrossRef]
- Romanos, J.; Beckner, M.; Rash, T.; Firlej, L.; Kuchta, B.; Yu, P.; Suppes, G.; Wexler, C.; Pfeifer, P. Nanospace engineering of KOH activated carbon. Nanotechnology 2011, 23, 015401. [Google Scholar] [CrossRef]
- Nowicki, P.; Pietrzak, R. Węgle aktywne wzbogacone w azot-otrzymywanie, właściwości i potencjalne zastosowania. Adsorbenty I Katal. 2012, 7, 129–144. [Google Scholar]
- Zhang, J.; Qu, L.; Shi, G.; Liu, J.; Chen, J.; Dai, L. N,P-codoped carbon networks as efficient metal-free bifunctional catalysts for oxygen reduction and hydrogen evolution reactions. Angew. Chem. 2016, 128, 2270–2274. [Google Scholar] [CrossRef]
- Ilnicka, A.; Kamedulski, P.; Skorupska, M.; Lukaszewicz, J.P. Metal-free nitrogen-rich carbon foam derived from amino acids for the oxygen reduction reaction. J. Mater. Sci. 2019, 54, 14859–14871. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Chen, D.; Lu, C.; Zhang, C.; Qiu, F.; Han, S.; Zhuang, X. Rational synthesis of N/S-doped porous carbons as high efficient electrocatalysts for oxygen reduction reaction and Zn-Air batteries. Electrochim. Acta 2018, 266, 17–26. [Google Scholar] [CrossRef]
- Kaur, P.; Kim, D.-E.; Verma, G.; Park, J.-S.; Sekhon, S.S. Facile and scalable functionalization of carbon nanofibers for oxygen reduction reaction: Role of nitrogen precursor and non-ionic dispersant. J. Ind. Eng. Chem. 2021, 96, 307–314. [Google Scholar] [CrossRef]
- Massaglia, G.; Margaria, V.; Sacco, A.; Castellino, M.; Chiodoni, A.; Pirri, F.C.; Quaglio, M. N-doped carbon nanofibers as catalyst layer at cathode in single chamber Microbial Fuel Cells. Int. J. Hydrogen Energy 2019, 44, 4442–4449. [Google Scholar] [CrossRef]
- Huang, R.; Cao, C.; Liu, J.; Sun, D.; Song, W. N-Doped carbon nanofibers derived from bacterial cellulose as an excellent metal-free catalyst for selective oxidation of arylalkanes. Chem. Commun. 2019, 55, 1935–1938. [Google Scholar] [CrossRef]
- Boskovic, B.O.; Stolojan, V.; Khan, R.U.; Haq, S.; Silva, S.R.P. Large-area synthesis of carbon nanofibres at room temperature. Nat. Mater. 2002, 1, 165–168. [Google Scholar] [CrossRef]
- Buan, M.E.; Cognigni, A.; Walmsley, J.C.; Muthuswamy, N.; Rønning, M. Active sites for the oxygen reduction reaction in nitrogen-doped carbon nanofibers. Catal. Today 2020, 357, 248–258. [Google Scholar] [CrossRef]
