Recent Advances in Creating Biopreparations to Fight Oil Spills in Soil Ecosystems in Sharply Continental Climate of Republic of Kazakhstan
Abstract
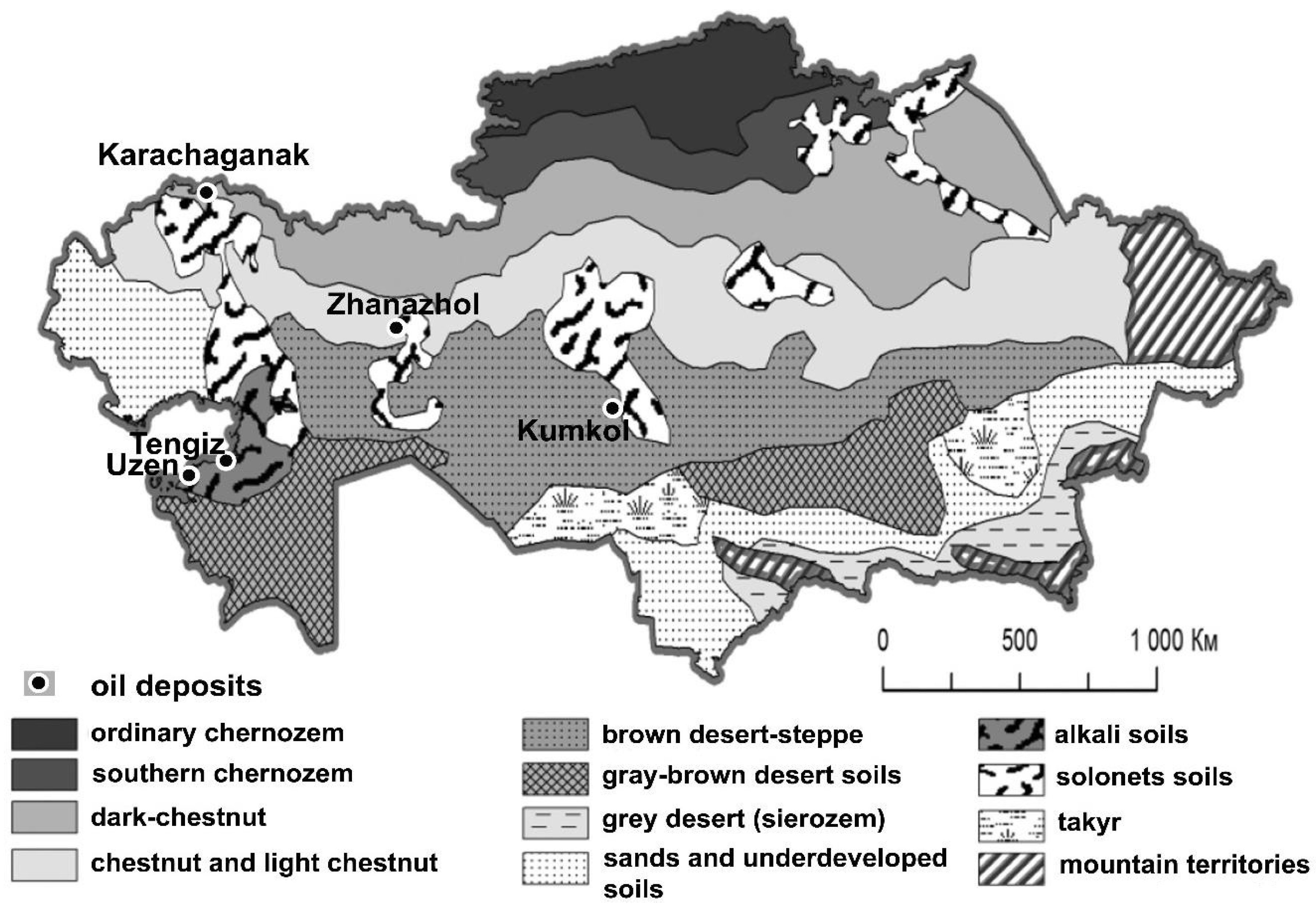
1. Natural Conditions, Soils, and Hydrocarbon Production in Kazakhstan
2. Up-to-Date Studies Focusing on Hydrocarbon-Degrading Microorganisms in Kazakhstan
3. Bioremediation Approaches
4. Enzymatic Activity of Oil-Degrading Microbes
5. Microorganisms and Biopreparations to Remediate Oil-Spilled Soils in Deserts of Kazakhstan
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veerasingam, S.; Vethamony, P.; Mani Murali, R.; Babu, M.T. Sources, vertical fluxes and accumulation of petroleum hydrocarbons in sediments from the mandovi estuary, west coast of India. Int. J. Environ. Res. 2015, 9, 179–186. [Google Scholar]
- Kaiser, M.J.; Pulsipher, A.G. A review of the oil and gas sector in Kazakhstan. Energy Policy 2007, 35, 1300–1314. [Google Scholar] [CrossRef]
- Caspian Sea: The Tehran Convention. Available online: https://www.unep.org/explore-topics/oceans-seas/what-we-do/working-regional-seas/regional-seas-programmes/caspian-sea; https://web.archive.org/web/20220301151032/https%3A%2F%2Fwww.unep.org%2Fexplore-topics%2Foceans-seas%2Fwhat-we-do%2Fworking-regional-seas%2Fregional-seas-programmes%2Fcaspian-sea (accessed on 6 February 2022).
- Argentina–America–Oil Deposits [Argentina–America–Mestorozhdeniya Nefti I Gaza]. Available online: http://www.nftn.ru/oilfields/america/argentina/73 (accessed on 15 November 2021).
- Klebanovich, N.V.; Efimova, I.A.; Prokopovich, S.N. Soil and Agrarian Resources of Kazakhstan; Belarus State University Publishing: Minsk, Belarus, 2016; 46p. [Google Scholar]
- Dosbergenov, S.N. Environmental problems of oil-contaminated soils in the oil production areas of western Kazakhstan and ways to solve them. Hydrometeorol. Ecol. 2010, 3, 111–122. [Google Scholar]
- National Environmental Strategy of the Republic of Kazakhstan. Available online: http://savesteppe.org/ru/archives/5296 (accessed on 6 February 2022).
