Assessing the Environmental and Economic Sustainability of Functional Food Ingredient Production Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Recovery of Phenolic-Rich Extract from Seaweed
2.2. Environmental Assessment
2.2.1. Goal and Scope Definition
2.2.2. Life Cycle Inventory
2.2.3. Life Cycle Impact Assessment
2.3. Economic Assessment
- COM = Cost of manufacturing
- X1 = Operational cost of workers
- X2 = Raw material cost
- X3 = Extraction cost
- X4 = Filtration cost
- X5 = Extract drying cost
- X6 = Waste treatment cost
- W= Number of workers
- Rh = Hourly rate of workers (EUR/h)
- HW = Number of hours worked
- RM = Raw material quantity k (kg)
- RRM = Rate of raw material (EUR/kg)
- CDO = Cost of dryer oven of size k (EUR)
- WDO = Wattage of dryer oven (kW)
- RT = Runtime (h)
- RE = Electricity rate (EUR/kWh)
- CMM = Cost of milling machine of size k (EUR)
- WMM = Wattage of milling machine (kW)
- S1 = Solvent 1 volume k (kg)
- RS1 = Rate of Solvent 1 volume k (EUR/kg)
- S2 = Solvent 2 (kg)
- RS2 = Rate of Solvent 2 (EUR/kg)
- CV = Cost of vessel of size k (EUR)
- WV = Wattage of vessel (kW)
- RT = Runtime (h)
- RE = Electricity rate (EUR/kWh)
- CC = Cheesecloth of length k (m)
- RCC = Rate of cheesecloth (EUR/m)
- CVP = Cost of vacuum pump of size k (EUR)
- WVP = Wattage of vacuum pump (kW)
- RT = Runtime (h)
- RE = Electricity rate (EUR/kWh)
- CFD = Cost of freeze dryer of size k (EUR)
- WFD = Wattage of freeze dryer (kW)
- CVP = Cost of vacuum pump of size k (EUR)
- WVP = Wattage of vacuum pump (kW)
- RT = Runtime (h)
- RE = Electricity rate (EUR/kWh)
2.3.1. Lab Scale
2.3.2. Large Scale
3. Results and Discussion
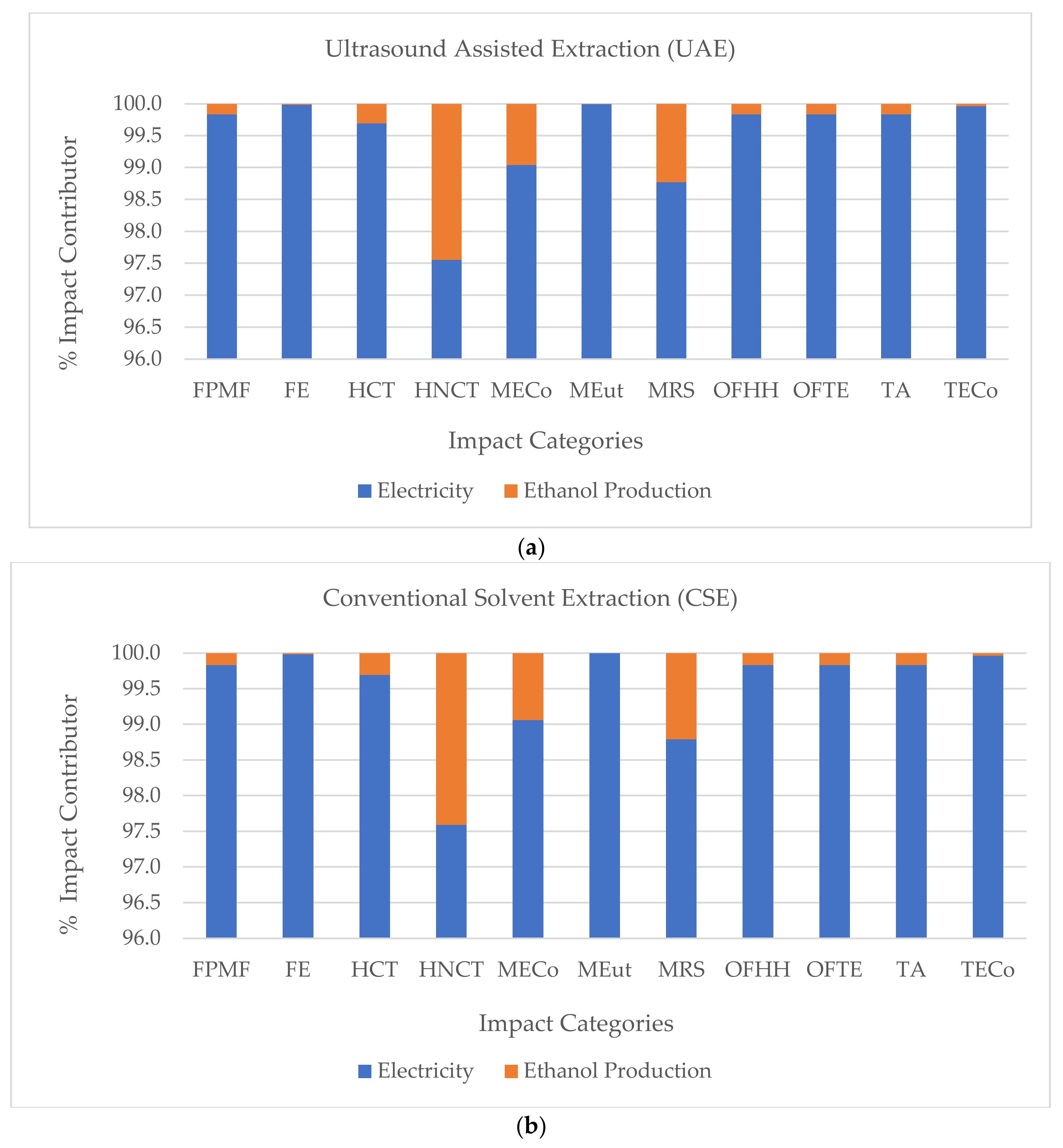
3.1. Environmental Assessment of the Extraction Processes
3.2. Economic Assessment of the Extraction Processes using Extraction Yield
3.3. Economic Assessment of the Extraction Processes Using Total Phenolic Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S. Strategic design of delivery systems for nutraceuticals. In Nanotechnology Applications in Food; Grumezescu, A.M., Oprea, A.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–86. [Google Scholar]
