Abstract
In this work, Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) alloys are prepared and used as the anode of an Al-air battery (AAB). We use scanning electron microscopy (SEM) with energy-dispersive X-ray spectroscopy (EDS) and optical microscopy (OM) to analyze the microstructures of the alloys. The hydrogen evolution rate, electrochemical performance (including polarization curves), electrochemical impedance spectroscopy (EIS), and battery performance of the samples are examined in the 4 M NaOH electrolyte. The experimental data display that the average grain size is significantly refined after adding manganese into the Al-1Zn-0.1In-0.1Sn-0.5Mg alloy, with a decrease in grain size from over 100 μm to about 10 μm. The improved activity of the aluminum anode in the AAB can be attributed to the introduction of manganese. The Al-1Zn-0.1In-0.1Sn-0.5Mg-0.1Mn alloy possesses the optimal overall performance with a lower self-corrosion rate (0.128 mL∙cm−2∙min−1), the highest working potential (1.630 V) and energy density (2415 mWh·g−1), a higher capacity (1481 mAh·g−1) and anodic utilization (49.75%).
1. Introduction
Environmental contamination and conventional fossil energy shortage have gradually become serious obstacles to the sustainable development of the economy and society. Therefore, searching for clean energy to replace traditional fossil fuels has become more and more important in the world. With the unique advantages of high efficiency and low pollution, the fuel cell has become the focus of clean energy research in recent years. Particularly, as a new type of fuel cell, the metal-air battery (MAB), with the advantages of high specific capacity, flat discharge voltage, and low manufacturing cost, are of great commercial value in the fields of civil and military power supply [1,2,3,4,5]. Owing to higher theoretical capacity (2.98 Ah·g−1) and lower cost compared to other metal anode materials, for instance, Mg (2.20 Ah·g−1) and Zn (0.82 Ah·g−1), the second-highest energy density (8.1 kWh·kg−1) after Li (13.3 kWh·kg−1), and its low negative electrochemical potential (−1.66 V vs. NHE), aluminum shows great potential for its application in MAB as the negative electrode material. The additional advantages of aluminum include its abundance, rapid mechanical rechargeability, environmental friendliness, and ease of recycling [6,7,8,9].
While in neutral solutions, a passive film is easily formed on the surface of pure aluminum, causing the activation of aluminum to be significantly slowed down. In addition, aluminum is prone to self-corrosion in alkaline electrolytes, which leads to the degradation of the energy density of AAB and a reduction in anode utilization efficiency, along with an increase in fuel loss during battery standby. The above phenomenon indicates that pure aluminum is not directly suitable as the anode of the MAB [10,11,12].
Alloying has been widely used to overcome the above problems, and the known additive elements are indium, germanium, zinc, magnesium, thallium, tin, etc. [13,14,15,16,17,18]. The elements currently used to form alloys with aluminum are mainly Mg, Zn, Sn, and In, since they have higher hydrogen evolution potentials in an alkaline solution and lower melting points compared with aluminum [19,20,21]. More specifically, adding a small amount of magnesium to aluminum can improve its mechanical tensile strength and impurity tolerance [22]. The presence of Zn can increase the surface defects of the aluminum anodized film and reduce the hydrogen evolution rate of the aluminum anode [23,24]. The addition of In can reduce the rate of pitting of the aluminum anode, resulting in uniform surface corrosion and a negative shift in the potential of the aluminum anode [25]. Synergies have been found between In and Zn in the activation of aluminum anodes [26]. The addition of Sn not only breaks the passive layer and reduces the potential of aluminum, but also refines the grain and reduces the intergranular segregation phase [27]. Previous studies have shown that the addition of Mn to the matrix alloy facilitates the refinement of the grain size through the formation of fine dispersions [28,29,30,31,32]. In addition, the corrosion resistance of the alloy can be improved by reducing the grain size [33,34,35,36]. However, the refinement of aluminum anode grains by doping with Mn has rarely been reported in AABs.
In this paper, we used the vacuum induction melting method to synthesize the multi-component Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) alloys, aiming to study the effect of the addition of manganese on the structural and electrochemical performances of Al-Zn-In-Sn-Mg anode alloys for AAB.
