Abstract
The problem of cleaning and disinfecting surfaces has become extremely important in the context of the ongoing SARS-CoV-2 coronavirus pandemic. However, it should be considered that, in everyday life, we come into contact with many other viruses, as well as pathogenic bacteria and fungi, that may cause infections and diseases. Hence, there is a continuous need to search for new and more effective methods of fighting pathogens. Due to their documented antimicrobial activity, silver nanoparticles may be an interesting alternative to the commonly used surface cleaners and disinfectants. Therefore, the present study aimed to evaluate the bactericidal properties of silver nanoparticles obtained with the use of nontoxic plant waste biomass against bacteria isolated from the environment. Silver nanoparticles with the desired physicochemical characteristics were obtained by a simple and rapid chemical reduction method using plant waste such as unused parsley stems and potato peels (the biogenic method). A nanosilver colloid was also prepared by the chemical reduction method, but with reducing and stabilizing chemical substances (the chemical method) used as a control. The bacterial susceptibility to nanosilver synthesized using both methods was evaluated using the disk-diffusion method. The sensitivity of particular Escherichia coli and Staphylococcus aureus isolates to nanosilver varied considerably, and the strongest antimicrobial effect was found in the case of nanoparticles synthesized by the chemical method using a strong chemical reducing agent and a polymeric stabilizing substance, while nanosilver obtained using the biogenic method, using phytochemicals, also had a strong antimicrobial effect, which was found to be extremely satisfactory. Thus, it can be strongly concluded that the biogenic, pro-ecological method of synthesis with the use of plant waste biomass presented in this work allows the application of biogenic nanosilver as a component of agents for washing and disinfection of public utility surfaces.
1. Introduction
The demand for disinfectants and cleansers has been continuously increasing and often far exceeds their supply. This phenomenon was particularly noticeable after the outbreak of the SARS-CoV-2 pandemic when disinfectants and cleaning agents became scarce in many countries [,]. There is also a growing public consciousness of the fact that cleanliness and hygiene in areas used by people contribute directly to inhibiting the spread of diseases of viral and microbiological etiology, thus affecting the health safety of the entire population. This is why much more attention is being paid to cleaning and disinfection procedures on surfaces in these areas. In addition to traditional methods of cleaning and disinfection using agents based on sodium dichloroisocyanurate, potassium peroxymonosulfate, aromatic oils, and organic acids, preparations containing nanosilver (AgNPs) are also being sought due to their proven bactericidal properties [,]. Nanosilver is most commonly obtained by chemical, physical, or physico-chemical synthesis [,,,]; however, the aforementioned methods of synthesis, although effective and well-known, also have drawbacks that are problematic in the context of the wider application of nanosilver in that they pose certain difficulties. These complications are related to the safety of nanosilver application due to its toxicity to higher organisms, including humans []. The toxicity of nanosilver synthesized by these methods results from the use of toxic reagents in the process of its preparation, the formation of harmful by-products during the process, and the lack of appropriate methods of separation of nanostructures from the remaining, often toxic, reagents []. Chemical and physical methods are also cost-intensive and the amount of pollution generated puts a major strain on the environment []. Therefore, new, less toxic, and environmentally friendly methods of nanoparticle synthesis are constantly being developed [,]. An extremely promising alternative to chemical and physical methods is the biological synthesis of nanoparticles, called “green synthesis” []. The “green” approach to nanoparticle synthesis has strong potential due to its ease of fabrication, economic viability, availability of environmentally friendly substrates, and increased applicability, especially in the biomedicine and food industries [].
Syntheses are usually performed under mild conditions such as atmospheric pressure, with no need for an inert environment, and a temperature not exceeding 100 °C, allowing these processes to be classified as low energy and non-labor-intensive. The desired effect of the application of biological synthesis on nanosilver preparation is the complete elimination or significant minimization of the waste generated and to strive to ensure that the processes are carried out in an environmentally friendly manner [,,].
Evaluating the routes for the green synthesis of silver nanoparticles and possible sources of natural substances promoting the synthesis of nanoparticles, it was concluded that plant extracts appear to be advantageous compared to the use of microorganisms or fungi as they usually do not require toxic reducing agents and stabilizers. In addition, the use of radiation, high-temperature, microbial or fungal strains, and thus expensive growth media for their growth and maintaining conditions for optimal growth under sterile conditions is not required. The risk of infection or contamination during synthesis and application is also diminished. From an economic point of view, plant extracts should become a priority due to their ubiquity and ease of culture [,].
Plant parts such as leaves, stems, roots, fruits, shoots, flowers, bark, seeds, and their metabolites have so far been successfully used for efficient biosynthesis of nanoparticles []. For example, Moldovan et al. [] reported the synthesis of spherical silver nanoparticles from an extract of elderberry, Sambucus nigrafruit []. Interestingly, their in vitro studies conducted on laboratory rats suggested that the functionalization of silver nanoparticles with natural phytochemicals could protect cellular proteins from the generation of reactive oxygen species []. They also reported the biosynthesis of silver nanoparticles using an aqueous extract obtained from the Alternanthera sessilis plant. The authors showed that the extract containing alkaloids, tannins, ascorbic acid, carbohydrates, and proteins serve as reducing agents and limit excessive particle growth beyond the nanometer scale. The biomolecules in the extract also acted as stabilizers for the silver nanoparticles obtained over time []. Logaranjan et al. [] described the controlled shape- and size-dependent synthesis of silver nanoparticles from a plant extract of Aloe vera and demonstrated a four-times-higher antimicrobial activity of the nanosilver compared to commonly available antibiotics. In the paper [], Roy et al. described the biosynthesis of silver nanoparticles using the Cucumis sativus cucumber extract—the photocatalytic properties of the obtained nanostructures suggested the high efficiency of these biosynthesized nanoparticles in the degradation of organic dyes under sunlight. Moreover, the result of the antimicrobial test against Staphylococcus aureus, Klebsiella pneumoniae, and Escherichia coli showed that these nanoparticles have effective antibacterial properties. Another example of the use of raw plant materials in the synthesis of nanometals is in the study by Karatoprak et al. [], who demonstrated that the extract of Pelargonium endlicherianum contains gallic acid, apocyanin, and quercetin, which act effectively as reducing agents for silver nanoparticles. In the paper [], a study on the biosynthesis and antimicrobial activity of silver nanoparticles obtained using Asian basil Ocimum sanctum leaf extract was made public. The biosynthesized silver nanoparticles were in the size range of 4–30 nm and exhibited an antimicrobial activity that was greater against Gram-negative than Gram-positive microorganisms. They also showed that the nanoparticles they synthesized had stronger activity than silver nitrate salt and the commonly used antibiotic—ciprofloxacin.