- Minea, T.; Point, S.; Granier, A.; Touzeau, M. Room temperature synthesis of carbon nanofibers containing nitrogen by plasma-enhanced chemical vapor deposition. Appl. Phys. Lett. 2004, 85, 1244–1246. [Google Scholar] [CrossRef]
- Hofmann, S.; Ducati, C.; Robertson, J.; Kleinsorge, B. Low-temperature growth of carbon nanotubes by plasma-enhanced chemical vapor deposition. Appl. Phys. Lett. 2003, 83, 135–137. [Google Scholar] [CrossRef]
- Kong, Y.; Li, Y.; Yang, B.; Li, Z.; Yao, Y.; Lu, J.; Lei, L.; Wen, Z.; Shao, M.; Hou, Y. Boron and nitrogen co-doped porous carbon nanofibers as metal-free electrocatalysts for highly efficient ammonia electrosynthesis. J. Mater. Chem. A 2019, 7, 26272–26278. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, M.; Cao, S.; Hu, J.; Huang, L.; Yu, S.; Ragauskas, A.J. Chitosan-based layered carbon materials prepared via ionic-liquid-assisted hydrothermal carbonization and their performance study. J. Taiwan Inst. Chem. Eng. 2019, 101, 231–243. [Google Scholar] [CrossRef]
- Tong, X.; Chen, Z.; Zhuo, H.; Hu, Y.; Jing, S.; Liu, J.; Zhong, L. Tailoring the physicochemical properties of chitosan-derived N-doped carbon by controlling hydrothermal carbonization time for high-performance supercapacitor application. Carbohydr. Polym. 2019, 207, 764–774. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, F.; Smith, R.L., Jr.; Qi, X. High-Performance Supercapacitor Electrode Materials from Chitosan via Hydrothermal Carbonization and Potassium Hydroxide Activation. Energy Technol. 2017, 5, 452–460. [Google Scholar] [CrossRef]
- Simsir, H.; Eltugral, N.; Karagoz, S. Hydrothermal carbonization for the preparation of hydrochars from glucose, cellulose, chitin, chitosan and wood chips via low-temperature and their characterization. Bioresour. Technol. 2017, 246, 82–87. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, J.; Zhang, X.; Song, Q.; Wu, J.; Ding, L.; Zhang, C.; Zhu, H.; Cui, H. Template-free preparation of nitrogen-doped activated carbon with porous architecture for high-performance supercapacitors. Microporous Mesoporous Mater. 2019, 276, 280–291. [Google Scholar] [CrossRef]
- Shi, J.; Yan, N.; Cui, H.; Liu, Y.; Weng, Y.; Li, D.; Ji, X. Nitrogen doped hierarchically porous carbon derived from glucosamine hydrochloride for CO2 adsorption. J. CO2 Util. 2017, 21, 444–449. [Google Scholar] [CrossRef]