2. Experimental
2.1. Sample Preparation and Structural Characterizations
The raw materials used are tin particles (99.99 wt.%, Aladdin, Shanghai, China), indium particles (99.99 wt.%, Aladdin, Shanghai, China), zinc particles (99.99 wt.%, Aladdin, Shanghai), Al-50 wt.% Mg, Al-20 wt.% Mn master alloy (China New Metal Materials Technology Co., Ltd., Beijing), and pure aluminum ingots (>99.9 wt.%, Huaxiahuiying Technology Development Co., Beijing, China). The theoretical design compositions of the alloys are Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) (wt.%). The alloys were melted in cold crucible vacuum induction melting equipment under an argon atmosphere. Each sample was melted repeatedly for not less than four times to obtain a homogeneous composition. We then processed the as-cast alloys into 1 cm2 cylindrical pieces using wire cut electrical discharge machining (WEDM). The actual compositions of the fabricated samples were examined by X-ray fluorescence analysis (XRF, ARL ADV ANT’XP). Table 1 shows that the differences between the actual and theoretical compositions of the samples are small, indicating that the designed alloys were successfully prepared. Moreover, we used an SEM with EDS (JSM-6360LV) and an OM (Scope.A1) to analyze the microstructures and phase compositions of the samples.

Table 1.
The element compositions of the as-cast Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) (wt.%) alloys.
2.2. Electrochemical Measurements
The electrochemical characterization of the samples was carried out by the CHI660E Electrochemical Workstation (Chenhua, Shanghai, China) using a three-electrode system. The prepared aluminum alloys (0.54 g), a Pt sheet (15 × 15 mm), and an Hg/HgO electrode were used as the working electrode, counter electrode, and reference electrode, respectively. The open circuit potential (OCP) of the anode was measured after immersing the three-electrode system in a 4M NaOH electrolyte for one hour to ensure stability. The potentiodynamic polarization measurements were performed at a scan rate of 1 mV/s between −1.0 to 1.0 V vs. OCP. The electrochemical impedance spectroscopy (EIS) was obtained at OCP with 5 mV perturbation and 100 kHz to 0.01 Hz frequency range. ZSimpWin EIS software was used to fit and analyze the data.
2.3. Self-Corrosion
The self-corrosion rate of the alloys in the 4 M NaOH electrolyte was measured via the hydrogen gas collection method. The test alloys were fixed with PVC hose and epoxy resin, and only a 1 cm2 circular plane was exposed as the working surface. We used emery paper (800–2000 grade) to grind the working surface of the samples in turn, and then polished the surface of the samples to a mirror surface with carborundum paste. Finally, the samples were immersed in the electrolyte for one hour. The hydrogen evolution rate of the samples is obtained as follows:
2.4. Battery Performance
An AAB unit similar to that in the literature study [37] was assembled for the battery performance test. The 4M NaOH solution, as-cast alloy (0.54 g), and commercial air cathode with MnO2 (70 mg) as catalyst were used as the electrolyte, the anode, and the air cathode, respectively. Galvanostatic discharge tests of the AABs were collected and analyzed using a battery test system (Landt CT2001A) under the current density of 20 mA·cm−2 for 4 h. The weight difference of the anode alloy before and after the discharge was measured as the anode consumption. The anode utilization, energy density, and capacity were obtained according to the following formulas:
where η is the anode utilization, %; t is the time, s; I is the current, A; Δm the weight loss of the alloy, g; F the Faraday constant; U the average voltage, V.
To ensure the reproducibility of the experimental results, all the above tests were repeated at least three times.
3. Results and Discussion
3.1. Microstructure
The microstructures of the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) alloys are displayed in Figure 1. The crystal grain of x = 0 alloy is coarse and its average size is above 100 μm. The crystal grain is obviously refined after the addition of Mn (Figure 1b–d), in which the average size is about 10 μm. The reason for the grain refinement is that Mn and Al formed intermetallic MnAl6; in addition, the impurity of Fe decreased the solid solubility of Mn in Al and formed the (Mn, Fe)Al6 compound. The segregation of fine MnAl6 and (Mn, Fe)Al6 may hinder the grain growth by pinning the grain boundaries [29,31]. The refined grain can not only facilitate the homogeneous dissolution of the alloy, but also may change the distribution of the segregation phase.
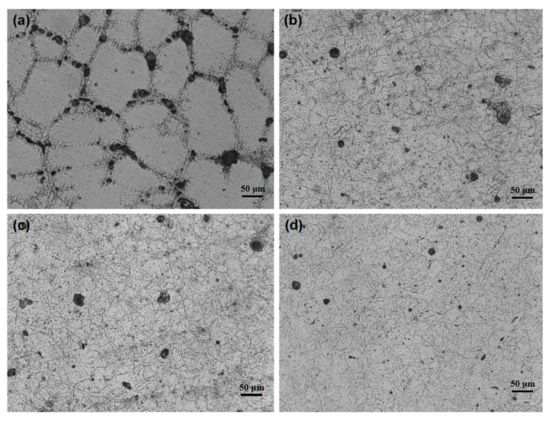
Figure 1.
Micrographs of the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn alloys: (a) x = 0, (b) x = 0.1, (c) x = 0.2, (d) x = 0.3.