Kaviya et al. performed biosynthesis of silver nanoparticles using Citrus sinensis peel extract. The peel extract of the aforementioned citrus can be used for biosynthesis because it contains many active ingredients and is rich in vitamin C, flavonoids, acids, and volatile oils, which allow the bioreduction of silver ions. In addition to the physicochemical characteristics of the nanoparticles, antimicrobial properties against Escherichia coli, Pseudomonas aeruginosa (Gram-negative bacteria), and Staphylococcus aureus (Gram-positive bacteria) have also been investigated, indicating that nanoparticles synthesized using plants act as an effective antimicrobial agent []. On the other hand, Roy et al. [,] conducted studies on the synthesis of silver nanoparticles using both the tuber infusion of the common potato (Solanum tuberosum) and the leaf extract of parsley (Petroselinum crispum); however, they were not waste agricultural biomass, which was successfully used by the authors in present research, but rather full-value plant materials usually used for food processing. Potatoes are one of the most important food crops, the edible part of which is the tuber, rich in essential amino acids such as leucine, lysine, phenylalanine, and threonine, which are not synthesized by the human body. It also contains proteins, thiamine, and ascorbic acid, making potatoes a potentially good reducing and stabilizing raw material for the preparation of silver nanoparticles [,]. Parsley is a biennial plant that belongs to the celery family. It is characterized by a bare stem and limp and pinnate leaves, while its white root has a spicy and delicate smell. Parsley roots contain about 4.6 g of total sugars, 0.8 g of protein, 39 mg of vitamin C, and a small amount of provitamin A in 100 g fresh weight, which means many researchers emphasize that the content of these compounds has a beneficial effect on the synthesis of nanoparticles by biological methods [,,,].
In this regard, silver nanoparticles are increasingly being used as ingredients in cleaning and disinfection formulations because of their bactericidal properties. Therefore, the development of an environmentally friendly method for the biological synthesis of nanosilver would increase the production and application of AgNPs in this area. Taking into account the fact that the demand for agents for the washing and disinfection of hands and surfaces is growing intensively in Poland, and the world, it is clear that the requirement for such formulations will increase. With unquestionable advantages resulting from the potential wide range of application possibilities of biologically synthesized nanosilver, it is also important to realize that the common use of AgNPs may promote the development of resistance of microorganisms to this metal. Microorganisms have a broad ability to adapt to unfavorable environmental conditions; therefore, there is always a danger that uncontrolled and excessive use of nanosilver as a component of cleaning and disinfecting agents will, in the long run, lead to the emergence of microbial resistance to nanoparticles, analogous to what happens with antibiotics [].
For this reason, the study aimed to determine whether silver nanoparticles synthesized with components naturally occurring in waste plant tissues have a bactericidal effect on S. aureus and E. coli strains isolated from surfaces in public areas. Moreover, the spectrum of bactericidal activity of the silver colloid obtained with the use of a strong chemical reductant (sodium borohydride) and a polymeric stabilizer (polyvinylpyrrolidone) was compared with the colloids obtained by the pro-ecological method, i.e., with the use of infusions obtained from plant waste biomass, without additional chemical components. Nanosilver synthesized according to an innovative, environmentally friendly method could be used in the future as a component of preparations used for washing, disinfection, and sanitization of large facilities.
2. Materials and Methods
2.1. Preparation of AgNPs by Classic and Biogenic Methods
2.1.1. Classic Synthesis Method of AgNPs Using Commercial Chemicals
To prepare 100 mL of a 500 ppm AgNPs suspension, 0.0787 g of AgNO3 (POCH S.A., p.a.) was added to 90 mL of a 3% aqueous solution of polyvinylpyrrolidone (PVP, M = 8000 g/mol, Acros Organics, p.a.) under continuous stirring on a magnetic stirrer at a stirring speed of 500 rpm (solution A). Then, 0.0017 g of NaBH4 (POCH S.A., p.a.) was dissolved in 10 mL of 3% PVP solution (solution B). After complete dissolution of the silver salt, solution B was added dropwise to solution A. After dropping, the mixture was stirred for 15 min at room temperature. The obtained nanoparticles were stored at room temperature.
2.1.2. Biogenic Synthesis Method of AgNPs Using Plant Waste Biomass
An amount of 10 g of finely chopped plant waste biomass (potato peelings or inedible parsley stems) was weighed, and then the biomass was poured into 100 mL of boiling distilled water. The infusion time was 30 min while covered. After the specified time, the infusion was filtered through a kitchen strainer to separate the solids from the liquid. Then, to obtain a 500 ppm AgNPs solution, 0.0787 g of AgNO3 was added to 90 mL of 3% PVP solution under continuous stirring on a magnetic stirrer at a stirring speed of 500 rpm. Once the silver salt was completely dissolved, 10 mL of parsley or potato waste infusion was dropped in. After dropping in, the resulting mixture was stirred for 3 h at room temperature. The final solutions were stored at room temperature.
2.2. Total Phenolic Content (TPC) and Antioxidant Capacity of Agricultural Waste Infusions
The total free radical scavenging capacity of the prepared plant infusions was examined by decolorizing the ethanolic solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH), which generates a purple color in the ethanol solution and changes to a yellow color in the presence of antioxidants. Briefly, the radical solution was prepared by dissolving 19.71 mg of DPPH (Sigma-Aldrich, St. Louis, MO, USA) in 100 mL of 96% ethanol. This prepared solution was diluted with ethanol to reach an absorbance signal of approximately 0.9 at 517 nm. An amount of 1.0 mL of this solution was mixed with 0.5 mL of each infusion at different concentrations (0.63–25 mg d.w./mL) and left in the dark for 10 min. The control sample (A0) was obtained by adding 0.5 mL of ethanol to 1.0 mL of DPPH solution. The absorbance of the mixtures was measured spectrophotometrically at 517 nm against ethanol as a blank sample. The measurements were carried out fivefold and the average value (A) was determined. The percentage of DPPH radical scavenging activity was calculated using the following equation:
and expressed as the extract concentration necessary to neutralize 50% of free DPPH radicals (IC50). The IC50 was calculated by plotting the correlation between the concentration of the extract (mg d.w./mL) and inhibitory concentration (IC, %).