- Qu, H.; Zhang, X.; Zhan, J.; Sun, W.; Si, Z.; Chen, H. Biomass-Based Nitrogen-Doped Hollow Carbon Nanospheres Derived Directly from Glucose and Glucosamine: Structural Evolution and Supercapacitor Properties. ACS Sustain. Chem. Eng. 2018, 6, 7380–7389. [Google Scholar] [CrossRef]
- Pei, S.; Zhang, J.; Gao, M.; Wu, D.; Yang, Y.; Liu, R. A facile hydrothermal approach towards photoluminescent carbon dots from amino acids. J. Colloid Interface Sci. 2015, 439, 129–133. [Google Scholar] [CrossRef]
- Song, L.-T.; Wu, Z.-Y.; Liang, H.-W.; Zhou, F.; Yu, Z.-Y.; Xu, L.; Pan, Z.; Yu, S.-H. Macroscopic-scale synthesis of nitrogen-doped carbon nanofiber aerogels by template-directed hydrothermal carbonization of nitrogen-containing carbohydrates. Nano Energy 2016, 19, 117–127. [Google Scholar] [CrossRef]
- Gorgieva, S.; Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Shao, X.; Li, S.; Cheng, P.; Hu, Z.; Yuan, D. Metal-free N-doped carbon nanofibers as an efficient catalyst for oxygen reduction reactions in alkaline and acid media. Nanotechnology 2016, 27, 505402. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-W.; Wu, Z.-Y.; Chen, L.-F.; Li, C.; Yu, S.-H. Bacterial cellulose derived nitrogen-doped carbon nanofiber aerogel: An efficient metal-free oxygen reduction electrocatalyst for zinc-air battery. Nano Energy 2015, 11, 366–376. [Google Scholar] [CrossRef]
- Park, G.S.; Lee, J.-S.; Kim, S.T.; Park, S.; Cho, J. Porous nitrogen doped carbon fiber with churros morphology derived from electrospun bicomponent polymer as highly efficient electrocatalyst for Zn-air batteries. J. Power Source 2013, 243, 267–273. [Google Scholar] [CrossRef]
- Maldonado, S.; Stevenson, K.J. Influence of Nitrogen Doping on Oxygen Reduction Electrocatalysis at Carbon Nanofiber Electrodes. J. Phys. Chem. B 2005, 109, 4707–4716. [Google Scholar] [CrossRef]
- Yin, J.; Qiu, Y.; Yu, J.; Zhou, X.; Wu, W. Enhancement of electrocatalytic activity for oxygen reduction reaction in alkaline and acid media from electrospun nitrogen-doped carbon nanofibers by surface modification. RSC Adv. 2013, 3, 15655–15663. [Google Scholar] [CrossRef]