Figure 2 shows the SEM images of the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) alloys and Table 2 summarizes the results of the EDS analysis. All the as-cast samples include a main alpha (Al) phase and a minor segregation phase. The number of segregated phases in aluminum alloys increases and the shape transforms gradually from nearly circle-like to rod-like with an increase in the addition of Mn. For aluminum alloy anodes in AAB, the segregated phases are both active points and hydrogen evolution points [23]. On one hand, the segregated phase can further activate the alloy; on the other hand, an excessive segregated phase may precipitate along the grain boundaries, leading to the aggravation of intergranular corrosion and a higher self-corrosion rate. In particular, the rod-shaped precipitates can aggravate corrosion along the grain boundaries and reduce the current efficiency [38]. The EDS results show that the Zn and Sn contents of the segregated phase in the alloy of x = 0 are larger than other alloys with the Mn addition, indicating that Mn facilitates the homogeneous dispersion of Zn and Sn in the alloys. The homogeneous dispersion of Zn in the alloy is favorable to the homogeneous dissolution of the aluminum alloy, because Zn can increase the surface defects of the oxide film [22]. The homogeneous distribution of Sn in the alloy will lead to better corrosion resistance [39].
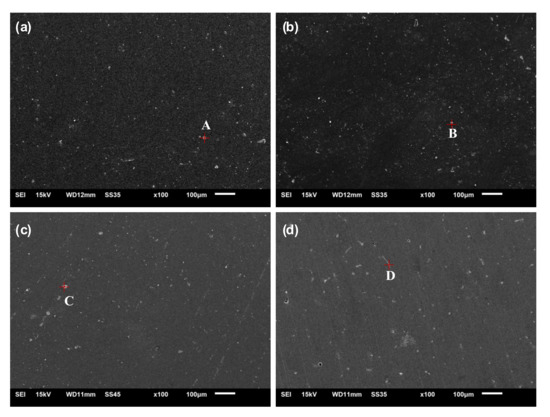
Figure 2.
SEM images of the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn alloys: (a) x = 0, (b) x = 0.1, (c) x = 0.2, (d) x = 0.3.

Table 2.
The composition measurements of the segregated phases of the as-cast Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) (wt.%) alloys.
3.2. Hydrogen Evolution Test
The hydrogen evolution rate of the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) alloys in the 4 M NaOH solution is displayed in Table 3. The results show that the self-corrosion rate of the aluminum alloy anodes increases continuously with the increase in Mn content, which can be attributed to the increase in the activation point of the hydrogen evolution caused by the precipitate phase forming corrosion micro cells with its surrounding Al. Moreover, the addition of Mn to the alloy leads to the grain refinement and an increase in grain boundaries, which also increases the hydrogen evolution rate. The increase in the corrosion rate means that the self-discharge is intensified, so the utilization ratio of the anode will decrease.

Table 3.
The corrosion parameters of the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) (wt.%) alloy anodes in the 4 M NaOH solution.
3.3. Potentiodynamic Polarization
The potentiodynamic polarization curves of the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) alloy anodes in the 4 M NaOH solution is shown in Figure 3; Table 3 is the summary of the corrosion parameters of the corresponding alloy anodes. Compared with the alloy of x = 0, the corrosion potential (Ecorr) shifts negatively after the addition of Mn and the alloy of x = 0.1 shows the most negative value of −1.776 V, which indicates that the Mn addition may increase the activity of the aluminum alloy. The corrosion current (Icorr) of the alloy increases continuously with the increase in the Mn content, which is similar to the influence of the Mn content on the corrosion rate of the alloy. The Rp of the x = 0 alloy is the largest, which is unfavorable for the aluminum alloy anode, while it is decreased sharply after adding Mn, contributing to higher surface activity and better anodic solubility. This is due to the fact that the Mn-added alloy has much smaller grain size and a large number of grain boundaries, with more crystal defects acting as channels for the anode reaction [40].
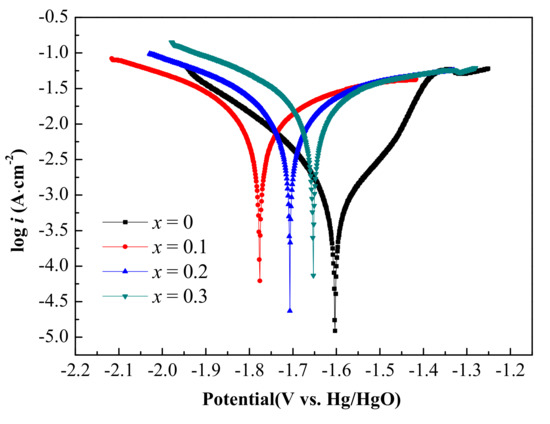
Figure 3.