IC [%] = (A0 − A)/A0 × 100
Total Phenolic Compounds (TPCs) in the plant infusion were quantified with the spectrophotometric Folin–Ciocalteau (F–C) method, which relies on the transfer of electrons from phenolic compounds to form a blue chromophore. The maximum absorption is proportional to the concentration of phenolic compounds. The content of phenolic compounds of the samples was expressed as gallic acid equivalents (GAE, mg·g−1 d.w.). Therefore, in this method, the gallic acid was used to set-up the standard curve in a concentration range of 0.05, 0.15, 0.25, 0.35, and 0.50 mg·mL−1 using gallic acid aqueous solution (5 mg·mL−1) as a working solution. Calibration curves were prepared by mixing 20 µL of individual calibration standards, 1.58 mL of double distilled water, and 100 µL of F–C reagent. Subsequently, 300 µL of a saturated solution of Na2CO3 was added, and then each sample was thermostatically maintained at 40 °C for 30 min. The tested plant infusions were prepared in the same manner using 20 µL of each infusion instead of a standard solution. All samples were analyzed in five replicates and absorbance measurements were performed at 765 nm against a blank sample containing no gallic acid.
2.3. Physicochemical Characterization of AgNPs
A Thermo Scientific UV-Vis Evolution 220 spectrophotometer was used to analyze the optical properties of the obtained nanostructures. The measurement was performed in 4 mL of polystyrene cuvettes in the wavelength range from 300 to 700 nm, and the spectral resolution was 2 nm.
The particle sizes and the size distribution of the nanoparticles were analyzed by DLS using a Zetasizer Nano ZS apparatus manufactured by Malvern Instruments Ltd. (Malvern, UK) and equipped with a 4 mW helium–neon laser that produces photons with a wavelength of 633 nm. Each of the samples was measured three times, each measurement consisting of six 10 s runs, which precisely determines the main values. The measurements were carried out at a temperature of 25 °C.
The nanosuspension morphology and the presence of silver in the samples were confirmed using the Hitachi S-4700 model Tabletop Microscope TM3000 equipped with a low-vacuum backscattered electron detector (BSE) and energy-dispersive X-ray microanalyzer (EDS) by ThermoNoran. The samples were mounted on a specialized holder adjusted in height to optimize operation.
2.4. Bacterial Cultures Used
Bacteria of the species S. aureus and E. coli used in the experiment came from the authors’ collection, and two reference strains of E. coli ATCC 25,922 and S. aureus ATCC 25,923 were also used. Environmental strains were isolated from the public surfaces by the imprinting method [] using Chapman’s medium (BTL, Warsaw, Poland) for S. aureus (cultured 37 °C, 24 h) and TBX agar (BTL, Warsaw, Poland) for E. coli (cultured 44 °C, 24 h). The bacteria were isolated from public surfaces, that is, the floor, door handles, cabinet handles, and handrails, located on the campus of the University of Agriculture in Krakow, Poland. The aim was to set a collection of several dozen isolates of environmental bacteria of the species S. aureus and E. coli, as representatives of Gram-positive and Gram-negative bacteria, respectively. The macroscopically characteristic colonies that grew on Chapman’s agar and TBX agar slides were prepared and stained using the Gram method, and microscopic observations were made. The final stage of identification was the MALDI-TOF MS technique (Bruker Daltonik, Bremen, Germany), which was performed according to the methodology recommended by the manufacturer and the guidelines contained in the following paper [].
2.5. Antibacterial Activity—Disk Diffusion Assay
To determine the susceptibility of the collected S. aureus and E. coli isolates to silver nanoparticles, the applied methodology was based on previous authors or other papers [,,,,]. The disc diffusion method was used to collect results in an accessible and easy-to-interpret way that can be compared with the analysis of drug resistance to antibiotics (control). The MHA medium (Mueller-Hinton agar, BTL, Warsaw, Poland) was used, onto which a suspension of the tested microorganisms of 0.5 McFarland Standard density was inoculated using a sterile cotton swab. The inoculum was prepared using a densitometer (DEN-1, Biosan, Józefów, Poland) in tubes with saline (0.9% NaCl). Then, sterile filter paper disks (Oxoid, Ireland) were placed on the prepared cultures using flame-sterilized forceps, and 10 µL of each concentration of 1-week-old suspension of silver nanoparticles (500, 250, 150, 100, 75, 50, and 25 μg/mL) was pipetted onto them. A deionized water disc was used as a negative control and an antibiotic disc with ampicillin (AMP, 10 µg) for E. coli and cefoxitin (FOX, 30 µg) for S. aureus (Oxoid, Ireland) was used as positive controls. The plates were incubated for 24 h at 37 °C, after which the zones of growth inhibition around the discs were read (mm). All tests for both the environmental strains isolated from public utility surfaces and the reference strains E. coli ATCC 25,922 and S. aureus ATCC 25,923 were set-up in triplicate. The results of the susceptibility assessment of the tested strains to ampicillin and cefoxitin were interpreted in accordance with EUCAST guidelines []. The Minimum Inhibitory Concentration (MIC) understood as the lowest concentration of an antimicrobial ingredient or agent that is bacteriostatic (prevents the visible growth of bacteria) has also been designated []. The diameter of the disc with the individual concentrations of nanosilver was 6 mm. No inhibition of bacterial growth was read as 6 mm, while any value >6 mm was measured and interpreted as inhibition of bacterial growth.
2.6. Statistical Analysis
Statistical analysis was conducted to evaluate the correlation between the concentration of AgNPs and the inhibition of bacterial growth. Statistical analysis was performed using Statistica v. 13.1 software (StatSoft, USA).
3. Results and Discussion
The evaluation of the bactericidal properties of silver nanoparticles obtained by chemical and biogenic methods was followed by physicochemical analysis of the obtained waste infusions and nanostructures. First, the antioxidant and reducing abilities of the infusions prepared from agrochemical plant waste were determined. Polyphenolic compounds contained in plant material exhibit oxygen capture properties and reduced activity [,]. Therefore, it was important to determine their total amount in the prepared plant infusions. Table 1 summarizes the results of the scavenging of DPPH radicals by antioxidants contained in the prepared infusions and TPC (expressed as milligrams per gram dry weight of the plant waste studied).