- Bokach, D.; ten Hoopen, S.; Muthuswamy, N.; Buan, M.E.; Rønning, M. Nitrogen-doped carbon nanofiber catalyst for ORR in PEM fuel cell stack: Performance, durability and market application aspects. Int. J. Hydrogen Energy 2016, 41, 17616–17630. [Google Scholar] [CrossRef]
- Muthuswamy, N.; Buan, M.E.; Walmsley, J.C.; Rønning, M. Evaluation of ORR active sites in nitrogen-doped carbon nanofibers by KOH post treatment. Catal. Today 2018, 301, 11–16. [Google Scholar] [CrossRef]
- Buan, M.E.; Muthuswamy, N.; Walmsley, J.C.; Chen, D.; Rønning, M. Nitrogen-doped carbon nanofibers on expanded graphite as oxygen reduction electrocatalysts. Carbon 2016, 101, 191–202. [Google Scholar] [CrossRef]
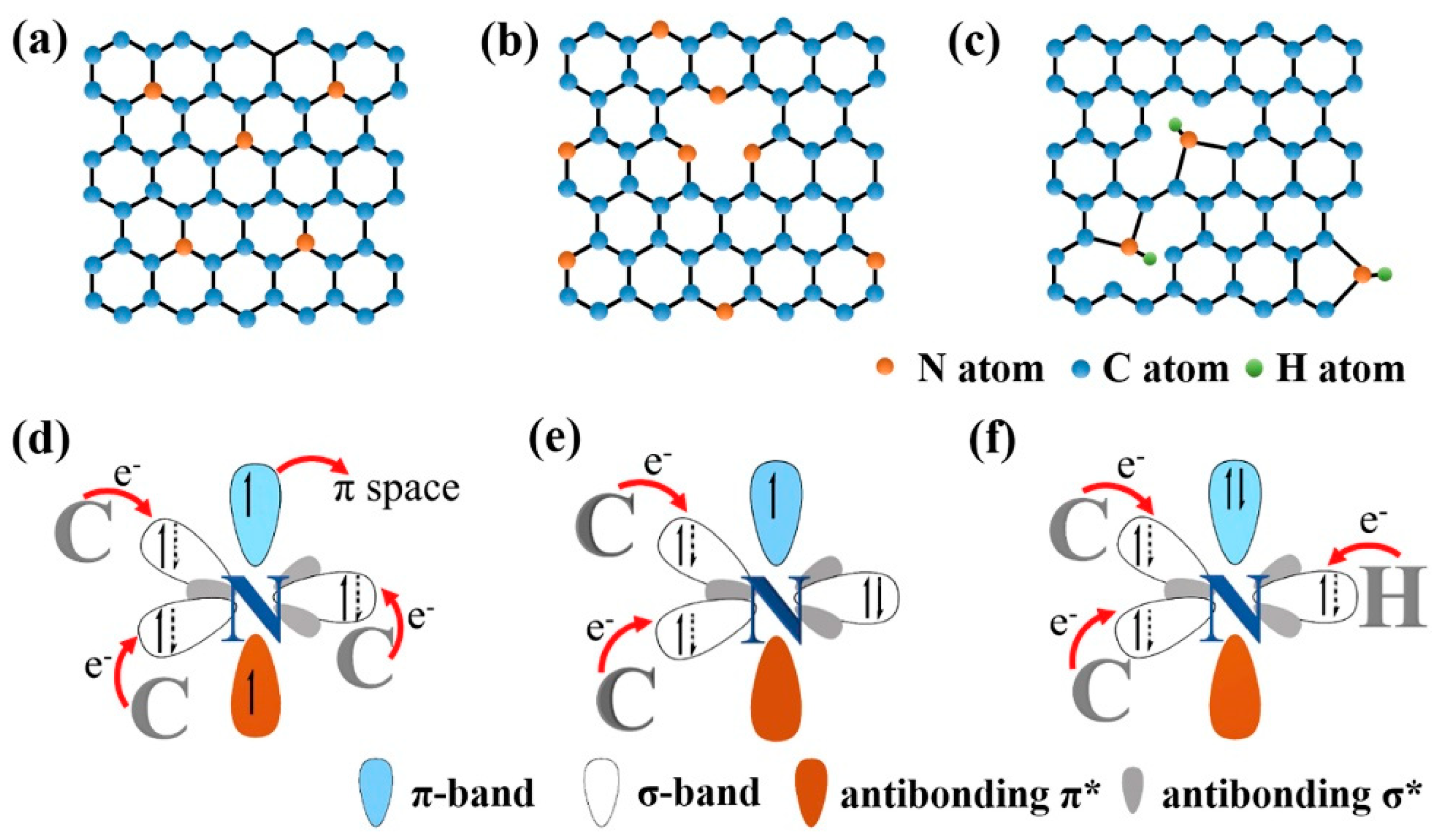
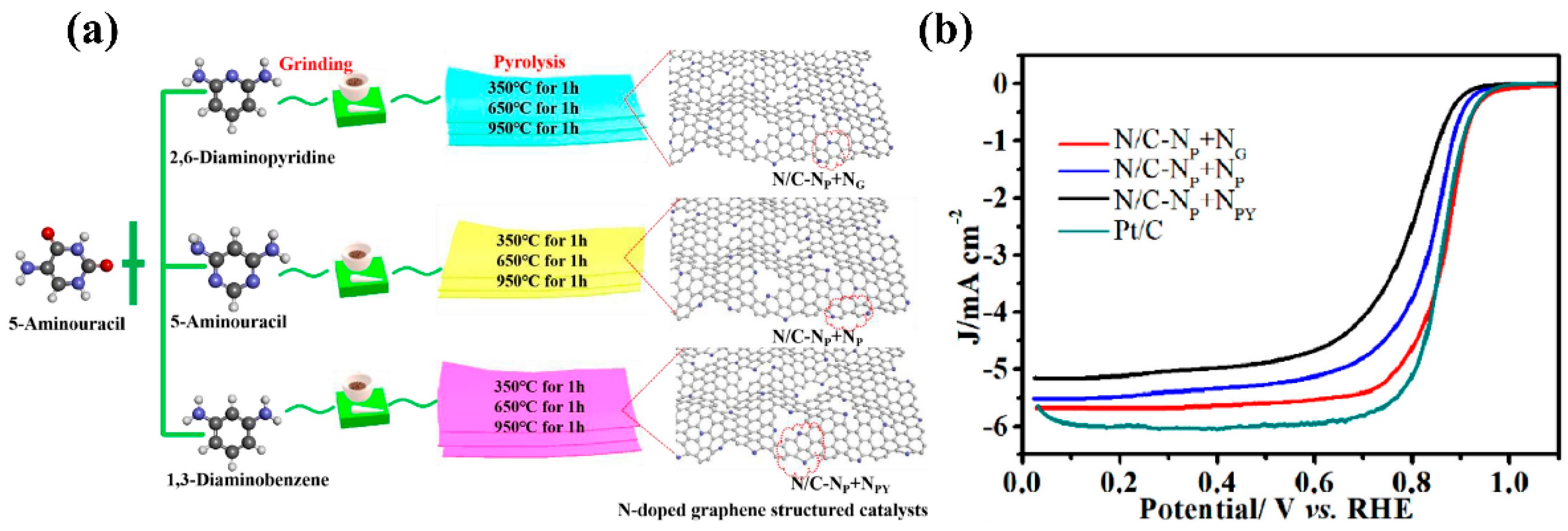
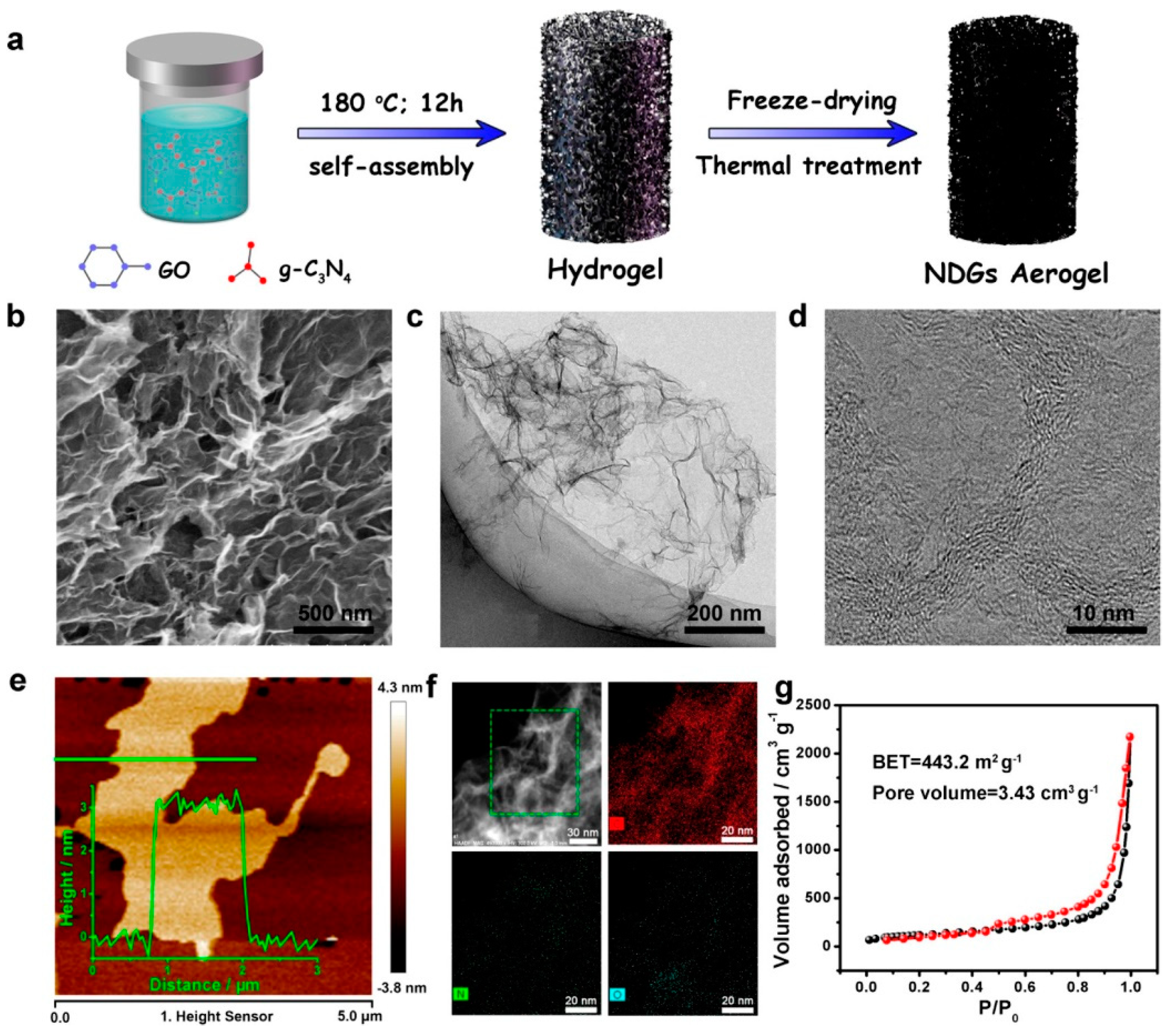
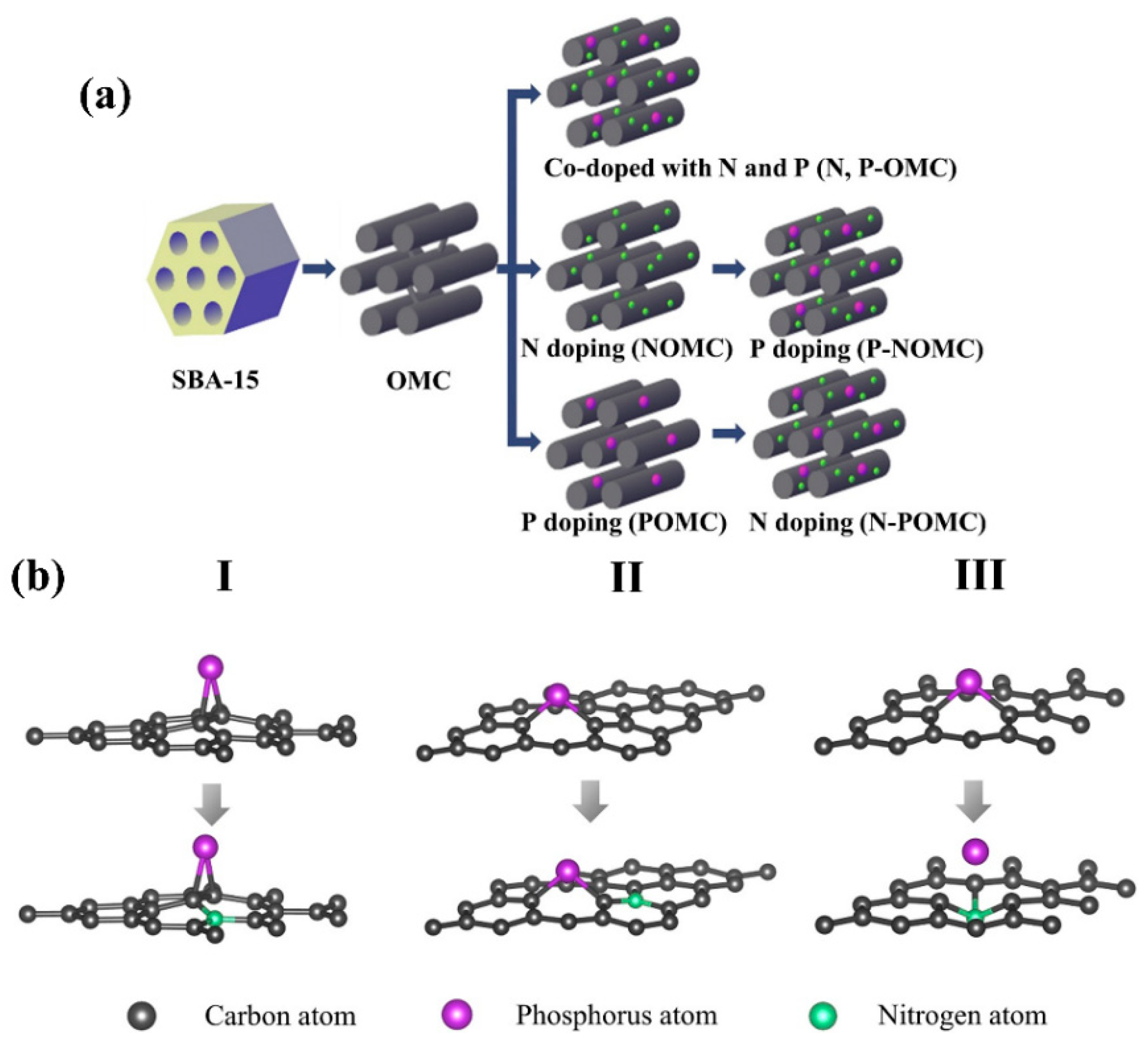
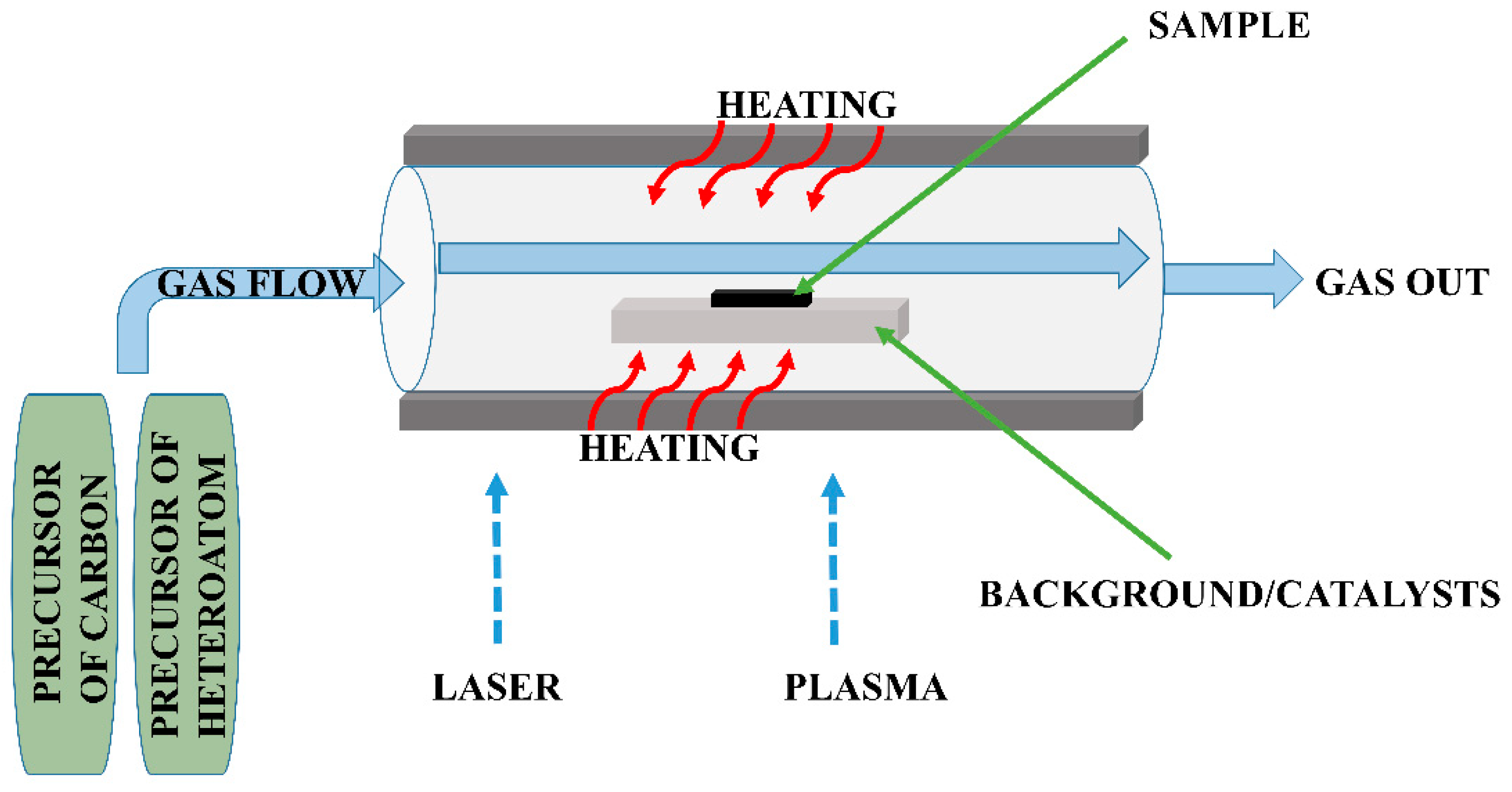
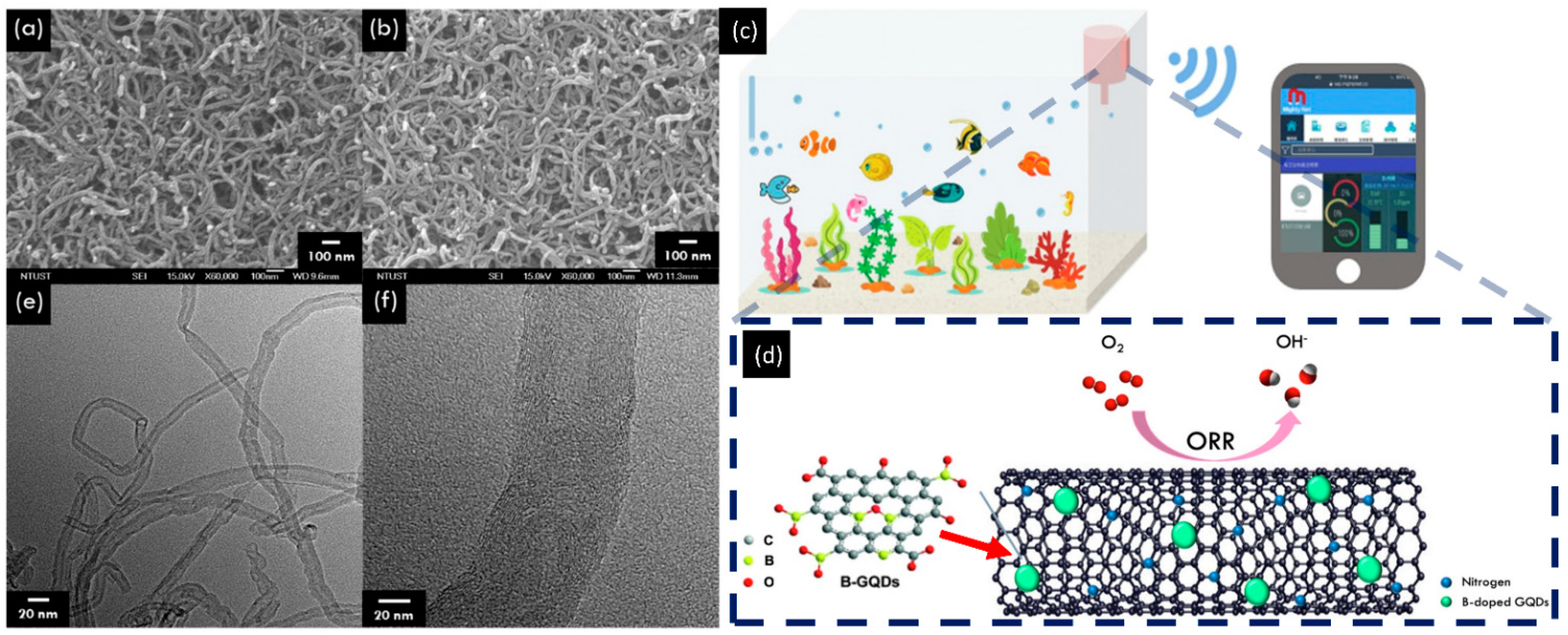
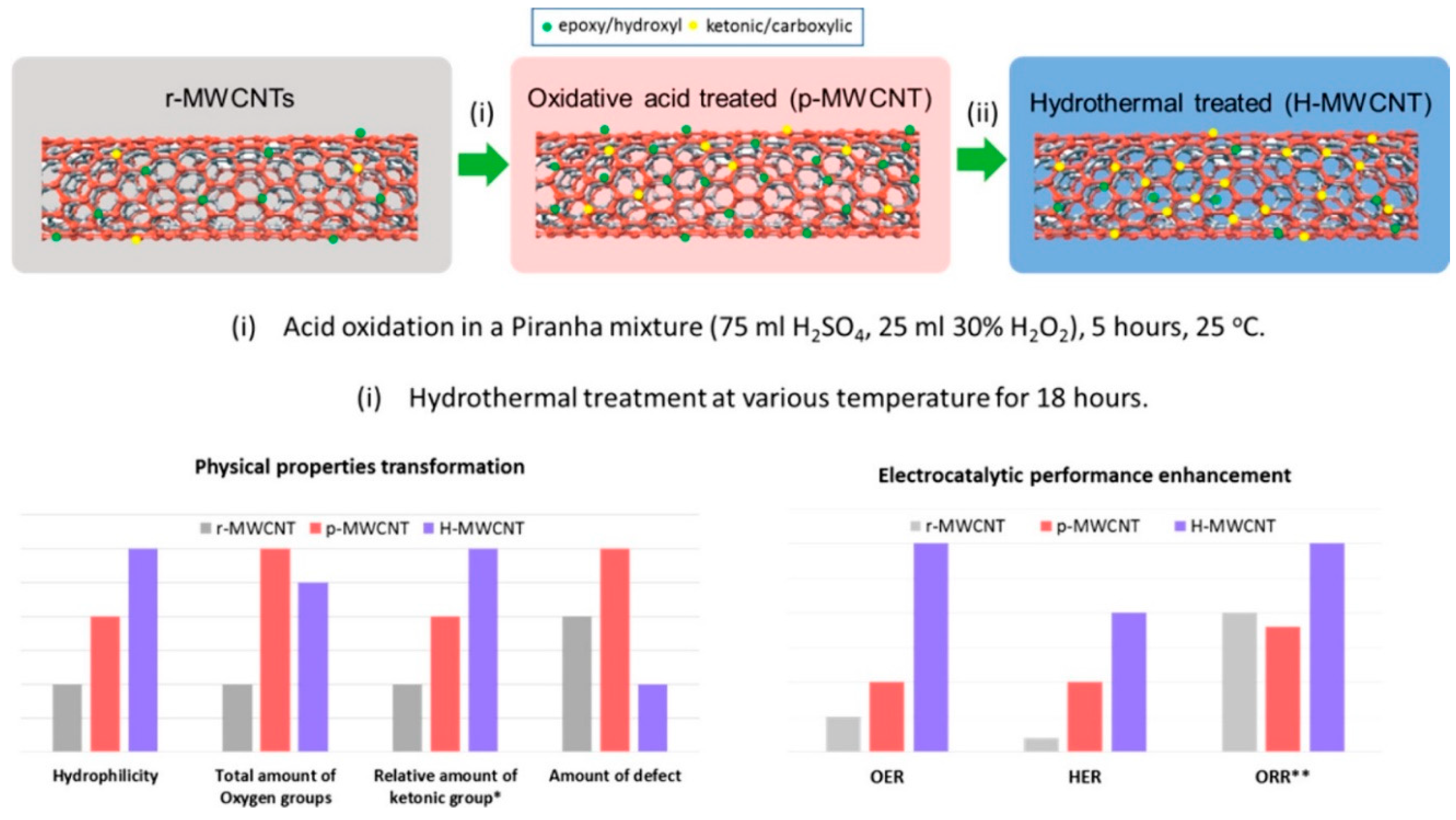
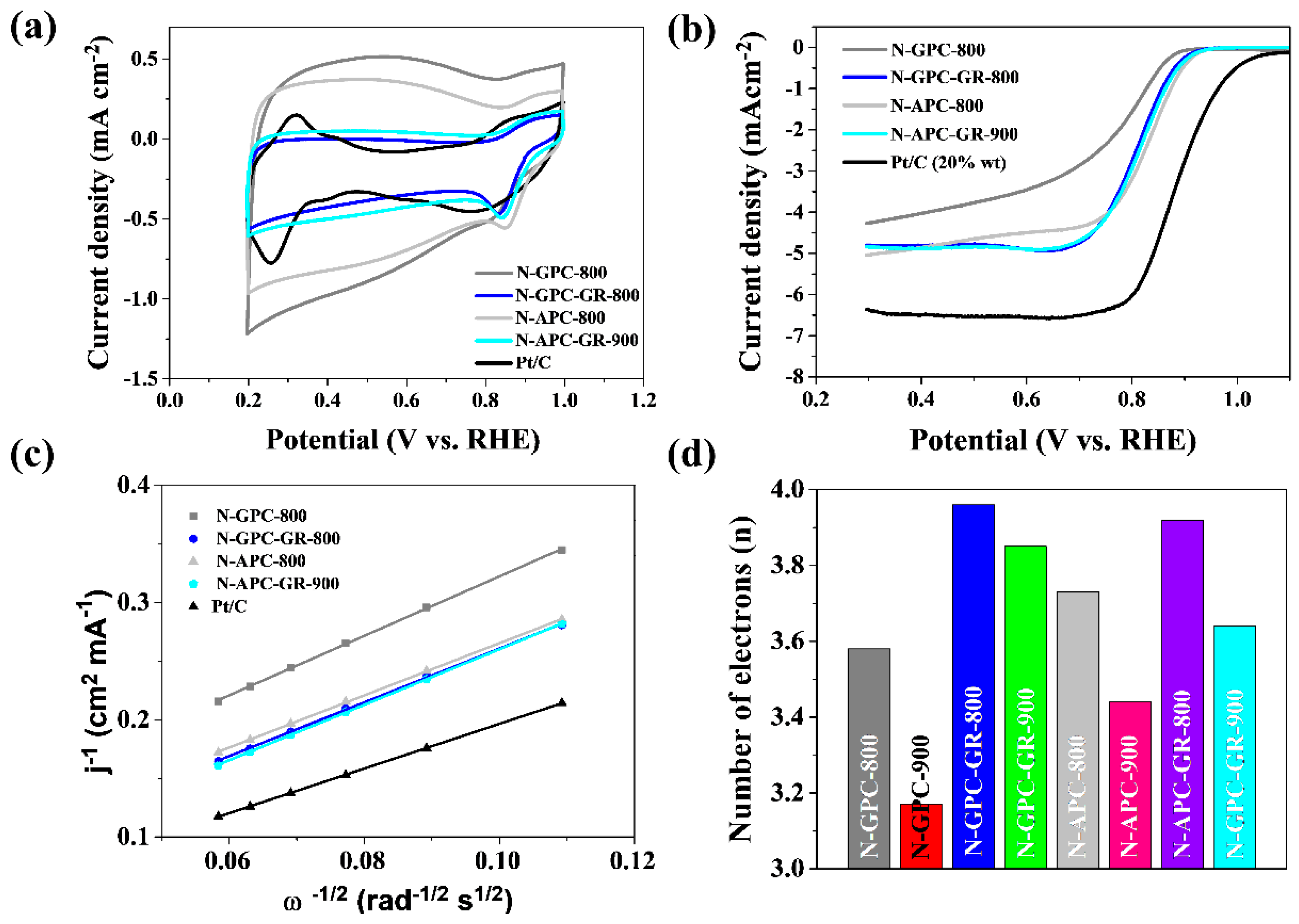
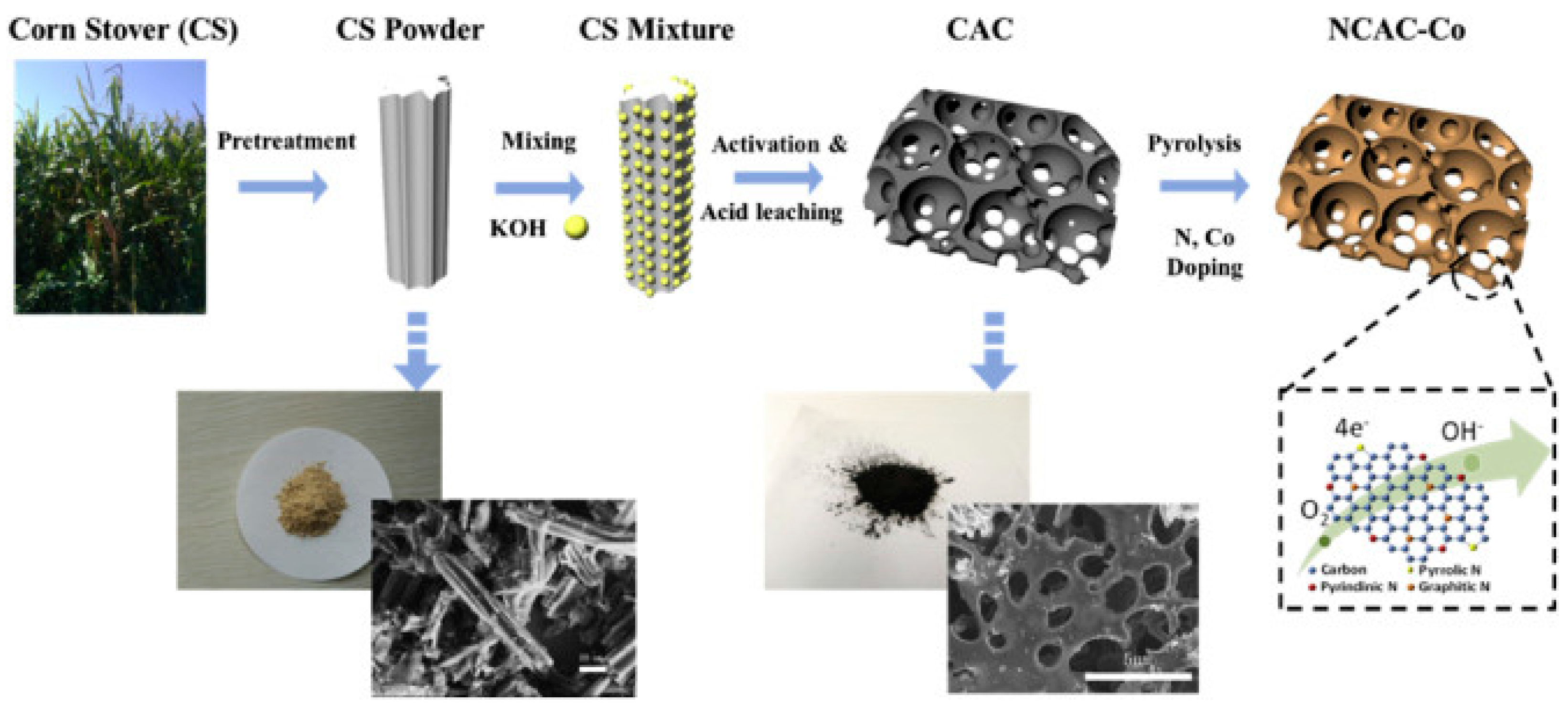
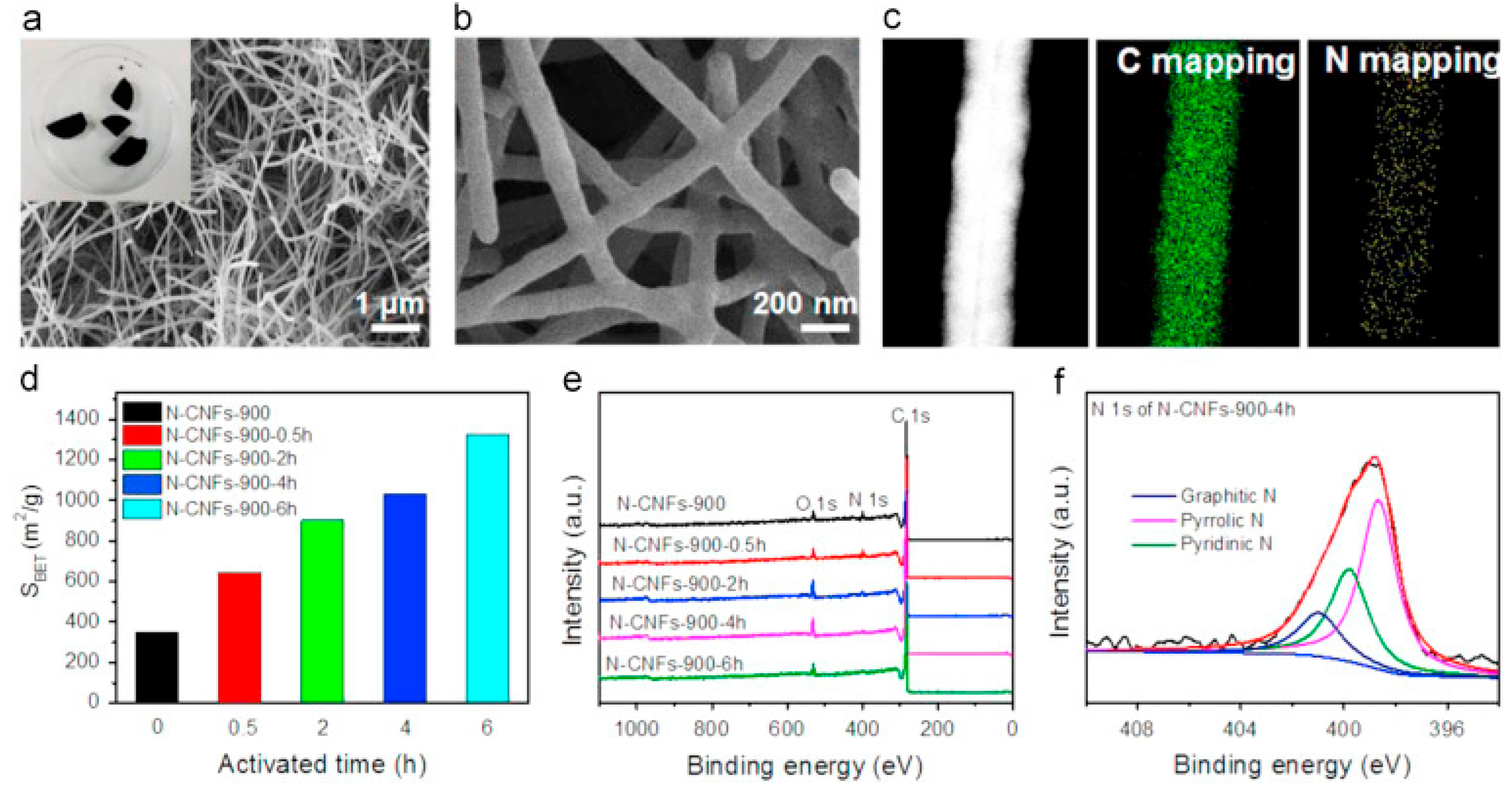
| Type of Sample | Precursor of Graphene | Precursor of Heteroatom(s) | SBET 1 (m2 g−1) | Electrolyte | Heteroatom Content | Electron Transfer Number | Ref. |
|---|---|---|---|---|---|---|---|
| N-graphene | GO 2 | PDA 3 | 62.6 | 0.1 M KOH | n.d. 4 | 3.98 | [8] |
| N-graphene | GO | BMIM BF4 5 | n.d. | 0.1 M KOH | 4.9–7.2% at. | 3.19 | [11] |
| N-graphene | GO | Melamine | 1599 | 0.1 M KOH | 2.8% at. | 2.9 | [36] |
| N-graphene | GO | PDA | 185 | 0.1 M KOH | 3.20% at. | 3.98 | [37] |
| N-graphene | Graphite | Chlorella vulgaris | n.d. | 0.1 M KOH | 1.6–2.2% at. | 3.17–3.78 | [46] |
| N-graphene | Expanded graphite | Chlorella vulgaris | n.d. | 0.1 M KOH | 0.10–2.44% at. | 2.55–3.46 | [47] |
| N-graphene | PG 6 | NH4 (AC) | 364.5 | 0.1 M KOH | n.d. | 3.4–4 | [49] |
| N-graphene | GO | urea | n.d. | 0.1 M KOH | 7.86% at. | 3.6–4 | [50] |
| N-graphene | GO | Dicyandiamide | 670 | 0.1 M KOH/0.1 M HClO4 | 5.07% at. | >3.9 | [52] |
| N-graphene | GO | NH3 | 816 | 0.1 M KOH | 2.4–4.6% at. | ~2.75–3.25 | [54] |
| N-graphene | GO | Urea | 3.15.43 | 0.1 M KOH | 3.46–6.65% at. | 3.92 | [62] |
| N-graphene | GO | Urea | n.d. | 0.1 M KOH | 8.59–20.59% at. | 2.3–2 | [64] |
| N-graphene | Graphene nanoplatelets | ADC 7 | 533–657 | 0.1 M KOH | 0.7–2.7% at. | 3.04–4 | [70] |