- Bayir, A.G.; Aksoy, A.N.; Koçyiğit, A. The Importance of Polyphenols as Functional Food in Health. Bezmialem Sci. 2019, 7, 157–164. [Google Scholar] [CrossRef]
- Bernal, J.; Mendiola, J.; Ibáñez, E.; Cifuentes, A. Advanced analysis of nutraceuticals. J. Pharm. Biomed. Anal. 2011, 55, 758–774. [Google Scholar] [CrossRef] [PubMed]
- FAO. Nutrition and Food Systems, A Report by High Level Panel of Experts on Food Security and Nutrition; Food and Agriculture Organization: Rome, Italy, 2016. [Google Scholar]
- Baghel, R.S.; Suthar, P.; Gajaria, T.K.; Bhattacharya, S.; Anil, A.; Reddy, C. Seaweed biorefinery: A sustainable process for valorising the biomass of brown seaweed. J. Clean. Prod. 2020, 263, 121359. [Google Scholar] [CrossRef]
- Binsi, P.; Zynudheen, A. Functional and nutraceutical ingredients from marine resources. In Value-Added Ingredients and Enrichments of Beverages; Grumezescu, A.M., Holban, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 101–171. [Google Scholar]
- Fuad, N.; Omar, R.; Kamarudin, S.; Harun, R.; Idris, A.; Wan Azlina, W.A.K.G. Mass harvesting of marine microalgae using different techniques. Food Bioprod. Process. 2018, 112, 169–184. [Google Scholar] [CrossRef]
- Sudhakar, M.; Kumar, B.R.; Mathimani, T.; Arunkumar, K. A review on bioenergy and bioactive compounds from microalgae and macroalgae-sustainable energy perspective. J. Clean. Prod. 2019, 228, 1320–1333. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Nutraceuticals from microbes of marine sources. In Nutraceuticals-Past, Present and Future; Hueda, M.C., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- European Commission. Food from the Oceans, High Level Group of Scientific Advisors, European Commission, Scientific Opinion; No. 3/2017; European Commission: Brussels, Belgium, 2017; pp. 1–80. [Google Scholar]
- DAFM. FoodWise 2025: Challenge, Ambition, Opportunity. Local Roots Global Reach; Department of Agriculture, Food and the Marine (DAFM): Dublin, Ireland, 2020.