- Accident at the 37th well of Tengiz: How It Occurred and What Is Still Being Kept Silent about. Available online: https://atpress.kz/9535-avariya-na-37-oy-skvadgine-tengiza-kak-eto-bylo-i-o-chem-do-sikh-por-umalchivayu (accessed on 6 February 2022).
- Azubuike, C.C.; Chikere, C.B.; Okpokwasili, G.C. Bioremediation techniques-classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar] [CrossRef] [PubMed]
- Foght, J.M.; McFarlane, D.M. Growth of extremophiles on petroleum. In Enigmatic Microorganisms and Life in Extreme Environments; Seckbach, J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 527–538. [Google Scholar]
- Margesin, R.; Schinner, F. Bioremediation (natural attenuation and biostimulation) of diesel-oil-contaminated soil in an alpine glacier skiing area. Appl. Microbiol. Biotechnol. 2001, 67, 3127–3133. [Google Scholar] [CrossRef] [PubMed]
- Abed, R.M.M.; Al-Thukair, A.; de Beer, D. Bacterial diversity of a cyanobacterial mat degrading petroleum compounds at elevated salinities and temperatures. FEMS Microbiol. Ecol. 2006, 57, 290–301. [Google Scholar] [CrossRef][Green Version]
- Feitkenhauer, H.; Muller, R.; Markl, H. Degradation of polycyclic aromatic hydro-carbons and long chain n-alkanes at 60–70 °C by Thermus and Bacillus spp. Biodegradation 2003, 1, 367–372. [Google Scholar] [CrossRef]
- Wu, Q.; Bedard, D.L.; Wiegel, J. Influence of incubation temperature on the microbial reductive dechlorination of 2,3,4,6-tetrachlorobiphenyl in two freshwater sediments. Appl. Environ. Microbiol. 1996, 62, 4174–4179. [Google Scholar] [CrossRef]
- Müller, R.; Antranikianm, G.; Maloney, S.; Sharp, R. Thermophilic degradation of environmental pollutants. In Biotechnology of Extremophiles: Advances in Biochemical Engineering/Biotechnology; Antranikian, G., Ed.; Springer-Verlag Telos: Dordrecht, The Netherlands, 1998; pp. 155–169. [Google Scholar]
- Annweiler, E.; Richnow, H.H.; Antranikian, G.; Hebenbrock, S.; Garms, C.; Franke, S.; Francke, W.; Michaelis, W. Naphthalene degradation and incorporation of naphthalene-derived carbon into biomass by the thermophilic Bacillus thermoleovorans. Appl. Environ. Microbiol. 2000, 66, 518–523. [Google Scholar] [CrossRef]
- Obuekwe, C.O.; Al-Jadi, Z.K.; Al-Saleh, E.S. Hydrocarbon degradation in relation to cell-surface hydrophobicity among bacterial hydrocarbon degraders from petroleum-contaminated Kuwait desert environment. Int. Biodeterior. Biodegrad. 2009, 63, 273–279. [Google Scholar] [CrossRef]
- Ibragimova, S.T.; Aitkeldieva, S.A.; Faizulina, E.R.; Sadanov, A.K.; Poputnikova, T.O.; Terekhova, V.A. Ecological estimation of oil polluted soils in Kazakhstan according to standard biotest systems. Rep. Eco Soil Sci. 2009, 1, 79–94. [Google Scholar]
- Sadanov, A.K.; Aitkeldieva, S.A.; Faizulina, E.R.; Auezova, O.N.; Kurmanbaev, A.A.; Tatarkina, L.G.; Zaitova, T.S.; Amirasheva, B.K. Assessment of destructive activity and phytotoxicity of strains of oil-oxidizing microorganisms isolated from oil-contaminated soils of the Kyzylorda region. Microbiol. Virol. 2013, 1–2, 11–15. [Google Scholar]
- Faizulina, E.R.; Auezova, O.N.; Tatarkina, L.G.; Svirko, E.A.; Dauletova, A.A.; Aitkeldieva, S.A. Oil-oxidizing activity and identification of microorganisms isolated from the Caspian Sea. News Nation Acad. Sci. Repub. Kazakhstan Biol. Med. Ser. 2014, 3, 25–29. [Google Scholar]
- Goncharova, A.; Karpenyuk, T.; Kalbayeva, A.; Mukasheva, T.; Bektileuova, N. Screening and characterization of emulsifying hydrocarbon-degrading bacteria from coastal waters of the Caspian sea. Naše More 2021, 68, 74–82. [Google Scholar] [CrossRef]
- Kebekbaeva, K.M.; Dzhakibaeva, G.G.; Dzhobulaeva, A.K. Physiological and biochemical properties of oil-oxidizing bacteria during storage by different methods. Microbiol. Virol. 2014, 1, 39–42. [Google Scholar]
- Safary, A.; Ardakani, M.R.; Suraki, A.A.; Khiavi, M.A.; Motamedi, H. Isolation and characterization of biosurfactant producing bacteria from Caspian Sea. Biotechnology 2010, 9, 378–382. [Google Scholar] [CrossRef]
- Hassanshahian, M.; Ravan, H. Screening and identification of biosurfactant producing marine bacteria from the Caspian Sea. Caspian J. Environ. Sci. 2018, 16, 179–189. [Google Scholar]
- Nazina, T.N.; Shumkova, E.S.; Sokolova, D.S.; Babich, T.L.; Zhurina, M.V.; Xue, Y.-F.; Osipov, G.A.; Poltaraus, A.B.; Turova, T.P. Identification of hydrocarbon-oxidizing bacteria of the genus Dietzia from oil reservoirs on the basis of phenotypic traits and analysis of 16S rRNA and gyrB genes. Microbiology 2015, 84, 377–388. [Google Scholar] [CrossRef]
- Khasenova, E.Z.; Moldagulova, N.B.; Berdimuratova, K.T.; Ayupova, A.Z.; Kurmanbaev, A.A. Study of the effectiveness of the use of a biological product based on oil-oxidizing psychrotrophic microorganisms for soil bioremediation in the field. Int. J. Appl. Fund. Res. 2017, 10, 279–282. [Google Scholar]