| N,S-graphene | GO | PDA/ 2-mercaptoethanol | 273.0 | 0.1 M KOH | N-4.1% at. S-6.1% at. | 3.52 | [8] |
| N,S-graphene | GO | Cysteine | n.d. | 0.1 M KOH | N-1.02% at. S-1.32% at. | 3.47–3.72 | [45] |
| B,N,P-graphene | GO | Boric acid/cyanamide/phenylphosphine | 443.0 | 0.1 M KOH/0.1 M HClO4 | N-6.45% at. B-9.98% at. P-0.6% at. | 3.5 | [69] |
| Type of Sample | Precursor of Graphene | Precursor of Heteroatom(s) | SBET (m2 g−1) | Electrolyte | Heteroatom Content | Electron Transfer Number | Ref. |
|---|---|---|---|---|---|---|---|
| N-graphene | GO | Dicyandiamide | 16.4–443.2 | 0.1 M KOH/ 0.5 M H2SO4 | 1.48–11.04% at. | ~4 | [72] |
| N-graphene | CCl4 1 | Pyrrole | 363.7–413.6 | 0.1 M KOH | 2.8–2.9% at. | 3.88–3.81 | [73] |
| N-graphene | GO | Urea | 177.6–280.4 | 0.1 M KOH | 3.98–6.56% at. | 3.27–3.89 | [74] |
| N-graphene | GO | Urea | 489–592 | 0.1 M KOH | 7.6–7.8% wt. | 2.62–3.11 | [75] |
| N-graphene | GO | Urea | 69 | 0.1 M KOH | n.d. 2 | 3.61 | [79] |
| N-graphene | GO | NH3 (gas) | n.d. | 0.1 M KOH | 2.8% at. | 2.63 | [83] |
| N,B-graphene | GO | NH3/boric acid | n.d. | 0.1 M KOH | N-16.57% at. B-14.37% at. | 3.97 | [83] |
| N,B-graphene | GO | (NH4)2B4O7 × 4H2O | n.d. | 0.1 M KOH | N-4.25–4.43% at. B-3.03–3.55% at. | 3.05–3.84 | [91] |
| N,F-graphene | GO | Urea/TFA 3 | 103 | 0.1 M NaOH | n.d. | 3.41 | [79] |
| N,S-graphene | GO | Urea/thiourea | 374–441 | 0.1 M KOH | N-1.5–1.9% wt. S-1.9–2.1% wt. | 3–4.05 | [75] |
| N,S-graphene | GO | Thiourea | 354.8 | 0.1 M KOH | N-3.13% at. S-1.31% at. | >3 | [87] |
| N,S-graphene | GO | NH4SCN | 220 | 0.1 M KOH | N-4–19.7% wt. S-4.1–28.7% wt. | 3.9 | [84] |
| N,B,P-graphene | GO | NH3/BPO4 | 93–372 | 0.1 M KOH | N-1.98–4.50% at. B-3.73–6.53% at. P-3.07–3.80% at. | 3.13–3.71 | [86] |
| N,P,S-graphene | GO | Thiourea/TPP 4 | 250.2–301.3 | 0.1 M KOH | N-2.88–4.34% at. S-0.89–1.24% at. P-0.96–1.31% at. | >3.4 | [87] |
| Type of Sample | Source of Carbon | Precursor of Heteroatom(s) | SBET (m2 g−1) | Electrolyte | Heteroatom Content | Electron Transfer Number | Ref. |
|---|---|---|---|---|---|---|---|
| N-graphene | CH4 | gC3N4/NH3 | 830 | 0.1 M KOH | 0.7–6.5% at. | 3.86–3.88 | [14] |
| N-graphene | CH4 | N2 (gas) | 311.7–400.2 | 0.1 M KOH/ 0.1 M HClO4 | 3.8–6.7% at. | >3.9 | [95] |
| N-graphene | CH4 | NH3 (gas) | n.d. 1 | 0.1 M HClO4 | n.d. | n.d. | [97] |