- Garcia-Vaquero, M.; Rajauria, G.; O’doherty, J.; Sweeney, T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res. Int. 2017, 99, 1011–1020. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ummat, V.; Tiwari, B.; Rajauria, G. Exploring Ultrasound, Microwave and Ultrasound–Microwave Assisted Extraction Technologies to Increase the Extraction of Bioactive Compounds and Antioxidants from Brown Macroalgae. Marine Drugs 2020, 18, 172–186. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Vardanega, R.; Meireles, M.A.A. Extraction of natural blue colorant from Genipa americana L. using green technologies: Techno-economic evaluation. Food Bioprod. Process. 2019, 114, 132–143. [Google Scholar] [CrossRef]
- Vauchel, P.; Colli, C.; Pradal, D.; Philippot, M.; Decossin, S.; Dhulster, P.; Dimitrov, K. Comparative LCA of ultrasound-assisted extraction of polyphenols from chicory grounds under different operational conditions. J. Clean. Prod. 2018, 196, 1116–1123. [Google Scholar] [CrossRef]
- Sanz, V.; Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. Clean technologies applied to the recovery of bioactive extracts from Camellia sinensis leaves agricultural wastes. Food Bioprod. Process. 2020, 122, 214–221. [Google Scholar] [CrossRef]
- Priyadarshini, A.; Rajauria, G.; O’Donnell, C.P.; Tiwari, B.K. Emerging food processing technologies and factors impacting their industrial adoption. Crit. Rev. Food Sci. Nutr. 2019, 59, 1–20. [Google Scholar] [CrossRef]
- Allen, D.T.; Shonnard, D.R. Green engineering: Environmentally conscious design of chemical processes and products. Am. Inst. Chem. Eng. AIChE J. 2001, 47, 1906. [Google Scholar] [CrossRef]
- Grossmann, I.E.; Westerberg, A.W. Research challenges in process systems engineering. AIChE J. 2000, 46, 1700–1703. [Google Scholar] [CrossRef]
- Burgess, A.A.; Brennan, D.J. Application of life cycle assessment to chemical processes. Chem. Eng. Sci. 2001, 56, 2589–2604. [Google Scholar] [CrossRef]
- Jacquemin, L.; Pontalier, P.-Y.; Sablayrolles, C. Life cycle assessment (LCA) applied to the process industry: A review. Int. J. Life Cycle Assess. 2012, 17, 1028–1041. [Google Scholar] [CrossRef]
- Gillani, S.T.; Belaud, J.-P.; Sablayrolles, C.; Vignoles, M.; Le Lann, J.-M. Review of life cycle assessment in agro-chemical processes. Chem. Prod. Process Modeling 2010, 5, 1–26. [Google Scholar] [CrossRef][Green Version]
- Aziz, N.I.H.A.; Hanafiah, M.M.; Gheewala, S.H. A review on life cycle assessment of biogas production: Challenges and future perspectives in Malaysia. Biomass Bioenergy 2019, 122, 361–374. [Google Scholar] [CrossRef]
- Crossin, E. Life cycle assessment of a mallee eucalypt jet fuel. Biomass Bioenergy 2017, 96, 162–171. [Google Scholar] [CrossRef]