- Aitkeldiyeva, S.A.; Faizulina, E.R.; Auezova, O.N.; Tatarkina, L.G.; Spankulova, G.A. Isolation and study of thermotolerant oil-oxidizing microorganisms. News Nation Acad. Sci. Repub. Kazakhstan Biol. Med. Ser. 2019, 2, 56–62. [Google Scholar] [CrossRef]
- Bisekenov, T. Model experiment on soil cleaning with a biological product based on hydrocarbon-oxidizing microorganisms. Bull. A Dosmukhamedov. Atyrau Univ. 2010, 4, 1–6. [Google Scholar]
- Esenamanova, M.S.; Esenamanova, Z.S.; Abuova, A.E.; Ryskalieva, D.K.; Bektemirov, D.S.; Ryszhan, A.E. Neutralization of contaminated soil with biopreparations. Mod. Probl. Sci. Educ. 2016, 6, 511–518. [Google Scholar]
- Aitkeldiyeva, S.A.; Faizulina, E.R.; Tatarkina, L.G.; Alimzhanova, M.B.; Daugaliyeva, S.T.; Auezova, O.N.; Alimbetova, A.V.; Spankulova, G.A.; Sadanov, A.K. Degradation of petroleum hydrocarbons with thermotolerant microorganisms. Rasayan J. Chem. 2020, 13, 1271–1282. [Google Scholar] [CrossRef]
- Sadanov, A.K.; Aitkeldieva, S.A.; Faizulina, E.R.; Auezova, O.N.; Tatarkina, L.G. The Bacterial Strain Arthrobacter luteus 43-a, Used for Cleaning Soil and Water from Oil and Oil Products. Kazakhstan Patent No. 29148, 17 November 2014. Bull. No. 11. [Google Scholar]
- Sadanov, A.K.; Aitkeldieva, S.A.; Faizulina, E.R.; Auezova, O.N.; Tatarkina, L.G. The Bacterial Strain Arthrobacter sp. 12T, used for Cleaning Soil and Water from Oil and Oil Products. Kazakhstan Patent No. 29025, 15 October 2014. Bull. No. 10. Available online: https://kzpatents.com/3-ip29025-shtamm-bakterijj-arthrobacter-sp-12t-ispolzuemyjj-dlya-ochistki-pochvy-i-vody-ot-nefti-i-nefteproduktov.html (accessed on 6 February 2022).
- Sadanov, A.K.; Aitkeldieva, S.A.; Faizulina, E.R.; Auezova, O.N.; Tatarkina, L.G. The Bacterial Strain Arthrobacter sp. 15T, Used for Cleaning Soil and Water from Oil and Oil Products. Kazakhstan Patent No. 29026, 15 October 2014. Bull. No. 10. Available online: https://kzpatents.com/3-ip29026-shtamm-bakterijj-arthrobacter-sp-15t-ispolzuemyjj-dlya-ochistki-pochvy-i-vody-ot-nefti-i-nefteproduktov.html (accessed on 6 February 2022).
- Khasenova, E.Z.; Ayupova, A.Z.; Berdimuratova, K.T.; Sarsenova, A.S.; Bayakenov, D.A.; Nagyzbekkyzy, E.; Moldagulova, N.B. Development of a biological product “Kazbiorem” for cleaning the soil from oil and oil products. Int. J. Appl. Fund. Res. Ser. Biol. Sci. 2018, 9, 111–114. [Google Scholar]
- Sadanov, A.K.; Aitkeldieva, S.A.; Faizulina, E.R.; Auezova, O.N.; Tatarkina, L.G. The bacterial strain Dietzia maris 84T, used for cleaning soil and water from oil and oil products. Kazakhstan Patent No. 29027, 15 October 2014. Bull. No. 10. [Google Scholar]
- Sadanov, A.K.; Aitkeldieva, S.A.; Auezova, O.N.; Faizulina, E.R.; Kurmanbaev, A.A. Consortium of strains Acinetobacter calcoaceticus 2a and Microbacterium lacticum 41-3, Used to Clean the Soil from Oil and Oil Products. Kazakhstan Patent No. 22177, 15 January 2010. Bull. No. 1. [Google Scholar]
- Sadanov, A.K.; Aitkeldieva, S.A.; Gavrilova, N.N.; Ratnikova, I.A. A Preparation “Bakoil-KZ” to Clean Up Soils from Crude Oil and Petroleum Products. Kazakhstan Patent No. 24879, 15 October 2011. Bull. No. 11. [Google Scholar]
- Shigaeva, M.K.; Mukasheva, T.D.; Sydykbekova, R.K.; Berzhanova, R.Z. Method of Cleaning Soil from Oil and Oil Products. Kazakhstan Patent No. 19257, 15 April 2008. Bull. No. 4. [Google Scholar]
- Ramankulov, E.M.; Moldagulova, N.B.; Sarsenova, A.S.; Ayupova, A.Z. Biological Preparation for Cleaning Oil-Contaminated Soils. Kazakhstan Patent No. 29948, 15 June 2015. Bull. No. 6. [Google Scholar]
- Narmanova, R.A.; Filonov, A.E.; Appazov, N.O.; Puntus, I.F.; Akhmetov, L.I.; Funtikova, T.V.; Turmanov, R.A.; Omarov, E.A.; Bazarbaev, B.M. Association (Consortium) of Bacterial Strains for the Removal of Oil and Oil Products from Soils and Waters in the Extreme Continental Climate of the Republic of Kazakhstan. Kazakhstan Patent No. 33715, 18 June 2019. Bull. No. 25. [Google Scholar]
- Idrisova, U.R.; Idrisova, D.Z.; Musaldinov, T.B.; Auezova, O.N.; Ashykbaev, N.S.; Kabdenov, Z.M.; Aitkeldieva, S.A.; Sadanov, A.K. Study of the degree of purification of oil-contaminated soil under the influence of zeolite and active oil-oxidizing microorganisms. Microbiol. Virol. 2014, 1, 46–50. [Google Scholar]
- Faizulina, E.R.; Aitkeldieva, S.A.; Tatarkina, L.G.; Alimzhanova, M.B.; Alimbetova, A.V.; Auezova, O.N.; Spankulova, G.A. Creation of consortia of hydrocarbon-oxidizing microorganisms that effectively degrade xylene isomers. Bull. Kazakh. Nation Univ. Ecol. Ser. 2020, 3, 35–44. [Google Scholar]