Table 1.
Antioxidant capacity and TPC of plant infusions.
As can be observed, the TPC results, as well as the antioxidant activity values, showed significant differences depending on the plant material from which the infusions were prepared. The infusion of parsley stem waste (Petroselinum crispum) proved to be a more effective scavenger of DPPH radicals compared to the infusion of potato peel, Solanum tuberosum, as only a concentration of 3.3 ± 0.1 mg d.w./mL was able to neutralize 50% of DPPH free radicals. The data also showed that the TPC of the Petroselinum crispum infusion was significantly higher than that obtained for Solanum tuberosum. The lower TPC value (0.51 ± 0.0 mg GAE·g−1 d.w.) along with the IC50 (17.6 ± 0.8 mg d.w./mL) of the potato infusion may be related to the selected solvent used for extraction, as reports can be found in the literature in which acetone aqueous solution was an effective extractant []. Furthermore, the analyses conducted suggest that the antioxidant activity in the prepared infusions was mainly influenced by the number of phenolic compounds, which is consistent with reports in the literature. Wang et al. reported a significant effect of the extraction method used on the content of polyphenolic compounds from potato peels [].
In the next part of the study, the presence of nanoparticles in the obtained mixtures was confirmed by UV-Vis absorption spectroscopy, as metallic nanoparticles show the ability to absorb visible and ultraviolet radiation. Colloidal solutions of metal nanoparticles, in the case of silver nanoparticles with a characteristic yellow-brown color with an orange afterglow, show strong absorption bands in the UV-Vis range caused by the surface plasmon resonance phenomenon (SPR).
The presence of silver nanoparticles is confirmed by the occurrence of an absorption maximum in the range of about 380–450 nm [,]. The analysis of the UV-Vis spectra of the nanosuspensions obtained directly after the synthesis, and then after 1 week and 2 months, confirmed the above theoretical assumptions, as shown in Figure 1. A clear difference was observed in the appearance of the spectra—shape, symmetry, peak width, as well as their location depending on the colloid tested. It has been demonstrated that the classical method, in which the reduction in silver occurs rapidly in time—at the point of mixing the metal ions with molecules of a strong reducing agent—leads to sharp, symmetric peaks with the absorption maximum shifted toward shorter wavelengths (λmax~400 nm), which indicates monodispersity and the achievement of nanometric sizes not exceeding several tens of nanometers. This tendency is maintained throughout the research—a characteristic, narrow peak is also present two months after the synthesis. The spectra are different in the case of biogenic synthesis, which uses raw plant materials without the use of additional reducing and stabilizing components. When plant infusions were used, silver nanoparticle colloids were successfully obtained, with asymmetric flattened and broadened peaks with absorption maxima shifted toward longer wavelengths, proving that larger and polydisperse nanoparticles were obtained in comparison to those acquired by the method of using a strong reducing and stabilizing component. According to the literature data and previous studies of the authors, it has been proven that with the increase of nanoparticles in suspension, more electrons can scatter the incident electromagnetic wave, which leads to disturbances in the absorption process. As a result of the so-called retardation of the electromagnetic wave, the spectral spectra obtained are slightly broadened compared to the spectra obtained after the irradiation of small particles and a redshift of λmax is observed, as is the case with the spectra of AgNPs potato and AgNPs parsley colloids [,]. Moreover, it should be emphasized that numerous chemical compounds are present in the infusions, which comprehensively reduce and stabilize the particles, causing the particle coated with anti-aggregation substances to have a larger diameter. The 2-month-old colloids show the expected stability over time. In the case of samples with infusions from plant waste, the older suspension gives the clearer spectrum, which means that in samples obtained with the biogenic method, the time of reducing ions to free atoms is extended and the reducing-stabilizing agents act less rapidly in comparison with a strong reducer (sodium borohydride) in the chemical method.
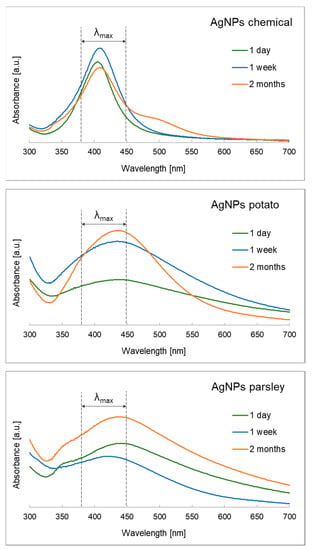
Figure 1.
The UV-Vis spectra of silver nanoparticle suspensions depending on time and preparation method.
The analysis of the average particle size and particle size distribution in the suspension supported the above observations (Figure 2). The silver nanoparticles obtained using the classical method exhibited the smallest average size and the narrowest particle size distribution of all the colloids tested—the average size was about 50 nm; however, it should be emphasized that almost all the nanoparticles formed adopted an average size below 10 nm, with a 0.1% contribution of particles beyond the nanometer scale, making the average size overestimated. The use of a suitable separation method would yield particles with sizes below 10 nm, eliminating the contribution of micrometric agglomerates. In contrast, the use of plant waste infusions as reducing and stabilizing agents led to larger particles with a significant proportion of particles of different sizes—polydisperse, which is consistent with the conclusions drawn from the absorption spectra. The use of raw plant materials being a mixture of many compounds, instead of a simple system of silver salt/reducer/stabilizer, as in the case of the classical method, will cause the obtained nanostructures to show larger sizes and a higher proportion of particles exceeding the nanometric scale. In the case of the potato peel infusion, the average particle size was 126.3 ± 17.2 nm with as much as a 7% share of particles larger than 4 μm—the largest nanoparticles among all the samples tested were obtained using this method. Alternatively, the infusion of the waste parts of parsley stalk made it possible to obtain particles with an average size larger than the nanostructures obtained with the potato infusion, reaching approximately 200 nm, with the proportion of particles with an average size of 13.98 nm being above 80%, while the remaining 17.5% of the yield of particles had an average size of ~300 nm.