- Modahl, I.S.; Brekke, A.; Valente, C. Environmental assessment of chemical products from a Norwegian biorefinery. J. Clean. Prod. 2015, 94, 247–259. [Google Scholar] [CrossRef]
- Siegl, S.; Laaber, M.; Holubar, P. Green electricity from biomass, part I: Environmental impacts of direct life cycle emissions. Waste Biomass Valorization 2011, 2, 267–284. [Google Scholar] [CrossRef]
- Barjoveanu, G.; Pătrăuțanu, O.-A.; Teodosiu, C.; Volf, I. Life cycle assessment of polyphenols extraction processes from waste biomass. Sci. Rep. 2020, 10, 1–12. [Google Scholar]
- Gonzalez-Garcia, S.; Gullón, B.; Moreira, M.T. Environmental assessment of biorefinery processes for the valorization of lignocellulosic wastes into oligosaccharides. J. Clean. Prod. 2018, 172, 4066–4073. [Google Scholar] [CrossRef]
- Gao, W.; Sun, Z.; Cao, H.; Ding, H.; Zeng, Y.; Ning, P.; Xu, G.; Zhang, Y. Economic evaluation of typical metal production process: A case study of vanadium oxide production in China. J. Clean. Prod. 2020, 256, 120217. [Google Scholar] [CrossRef]
- Liu, X.; Tanaka, M.; Matsui, Y. Economic evaluation of optional recycling processes for waste electronic home appliances. J. Clean. Prod. 2009, 17, 53–60. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Papadaki, S.; Krokida, M. Life cycle analysis of β-carotene extraction techniques. J. Food Eng. 2015, 167, 51–58. [Google Scholar] [CrossRef]
- Agnhage, T.; Perwuelz, A.; Behary, N. Towards sustainable Rubia tinctorum L. dyeing of woven fabric: How life cycle assessment can contribute. J. Clean. Prod. 2017, 141, 1221–1230. [Google Scholar] [CrossRef]
- Papadaki, S.; Kyriakopoulou, K.; Tzovenis, I.; Krokida, M. Environmental impact of phycocyanin recovery from Spirulina platensis cyanobacterium. Innov. Food Sci. Emerg. Technol. 2017, 44, 217–223. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and drawbacks of ultrasound-assisted extraction for the recovery of bioactive compounds from marine algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Carciochi, R.A.; Dieu, V.; Vauchel, P.; Pradal, D.; Dimitrov, K. Reduction of environmental impacts of caffeine extraction from guarana by using ultrasound assistance. Food Bioprod. Process. 2021, 127, 266–275. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, Y.; Han, Y.; Chemat, F.; Li, D.; Show, P.L. Insight into mass transfer during ultrasound-enhanced adsorption/desorption of blueberry anthocyanins on macroporous resins by numerical simulation considering ultrasonic influence on resin properties. Chem. Eng. J. 2020, 380, 122530. [Google Scholar] [CrossRef]
- Ummat, V.; Tiwari, B.K.; Jaiswal, A.K.; Condon, K.; Garcia-Vaquero, M.; O’Doherty, J.; O’Donnell, C.P.; Rajauria, G. Optimisation of Ultrasound Frequency, Extraction Time and Solvent for the Recovery of Polyphenols, Phlorotannins and Associated Antioxidant Activity from Brown Seaweeds. Mar. Drugs 2020, 18, 250–264. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management—Life Cycle Assessment—Part 1: Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006; 3, pp. 1–20.