The potentiodynamic polarization curves of the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) (wt.%) alloy anodes in the 4 M NaOH solution.
3.4. EIS Measurement
The EIS curves of the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) alloy anodes in the 4 M NaOH solution are presented in Figure 4. Figure 5 shows the equivalent circuits for simulating the EIS curve. Among them, Rs represents the solution resistance. It has been reported that the induction loops in the EIS curves reflect the hydrogen adsorption process in the hydrogen evolution reaction caused by the corrosion of the alloy, and the L1 in the equivalent circuit corresponds to the hydrogen evolution reaction parameter [41]. In addition, the high frequency capacitance loops in the EIS curves reflect the charge transfer reaction that occurs in an electric double layer formed between the corrosive medium generated during the etching process and the surface of the alloy. Similarly, the equivalent components reflected in the equivalent circuit are the constant phase component (CPE1) and the parallel charge transfer resistor (Rt). Furthermore, the dissolution-deposition of In and Sn ions during the alloy corrosion process is reflected in the low frequency capacitive loops. The corresponding dissolution-deposition parameters are CPE2 and R2 in the equivalent circuit. The intergranular corrosion is reflected in the low-frequency induction loop, and L2, R3 are the corresponding parameters [42,43].
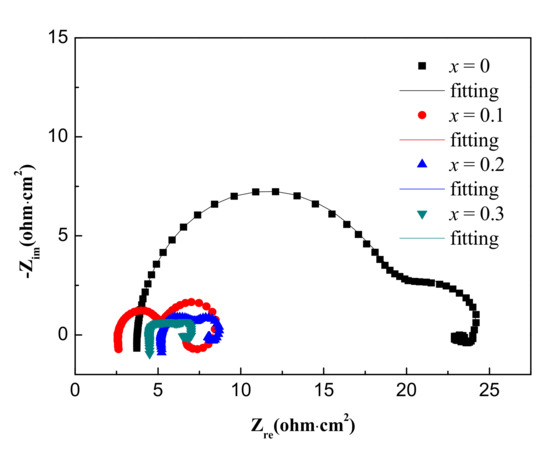
Figure 4.
EIS plots of the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) (wt.%) alloy anodes in the 4 M NaOH solution.
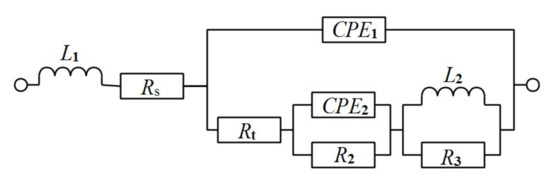
Figure 5.
The equivalent circuit of the EIS curve of the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) (wt.%) alloy anodes in the 4 M NaOH solution.
The fitting values of EIS are listed in Table 4. Among them, x2 represents the accuracy of the fitted data; the smaller the x2 value, the more accurate the fit. Table 4 shows that the x2 values of all the samples are less than 5 × 10−5, indicating that the fitted data are highly consistent with the real data. In addition, Rt is a very important factor in the dissolution of aluminum alloy anodes. The bigger the Rt value, the smaller the exchange current and the slower the corrosion rate. Table 4 shows that Rt decreases with the increasing Mn content in the alloy. The data indicate that the corrosion resistance of the sample decreases with the increasing Mn content, which is consistent with the results of hydrogen evolution and potentiodynamic polarization.

Table 4.
The EIS simulated values of the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) (wt.%) alloy anodes in the 4 M NaOH solution.
3.5. Battery Performance
The discharge curves of the prepared AAB in the 4M NaOH electrolyte at 20 mA·cm−2 current density for 4 h are shown in Figure 6. Owing to the internal resistance of the battery, the discharge voltage drops rapidly at first [44], gradually rising to an almost constant discharge voltage as the discharge time increases. All the alloy anodes exhibit a stable discharge process. The electrochemical parameters of the constant current discharge of the AABs using different Al alloy anodes are summarized in Table 5. It shows that the operating voltage of the x = 0 alloy is the lowest, while the alloys with Mn addition provide higher operating voltage, and the alloy of x = 0.1 shows the highest average voltage of 1.630 V. The anodic utilization (η) of the alloys reduces with the increasing Mn content. Compared with the alloy of x = 0, the anodic utilization of alloys of x = 0.1, 0.2 and 0.3 is reduced by 1.91%, 6.43%, and 10.96%, respectively. The capacity of the alloy anodes presents a similar variation with the anodic utilization. The energy densities of the alloy anodes are 2385, 2415, 2163, and 1916 mWh·g−1, respectively. The highest energy density of the x = 0.1 alloy is due to the highest average voltage and a relatively higher capacity. Table 6 shows the comparison of the battery performance with that reported in the literatures. The battery assembled using the Al-1Zn-0.1In-0.1Sn-0.5Mg-0.1Mn alloy has a higher discharge voltage.