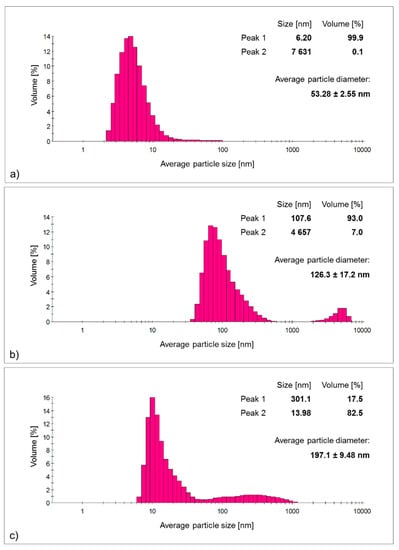
Figure 2.
The histograms of particle size distribution and average particle sizes of 1-week-old silver nanoparticle colloids obtained by: (a) classical method (AgNPs chemical); (b) bio-based method using potato waste (AgNPs potato); (c) bio-based method using parsley waste (AgNPs parsley).
In conclusion, it is possible to obtain nanoparticles with the expected physicochemical characteristics using waste raw material; however, they have a larger size distribution and particle size compared to the use of classical methods [,,].
The SEM and SEM-EDS analysis, along with the particle distribution determination, is shown in Figure 3 and Figure 4, respectively. In general, a uniform distribution of nanoparticles was observed in the lyophilizates studied; however, it is worth noting that in the case of the nanoparticles in the AgNPs parsley sample, the nanoparticles densely covered the sample surface, while the nanoparticles obtained using the potato peel infusion, in the AgNPs potato colloid, tended to accumulate and form prisms, agglomerates, and uneven surfaces as in the parsley nanoparticles.
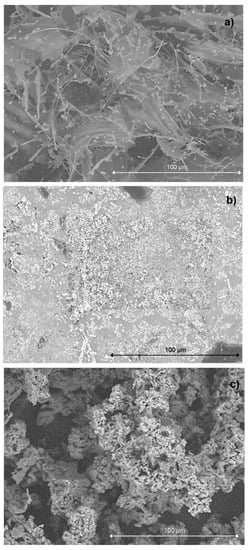
Figure 3.
The SEM images of freeze-dried 1-week-old silver nanoparticle colloids obtained by: (a) classical method (AgNPs chemical); (b) biogenic method using potato waste (AgNPs potato); (c) biogenic method using parsley waste (AgNPs parsley), magnification ×1000.
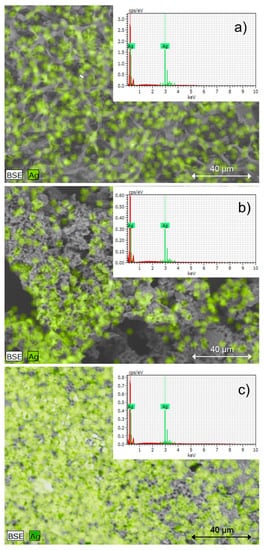
Figure 4.
The EDS-SEM images of freeze-dried 1-week-old silver nanoparticle colloids with silver distribution and confirmation of its presence obtained by: (a) classical method (AgNPs chemical); (b) biogenic method using potato waste (AgNPs potato); (c) biogenic method using parsley waste (AgNPs parsley), magnification ×1000.
Spot analysis of microscopic images using an energy-dispersive X-ray microanalyzer combined with a scanning electron microscope (SEM-EDS) confirmed the presence of silver in every colloid examined. This means that the nanostructures visible in the micro-images—uniformly distributed on the analyzed surfaces—were metallic nanoparticles.
In summarizing the obtained preliminary physicochemical studies of nanoparticles, it should be emphasized that the presence of characteristic absorption peaks, the lack of an excessive aggregation of nanoparticles, and the desired particle size confirmed the proper selection of substrates for nanostructures’ synthesis. It turns out that the use of safe, pro-ecological components derived from plant waste led to the production of silver nanoparticle suspensions with physicochemical parameters desirable for the control of selected pathogens.
As a result of the isolation of microorganisms from public utility surfaces, a collection of 160 bacterial strains was completed (80 E. coli and 80 S. aureus strains). The results of the average growth inhibition of environmental E. coli strains and E. coli reference strain ATCC 25,922 due to different types and concentrations of nanosilver are presented below (Table 2).

Table 2.
Antibacterial activity of nanosilver on E. coli and ampicillin as a positive control.
Based on the results, it should be stated that all types of AgNPs tested had antimicrobial properties that increased with their concentration. The minimum inhibitory concentration (MIC) of all E. coli isolates was achieved by 150 μg/mL (AgNPs potato), 75 μg/mL (AgNPs parsley), and 50 μg/mL (AgNPs chemical). Resistance to ampicillin was detected in 26 strains, 19 of which did not show growth inhibition around the antibiotic disc. Moreover, in 7 others, the zone of growth inhibition was less than 14 mm, which, according to EUCAST guidelines [], indicates resistance to this antibiotic. This is important as, despite resistance to ampicillin, the same E. coli isolates were sensitive to nanosilver, with 23 strains having a MIC of 100 μg/mL (AgNPs potato), 17 strains having a MIC of 50 μg/mL (AgNPs parsley), and 26 strains having a MIC of 50 μg/mL (AgNPs chemical).
The results of the average growth inhibition of environmental S. aureus strains and S. aureus reference strain ATCC 25,923 (Table 3) due to different types and concentrations of nanosilver are presented below.

Table 3.
Antibacterial activity of nanosilver on S. aureus and cefoxitin as a positive control.
The MIC of all S. aureus isolates was 150 μg/mL (AgNPs potato), 250 μg/mL (AgNPs parsley), and 250 μg/mL (AgNPs chemical). Among all strains tested, the zone of growth inhibition around the cefoxitin disc was greater than 22 mm, which, according to EUCAST guidelines [], indicates sensitivity to this antibiotic. Within the cefoxitin-sensitive S. aureus isolates, there were both resistant and sensitive strains to the nanoparticles used; therefore, on this basis, it can be concluded that there was no relationship between these two types of resistance.
The analysis of the dependence of the mean zone of inhibition of bacterial growth on the concentration of nanoparticles confirmed a remarkably high positive correlation between these two values. The value of Pearson’s r correlation coefficient for E. coli and S. aureus, respectively, was: 0.88 (AgNPs potato), 0.80 (AgNPs parsley), 0.86 (AgNPs chemical), 0.98 (AgNPs potato), 0.97 (AgNPs parsley), and 0.95 (AgNPs chemical) for p < 0.05.