- Kaplan, R.; Anderson, S. Time-Driven Activity-Based Costing. Harv. Bus. Rev. 2004, 11, 4–45. [Google Scholar] [CrossRef]
- Hoozée, S.; Bruggeman, W. Identifying operational improvements during the design process of a time-driven ABC system: The role of collective worker participation and leadership style. Manag. Account. Res. 2010, 21, 185–198. [Google Scholar] [CrossRef]
- Rajasekaran, V. Cost Accounting; Pearson Education India: Delhi, India, 2010. [Google Scholar]
- Demeere, N.; Stouthuysen, K.; Roodhooft, F. Time-driven activity-based costing in an outpatient clinic environment: Development, relevance and managerial impact. Health Policy 2009, 92, 296–304. [Google Scholar] [CrossRef]
- Piotrowska, A.; Rao, M.A.; Scotti, R.; Gianfreda, L. Changes in soil chemical and biochemical properties following amendment with crude and dephenolized olive mill waste water (OMW). Geoderma 2011, 161, 8–17. [Google Scholar] [CrossRef]
- Boechat, C.L.; Santos, J.A.G.; Accioly, A.M.d.A.; Bomfim, M.R.; dos Santos, A.C. Industrial and urban organic wastes increase soil microbial activity and biomass. Rev. Bras. Ciência Solo 2012, 36, 1629–1636. [Google Scholar] [CrossRef][Green Version]
- Farías-Campomanes, A.M.; Rostagno, M.A.; Meireles, M.A.A. Production of polyphenol extracts from grape bagasse using supercritical fluids: Yield, extract composition and economic evaluation. J. Supercrit. Fluids 2013, 77, 70–78. [Google Scholar] [CrossRef]
- Prado, J.M.; Dalmolin, I.; Carareto, N.D.; Basso, R.C.; Meirelles, A.J.; Oliveira, J.V.; Batista, E.A.; Meireles, M.A.A. Supercritical fluid extraction of grape seed: Process scale-up, extract chemical composition and economic evaluation. J. Food Eng. 2012, 109, 249–257. [Google Scholar] [CrossRef]
- Rosa, P.T.; Meireles, M.A.A. Rapid estimation of the manufacturing cost of extracts obtained by supercritical fluid extraction. J. Food Eng. 2005, 67, 235–240. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Gómez-Ordóñez, E.; Rupérez, P. Brown and red seaweeds as potential sources of antioxidant nutraceuticals. J. Appl. Phycol. 2012, 24, 1123–1132. [Google Scholar] [CrossRef]
- Montero, L.; del Pilar Sánchez-Camargo, A.; Ibáñez, E.; Gilbert-López, B. Phenolic compounds from edible algae: Bioactivity and health benefits. Curr. Med. Chem. 2018, 25, 4808–4826. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Saravacos, G.; Maroulis, Z. Food Process Economics. In Food Engineering Interfaces; Aguilera, J.M., Simpson, R., Welti-Chanes, J., Eds.; Springer: New York, NY, USA, 2010; pp. 219–236. [Google Scholar]