- Zhubanova, A.A.; Ernazarova, A.K.; Kayyrmanova, G.K.; Zayadan, B.K.; Savitskaya, I.S.; Abdieva, G.Z.; Kistaubaeva, A.S.; Akimbekov, N.S. Construction of a cyano-bacterial consortium based on axenic cultures of cyanobacteria and heterotrophic bacteria for bioremediation of oil-contaminated soils and water bodies. Plant Physiol. 2013, 60, 588–595. [Google Scholar]
- Bishimbaev, V.K.; Issaeva, A.U.; Uspabayeva, A.A.; Makulbekova, M.M.; Sulima, V.O. Method of Cleaning Soil from Oil and Oil Products Pollution. Kazakhstan Patent No. 25271, 15 December 2011. Bull. No. 12. [Google Scholar]
- Mukhamedova, N.S.; Islambekuly, B.; Idrisova, D.T.; Tapalova, A.S.; Zhumadilova, Z.S.; Appazov, N.O.; Shorabaev, E.Z. Study of the destruction of oil during the treatment of oil-contaminated soil with organic-mineral fertilizers. Izv. NAN Repub. Kazakhstan Chem. Technol. Ser. 2014, 4, 39–43. [Google Scholar]
- Idrisova, D.T.; Mukhamedova, N.S.; Zhumadilova, Z.S.; Abdieva, K.M.; Shorabaev, E.Z.; Sadanov, A.K. Investigation of the processes of bioremediation of soils with different degrees of oil pollution in the Kyzylorda region in field conditions. Fundam. Stud. 2014, 12, 1669–1671. [Google Scholar]
- Idrisova, D.T.; Mukhamedova, N.S.; Zhumadilova, Z.S.; Shorabaev, E.Z.; Sadanov, A.K. Influence of organo-mineral fertilizers on bioremediation of oil-contaminated soils. Fundam Stud. 2014, 9, 2246–2249. [Google Scholar]
- Idrisova, D.T.; Mukhamedova, N.S.; Zhusupova, B.K.; Zhumadilova, Z.S.; Shorabaev, E.Z. Study of the influence of organomineral fertilizers on the cleaning of soils with different degrees of oil pollution in laboratory conditions. Mod. Probl. Sci. Educ. 2014, 6, 1421. Available online: http://www.science-education.ru/120-r16564 (accessed on 6 February 2022).
- Kurmanbaev, A.A.; Baigonusova, Z.A.; Aitkeldieva, S.A. Biological activity of soils with different degrees of oil pollution. In Proceedings of the International Scientific-Practical Conference “The Current Ecological State of the Aral Sea Region, the Prospects for Solving Problems”, Kyzylorda, Kazakhstan, 7 October 2011; Korkyt Ata Kyzylorda University Publishing: Kyzylorda, Kazakhstan, 2011; pp. 46–51. [Google Scholar]
- Uspabayeva, A.A.; Issaeva, A.U.; Ilyaletdinov, A.N. Participation of hydrocarbon-oxidizing bacteria in the removal of hydrocarbons in the soil of Southern Kazakhstan. News Nation Acad. Sci. Repub. Kazakhstan Biol. Ser. 2004, 6, 81–87. [Google Scholar]
- Bishimbaev, V.K.; Issaeva, A.U.; Uspabayeva, A.A.; Rysbaeva, G.A. Distribution of oil-degrading microorganisms in the soils of Southern Kazakhstan. In Proceedings of the International Scientific and Practical Conference “Valikhanov Readings-9”, Kokchetau, Kazakhstan, 22–24 April 2004; Sh. Valikhanov Kokchetau State University Publishing: Kokchetau, Kazakhstan, 2004; pp. 54–55. [Google Scholar]
- Issaeva, A.U.; Bishimbaev, V.K.; Pirmanova, M.; Makhanova, U. Influence of soil pollution with oil products on the formation of artificial phytocenoses. Poisk 2003, 2, 117–120. [Google Scholar]
- Idrisova, U.R.; Musaldinov, T.B.; Auezova, O.N.; Myrzadauleutova, I.T.; Kurmanbaev, A.A.; Aitkeldieva, S.A.; Idrisova, D.Z.; Sadanov, A.K. Determination of the optimal dose and fraction of zeolite to increase the activity of the microflora of oil-contaminated soil. Microbiol. Virol. 2013, 1–2, 21–25. [Google Scholar]
- Idrisova, U.R.; Sadanov, A.K.; Musaldinov, T.B.; Idrisova, D.Z.; Aytkeldieva, S.A.; Auezova, O.N. The method of biological recultivation for the soil spilled with crude oil and petroleum products. Kazakhstan Patent No. 30176, 15 July 2015. Bull. No. 7. [Google Scholar]
- Bishimbaev, V.K.; Issaeva, A.U.; Issaev, E.B.; Baizhanova, D.N.; Uspabayeva, A.A. Flora of oil-contaminated soils of South Kazakhstan. News Nation Acad. Sci. Repub. Kazakhstan 2003, 3, 33–39. [Google Scholar]
- Oleinikova, E.A.; Kuznetsova, T.V.; Raiymbekova, L.T.; Aitkeldieva, S.A.; Kurmanbaev, A.A.; Faizulina, E.R. Evaluation of the effectiveness of stimulation of soil microflora and self-purification of oil-contaminated soils in model experiments with composting in piles. In Proceedings of the International Scientific-Practical, Conference Dedicated to the 100th Anniversary of Acad. Kh. Zh. Zhumatov “Actual problems of microbiology and virology”, Almaty, Kazakhstan, 12 October 2012; pp. 139–141. [Google Scholar]
- Oleinikova, E.A.; Kuznetsova, T.V.; Kulnazarov, B.A.; Kurmanbaev, A.A.; Aitkeldieva, S.A. Evaluation of the effectiveness of stimulation of soil microflora and remediation of oil-contaminated soil by composting in the conditions of the Kyzylorda region. Bull. Nation Acad. Sci. Repub. Kazakhstan Biol. Med. Ser. 2013, 5, 42–45. [Google Scholar]
- Kireeva, N.A.; Onegova, T.S.; Grigoriadi, A.S. Characteristics of Belvitamil used for the remediation of oil-contaminated natural objects. Bull. Bashkir Univ. 2008, 13, 279–281. [Google Scholar]
- Ismailov, N.M. Microbiology and enzymatic activity of oil-contaminated soils. In Restoration of Oil-Contaminated Soil Ecosystems; Glazovskaya, M.A., Ed.; Nauka: Moscow, Russia, 1988; pp. 42–57. [Google Scholar]
- Galiulin, R.V.; Galiulina, R.A.; Bashkin, V.N.; Akopova, G.S.; Listov, E.L.; Balakirev, I.V. Comparative assessment of the decomposition of hydrocarbons of gas condensate and oil in the soil under the influence of biological agents. Agrochemistry 2010, 10, 52–58. [Google Scholar]
- Novoselova, E.I.; Kireeva, N.A. Enzymatic activity of soils under pollution and its biodiagnostic importance. Theor. Appl. Ecol. 2009, 2, 4–12. [Google Scholar]
- Kireeva, N.A.; Novoselova, E.I.; Khaziev, F.K. The use of activated sludge in soil recultivation. Soil Sci. 1996, 11, 1399–1403. [Google Scholar]
- Medvedeva, E.I. Biological Activity of Oil-Contaminated Soils in the Conditions of the Middle-Volga Region. Ph.D. Thesis, Nayanova Samara Municipal University, Samara, Russia, 2002; 211p. [Google Scholar]
- Skujins, J. History of abiotic soil enzyme research. In Soil Enzymes; Burns, R.G., Ed.; Academic Press Inc.: New York, NY, USA, 1978; pp. 1–19. [Google Scholar]
- Frankenberger, W.T.; Johanson, J.B. Influence of crude oil and refined petroleum products on soil dehydrogenase activity. J. Environ. Qual. 1982, 4, 602–607. [Google Scholar] [CrossRef]
- Ismailov, N.M.; Gadzhiev, V.I.; Gasanov, M.G. Hydrocarbon mineralization coefficient as an indicator of the self-purification ability of oil-contaminated soils and the effectiveness of the applied methods of their remediation. Bull. Azerbaijan SSR Ser. Biol. Sci. 1984, 6, 76–85. [Google Scholar]
- Kireeva, N.A.; Vodopyanov, V.V.; Miftakhova, A.M. Biological Activity of Oil-Contaminated Soils; Gilem: Ufa, Russia, 2001; 376p. [Google Scholar]
- Kireeva, N.A.; Novoselova, E.I.; Khaziev, F.K. Enzymes of nitrogen metabolism in oil-contaminated soils. Biol. Bull. 1997, 24, 625–628. [Google Scholar]
- Khabirov, I.K.; Gabbasova, I.M.; Khaziev, F.K. Stability of Soil Processes; Bashkir State Agrarian University Publishing: Ufa, Russia, 2001; 327p. [Google Scholar]
- Gabbasova, I.M. Degradation and Remediation of Soils in the Southern Urals. Ph.D. Thesis, Institute of Biology, Ufa Scientific Centre, Ufa, Russia, 2001; 453p. [Google Scholar]
- Chukparova, A.U. Microbiological state and enzymatic activity of oil-contaminated soils. Bull. Karaganda State Univ. Ser. Biol. Med. Geog. 2011, 1, 25–31. [Google Scholar]
- Shkidchenko, A.N.; Arinbasarov, M.U. Study of the oil-degrading activity of Caspian shore microflora. Appl. Biochem. Microbiol. 2002, 38, 433–436. [Google Scholar] [CrossRef]
- Filonov, A.; Akhmetov, L.; Puntus, I.; Solyanikova, I. Removal of oil spills in temperate and cold climates of Russia: Experience in the creation and use of biopreparations based on effective microbial consortia In Biodegradation, Pollutants and Bioremediation Principles; Bidoia, E.D., Motagnolli, R.N., Eds.; Taylor’s & Francis CRC Press: Boca Raton, FL, USA, 2021; pp. 137–159. [Google Scholar]
- Miko-Oil—A Biopreparation for Oil-Spilled Soils Clean-Up [Miko-Oil—Biopreparat Dlya Ochistki Pochv ot Neftyanykh Zagriazneniy]. Available online: https://web.archive.org/web/20211115124503/http://kazecosolutions.kz/ru/content/biopreparat-miko-oyl (accessed on 15 November 2021).
- Shigaeva, M.K.; Mukasheva, T.D.; Sydykbekova, R.K.; Berzhanova, R.Z. Biopreparation for Cleaning Oil-Contaminated Ecosystems from Oil. Kazakhstan Patent No. 19425, 15 May 2008. Bull. No. 5. [Google Scholar]
- Bishimbaev, V.K.; Issaeva, A.U.; Uspabayeva, A.A.; Rysbaeva, G.A.; Saparbekova, A.A.; Ilyaletdinov, A.N.; Emberdiev, A.Z.; Zharkimbekov, S.U.; Manapova, N.M. Consortium of Microorganisms “Peroil” Used to Clean Water and Soil from Oil and Oil Pollution. Kazakhstan Patent No. 14923, 15 October 2004. Bull. No. 10. [Google Scholar]
- Myrhalykov, Z.U.; Issaeva, A.U.; Uspabaeva, A.A. Method for Cleaning Soil from Oil and Oil Products. Kazakhstan Patent No. 18192, 17 November 2014. Bull. No. 11. [Google Scholar]
- Khasenova, E.Z.; Sharipova, G.Z.; Moldagulova, N.B. Characteristics of the active psychrotrophic strain-oil destructor Rhodococcus erythropolis DH1. In Proceedings of the Materials of the 18th International Pushchino School-Conference of Young Scientists “Biology—The Leading Science of the XXI Century”, Pushchino, Russia, 21–25 April 2014; pp. 49–50. [Google Scholar]
- Sirenko, L.A.; Sakevich, A.I.; Osipov, L.F.; Lukina, L.F.; Kuzmenko, M.I.; Kozitskaya, V.N.; Velichko, I.M.; Myslovich, V.O.; Gavrilenko, M.Y.; Arendarchuk, V.V.; et al. Methods of Physiological and Biochemical Research of Algae in Hydrobiological Practice; Naukova Dumka: Kiev, Ukraine, 1975; 247p. [Google Scholar]
- The Ten Largest Oil Deposits in the World. Available online: https://www.businessinsider.com/the-ten-largest-oil-deposits-in-the-world-2011-9 (accessed on 6 February 2022).
- Delegan, Y.; Vetrova, A.; Titok, M.; Filonov, A. Development of thermotolerant bacterial consortium, the basis for biopreparation for remediation of petroleum-contaminated soils and waters in hot climates. Appl. Biochem. Microbiol. 2016, 52, 828–836. [Google Scholar] [CrossRef]
- Issaeva, A.; Uspabaeva, A.; Bishimbaev, V.; Issayev, E.; Sattarova, A. Bioremediation of oil-contaminated soils of South Kazakhstan. Mod. Appl. Sci. 2015, 9, 97–103. [Google Scholar] [CrossRef]
| Microbial Group | Genus, Species, Strain | Source | Reference |
|---|---|---|---|
| Prokaryotes (Bacteria) | |||
| Phylum Bacteroidetes | |||
| Sphingobacterium kitahiroshimense wkar54 | Caspian Sea | [21] | |
| Phylum Firmicutes, Class Bacilli | |||
| Bacillus amyloliquefaciens I-15 | soil/sewage | [26] | |
| B. cereus SBUG2056 | Caspian Sea | [21] | |
| B. aerius KB-36 | [27] | ||
| B. cereus IP-40-4 | |||
| B. cereus P1-40-8 | |||
| B. megaterium P1-35-2 | |||
| B. subtilis 72 | |||
| B. subtilis 109KC | soil | [28,29] | |
| Brevibacillus borstelensis P2-50-2 | soil | [30] | |
| B. borstelensis P2-50-5 | [27] | ||
| Phylum Actinobacteria | |||
| Arthrobacter luteus 43-A | soil | [31] | |
| Arthrobacter sp. 12T | [32] | ||
| Arthrobacter sp. 15T | [33] | ||
| Dietzia maris 12K | soil | [19] | |
| D. maris 22K | soil | [34] | |
| D. maris 84T | [35] | ||
| D. schimae 22K | soil | [19] | |
| Gordonia alkanivorans 25K | soil | [19] | |
| G. amicalis P1-35-14 | soil | [30] | |
| G. lacunae 15K | soil | [19] | |
| Microbacterium foliorum 29K | soil | [19] | |
| M. lacticum 41-3 | soil | [36] | |
| Micrococcus roseus 34 | soil | [37] | |
| M. roseus 40 | soil | [37] | |
| Mycobacterium thermoresistible sp. 119-3GM | [38] | ||
| Rhodococcus equi 51K | [38] | ||
| R. erythreus AT7 | soil | [34] | |
| R. erythropolis 7A | soil | [37] | |
| R. erythropolis 14K | soil | [19] | |
| R. erythropolis B12 | soil | [39] | |
| R. erythropolis DH-1 | soil | [26] | |
| R. erythropolis 119GM | soil | [29] | |
| Rhodococcus erythropolis KZ1 | soil | [40] | |
| R. erythropolis KZ2 | soil | [40] | |
| R. erythropolis SBUG2052 | Caspian Sea | [21] | |
| R. fascians K3 (similarity 99%) | soil | [30] | |
| R. globerulus 51KC | soil | [29] | |
| R. jialingiae 4/5 R. jialingiae 22PK | [27] | ||
| R. maris 65 | soil | [41] | |
| Phylum Proteobacteriae | |||
| α-proteobacteriae | Roseomonas sp. wkal24 | Caspian Sea | [21] |
| Ochrobactrum sp. wkal48 | Caspian Sea | [21] | |
| β-proteobacteriae | Achromobacter xylosoxidans P2-35-9 A. pestifer 25SH | [27] | |
| Achromobacter sp. wkar55 | Caspian Sea | [21] | |
| Tetrathiobacter mimigardefordensis (now Advenella mimigardefordensis) strains 24SH, 25SH, 26SH | Caspian Sea | [22] | |
| γ-proteobacteriae | Acinetobacter calcoaceticum 2A | soil | [36] |
| Azotobacter chroococcum | [41] | ||
| Enterobacter sp. 23SH | Caspian Sea | [42] | |
| Pseudomonas sp. 16-SH | Caspian Sea | [41] | |
| Pseudomonas aeruginosa 122AC | soil | [28,29] | |
| P. azotifigens 20K | soil | [19] | |
| P. putida KZ3 | soil | [40] | |
| P. xanthomarina 17K | soil | [19] | |
| P. stutzeri A1 | artificial pond | [43] | |
| Pseudomonas sp. N2 | |||
| P. alcaligenes A5 | |||
| Serratia marcescens N3K | soil/sewage | [26] | |
| Stenotrophomonas chelatiphaga wkal49, S. chelatiphaga wkal51 | Caspian Sea | [21] | |
| Eukaryotes | |||
| yeasts | Trichosporonoides sp. V1 | soil | [28,29] |
| Trichosporon cutaneum R20CO2 | |||
| T. jirovecii V2 | |||
| mold fungi | Penicillium sp. | [44] | |
| Mucor sp. | |||
| Endogone sp. | |||
| Alternaria sp. | |||
| Fusarium sp. | |||
| Order Dothideales | Aureobasidium pullulans P7 | soil | [29] |
| Deposit | Enzyme | Clean Control | Medium Contamination 0.5–0.9% | Heavy Contamination 2.8–3.4% |
|---|---|---|---|---|
| Suhltanat Balgimbaev | UA | 4.4 | 3.1 | 1.1 |
| Zhanatalap | 5.5 | 3.3 | 0.8 | |
| Teren-Uzek | 3.7 | 2.1 | 0 | |
| Suhltanat Balgimbaev | DHA | 0.4 | 0.7 | 1.2 |
| Zhanatalap | 0.4 | 0.5 | 0.6 | |
| Teren-Uzek | 0.2 | 0.6 | 0.8 | |
| Suhltanat Balgimbaev | CA | 12.9 | 11.3 | 10.6 |
| Zhanatalap | 12.7 | 12.4 | 10.7 | |
| Teren-Uzek | 10.6 | 11.0 | 7.8 |
| Experiments in the Soil at Akshabulak Oil Deposit | ||||||||
| Experiment Conditions | Enzyme | Before | After Four Months | |||||
| Clean Control | Oil Control 3% | Oil Control | Oil Fertilizers | |||||
| Small field trials using organic and mineral fertilizers [46] | UA | 0.66 | 0 | 0.155 | 0.2–0.9 | |||
| DHA | 0.131 | 0.085 | 0.214 | 0.6–0.8 | ||||
| Before | After three months | |||||||
| Clean control | Oil control | Oil control | Oil fertilizers | |||||
| 5% | 7% | 5% | 7% | 5% | 7% | |||
| Small field trials using organic and mineral fertilizers [47] | UA | 0.66 | - | - | 0.35 | 0.25 | 1.75 | 0.5 |
| DHA | 0.131 | - | - | 1.58 | 0.89 | 2.08 | 1.9 | |
| Experiments with the soil from K-Kurylys oil deposit | ||||||||
| Before | After two months | |||||||
| Clean control | Oil control 10% | Oil control 10% | Oil fertilizers | |||||
| Lab test At certain time intervals, loosening was carried out and the soil moisture was maintained at a level of 60% [54] | DHA | 1.25 | 0.48 | ~1 | ||||
| CA | 3.9 | 1.3 | 2.9–3.6 | |||||
| Before | After two months | |||||||
| Clean control | Oil control 10% | Oil control 10% | Oil fertilizers | |||||
| Small field trials using zeolite, vermicompost, nitroammophos, and “Bakoil-KZ” [54] | DHA | 1.25 | - | 1.38 | ~1 | |||
| CA | 3.9 | - | 4.7 | 3.1–4.2 | ||||
| Biopreparation | Strain | Form | Application (if Mentioned) | Organization, Reference |
|---|---|---|---|---|
| Consortium | A. calcoaceticum 2A M. lacticum 41-3 | to clean up soils from crude oil and petroleum products | Institute of Microbiology and virology [36,37] | |
| “Bakoil-KZ” | M. roseus 40 M. roseus 34 R. erythropolis 7A bentonite as sorbent | paste | to clean up soils from crude oil and petroleum products | Institute of Microbiology and virology [37] |
| “Miko-Oil” | eight strains bacteria B. subtilis 109KC P. aeruginosa 122AC R. erythropolis 11GM R. globerulus 51KC yeasts T. jirovecii V2 Trichosporonoides sp. V1 T. cutaneum R20CO2 A. pullulans R7 | paste |
| Al-Farabi Kazakh National University, KazEcoSolutions Inc. [28,29,74] |
| Consortium | Penicillium sp. Mucor sp. Endogone sp. Alternaria sp. Fusarium sp. | cell biomass, 108–109 cells/ml | soil | M.Auezov South-Kazakhstan State University [44] |
| Arthrobacter sp. 12T | to clean up soils and waters from crude oil and petroleum products 59.3–85.2% | Institute of Microbiology and virology [32] | ||
| Arthrobacter sp. 15T | to clean up soils and waters from crude oil and petroleum products by 58.4–84.8% | Institute of Microbiology and virology [33] | ||
| D. maris 84T | to clean up soils and waters from crude oil and petroleum products by 72.4–90.6% | Institute of Microbiology and virology [35] | ||
| A. luteus 43-A | to clean up soils and waters from crude oil and petroleum products by 56.4–80.1%. | Institute of Microbiology and virology [31] | ||
| Consortium | host cyanobacterium Phormidium sp. K11 symbiotic degrader bacteria P. stutzeri A1 Pseudomonas sp. N2 P. alcaligenes A5 | to clean up soils and waters from crude oil and petroleum products | Al-Farabi Kazakh National University [43] | |
| “Enoil” | R.erythropolis DN-1 B. amyloliquefaciens I-15 S. marcescens N3K | Republic State Enterprise “National Center of Biotechnology” [26] | ||
| R. erythropolis B12 | biomass in water glycerol 1% and sodium chloride (10%) solution | to clean up soil and water ecosystems | Republic State Enterprise “National Center of Biotechnology” [39] | |
| “Mikot-rikh” | M. thermoresistible 119-ZGM R. equi 51KT T. cutaneum R20CO2 | concentrated biomass | Al-Farabi Kazakh National University [38] | |
| concentrated paste | to clean up oil- and oil-product-spilled ecosystems and containers | Al-Farabi Kazakh National University [75] | ||
| Consortium | R. erythropolis KZ1 R. erythropolis KZ2 P. putida KZ3 | soil | Korkyt Ata Kyzylorda State University [40] | |
| “Peroil” | R. erythropolis DP 304 M. luteus B1Ag8G | lyophilizate | M.Auezov South Kazakhstan State University [76] | |
| lyophilizate and bentonite | [77] | |||
| “Kazbiorem” | R. erythreus AT7 D. maris 22K | Ecostandard.kz Ltd. [78] |
| Strain/Biopreparation | Test Place, Reference | Introduction Features | Pollutant, Concentration and Remediation Efficiency | |||||
|---|---|---|---|---|---|---|---|---|
| “Bakoil-KZ” | Kyzylorda Region [54] Small field trials on the landfill «K-Kurylys» plots 2 × 1 m (triple) periodic loosening and watering two months exposure | soil moisture 60% nitroammophos, biovermicompost, zeolite | Oil 10% | |||||
| decrease by 68.3–82.2% (control 18.3%) | ||||||||
| Atyrau Region [37] landfill of “West Dala” LLC
| first inoculation—10 kg with 600 L water second inoculation—5 kg with 600 L water
| decrease by 80% (control 18%) | ||||||
| “Bakoil-KZ” | Atyrau Region [29] oil deposits one month exposure | one inoculation | Oil | |||||
| “Bakoil-KZ”: decrease by 73% | ||||||||
| “Miko-Oil” | double inoculation | “Miko-Oil”: decrease by 99% | ||||||
| “Miko-Oil” | Mangystau Region [74] test plot on “Uzen” oil deposit plowing, loosening | double inoculation application of organic and mineral fertilizers | oil | |||||
| efficiency more than 93% | ||||||||
| “Miko-Oil” | Atyrau Region [74] Oil Producing Companies “DossorMunaiGas”, “Zhaikmunaigas”, “ZhylyoiMunaiGas», JSC “EmbaMunaiGas” territories (four hectares) and oil sludge | Oil sludges (20,000 tons) and fuel oily soils | ||||||
| Consortium of five molds | the city of Shymkent [44] trials on a territory of Research Institute of Problems in Biological Safety Bishimbaev ten plots of 1 m2 each, artificial black oil pollution. One month | ammophos 1% | Oil 10% | Fuel oil 10% | ||||
| Decrease by 75.1% | Decrease by 62.3% | |||||||
| the city of Shymkent [44] Oil chronically spilled soils on a territory of “PetroKazasktan Oil Products” Ltd. Total area of spills was 325 m2. Two weeks Plowing was made | ammophos 1% | ~Oil 5.4% | ||||||
| Decrease by 69.8% | ||||||||
| Bacterial-cyanobacterial consortium | Aktau Region [43] field trials on a storage landfill of “KhimPromService—Aktobe”
|
| fuel oil | |||||
| decrease by 80% | ||||||||
| “Enoil” | Zhambyl Region [26] a base of Eastern branch of “Fuel and Energy Complex—Kazakhstan”, the city of Tharaz two plots with chronic and emergency petroleum products spills; soil was loosened to a depth of 10–15 cm Soil was watered and mixed before and after biopreparation inoculation into the soil Four months | Concentrated suspension 1010 cells/mL was added into the soil until the final titer 106 cells/g soil biopreparation was started in vessels of 200 L each with tap water, mineral medium, and diesel fuel. Nitroammophos was added | Oil 0.2% | Oil 0.3% | ||||
| Decrease by 76.2% | Decrease by 82% | |||||||
| “Mikotrikh” | Site unknown [38] three plots of 6 m2 each and 0.5 m in depth with high content of oil products (diesel fuel and black oil), regular plowing three times every month | Bacterial and yeasts cultures were mixed in the ratio 1:1. Concentrated biomass was diluted in water and added together with nitroammophos and sawdust | Diesel fuel | Fuel oil | ||||
| 86% control by 17% | 37% | |||||||
| “Mikotrikh” | the city of Almaty [75] small scale field trials on chronically fuel oiled soils (spill depth 0.5 m) of locomotive and carriage depots, temperature 28–35 °C, no rains | Concentrated biomass of single strains was mixed in the ratio 1:1:1 before introduction | Fuel oil (10%) | |||||
| Decrease by 89.5% (control by 13.2) | ||||||||
| “Peroil” | Site unknown [51] plowing every month and regular watering six sites with regular and single pollutions: Total area of territories under recultivation was three hectares, period—1.5 months | soil moisture 50–60% 1% ammophos | Oil | Diesel fuel | Fuel oil | Sludge-like waste | ||
| 0.5% | 1.0% | 2.5% | 2.3% | >10% | 9.9% | |||
| Content after bioremediation | ||||||||
| 0.05% | 0.51% | 0.27% | 0.33% | 0.99% | 0.98% | |||
| “Peroil” | the city of Shymkent [77] Territory of JSC “PetroKazakhstan Oil Products”. Total area was 6 hectares, the soil volume was 25 tons bentonite as an immobilizer with water suspension of lyophilized form | Oil 5.4% | Fuel oil 4.7% | |||||
| content decreased by 66.2% in 15 days | content decreased by 73.1% in 20 days | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmetov, L.I.; Puntus, I.F.; Narmanova, R.A.; Appazov, N.O.; Funtikova, T.V.; Regepova, A.A.; Filonov, A.E. Recent Advances in Creating Biopreparations to Fight Oil Spills in Soil Ecosystems in Sharply Continental Climate of Republic of Kazakhstan. Processes 2022, 10, 549. https://doi.org/10.3390/pr10030549
Akhmetov LI, Puntus IF, Narmanova RA, Appazov NO, Funtikova TV, Regepova AA, Filonov AE. Recent Advances in Creating Biopreparations to Fight Oil Spills in Soil Ecosystems in Sharply Continental Climate of Republic of Kazakhstan. Processes. 2022; 10(3):549. https://doi.org/10.3390/pr10030549
Chicago/Turabian StyleAkhmetov, Lenar I., Irina F. Puntus, Roza A. Narmanova, Nurbol O. Appazov, Tatiana V. Funtikova, Ainur A. Regepova, and Andrey E. Filonov. 2022. "Recent Advances in Creating Biopreparations to Fight Oil Spills in Soil Ecosystems in Sharply Continental Climate of Republic of Kazakhstan" Processes 10, no. 3: 549. https://doi.org/10.3390/pr10030549
APA StyleAkhmetov, L. I., Puntus, I. F., Narmanova, R. A., Appazov, N. O., Funtikova, T. V., Regepova, A. A., & Filonov, A. E. (2022). Recent Advances in Creating Biopreparations to Fight Oil Spills in Soil Ecosystems in Sharply Continental Climate of Republic of Kazakhstan. Processes, 10(3), 549. https://doi.org/10.3390/pr10030549