The exemplary nanosilver MICs for E. coli (Table 4) and S. aureus (Table 5) isolates available in the literature are summarized below.

Table 4.
Selected studies on the antibacterial activity of silver nanoparticles to E. coli.

Table 5.
Selected studies on the antibacterial activity of silver nanoparticles to S. aureus.
As shown in Table 3 and Table 4, the inhibition of E. coli and S. aureus growth was possible in a broad spectrum of nanosilver concentrations ranging from 6.75 to 400 μg/mL and from 13.5 to 430 μg/mL, respectively. However, it should be noted that no significant effect of nanoparticle origin (chemical or biogenic) or particle size (below or above the conventional scale of 100 nm) was observed, which means that nanomaterials based on agrochemical waste products can be used successfully and with similar efficacy in place of their chemical equivalents.
The results obtained in the present work, but also in earlier research by the authors [,,], showed that nanosilver prepared with an environmentally friendly method with the use of plant waste biomass has bactericidal properties that are most evident at higher nanoparticle concentrations than those described in Table 3 and Table 4. This state of affairs should not be surprising considering that the differences in bactericidal activity of nanosilver are due to many factors such as the synthesis model (chemical, physical, physicochemical, and biological), nanoparticle size (smaller diameter—higher biological activity), shape (trigonal, spherical, elongated, and rod-shaped), the concentration of nanoparticles in solution, and the contact time between the nanostructure and microorganism, as well as the species of microorganism and its individual (strain) sensitivity to silver [,,,].
Analysis of the susceptibility of isolates from E. coli and S. aureus species to AgNPs showed that strains isolated from public utility surfaces and representing the same species were characterized by a different resistance to particular concentrations of nanostructures and had different MIC values. The differences in the zones of bacterial growth inhibition of S. aureus and E. coli bacteria indicated that the resistance to particular concentrations was a strain-specific feature and was not related to belonging to a particular species (differences between individual strains were observed; for example, within E. coli, there were strains sensitive and resistant to nanosilver).
Examination of the diameters of the zones of bacterial growth inhibition proved that Gram-positive S. aureus bacteria were more resistant to the applied nanosilver (higher MIC for the applied AgNPs). The reasons for this phenomenon should be attributed to morphological and physiological differences between Gram-positive and Gram-negative bacteria []. Previous studies by the authors [,,] and Mosallam et al. [], Kawahara et al. [], Rhim et al. [], Kim et al. [], and Egger et al. [] showed that a higher resistance to nanosilver is presented by Gram-positive bacteria. The cell wall of these bacteria is saturated with peptidoglycan, which is multilayered and negatively charged. Thus, the positively charged silver cations released from the coated nanoparticles are more efficiently captured and bound by the negative charge found on the cell walls of Gram-positive bacteria. This immobilized the AgNPs and kept them outside the cell longer []. In addition, peptidoglycan forms a stable complex with thymic acids and lipoteichoic acids, which hinder the penetration of nanosilver into the cell.
The bactericidal properties of AgNPs make nanosilver a component of soaps and disinfectants considered to be extremely effective in eliminating microorganisms [,,]. The described antimicrobial properties of AgNPs result from the ability of Ag+ ions to bind to cellular envelopes, disrupt their function, inactivate key enzymes, interfere with metabolic and respiratory processes, interact with nucleic acids, and generate free radicals [,,]. On the other hand, the level of activity of AgNPs against microorganisms depends on the shape and size of the nanostructures, the concentration of nanoparticles in solution, and the sensitivity to the silver characteristic of each microorganism []. In the present study, the sensitivity to AgNPs was dependent on the type of nanosilver (AgNPs chemical > AgNPs parsley > AgNPs potato for E. coli and AgNPs chemical = AgNPs parsley > AgNPs potato for S. aureus). The nanosilver used was synthesized by an environmentally friendly method using plant components derived from waste biomass (AgNPs potato, AgNPs parsley) and by a chemical method (AgNPs chemical) using typical chemical reactants.
The collected results indicate that all three types of silver nanoparticles tested possessed strong antibacterial properties against both Gram-positive and Gram-negative bacteria and can be used as components of washing and disinfecting agents. On the other hand, taking into account the profit and loss calculations, it should be concluded that the ecological aspect combined with the effectiveness of the bactericidal action of silver nanoparticles favors the application of an environmentally friendly method of AgNPs synthesis. Thus, it can be strongly concluded that the pro-ecological, bio-based method of AgNPs synthesis, presented in this work, with the use of nontoxic plant components, best allows the use of nanosilver as a component of agents for cleaning and disinfecting surfaces of public use.
4. Conclusions
The main conclusion of the study is to confirm the initial assumption that it is possible to use plant waste biomass to obtain stable silver nanoparticles with strong antimicrobial activity to fight microbes that threaten health and life during daily activities. The studies carried out in this work further allow the conclusion that nanosilver with favorable physicochemical characteristics has strong antimicrobial properties against E. coli and S. aureus bacteria isolated from public utility surfaces. Thus, it can be seen that silver nanoparticles synthesized by a biogenic, pro-ecological method with the use of nontoxic substrates derived from plant waste biomass with confirmed reducing and stabilizing properties could be effectively used as components of preparations for decontamination, washing, and disinfection of surfaces and rooms. This is a response to the rapidly emerging and growing resistance of microorganisms to various bactericides and bacteriostatic agents, i.e., antibiotics, chemotherapeutics, and disinfectants currently being used. However, due to the ability of microorganisms to adapt to unfavorable environmental conditions, the potential resistance of microorganisms to nanosilver should be monitored.
Our team’s ongoing research is focused on developing more time-stable nanometal colloids prepared using waste nontoxic biomass, more monodisperse with smaller size distributions, and which have a broader spectrum of antimicrobial activity. This research should be continued in terms of the application of nanosilver in commercial ready-made preparations for cleaning and disinfecting surfaces. In these studies, we tested the active ingredient in a laboratory, which may be a first step toward the development of new, sustainable disinfectants. Therefore, the further use of ready-made formulations in the form of washing preparations will answer the question about their target activity in terms of bacteria.