- Gwee, Y.L.; Yusup, S.; Tan, R.R.; Yiin, C.L. Techno-economic and life-cycle assessment of volatile oil extracted from Aquilaria sinensis using supercritical carbon dioxide. J. CO2 Util. 2020, 38, 158–167. [Google Scholar] [CrossRef]
- Ochoa, S.; Durango-Zuleta, M.M.; Osorio-Tobón, J.F. Techno-economic evaluation of the extraction of anthocyanins from purple yam (Dioscorea alata) using ultrasound-assisted extraction and conventional extraction processes. Food Bioprod. Process. 2020, 122, 111–123. [Google Scholar] [CrossRef]
- Galviz-Quezada, A.; Ochoa-Aristizábal, A.M.; Zabala, M.E.A.; Ochoa, S.; Osorio-Tobón, J.F. Valorization of iraca (Carludovica palmata, Ruiz & Pav.) infructescence by ultrasound-assisted extraction: An economic evaluation. Food Bioprod. Process. 2019, 118, 91–102. [Google Scholar]
- Zabot, G.L.; Bitencourte, I.P.; Tres, M.V.; Meireles, M. Process intensification for producing powdered extracts rich in bioactive compounds: An economic approach. J. Supercrit. Fluids 2017, 119, 261–273. [Google Scholar] [CrossRef]
- Johner, J.C.; Hatami, T.; Zabot, G.L.; Meireles, M.A.A. Kinetic behavior and economic evaluation of supercritical fluid extraction of oil from pequi (Caryocar brasiliense) for various grinding times and solvent flow rates. J. Supercrit. Fluids 2018, 140, 188–195. [Google Scholar] [CrossRef]
- Tufvesson, P.; Ekman, A.; Sardari, R.R.; Engdahl, K.; Tufvesson, L. Economic and environmental assessment of propionic acid production by fermentation using different renewable raw materials. Bioresour. Technol. 2013, 149, 556–564. [Google Scholar] [CrossRef]

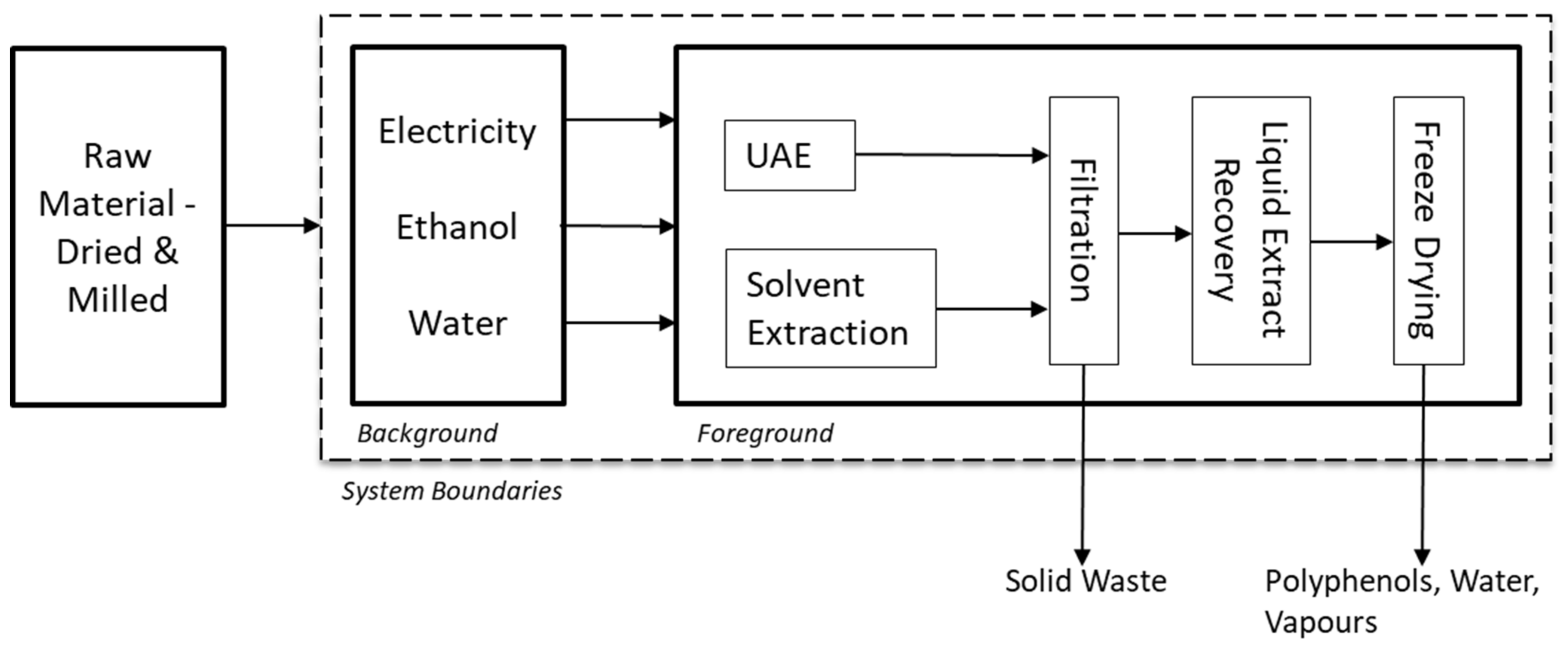

| Inventory | Unit | CSE | UAE | Data Source/Remark |
|---|---|---|---|---|
| Inputs | ||||
| Seaweed | gm | 28.79 | 4.98 | Measured |
| Ethanol | gm | 115.17 | 19.91 | Measured |
| Water | L | 0.14 | 0.02 | Measured |
| Electricity for extraction | kWh | 2.76 | 0.01 | Measured |
| Electricity for filtration | kWh | 0.02 | 0.002 | Measured |
| Electricity for freeze drying | kWh | 138.89 | 24.01 | Measured |
| Outputs | ||||
| Polyphenols | g | 1 | 1 | |
| Evaporated ethanol | g | 11.52 | 1.99 | Calculated for experiments from mass balance. Assuming 10% loss |
| Recovered ethanol | g | 103.65 | 17.92 | Considers a 90% ethanol recovery rate |
| Solid waste | g | 25.57 | 3.23 | Calculated from mass balance |
| Wastewater | L | 0.14 | 0.02 | Assuming all water used was finally considered as wastewater |
| Parameters | Rate (Ex VAT) |
|---|---|
| Pre-treated raw material (dried, milled and transportation) a | EUR 27.72/kg |
| Industrial ultrasound units/vessels a | |
| 50 L (0.05 m3) | EUR 12,360 |
| 100 L (0.10 m3) | EUR 15,175 |
| 150 L (0.15 m3) | EUR 16,975 |
| Labour b | EUR 9.80/h |
| For 50 L | 1 operator |
| For 100 L | 2 operators |
| For 150 L | 3 operators |