| N-graphene | CH4 | NH3 (gas) | n.d. | 0.1 M KOH | n.d. | 3.6–4 | [101] |
| N-graphene | CH4 | NH3 (gas) | 1440 | 0.1 M KOH | 3.41% at. | >3 | [105] |
| N-graphene | CH4 | Pyridine | 1531–1732 | 0.1 M KOH | 1.18–1.81% at. | 3.34 | [106] |
| N-graphene | Pyridine | Pyridine | 1000 | 0.1 M KOH | 2.26–4.95% at. | 1.1–3.9 | [109] |
| N,S-graphene | C2H5OH | Thiourea | 43.8–119.5 | 0.1 M KOH | N-4.50% at. S-0.77% at. | 3.6–3.8 | [102] |
| N,S-graphene | Pyrimidine/thiophene | Pyrimidine/thiophene | 640 | 0.1 M NaOH | N-2–9.6% at. S-0.7–3.2% at. | 3.2–4.1 | [103] |
| Type of Sample | Type of Carbon | Precursor of Heteroatom(s) | SBET (m2 g−1) | Electrolyte | Heteroatom Content | Electron Transfer Number | Ref. |
|---|---|---|---|---|---|---|---|
| N-CNT | CNT | urea/DCDA 1 | n.d. | 0.1 M KOH | 4–6% at. | >3.5 | [15] |
| N-CNT | CNT | melamine | 104.7 | 0.1 M KOH | 0.193% at. | 2.77–2.80 | [51] |
| N-CNT | CNT | NH3/aniline | n.d. | 0.1 M KOH | n.d. | 2.5–4 | [20] |
| N-CNT | FePc 2 | NH3 (gas) | n.d. | 0.1 M KOH | 3.6–5.6% at. | 1.8–3.9 | [113] |
| N-CNT | CNT | urea | 230–284 | 0.1 M KOH | 0.5–1.7% at. | 2.9–3.55 | [114] |
| N-CNT | CNT | urea/NH3 | n.d. | 0.1 M KOH | n.d. | >3.7 | [115] |
| N-CNT | CNT/GO | PEI 3/DCDA | n.d. | 1 M HClO4 | 1.5–3.1% at. | 3.58–3.94 | [120] |
| N-CNT | C2H4 | pyrrole | 149.46–192.47 | 0.1 M KOH | 5.69–6.90% at. | 3.03–3.94 | [131] |
| N-CNT | CNT | melamine | 101–479 | 0.1 M KOH | 3.54–15.76% at. | ~4 | [133] |
| N-CNT | CNT | CM 4/DCDA | n.d. | 0.1 M KOH | 2.3–3.7% at. | 4 | [23] |
| N-CNT | CNT | NH3 (gas) | n.d. | 0.1 M KOH/ 0.5 M H2SO4 | 3.09% at. | 3.88–3.96/ 3.19–3.96 | [134] |
| N,B-graphene | CNT/ BGQDs | NH3 (gas)/ Boric acid | n.d. | 0.1 M KOH/0.01 M PBS/0.1 M HClO4 | N-0.5% wt. B-4.86% at. | 3.2–3.5 | [123] |
| N,S,F-graphene | CNT | BMITFSI 5 | 293–489 | 0.1 M KOH | N-4.67.5% at. S-0.6%–1.1% at. F-0.7%–1.2% at. | 3.4–4 | [115] |
| Type of Sample | Type of Carbon | Precursor of Heteroatom(s) | SBET (m2 g−1) | Electrolyte | Heteroatom Content | Electron Transfer Number | Ref. |
|---|---|---|---|---|---|---|---|
| N-CNT | CNT/CQDs | EDA 1 | n.d. | 0.1 M KOH | n.d. | 3.78–3.85 | [139] |
| N-CNT | CNT | NH4OH | 388 | 0.1 M KOH | 1.32% at. | 3.7–3.8 | [140] |
| N-CNT | Acetonitrile | Acetonitrile | n.d. | 0.1 M KOH/ 0.5 M H2SO4 | 3% at. | 2–3.5/2–4 | [142] |