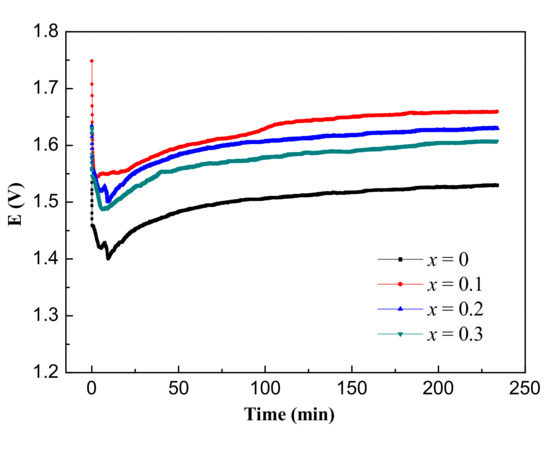
Figure 6.
The discharge curves of the AAB based on the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) (wt.%) alloy anodes at 20 mA·cm−2 current density for 4 h in the 4 M NaOH solution.

Table 5.
The electrochemical parameters of the AABs using the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) (wt.%) alloy anodes discharged at 20 mA·cm−2 current density for 4 h in the 4 M NaOH solution.

Table 6.
The comparison of the battery performance with that reported in the literature.
3.6. Surface Analysis after Discharge
The surface morphologies of the samples after discharge are shown in Figure 7. It shows the typical crystalline corrosion of Al alloys in an alkaline electrolyte. The morphology of the alloys shows intense corrosion on the entire surface. The corrosion morphology of the x = 0 alloy is coarse with reticulate crack structures and pitting pores due to the corrosion along the grain boundaries and the segregation phase, while the alloy of x = 0.1 presents a relatively flat morphology owing to the homogeneous distribution of the segregation phase and an increase in the anodic reaction channels derived from the additional grain boundaries. However, the alloys of x = 0.2 and 0.3 both display a large-pore surface structure formed via galvanic corrosion between aluminum and the segregation phases or shed grains.
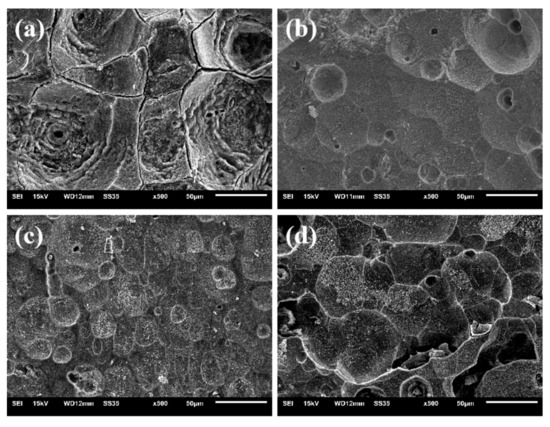
Figure 7.
The surface morphologies of the Al-1Zn-0.1In-0.1Sn-0.5Mg-xMn (x = 0, 0.1, 0.2, 0.3) (wt.%) alloy anodes after discharging at 20 mA·cm−2 for 4 h in the 4 M NaOH solution; (a) x = 0, (b) x = 0.1, (c) x = 0.2, (d) x = 0.3.
4. Conclusions
- (1)
- By doping Mn in the Al-1Zn-0.1In-0.1Sn-0.5Mg alloy, the average grain size is significantly refined, showing a decrease in size from over 100 μm to about 10 μm. The segregated phases in the as-cast alloys increase with the increasing content of Mn; meanwhile, the shape of the segregation phases transforms from nearly circle-like to rod-like.
- (2)
- After Mn doping, the alloy corrosion potential shifts negatively, while the hydrogen evolution rate and corrosion current of the alloy both increase continuously.
- (3)
- The Al-1Zn-0.1In-0.1Sn-0.5Mg-0.1Mn alloy possesses the optimal overall performance, with a lower self-corrosion rate (0.128 mL∙cm−2∙min−1), the highest working voltage (1.630 V) and energy density (2415 mWh·g−1), and higher capacity (1481 mAh·g−1) and anodic utilization (49.75%) in the 4 M NaOH solution.