| Extraction solvents a | |
| Ethanol | EUR 15.10/kg |
| Water c | EUR 1.21/kg |
| Electricity cost d | EUR 0.2122/kWh |
| Filtration cost (including cheese cloth, vacuum pump, and electricity cost) a | |
| Cheese cloth | EUR 2.5/metre |
| Vacuum pump | EUR 1893 |
| Freeze drying cost (including 6L freeze dryer, vacuum pump, and electricity cost) | |
| Freeze dryer (6L, fully assembled unit) | EUR 18,495 |
| Vacuum pump | EUR 2206 |
| Waste treatment cost e | Nil |
| Impact Category | Unit | CSE | UAE |
|---|---|---|---|
| Fine particulate matter formation (FPMF) | kg PM2.5 eq | 7.55 × 10−5 | 1.28 × 10−5 |
| Freshwater ecotoxicity (FE) | kg 1,4-DCB | 2.94 × 10−7 | 4.98 × 10−8 |
| Human carcinogenic toxicity (HCT) | kg 1,4-DCB | 2.07 × 10−6 | 3.52 × 10−7 |
| Human non-carcinogenic toxicity (HNCT) | kg 1,4-DCB | 1.75 × 10−4 | 2.97 × 10−5 |
| Marine ecotoxicity (MECo) | kg 1,4-DCB | 9.72 × 10−4 | 1.65 × 10−4 |
| Marine eutrophication (MEut) | kg N eq | 6.66 × 10−9 | 1.13 × 10−9 |
| Mineral resource scarcity (MRS) | kg Cu eq | 2.31 × 10−3 | 3.92 × 10−4 |
| Ozone formation, Human health (OFHH) | kg NOx eq | 6.80 × 10−4 | 1.15 × 10−4 |
| Ozone formation, Terrestrial ecosystems (OFTE) | kg NOx eq | 6.80 × 10−4 | 1.15 × 10−4 |
| Terrestrial acidification (TA) | kg SO2 eq | 2.45 × 10−4 | 4.16 × 10−5 |
| Terrestrial ecotoxicity (TECo) | kg 1,4-DCB | 1.44 × 10−4 | 2.45 × 10−5 |
| Water consumption (WC) | m3 | 1.44 × 10−4 | 2.49 × 10−5 |
| Extraction Yield (%) | TPC (mg/g) | Vessel Capacity (m3) | COM of Extract (EUR/kg) | COM of Total Polyphenols (EUR/kg) | |
|---|---|---|---|---|---|
| Lab Scale | |||||
| CSE | 11.2 | 310.11 | 0.01 | 8981 | 28,961 |
| UAE | 35.1 | 572.33 | 0.01 | 922 | 1611 |
| Large Scale | |||||
| UAE | -- | -- | 0.05 | 345 | 603 |
| UAE | -- | -- | 0.10 | 340 | 595 |
| UAE | -- | -- | 0.15 | 443 | 774 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priyadarshini, A.; Tiwari, B.K.; Rajauria, G. Assessing the Environmental and Economic Sustainability of Functional Food Ingredient Production Process. Processes 2022, 10, 445. https://doi.org/10.3390/pr10030445
Priyadarshini A, Tiwari BK, Rajauria G. Assessing the Environmental and Economic Sustainability of Functional Food Ingredient Production Process. Processes. 2022; 10(3):445. https://doi.org/10.3390/pr10030445
Chicago/Turabian StylePriyadarshini, Anushree, Brijesh K. Tiwari, and Gaurav Rajauria. 2022. "Assessing the Environmental and Economic Sustainability of Functional Food Ingredient Production Process" Processes 10, no. 3: 445. https://doi.org/10.3390/pr10030445
APA StylePriyadarshini, A., Tiwari, B. K., & Rajauria, G. (2022). Assessing the Environmental and Economic Sustainability of Functional Food Ingredient Production Process. Processes, 10(3), 445. https://doi.org/10.3390/pr10030445