Author Contributions
Conceptualization, K.W.-K. and D.M.; methodology, K.W.-K. and D.M.; software, A.S.; validation, A.S., D.M. and K.P.; formal analysis, A.S., D.M. and K.P.; investigation, Z.W.; resources, K.W.-K., A.S., D.M. and K.P.; data curation, K.W.-K., A.S., D.M. and K.P.; writing—original draft preparation, K.W.-K., D.M. and K.P.; writing—review and editing, K.W.-K., D.M. and A.S.; visualization, K.W.-K. and D.M.; supervision, Z.W.; funding acquisition, K.W.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a subsidy of the Ministry of Education and Science for Cracow University of Technology and University of Agriculture in Krakow for the 2021 year.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kampf, G.; Scheithauer, S.; Lemmen, S.; Saliou, P.; Suchomel, M. COVID-19-associated shortage of alcohol-based hand rubs, face masks, medical gloves, and gowns: Proposal for a risk-adapted approach to ensure patient and healthcare worker safety. J. Hosp. Infect. 2020, 105, 424–427. [Google Scholar] [CrossRef]
- Berardi, A.; Perinelli, D.R.; Merchant, H.A.; Bisharat, L.; Basheti, I.A.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. Hand sanitisers amid CoViD-19: A critical review of alcohol-based products on the market and formulation approaches to respond to increasing demand. Int. J. Pharm. 2020, 584, 119431. [Google Scholar] [CrossRef] [PubMed]
- Wolny-Koładka, K.; Malina, D. Toxicity assessment of silver nanoparticles against Escherichia coli strains isolated from horse dung. Micro Nano Lett. 2017, 12, 772–776. [Google Scholar] [CrossRef]
- Wolny-Koładka, K.; Malina, D. Silver nanoparticles toxicity against airborne strains of Staphylococcus spp. J. Environ. Sci. Heal A 2017, 52, 1247–1256. [Google Scholar] [CrossRef]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.; Tam, P.K.; Chiu, J.F.; Chen, C.M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S.M.S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomedicine 2007, 3, 168–171. [Google Scholar] [CrossRef]
- Gudikandula, K.; Maringanti, S.C. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Gandhi, H.; Khan, S. Biological synthesis of silver nanoparticles and its antibacterial activity. J. Nanomed. Nanotechnol. 2016, 7, 366. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, H.; Chen, Z.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 2011, 1687–4110. [Google Scholar] [CrossRef] [Green Version]
- Marzec, A. Biochemical methods for synthesis of metal nanoparticles as a pro-ecological alternative to traditional means. Przem. Chem. 2013, 92, 1000–1005. (In Polish) [Google Scholar]
- Egorova, E.M.; Kubatiev, A.A.; Schvets, V.I. Biological Effects of Metal Nanoparticles; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-30906-4. [Google Scholar]
- Wolny-Koładka, K.; Malina, D. Eco-friendly approach to the synthesis of silver nanoparticles and their antibacterial activity against Staphylococcus spp. and Escherichia coli. J. Environ. Sci. Health A 2018, 53, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Deepak, V.; Ram Kumar Pandian, S.; Kottaisamy, M.; Barathmani Kanth, S.; Kartikeyan, B. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf. B Biointerfaces 2010, 77, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Florkiewicz, W.; Pluta, K.; Malina, D.; Rudnicka, K.; Żywicka, A.; Guigou, M.D.; Tyliszczak, B.; Sobczak-Kupiec, A. Investigation on Green Synthesis, Biocompatibility, and Antibacterial Activity of Silver Nanoparticles Prepared Using Cistus incanus. Materials 2021, 14, 5028. [Google Scholar] [CrossRef] [PubMed]
- Husen, A. Gold nanoparticles from plant system: Synthesis, characterization and their application. In Nanoscience and Plant–Soil Systems; Ghorbanpourn, M., Manika, K., Varma, A., Eds.; Springer International Publication: Cham, Switzerland, 2017; Volume 48, pp. 455–479. [Google Scholar]
- Moldovan, B.; David, L.; Achim, M.; Clichici, S.; Filip, G.A. A green approach to phytomediated synthesis of silver nanoparticles using Sambucus nigra L fruits extract and their antioxidant activity. J. Mol. Liq. 2016, 221, 271–278. [Google Scholar] [CrossRef]
- Niraimathi, K.L.; Sudha, V.; Lavanya, R.; Brindha, P. Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Coll Surf. B Biointerface 2013, 102, 288–291. [Google Scholar] [CrossRef]
- Logaranjan, K.; Raiza, A.J.; Gopinath, S.C.B.; Chen, Y.; Pandian, K. Shape- and sizecontrolled synthesis of silver nanoparticles using Aloe vera plant extract and their antimicrobial activity. Nano Res. Lett. 2016, 11, 520. [Google Scholar] [CrossRef] [Green Version]
- Roy, K.; Sarkar, C.K.; Ghosh, C.K. Single-step novel biosynthesis of silver nanoparticles using Cucumis sativus fruit extract and study of its photcatalytic and antibacterial activity. Dig. J. Nanomater. Biostructure 2015, 10, 107–115. [Google Scholar]
- Karatoprak, G.S.; Aydin, G.; Altinsoy, B.; Altinkaynak, C.; Kos, M.; Ocsoy, I. The Effect of Pelargonium endlicherianum Fenzl. root extracts on formation of nanoparticles and their antimicrobial activities. Enzym. Microb. Technol. 2017, 97, 21–26. [Google Scholar] [CrossRef]
- Singhal, G.; Bhavesh, R.; Kasariya, K.; Sharma, A.R.; Singh, R.P. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J. Nanopart. Res. 2011, 13, 2981–2988. [Google Scholar] [CrossRef]
- Kaviya, S.; Santhanalakshmi, J.; Viswanathan, B.; Muthumary, J.; Srinivasan, K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 594–598. [Google Scholar] [CrossRef]
- Roy, K.; Sarkar, C.K.; Ghosh, C.K. Photocatalytic activity of biogenic silver nanoparticles synthesized using potato (Solanum tuberosum) infusion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 146, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Sarkar, C.K.; Ghosh, C.K. Plant-mediated synthesis of silver nanoparticles using parsley (Petroselinum crispum) leaf extract: Spectral analysis of the particles and antibacterial study. Appl. Nanosci. 2015, 5, 945–951. [Google Scholar] [CrossRef] [Green Version]
- Zarzecka, K.; Gugała, M.; Zarzecka, M. Potatoes as a good source of nutrients. Borgis 2013, 3, 191–194. (In Polish) [Google Scholar]
- Górnicki, K.; Kaleta, A.; Golisz, E.; Kukiełko, E.; Bryś, A.; Bryś, J.; Sojak, M.; Głowacki, S.; Jaros, M.; Bazylak, W. Characteristics of Selected Raw Materials and Plant Products in Terms of Their Preservation by Various Methods; SGGW Publisher: Warsaw, Poland, 2017. [Google Scholar]
- Polish Standards No. PN-EN ISO 18593:2018-08; Microbiology of the Food Chain—Horizontal Methods for Surface Sampling. Available online: https://www.iso.org/standard/64950.html (accessed on 20 October 2021).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0. 2021. Available online: http://www.eucast.org (accessed on 20 October 2021).
- Agourram, A.; Ghirardello, D.; Rantsiou, K.; Zeppa, G.; Belviso, S.; Romane, A.; Oufdou, K.; Giordano, M. Phenolic content, Antioxidant Potential, and Antimicrobial Activities of Fruit and Vegetable By-Product Extracts. Int. J. Food Prop. 2013, 16, 1092–1104. [Google Scholar] [CrossRef]
- Wang, S.; Hui-Mei, A.; Han, Q.; Xu, Q. Evaluation of Direct Ultrasound-Assisted Extraction of Phenolic Compounds from Potato Peels. Processes 2020, 8, 1665. [Google Scholar] [CrossRef]
- Sönnichsen, C.; Franzl, T.; Wilk, T.; Plessen, G.; Feldmann, J. Plasmon resonances in large noble-metal clusters. New J. Phys. 2002, 4, 93.1–93.8. [Google Scholar] [CrossRef] [Green Version]
- Slistan-Grijalva, A.; Herrera-Urbina, R.; Rivas-Silva, J.F.; Avalos-Borja, M.; Castillon-Barraza, F.F.; Posada-Amarillas, A. Classical theoretical characterization of the surface plasmon absorption band for silver spherical nanoparticles suspended in water and ethylene glycol. Physica E 2005, 27, 104–112. [Google Scholar] [CrossRef]
- Dugal, S.; Chakraborty, S. Biogenic synthesis of nanosilver and its antibacterial effect against resistant Gram negative pathogens. Int. J. Pharm. Pharm. Sci. 2013, 5, 498–501. [Google Scholar]
- Quelemes, P.V.; Araruna, F.B.; de Faria, B.E.; Kuckelhaus, S.A.; da Silva, D.A.; Mendonça, R.Z.; Eiras, C.; Soares, M.J.; Leite, J.R. Development and antibacterial activity of cashew gum-based silver nanoparticles. Int. J. Mol. Sci. 2013, 14, 4969–4981. [Google Scholar] [CrossRef] [Green Version]
- Arokiyaraj, S.; Arasu, M.V.; Vincent, S.; Prakash, N.U.; Choi, S.H.; Oh, Y.K.; Choi, K.C.; Kim, K.H. Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L and its antibacterial and cytotoxic effects: An in vitro study. Int. J. Nanomed. 2014, 9, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Ghodake, G.; Lim, S.R.; Lee, D.S. Casein hydrolytic peptides mediated green synthesis of antibacterial silver nanoparticles. Colloids. Surf. B. Biointerfaces 2013, 108, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Sharma, S.; Singh, V.N.; Shamsi, S.F.; Fatma, A.; Mehta, B.R. Biosynthesis of silver nanoparticles from Desmodium triflorum: A novel approach towards weed utilization. Biotechnol. Res. Int. 2011, 2011, 454090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Ajetomobi, F.E.; Gueguim-Kana, E.B.; Beukes, L.S. Cola nitida-mediated biogenic synthesis of silver nanoparticles using seed and seed shell extracts and evaluation of antibacterial activities. BioNanoScience 2015, 5, 196–205. [Google Scholar] [CrossRef]
- Al-Ogaidi, I. Detecting the antibacterial activity of green synthesized silver (Ag) nanoparticles functionalized with ampicillin (Amp). Baghdad Sci. J. 2017, 14, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Mosallam, F.M.; Helmy, E.A.; Bendary, M.M.; El-Batal, A.I. Potency of a novel synthesized Ag-eugenol nanoemulsion for treating some bacterial and fungal pathogens. J. Mater. Res. 2021, 36, 1524–1537. [Google Scholar] [CrossRef]
- Jang, H.; Lim, S.H.; Choi, J.S.; Park, Y. Antibacterial properties of cetyltrimethylammonium bromide-stabilized green silver nanoparticles against methicillin-resistant Staphylococcus aureus. Arch. Pharm. Res. 2015, 38, 1906–1912. [Google Scholar] [CrossRef]
- Soleimani, M.; Habibi-Pirkoohi, M. Biosynthesis of silver nanoparticles using Chlorella vulgaris and evaluation of the antibacterial efficacy against Staphylococcus aureus. Avicenna J. Med. Biotechnol. 2017, 9, 120–125. [Google Scholar]
- Moideen, S.; Prabha, A.L. Green synthesis of silver nanoparticles using Luffa acutangula Roxb. Var. Amara. Lin and it’s antibacterial activity. Int. J. Pharm. Bio Sci. 2014, 5, 1051–1061. [Google Scholar]
- Kędziora, A.; Gorzelańczyk, K.; Bugla Płoskońska, G. Positive and negative aspects of silver nanoparticles usage. Biol. Int. 2013, 53, 67–76. [Google Scholar]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [Green Version]
- Egger, S.; Lehmann, R.P.; Height, M.J.; Loessner, M.J.; Schuppler, M. Antimicrobial properties of a novel silver-silica nanocomposite material. Appl. Environ. Microbiol. 2009, 75, 2973–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, K.; Tsuruda, K.; Morishita, M.; Uchida, M. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent. Mater. 2000, 16, 452–455. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Park, H.M.; Ng, P.K. Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity. J. Agric. Food. Chem. 2006, 54, 5814–5822. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, L. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Pratsinis, S.E. Antibacterial activity of nanosilver ions and particles. Environ. Sci. Technol. 2010, 44, 5649–5654. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).