Author Contributions
Conceptualization, T.C., Y.Z. and T.Y.; formal analysis, T.C. and X.Y.; funding acquisition, T.C. and Y.Z.; investigation, W.Z.; methodology, W.Z., T.H. and L.L.; project administration, Y.Z. and T.Y.; resources, Y.Z. and T.Y.; writing—original draft, W.Z.; writing—review and editing, T.H. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (52071177), the Six Talent Peaks Project in Jiangsu Province (2018, XNY-020), the Postgraduate Research and Practice Innovation Program of Jiangsu Province (SJCX21_0499), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buckingham, R.; Asset, T.; Atanassov, P. Aluminum-air batteries: A review of alloys, electrolytes and design. J. Power Sources 2021, 498, 229762. [Google Scholar] [CrossRef]
- Yang, L.X.; Wu, Y.T.; Chen, S.; Xiao, Y.Q.; Chen, S.; Zheng, P.H.; Wang, J.X.; Qu, J.E. A promising hybrid additive for enhancing the performance of alkaline aluminum-air batteries. Mater. Chem. Phys. 2021, 257, 123787. [Google Scholar] [CrossRef]
- Munoz-Torrero, D.; Leung, P.; Garcia-Quismondo, E.; Ventosa, E.; Anderson, M.; Palma, J.; Marcilla, R. Investigation of different anode materials for aluminum rechargeable batteries. J. Power Sources 2018, 374, 77–83. [Google Scholar] [CrossRef]
- Sumboja, A.; Ge, X.M.; Zong, Y.; Liu, Z.L. Progress in development of flexible metal-air batteries. Funct. Mater. Lett. 2016, 9, 1630001. [Google Scholar] [CrossRef]
- Sun, S.S.; Xue, Y.J.; Wang, Q.; Huang, H.R.; Miao, H.; Liu, Z.P. Cerium ion intercalated MnO2 nanospheres with high catalytic activity toward oxygen reduction reaction for aluminum-air batteries. Electrochim. Acta 2018, 263, 544–554. [Google Scholar] [CrossRef]
- Mori, R. Recent Developments for Aluminum-Air Batteries. Electrochem. Energy Rev. 2020, 3, 344–369. [Google Scholar] [CrossRef]
- Xie, J.D.; He, P.; Zhao, R.J.; Yang, J.H. Numerical Modeling and Analysis of the Performance of an Aluminum-Air Battery with Alkaline Electrolyte. Processes 2020, 8, 658. [Google Scholar] [CrossRef]
- Huang, L.S.; Zang, W.J.; Ma, Y.Y.; Zhu, C.Y.; Cai, D.M.; Chen, H.; Zhang, J.Z.; Yu, H.; Zou, Q.C.; Wu, L.M.; et al. In-situ formation of isolated iron sites coordinated on nitrogen-doped carbon coated carbon cloth as self-supporting electrode for flexible aluminum-air battery. Chem. Eng. J. 2021, 421, 129973. [Google Scholar] [CrossRef]
- Cheng, R.Q.; Wang, F.; Jiang, M.; Li, K.Q.; Zhao, T.S.; Meng, P.Y.; Yang, J.; Fu, C.P. Plasma-Assisted Synthesis of Defect-Rich O and N Codoped Carbon Nanofibers Loaded with Manganese Oxides as an Efficient Oxygen Reduction Electrocatalyst for Aluminum-Air Batteries. ACS Appl. Mater. Interfaces 2021, 13, 37123–37132. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.Q.; Yin, X.; Yan, Y.; Dai, Y.L.; Fan, S.F.; Qiao, X.Y.; Yu, K. Corrosion and Discharge Behaviors of Al-Mg-Sn-Ga-In in Different Solutions. J. Mater. Eng. Perform. 2016, 25, 3456–3464. [Google Scholar] [CrossRef]
- Ma, J.L.; Ren, F.Z.; Wang, G.X.; Xiong, Y.; Li, Y.Q.; Wen, J.B. Electrochemical performance of melt-spinning Al-Mg-Sn based anode alloys. Int. J. Hydrogen Energy 2017, 42, 11654–11661. [Google Scholar]
- Wang, J.B.; Wang, J.M.; Shao, H.B.; Chang, X.T.; Wang, L.; Zhang, J.Q.; Cao, C.N. The corrosion and electrochemical behavior of pure aluminum in additive-containing alkaline methanol-water mixed solutions. Mater. Corros. 2009, 60, 269–273. [Google Scholar] [CrossRef]
- Fan, L.; Lu, H.M.; Leng, J.; Sun, Z.G. Performance of Al-0.6 Mg-0.05 Ga-0.1 Sn-0.1 In as Anode for Al-Air Battery in KOH Electrolytes. J. Electrochem. Soc. 2015, 162, A2623–A2627. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.G.; Wang, R.C.; Peng, C.Q.; Liu, L. Influence of cerium on microstructures and electrochemical properties of Al-Mg-Sn-Hg anode materials for seawater battery. J. Rare Earths 2015, 33, 1010–1016. [Google Scholar] [CrossRef]
- Gudic, S.; Smoljko, I.; Kliskic, M. Electrochemical behaviour of aluminium alloys containing indium and tin in NaCl solution. Mater. Chem. Phys. 2010, 121, 561–566. [Google Scholar] [CrossRef]
- Ma, J.L.; Wen, J.B.; Li, Q.A.; Zhang, Q. Electrochemical polarization and corrosion behavior of Al-Zn-In based alloy in acidity and alkalinity solutions. Int. J. Hydrogen Energy 2013, 38, 14896–14902. [Google Scholar] [CrossRef]
- Wang, J.B.; Wang, J.M.; Shao, H.B.; Zhang, J.Q.; Cao, C.N. The corrosion and electrochemical behaviour of pure aluminium in alkaline methanol solutions. J. Appl. Electrochem. 2007, 37, 753–758. [Google Scholar] [CrossRef]
- Varnell, J.A.; Bakir, M.; DiAscro, A.M.; Chen, X.Y.; Nilufar, S.; Jasiuk, I.; Gewirth, A.A. Understanding the influence of carbon addition on the corrosion behavior and mechanical properties of Al alloy “covetics”. J. Mater. Sci. 2019, 54, 2668–2679. [Google Scholar] [CrossRef]
- Sun, Z.G.; Lu, H.M.; Fan, L.; Hong, Q.S.; Leng, J.; Chen, C.B. Performance of Al-Air Batteries Based on Al-Ga, Al-In and Al-Sn Alloy Electrodes. J. Electrochem. Soc. 2015, 162, A2116–A2122. [Google Scholar] [CrossRef]
- Sun, Z.G.; Lu, H.M. Performance of Al-0.5In as Anode for Al-Air Battery in Inhibited Alkaline Solutions. J. Electrochem. Soc. 2015, 162, A1617–A1623. [Google Scholar] [CrossRef]
- Ma, J.L.; Wen, J.B.; Gao, J.W.; Li, Q.A. Performance of Al-0.5 Mg-0.02 Ga-0.1 Sn-0.5 Mn as Anode for Al-Air Battery. J. Electrochem. Soc. 2014, 161, A376–A380. [Google Scholar] [CrossRef]
- Ma, J.L.; Wen, J.B.; Gao, J.W.; Li, Q.A. Performance of Al-1Mg-1Zn-0.1Ga-0.1Sn as anode for Al-air battery. Electrochim. Acta 2014, 129, 69–75. [Google Scholar] [CrossRef]
- Tang, Y.G.; Lu, L.B.; Roesky, H.W.; Wang, L.W.; Huang, B.Y. The effect of zinc on the aluminum anode of the aluminum-air battery. J. Power Sources 2004, 138, 313–318. [Google Scholar] [CrossRef]
- Saidman, S.B.; Munoz, A.G.; Bessone, J.B. Electrodeposition of indium and zinc on aluminium. J. Appl. Electrochem. 1999, 29, 245–251. [Google Scholar] [CrossRef]
- Pourgharibshahi, M.; Lambert, P. The role of indium in the activation of aluminum alloy galvanic anodes. Mater. Corros. 2016, 67, 857–866. [Google Scholar] [CrossRef]
- Puridetvorakul, C.; Poolthong, N.; Tareelap, N. Corrosion Behavior of Al-Zn-In Sacrificial Anode Alloys Produced by Conventional Casting and Semi-Solid Metal Casting Processes. Key Eng. Mater. 2017, 751, 101–106. [Google Scholar] [CrossRef]
- Khireche, S.; Boughrara, D.; Kadri, A.; Hamadou, L.; Benbrahim, N. Corrosion mechanism of Al, Al-Zn and Al-Zn-Sn alloys in 3 wt.% NaCl solution. Corros. Sci. 2014, 87, 504–516. [Google Scholar] [CrossRef]
- Ge, L.; Cheng, R.; Lu, Z.; Zhou, C.; Cao, L. Effects of Mn content on microstructure and mechanical properties of Al-Mg alloy plate. Heat Treat. Met. 2017, 42, 14–17. [Google Scholar]
- Yoo, H.S.; Kim, Y.H.; Jung, C.G.; Lee, S.C.; Lee, S.H.; Son, H.T. Effects of Mn Addition on Microstructure and Mechanical Properties of the Al-Si-Fe-Cu-Zn Based Alloys. J. Nanosci. Nanotechnol. 2017, 17, 7619–7622. [Google Scholar] [CrossRef]
- He, Y.D.; Zhang, X.M.; Cao, Z.Q. Effect of Minor Cr, Mn, Zr, Ti and B on Grain Refinement of As-Cast Al-Zn-Mg-Cu Alloys. Rare Metal Mater. Eng. 2010, 39, 1135–1140. [Google Scholar]