| N-CNT | FePc | Aniline/DEA 2/EDA | n.d. | 0.5 M H2SO4 | 4.33–6.58% at. | 3–3.6 | [144] |
| N-CNT | Pyridine | Pyridine | n.d. | 0.5 M H2SO4 | 4.29–5.6% wt. 1.8–2.5% at. | n.d. | [145] |
| N,B-CNT | CNT | L-aspartic/orthoboric acid | 136.2 | 0.1 M KOH | N-1.19% at. B-0.51% at. | 3.48 | [135] |
| N,B-CNT | CNT | BN 3 | n.d. | 0.1 M KOH | n.d. | 3.9 | [136] |
| N,S-CNT | CNT | (NH4)2 S | n.d. | 0.1 M KOH | N-2.65% at. S-0.76% at. | n.d. | [140] |
| Type of Sample | Precursor of C and N | SBET (m2 g−1) | Electrolyte | N Content | Electron Transfer Number | Ref. |
|---|---|---|---|---|---|---|
| N-C 1 | Seaweed | 674.63–1217.78 | 0.1 M KOH | 1.8–5.21% at. | 3.7 | [153] |
| N-C | Spiral seaweed | 199.2–1610.3 | 0.1 M KOH | 4.5–5.1% wt. | 4 | [154] |
| N-C | Sargassum spp. | 3.84–188.87 | 0.5 M KOH | 0.56–0.95% wt. | n.d. 2 | [155] |
| N-C | Shrimp shell | 647.7 | 0.1 M KOH | 7.44% at. | 3.8 | [156] |
| N-C | Shrimp shell | 13.4–360.2 | 0.1 M KOH | 6.8–11.8% at. | 1.36–2.95 | [157] |
| N-C | Fish bone | n.d. | 0.1 M KOH | 6.02% at. | n.d. | [158] |
| N,S-C 3 | Algae, MEL 4 | 538 | 0.1 M KOH | 2.63% at. | 3.97 | [159] |
| N-C | Green algae | 366–623 | 0.1 M KOH | 2.16–7.09% wt. | 3.44–3.84 | [160] |
| N,Co-C | Alginate | 252 | 0.1 M KOH | 2–5.5% at. | >4 | [162] |
| N-C | Alginate | 470.9 | 0.1 M KOH | 1% at. | 4 | [161] |
| N-C | Chitin, chitosan | 625–1801 | 0.1 M KOH | 4.85–10.85% wt. | 2.17–3.76 | [163] |
| N-C | Chitosan | 907 | 0.1 M KOH | n.d. | 3.9–4 | [164] |
| N-C | Chitosan | 78–1317.97 | 0.1 M KOH | 4.7–8.1% wt. | 2.2–3.5 | [165] |
| N-C | Chitosan | n.d. | 0.1 M KOH | n.d. | 3.9–4.1 | [166] |
| N-C | Chitosan | 285 | 0.1 M KOH | 3.3–4.5% wt. | n.d. | [167] |
| N-C | Gelatin | 376–839 | 0.1 M KOH | 4.69–5.73% at. | 3.41–4.14 | [171] |
| N-C | Gelatin | 739.5–933.9 | 0.1 M KOH | 1.19–1.79% at. | 3.7–3.85 | [172] |
| N-C | Gelatin | 189.8–1215.4 | 0.1 M KOH | 3.6–4.3% at. | 3.9–4 | [173] |
| N-C | Gelatin | 360–880 | 0.1 M KOH | 3.24–10.08% wt. | 3.17–3.85 | [160] |
| N,Fe,Mg-C | Gelatin | 370–650 | 0.1 M KOH | 5.8–6.8% wt. | 3.9–4.2 | [174] |
| N,Co-C 6 | Corn stover, urea, CoCl2 | 1877.3 | 0.1 M KOH | 2.56% at. | 3.87 | [175] |
| N-C | Ginkgo leaves, NH3 | 1436.02 | 0.1 M KOH | 1.59% at. | 3.7 | [176] |
