Abstract
Microcellular nanocomposite foams functionalized with cinnamaldehyde (Ci) were obtained through two-step supercritical foaming and impregnation processing. PLA nanocomposite foams with different C30B concentrations (1, 2, and 3 wt.%) were obtained by foaming with scCO2 at 25 MPa and 135 °C and impregnated with Ci at 12 MPa and 40 °C. The effect of the C30B content and Ci incorporation on the morphological, structural, thermal, and release properties of the developed foams were investigated. The incorporation of Ci was not influenced by C30B’s addition. The presence of C30B and Ci incorporation reduced the average pore diameter slightly and the crystallinity degree of the foams extensively. Simultaneously, the experimental and theoretical characterization of the Ci release from the PLA nanocomposite foams in EtOH 50% was analyzed. The mechanism of Ci release from the foams was defined as a quasi-Fickian diffusion process that could be successfully described using the Korsmeyer–Peppas model. The active PLA foams presented a higher potential of migration and faster release when compared with that reported in commonly used PLA films, showing that biopolymeric foams could be potentially used as active food packaging to improve the migration of active compounds with low migration potentials in order to improve their biological activity in foods.
Keywords:
supercritical CO2; PLA; nanoclay; nanocomposite foam; foaming; impregnation; cinnamaldehyde release 1. Introduction
The concept of active packaging has emerged in the last few decades as an innovative strategy to increase the shelf-life and safety and enhance the sensory properties of minimally processed foods while preserving their quality. Among the different kinds of active packaging, those that possess the ability to release antimicrobial agents have gained great attention. Plant extracts, essential oils, and their derivatives have been extensively incorporated to afford antimicrobial activities in polymers for food packaging due to their recognition as being safe for human consumption and the wide antimicrobial spectrum [1,2]. In particular, cinnamaldehyde (Ci), which is the main compound presented in the essential oil of the genus Cinnamomun, has been widely used to develop antimicrobial structures for food packaging by using different processing techniques thanks to its great activity against bacteria, yeast, and filamentous fungi [3,4].
The term polymeric foam (or porous polymer) is defined as a gas–polymer two-phase system [5]. Polymeric foams can be defined as (1) open foams, when the gas phase is continuous and cells are connected with each other, and (2) closed cell foams, when the gas phase is dispersed in the polymer, and therefore, foam cells are isolated from each other, and cavities are surrounded by complete cell walls. Moreover, polymeric foams can be defined with respect to their hierarchical organization structure (cell size of the foam) at four successive levels, such as macrocellular (>100 µm), microcellular (1–100 µm), ultramicrocellular (0.1–1 µm) and nanocellular (0.1–100 nm) [5]. This wide window of foam morphologies has opened great possibilities in the last decade for the development of delivery polymer matrices with tailored mass transport properties as a function of the foam morphology. This opportunity has been exploited to date mainly to develop polymer drug delivery systems with tailored drug release characteristics according to the release profile requirements [6]. