Phenolic Characterization and Bioactivity of Fennel Seed (Foeniculum vulgare Mill.) Extracts Isolated by Microwave-Assisted and Conventional Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Standards
2.3. Microbial Strains and Growth Conditions
2.4. Phenolics Extraction and Analysis
2.4.1. Microwave-Assisted Extraction (MAE)
2.4.2. Conventional Heat-Reflux Extraction (CE)
2.4.3. Total Phenolic Content (TPC)
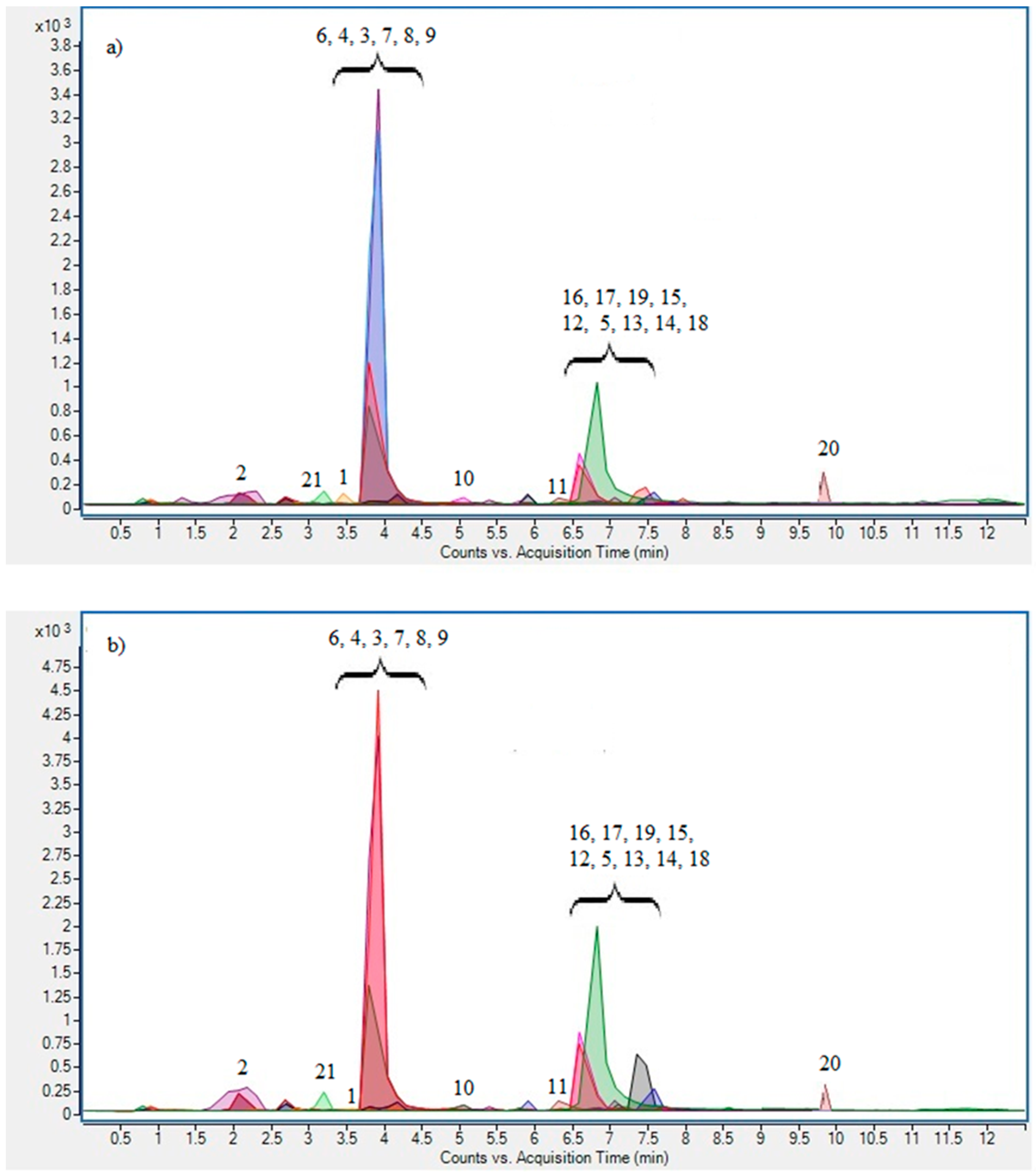
2.4.4. UPLC/ESI MS2 Analysis
2.4.5. Antioxidant Capacity (Oxygen Radical Antioxidant Capacity—ORAC)
2.5. Antimicrobial Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. MAE Optimization
3.2. Phenolic Characterization and Antioxidant Capacity of MAE and CE Extracts
3.3. Antimicrobial Activity of Fennel Seeds Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Akhtar, I.; Javad, S.; Ansari, M.; Ghaffar, N.; Tariq, A. Process optimization for microwave assisted extraction of Foeniculum vulgare Mill using response surface methodology. J. King Saud. Univ. 2020, 32, 1451–1458. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef] [Green Version]
- Balbino, S.; Repajić, M.; Obranović, M.; Medved, A.M.; Tonković, P.; Dragović-Uzelac, V. Characterization of lipid fraction of Apiaceae family seed spices: Impact of species and extraction method. J. Appl. Res. Med. Aromat. Plants 2021, 25, 100326. [Google Scholar] [CrossRef]
- Parejo, I.; Jauregui, O.; Sánchez-Rabaneda, F.; Viladomat, F.; Bastida, J.; Codina, C. Separation and characterization of phenolic compounds in fennel (Foeniculum vulgare) using liquid chromatography−negative electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2004, 52, 3679–3687. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Manousi, N.; Sarakatsianos, I.; Samanidou, V. Extraction techniques of phenolic compounds and other bioactive compounds from medicinal and aromatic plants. In Engineering Tools in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–314. [Google Scholar]
- Lovrić, V.; Putnik, P.; Bursać Kovačević, D.; Jukić, M.; Dragović-Uzelac, V. The Effect of Microwave-Assisted Extraction on the Phenolic Compounds and Antioxidant Capacity of Blackthorn Flowers. Food Technol. Biotechnol. 2017, 55, 243–250. [Google Scholar] [CrossRef]
- Ismail-Suhaimy, N.W.; Gani, S.S.A.; Zaidan, U.H.; Halmi, M.I.E.; Bawon, P. Optimizing conditions for microwave-assisted extraction of polyphenolic content and antioxidant activity of Barleria lupulina lindl. Plants 2021, 10, 682. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jiménez, A.; Garrigós, M.C. Optimization of microwave-assisted extraction of cocoa bean shell waste and evaluation of its antioxidant, physicochemical and functional properties. LWT 2020, 127, 109361. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Repajic, M.; Elez Garofulić, I.; Tuđen, L.; Dragović-Uzelac, V.; Levaj, B. Comparison of Different Extraction Methods for the Recovery of Olive Leaves Polyphenols. Processes 2020, 8, 1008. [Google Scholar] [CrossRef]
- Dobroslavić, E.; Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Polyphenolic Characterization and Antioxidant Capacity of Laurus nobilis L. Leaf Extracts Obtained by Green and Conventional Extraction Techniques. Processes 2021, 9, 1840. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Dragović-Uzelac, V.; Režek Jambrak, A.; Jukić, M. The effect of microwave assisted extraction on the isolation of anthocyanins and phenolic acids from sour cherry Marasca (Prunus cerasus var. Marasca). J. Food Eng. 2013, 117, 437–442. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Kruk, V.; Martić, A.; Martić, I.; Zorić, Z.; Pedisić, S.; Dragović, S.; Dragović-Uzelac, V. Evaluation of Polyphenolic Profile and Antioxidant Activity of Pistacia lentiscus L. Leaves and Fruit Extract Obtained by Optimized Microwave-Assisted Extraction. Foods 2020, 9, 1556. [Google Scholar] [CrossRef] [PubMed]
- Elez Garofulić, I.; Malin, V.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Smole Možina, S.; Dragović-Uzelac, V. Phenolic Profile, Antioxidant Capacity and Antimicrobial Activity of Nettle Leaves Extracts Obtained by Advanced Extraction Techniques. Molecules 2021, 26, 6153. [Google Scholar] [CrossRef] [PubMed]
- Dragović-Uzelac, V.; Garofulić, I.E.; Jukić, M.; Penić, M.; Dent, M. The influence of microwave-assisted extraction on the isolation of sage (Salvia officinalis L.) Polyphenols. Food Technol. Biotechnol. 2012, 50, 377–383. [Google Scholar]
- Rezaei, S.; Ebadi, M.-T.; Ghobadian, B.; Ghomi, H. Optimization of DBD-Plasma assisted hydro-distillation for essential oil extraction of fennel (Foeniculum vulgare Mill.) seed and spearmint (Mentha spicata L.) leaf. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100300. [Google Scholar] [CrossRef]
- Koşar, M.; Özek, T.; Kürkçüoglu, M.; Başer, K.H.C. Comparison of microwave-assisted hydrodistillation and hydrodistillation methods for the fruit essential oils of Foeniculum vulgare. J. Essent. Oil Res. 2007, 19, 426–429. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Salgado, J.M.; Domínguez, J.M.; Cortés-Diéguez, S. Characterization of fennel extracts and quantification of estragole: Optimization and comparison of accelerated solvent extraction and Soxhlet techniques. Ind. Crops Prod. 2014, 52, 528–536. [Google Scholar] [CrossRef]
- Boudraa, H.; Kadri, N.; Mouni, L.; Madani, K. Microwave-assisted hydrodistillation of essential oil from fennel seeds: Optimization using Plackett–Burman design and response surface methodology. J. Appl. Res. Med. Aromat. Plants 2021, 23, 100307. [Google Scholar] [CrossRef]
- Mirdehghan Ashkezari, S.M.; Bahmanyar, H.; Azizpour, H.; Mohammadi, M.; Najafipour, I. Investigation of Operating Parameters on Ultrasound-Assisted Extraction of Anethole in Fennel Essential oil. J. Chem. Pet. Eng. 2021, 55, 339–351. [Google Scholar]
- Mokhtari, L.; Ghoreishi, S.M. Supercritical carbon dioxide extraction of trans-anethole from Foeniculum vulgare (fennel) seeds: Optimization of operating conditions through response surface methodology and genetic algorithm. J. CO2 Util. 2019, 30, 1–10. [Google Scholar] [CrossRef]
- Hatami, T.; Johner, J.C.F.; Meireles, M.A.A. Extraction and fractionation of fennel using supercritical fluid extraction assisted by cold pressing. Ind. Crops Prod. 2018, 123, 661–666. [Google Scholar] [CrossRef]
- Hatami, T.; Johner, J.C.F.; Meireles, M.A.A. Investigating the effects of grinding time and grinding load on content of terpenes in extract from fennel obtained by supercritical fluid extraction. Ind. Crops Prod. 2017, 109, 85–91. [Google Scholar] [CrossRef]
- Shortle, E.; O’grady, M.N.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J.P. Influence of extraction technique on the anti-oxidative potential of hawthorn (Crataegus monogyna) extracts in bovine muscle homogenates. Meat Sci. 2014, 98, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Brnčić, M.; Dragović-Uzelac, V. UPLC-MS2 Profiling of Blackthorn Flower Polyphenols Isolated by Ultrasound-Assisted Extraction. J. Food Sci. 2018, 83, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef]
- Zhao, C.N.; Zhang, J.J.; Li, Y.; Meng, X.; Li, H. Bin Microwave-assisted extraction of phenolic compounds from melastoma sanguineum fruit: Optimization and identification. Molecules 2018, 23, 2498. [Google Scholar] [CrossRef] [Green Version]
- Dent, M.; Dragovic-Uzelac, V.; Garofulic, I.E.; Bosiljkov, T.; Ježek, D.; Brncic, M. Comparison of conventional and ultrasound-assisted extraction techniques on mass fraction of phenolic compounds from sage (Salvia officinalis L.). Chem. Biochem. Eng. Q. 2015, 29, 475–484. [Google Scholar] [CrossRef]
- Dent, M.; Dragović-Uzelac, V.; Penić, M.; Bosiljkov, T.; Levaj, B. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage (Salvia officinalis L.) extracts. Food Technol. Biotechnol. 2013, 51, 84–91. [Google Scholar]
- Kaderides, K.; Papaoikonomou, L.; Serafim, M.; Goula, A.M. Microwave-assisted extraction of phenolics from pomegranate peels: Optimization, kinetics, and comparison with ultrasounds extraction. Chem. Eng. Process. Process Intensif. 2019, 137, 1–11. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- Repajić, M.; Ekić, S.; Kruk, V.; Dragović-Uzelac, V. Effect of accelerated solvent extraction conditions on the isolation of bioactive compounds from fennel (Foeniculum vulgare Mill.) seeds. Hrvat. Časopis Prehrambenu Tehnol. Biotehnol. Nutr. 2020, 15, 102–106. [Google Scholar] [CrossRef]
- Anwar, F.; Ali, M.; Hussain, A.I.; Shahid, M. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare Mill.) seeds from Pakistan. Flavour Fragr. J. 2009, 24, 170–176. [Google Scholar] [CrossRef]
- Salami, M.; Rahimmalek, M.; Ehtemam, M.H.; Sabzalian, M.R. Variation in bioactive contents and anatomical characteristics of different fennel (Foeniculum vulgare Mill.) populations as affected by self-pollination. J. Appl. Bot. Food Qual. 2016, 89, 38–48. [Google Scholar]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; Núñez, M.J.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Rocha-Guzmán, N.E.; Herzog, A.; González-Laredo, R.F.; Ibarra-Pérez, F.J.; Zambrano-Galván, G.; Gallegos-Infante, J.A. Antioxidant and antimutagenic activity of phenolic compounds in three different colour groups of common bean cultivars (Phaseolus vulgaris). Food Chem. 2007, 103, 521–527. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Angelov, G.; Boyadzhieva, S. Extraction of fennel (Foeniculum vulgare) seeds: Process optimization and antioxidant capacity of the extracts. Chem. Biochem. Eng. Q. 2016, 30, 245–253. [Google Scholar] [CrossRef]
- Repajić, M.; Cegledi, E.; Kruk, V.; Pedisić, S.; Çinar, F.; Kovačević, D.B.; Žutić, I.; Dragović-Uzelac, V. Accelerated solvent extraction as a green tool for the recovery of polyphenols and pigments fromwild nettle leaves. Processes 2020, 8, 803. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ali, H.A.; Zain, N.M. Microwave-assisted extraction of phenolic compounds from Carica papaya leaves: An optimization study and LC-QTOF-MS analysis. Futur. Foods 2021, 3, 100035. [Google Scholar] [CrossRef]
- Upadhyay, R.; Ramalakshmi, K.; Rao, L.J.M. Microwave-assisted extraction of chlorogenic acids from green coffee beans. Food Chem. 2012, 130, 184–188. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, X.; Xu, H.; Gong, Y.; Yuan, F.; Gao, Y. On-line HPLC-ABTS screening and HPLC-DAD-MS/MS identification of free radical scavengers in Gardenia (Gardenia jasminoides Ellis) fruit extracts. Food Chem. 2010, 123, 521–528. [Google Scholar] [CrossRef]
- Zorić, Z.; Repajić, M.; Kruk, V.; Levaj, B.; Kovačević, D.B.; Jurčević, I.L.; Dragović-Uzelac, V. UPLC-MS/MS characterization of phenolic constituents in fennel seed extracts (Foeniculum vulgare Mill.). Proc. Nutr. Soc. 2020, 79. [Google Scholar] [CrossRef]
- Leal, P.F.; Almeida, T.S.; Prado, G.H.C.; Prado, J.M.; Meireles, M.A.A. Extraction kinetics and anethole content of fennel (Foeniculum vulgare) and anise seed (Pimpinella anisum) extracts obtained by soxhlet, ultrasound, percolation, centrifugation, and steam distillation. Sep. Sci. Technol. 2011, 46, 1848–1856. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- El-Hamidi, M.; Zaher, F.A.; El-Shami, S.M. Interaction of oilseed pigments and phospholipids in the determination of total phenolic compounds using the Folin-Ciocalteu reagent. Int. J. PharmTech Res. 2016, 9, 207–214. [Google Scholar]
- Prior, R.L. Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
- Moser, B.R.; Zheljazkov, V.D.; Bakota, E.L.; Evangelista, R.L.; Gawde, A.; Cantrell, C.L.; Winkler-Moser, J.K.; Hristov, A.N.; Astatkie, T.; Jeliazkova, E. Method for obtaining three products with different properties from fennel (Foeniculum vulgare) seed. Ind. Crops Prod. 2014, 60, 335–342. [Google Scholar] [CrossRef]
- Pacifico, S.; Galasso, S.; Piccolella, S.; Kretschmer, N.; Pan, S.-P.; Nocera, P.; Lettieri, A.; Bauer, R.; Monaco, P. Winter wild fennel leaves as a source of anti-inflammatory and antioxidant polyphenols. Arab. J. Chem. 2018, 11, 513–524. [Google Scholar] [CrossRef] [Green Version]
- Haytowitz, D.B.; Bhagwat, S. USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2; U.S. Department of Agriculture: New York, NY, USA, 2010; 3, pp. 10–48.
- Simoes, M.; Bennett, R.N.; Rosa, E.A.S. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 2009, 26, 746–757. [Google Scholar] [CrossRef]
- Huynh, N.T.; Smagghe, G.; Gonzales, G.B.; Van Camp, J.; Raes, K. Bioconversion of Kaempferol and Quercetin Glucosides from Plant Sources Using Rhizopus spp. Fermentation 2018, 4, 102. [Google Scholar] [CrossRef] [Green Version]
- Sterniša, M.; Bucar, F.; Kunert, O.; Možina, S.S. Targeting fish spoilers Pseudomonas and Shewanella with oregano and nettle extracts. Int. J. Food Microbiol. 2020, 328, 108664. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G.B.; Pendland, S.L.; Stoia, A.; Hamill, F.A.; Fabricant, D.; Dietz, B.M.; Chadwick, L.R. In vitro susceptibility of Helicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phyther. Res. An Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2005, 19, 988–991. [Google Scholar]
| Solvent | Temperature, °C | Microwave Power, W | Time, min | TPC, mg GAE/100 g |
|---|---|---|---|---|
| Water | 40 | 300 | 5 | 511.6 ± 12.8 |
| 10 | 381.2 ± 5.0 | |||
| 600 | 5 | 431.2 ± 10.6 | ||
| 10 | 455.0 ± 8.2 | |||
| 60 | 300 | 5 | 482.4 ± 18.7 | |
| 10 | 478.9 ± 9.4 | |||
| 600 | 5 | 508.0 ± 7.0 | ||
| 10 | 491.1 ± 20.5 | |||
| 80 | 300 | 5 | 605.2 ± 13.4 | |
| 10 | 486.5 ± 18.8 | |||
| 600 | 5 | 599.3 ± 1.0 | ||
| 10 | 465.1 ± 3.9 | |||
| Ethanol, 30% (v/v) | 40 | 300 | 5 | 316.4 ± 9.1 |
| 10 | 295.7 ± 0.9 | |||
| 600 | 5 | 305.3 ± 9.2 | ||
| 10 | 292.9 ± 3.9 | |||
| 60 | 300 | 5 | 267.2 ± 10.7 | |
| 10 | 252.8 ± 7.6 | |||
| 600 | 5 | 237.3 ± 10.5 | ||
| 10 | 372.2 ± 6.6 | |||
| 80 | 300 | 5 | 610.9 ± 8.1 | |
| 10 | 544.7 ± 4.8 | |||
| 600 | 5 | 513.4 ± 12.5 | ||
| 10 | 533.4 ± 1.9 | |||
| Acetone, 30% (v/v) | 40 | 300 | 5 | 541.1 ± 24.0 |
| 10 | 524.7 ± 2.8 | |||
| 600 | 5 | 525.8 ± 7.7 | ||
| 10 | 644.7 ± 15.1 | |||
| 60 | 300 | 5 | 676.9 ± 14.1 | |
| 10 | 553.5 ± 18.4 | |||
| 600 | 5 | 547.5 ± 16.8 | ||
| 10 | 706.2 ± 19.0 | |||
| 80 | 300 | 5 | 608.2 ± 4.9 | |
| 10 | 667.0 ± 27.2 | |||
| 600 | 5 | 565.3 ± 14.5 | ||
| 10 | 534.5 ± 1.0 |
| MAE Parameters | TPC, mg GAE/100 g |
|---|---|
| Solvent | p < 0.01 * |
| Water | 491.3 ± 12.7 a |
| Ethanol (30%, v/v) | 378.5 ± 26.6 a |
| Acetone (30%, v/v) | 591.3 ± 13.4 b |
| Temperature, °C | p < 0.01 * |
| 40 | 435.5 ± 23.5 a |
| 60 | 464.5 ± 31.1 a |
| 80 | 561.1 ± 11.1 b |
| Microwave power, W | p = 0.64 |
| 300 | 489.1 ± 22.1 a |
| 600 | 484.9 ± 20.1 a |
| Irradiation time, min | p = 0.49 |
| 5 | 491.8 ± 21.3 a |
| 10 | 482.2 ± 20.9 a |
| Solvent × temperature, °C | |
| p < 0.01 * | |
| Water × 40 | 444.7 ± 17.9 ab |
| Ethanol (30%, v/v) × 40 | 302.5 ± 3.9 a |
| Acetone (30%, v/v) × 40 | 559.1 ± 19.2 b |
| p < 0.01 * | |
| Water × 60 | 490.1 ± 5.9 ab |
| Ethanol (30%, v/v) × 60 | 282.4 ± 20.2 a |
| Acetone (30%, v/v) × 60 | 621.0 ± 27.3 b |
| p = 0.17 | |
| Water × 80 | 539.0 ± 24.3 a |
| Ethanol (30%, v/v) × 80 | 550.6 ± 14.0 a |
| Acetone (30%, v/v) × 80 | 593.7 ± 19.3 a |
| Solvent × microwave power, W | |
| p < 0.01 * | |
| Water × 300 | 490.9 ± 20.0 a |
| Ethanol (30%, v/v) × 300 | 381.3 ± 42.8 a |
| Acetone (30%, v/v) × 300 | 595.2 ± 18.5 b |
| p < 0.01 * | |
| Water × 600 | 491.6 ± 16.5 a |
| Ethanol (30%, v/v) × 600 | 375.7 ± 33.7 a |
| Acetone (30%, v/v) × 600 | 587.3 ± 20.1 b |
| Solvent × irradiation time, min | |
| p < 0.01 * | |
| Water × 5 | 522.9 ± 18.8 ab |
| Ethanol (30%, v/v) × 5 | 375.1 ± 41.6 a |
| Acetone (30%, v/v) × 5 | 577.5 ± 15.8 b |
| p < 0.01 * | |
| Water × 10 | 459.6 ± 11.5 a |
| Ethanol (30%, v/v) × 10 | 381.9 ± 35.2 a |
| Acetone (30%, v/v) × 10 | 605.1 ± 21.5 b |
| Temperature, °C × microwave power, W | |
| p < 0.01 * | |
| 40 × 300 | 428.4 ± 30.6 a |
| 60 × 300 | 451.9 ± 45.5 a |
| 80 × 300 | 587.1 ± 17.5 b |
| p = 0.08 | |
| 40 × 600 | 442.5 ± 36.8 a |
| 60 × 600 | 477.0 ± 44.0 a |
| 80 × 600 | 535.1 ± 12.7 a |
| Temperature, °C × irradiation time, min | |
| p < 0.01 * | |
| 40 × 5 | 438.6 ± 29.3 a |
| 60 × 5 | 453.2 ± 46.8 a |
| 80 × 5 | 583.7 ± 10.8 b |
| p = 0.11 | |
| 40 × 10 | 432.3 ± 38.0 a |
| 60 × 10 | 475.8 ± 42.7 a |
| 80 × 10 | 538.5 ± 19.6 a |
| Microwave power, W × irradiation time, min | |
| p = 0.42 | |
| 300 × 5 | 465.0 ± 30.3 a |
| 600 × 5 | 499.4 ± 29.0 a |
| p = 0.16 | |
| 300 × 10 | 513.3 ± 31.9 a |
| 600 × 10 | 470.3 ± 28.2 a |
| Precursor Ion m/z | Product Ion m/z | MAE (mg/100 g) | CE (mg/100 g) | ||
|---|---|---|---|---|---|
| Benzoic acids | |||||
| 1 | p-hydroxybenzoic acid-O-glucoside | 299 | 137 | 19.0 ± 0.2 | 17.0 ± 0.2 |
| Cinnamic acids | |||||
| 2 | Rosmarinic acid * | 359 | 197, 179 | 7.9 ± 0.1 | 18.3 ± 0.2 |
| 3 | Neochlorogenic acid | 353 | 191, 179 | 25.4 ± 0.6 | 78.8 ± 2.1 |
| 4 | Chlorogenic acid * | 353 | 191, 179 | 60.4 ± 1.2 | 81.6 ± 2.1 |
| 5 | Dicaffeoylquinic acid derivate | 515 | 353 | 20.5 ± 0.2 | 60.3 ± 1.8 |
| 6 | Caffeoylquinic acid derivate 1 | 353 | 191 | 20.0± 0.2 | 31.6 ± 1.2 |
| 7 | Caffeoylquinic acid derivate 2 | 353 | 191 | 60.2 ± 1.4 | 83.8 ± 2.4 |
| 8 | Feruloylquinic acid derivate 1 | 367 | 193 | 1.4 ± 0.0 | 3.8 ± 0.1 |
| 9 | Feruloylquinic acid derivate 2 | 367 | 193 | 6.8 ± 0.1 | 10.9 ± 0.2 |
| 10 | p-coumaroylquinic acid derivate | 337 | 191 | 4.6 ± 0.2 | 6.0 ± 0.2 |
| Flavonols | |||||
| 11 | Rutin * | 611 | 303 | 25.0 ± 0.6 | 54.3 ± 1.3 |
| 12 | Kaempferol-3-rutinoside * | 595 | 287 | 27.4 ± 0.8 | 53.3 ± 1.3 |
| 13 | Isorhamnetin hexoside 1 | 479 | 317 | 2.2 ± 0.1 | 3.4 ± 0.1 |
| 14 | Isorhamnetin hexoside 2 | 479 | 317 | 2.7 ± 0.1 | 4.0 ± 0.1 |
| 15 | Quercetin glucuronide | 479 | 303 | 52.4 ± 1.2 | 103.0 ± 4.6 |