- Nam, S.W.; Lee, D.H. The effect of Mn on the mechanical of Al alloys. Korean J. Met. Mater. 2000, 6, 13. [Google Scholar] [CrossRef]
- Liu, S.H.; Pan, Q.L.; Li, H.; Huang, Z.Q.; Li, K.; He, X.; Li, X.Y. Characterization of hot deformation behavior and constitutive modeling of Al-Mg-Si-Mn-Cr alloy. J. Mater. Sci. 2019, 54, 4366–4383. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005. [Google Scholar] [CrossRef]
- Jung, H.D.; Alfantazi, A. An electrochemical impedance spectroscopy and polarization study of nanocrystalline Co and Co-P alloy in 0.1 M H2SO4 esolution. Electrochim. Acta 2006, 51, 1806–1814. [Google Scholar] [CrossRef]
- Nie, F.L.; Wang, S.G.; Wang, Y.B.; Wei, S.C.; Zheng, Y.F. Comparative study on corrosion resistance and in vitro biocompatibility of bulk nanocrystalline and microcrystalline biomedical 304 stainless steel. Dent. Mater. 2011, 27, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Li, D.Y. Mechanical and electrochemical behavior of nanocrystalline surface of 304 stainless steel. Electrochim. Acta 2002, 47, 3939–3947. [Google Scholar] [CrossRef]
- Cho, Y.J.; Park, I.J.; Lee, H.J.; Kim, J.G. Aluminum anode for aluminum-air battery—Part I: Influence of aluminum purity. J. Power Sources 2015, 277, 370–378. [Google Scholar] [CrossRef]
- He, J.G.; Wen, J.B.; Li, X.D. Effects of precipitates on the electrochemical performance of Al sacrificial anode. Corros. Sci. 2011, 53, 1948–1953. [Google Scholar] [CrossRef]
- Srinivas, M.; Adapaka, S.K.; Neelakantan, L. Solubility effects of Sn and Ga on the microstructure and corrosion behavior of Al-Mg-Sn-Ga alloy anodes. J. Alloys Compd. 2016, 683, 647–653. [Google Scholar] [CrossRef]
- Fan, L.; Lu, H.M. The effect of grain size on aluminum anodes for Al-air batteries in alkaline electrolytes. J. Power Sources 2015, 284, 409–415. [Google Scholar] [CrossRef]
- Yin, X.; Yu, K.; Zhang, T.; Fang, H.J.; Dai, H.; Xiong, H.Q.; Dai, Y.L. Influence of Rolling Processing on Discharge Performance of Al-0.5Mg-0.1Sn-0.05Ga-0.05In Alloy as Anode for Al-air Battery. Int. J. Electrochem. Sci. 2017, 12, 4150–4163. [Google Scholar] [CrossRef]
- Ma, J.L.; Wen, J.B.; Ren, F.Z.; Wang, G.X.; Xiong, Y. Electrochemical Performance of Al-Mg-Sn Based Alloys as Anode for Al-Air Battery. J. Electrochem. Soc. 2016, 163, A1759–A1764. [Google Scholar] [CrossRef]
- Umoren, S.A.; Li, Y.; Wang, F.H. Effect of aluminium microstructure on corrosion and inhibiting effect of polyacrylic acid in H2SO4 solution. J. Appl. Electrochem. 2011, 41, 307–315. [Google Scholar] [CrossRef]
- Sovizi, M.R.; Afshari, M.; Jafarzadeh, K.; Neshati, J. Electrochemical and microstructural investigations on an as-cast and solution-annealed Al-Mg-Sn-Ga alloy as anode material in sodium chloride solution. Ionics 2017, 23, 3073–3084. [Google Scholar] [CrossRef]
- Ren, J.M.; Ma, J.B.; Zhang, J.; Fu, C.P.; Sun, B.D. Electrochemical performance of pure Al, Al-Sn, Al-Mg and Al-Mg-Sn anodes for Al-air batteries. J. Alloys Compd. 2019, 808, 151708. [Google Scholar] [CrossRef]
- Park, I.J.; Choi, S.R.; Kim, J.G. Aluminum anode for aluminum-air battery—Part II: Influence of In addition on the electrochemical characteristics of Al-Zn alloy in alkaline solution. J. Power Sources 2017, 357, 47–55. [Google Scholar] [CrossRef]
- Fan, L.; Lu, H.M.; Leng, J.; Sun, Z.G.; Chen, C.B. The Study of Industrial Aluminum Alloy as Anodes for Aluminum-Air Batteries in Alkaline Electrolytes. J. Electrochem. Soc. 2016, 163, A8–A12. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).