| N,P-C 5 | Coconut shells, H3PO4, urea | 1216 | 0.1 M KOH | 0.5–1.1% at. | 4 | [177] |
| N,P-C | Cellulose, MEL, PA 7 | 241–612 | 0.1 M KOH | 2.4–4.4% at. | 3.58–3.99 | [5] |
| N,P-C | Cellulose, (NH4)3PO4 | n.d. | PBS 8 | 2.17% at. | 3.5 | [178] |
| Type of Sample | Precursor of CNF | Precursor of Heteroatom | SBET (m2 g−1) | Electrolyte | N Content | Electron Transfer Number | Ref. |
|---|---|---|---|---|---|---|---|
| N-CNF | C6H13NO5xHCl | C6H13NO5xHCl | 643–1324 | 0.1 M KOH | 1.64–4.67% at. | 3.74–4.02 | [204] |
| N-CNF | Bacterial cellulose | C4H5N | 215–253 | 0.1 M KOH/ 0.1 M HClO4 | 4.90–8.33% at. | 3.7 | [206] |
| N-CNF | Bacterial cellulose | NH3 | 916 | 0.1 M KOH | 5.8% at. | 3.96 | [207] |
| N-CNF | PAN 1 | PAN | n.d. 2 | 0.1 M KOH | 2% at. | 3.9 | [189] |
| N-CNF | PAN, PS 3 | PAN | 905–1271 | 0.1 M KOH | n.d. | 3.7–3.8 | [208] |
| N-CNF | Xylene | pyridine | 130 | 0.5 M H2SO4/0.1 M KOH | 4% at. | 3.97 | [209] |
| N-CNF/Fe | Graphite | NH3 | 225 | 0.5 M H2SO4 | 3.3% at. | n.d. | [211] |
| N-CNF/Ni or Fe | Graphite | NH3 | 35–226 | 0.5 M H2SO4/0.5 M KOH | 1.5–3.9% at. | 2–4 | [213] |
| N-CNF | Graphite | NH3 | 152–210 | 0.5 M H2SO4 | 2.4–5.1% at. | n.d. | [192] |
| N-CNF | Graphite | NH3 | 270–1151 | 0.5 M H2SO4 | 0.24–4.7% at. | n.d. | [212] |
| N,P,S-CNF | Cellulose nanofibrils | C3H6N6, C6H18O24P6 | 565–1217 | 0.5 M H2SO4/0.1 M KOH | 2.4–4.3% wt. | 3.58–3.99 | [5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skorupska, M.; Ilnicka, A.; Lukaszewicz, J.P. Successful Manufacturing Protocols of N-Rich Carbon Electrodes Ensuring High ORR Activity: A Review. Processes 2022, 10, 643. https://doi.org/10.3390/pr10040643
Skorupska M, Ilnicka A, Lukaszewicz JP. Successful Manufacturing Protocols of N-Rich Carbon Electrodes Ensuring High ORR Activity: A Review. Processes. 2022; 10(4):643. https://doi.org/10.3390/pr10040643
Chicago/Turabian StyleSkorupska, Malgorzata, Anna Ilnicka, and Jerzy P. Lukaszewicz. 2022. "Successful Manufacturing Protocols of N-Rich Carbon Electrodes Ensuring High ORR Activity: A Review" Processes 10, no. 4: 643. https://doi.org/10.3390/pr10040643
APA StyleSkorupska, M., Ilnicka, A., & Lukaszewicz, J. P. (2022). Successful Manufacturing Protocols of N-Rich Carbon Electrodes Ensuring High ORR Activity: A Review. Processes, 10(4), 643. https://doi.org/10.3390/pr10040643