| 16 | Quercetin hexoside | 465 | 303 | 14.5 ± 0.2 | 32.4 ± 0.7 |
| 17 | Quercetin-3-glucoside * | 465 | 303 | 18.0 ± 0.2 | 37.5 ± 1.2 |
| 18 | Kaempferol glucuronide | 463 | 287 | 72.1 ± 1.8 | 155.6 ± 7.5 |
| 19 | Kaempferol hexoside | 449 | 287 | 12.3± 0.1 | 22.2 ± 0.7 |
| Flavones | |||||
| 20 | Apigenin glucuronide | 447 | 271 | 8.4 ± 0.1 | 6.0 ± 0.1 |
| Coumarins | |||||
| 21 | Esculetin hexoside | 339 | 177 | 3.8 ± 0.1 | 6.8 ± 0.2 |
| Total UPLC/ESI- MS2 identified compounds (mg/100 g) | 465.0 ± 0.9 a | 870.5 ± 0.2 b | |||
| TPC (mg GAE/100 g) | 608.2 ± 4.9 a | 878.6 ± 26.1 b | |||
| ORAC (µmol TE/100 g) | 830.2 ± 5.7 a | 838.0 ± 8.5 a |
| Microbial Strain | MIC (mg/mL) | MBC/MFC (mg/mL) |
|---|---|---|
| Staphylococcus aureus ATCC25923 | nd | nd |
| Listeria innocua ŽM39 | nd | nd |
| Escherichia coli ATCC11229 | nd | nd |
| Pseudomonas fragi ATCC4973 | 2 | nd |
| Shewanella putrefaciens ŽM654 | 4 | nd |
| Shewanella xiamenensis ŽM655 | nd | nd |
| Shewanella baltica NCTC10735 | nd | nd |
| Campylobacter jejuni NCTC11168 | 2 | 4 |
| Candida albicans ZIM2202 | nd | nd |
| Pichia anomala ZIM1769 | nd | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malin, V.; Elez Garofulić, I.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Smole Možina, S.; Dragović-Uzelac, V. Phenolic Characterization and Bioactivity of Fennel Seed (Foeniculum vulgare Mill.) Extracts Isolated by Microwave-Assisted and Conventional Extraction. Processes 2022, 10, 510. https://doi.org/10.3390/pr10030510
Malin V, Elez Garofulić I, Repajić M, Zorić Z, Pedisić S, Sterniša M, Smole Možina S, Dragović-Uzelac V. Phenolic Characterization and Bioactivity of Fennel Seed (Foeniculum vulgare Mill.) Extracts Isolated by Microwave-Assisted and Conventional Extraction. Processes. 2022; 10(3):510. https://doi.org/10.3390/pr10030510
Chicago/Turabian StyleMalin, Valentina, Ivona Elez Garofulić, Maja Repajić, Zoran Zorić, Sandra Pedisić, Meta Sterniša, Sonja Smole Možina, and Verica Dragović-Uzelac. 2022. "Phenolic Characterization and Bioactivity of Fennel Seed (Foeniculum vulgare Mill.) Extracts Isolated by Microwave-Assisted and Conventional Extraction" Processes 10, no. 3: 510. https://doi.org/10.3390/pr10030510
APA StyleMalin, V., Elez Garofulić, I., Repajić, M., Zorić, Z., Pedisić, S., Sterniša, M., Smole Možina, S., & Dragović-Uzelac, V. (2022). Phenolic Characterization and Bioactivity of Fennel Seed (Foeniculum vulgare Mill.) Extracts Isolated by Microwave-Assisted and Conventional Extraction. Processes, 10(3), 510. https://doi.org/10.3390/pr10030510











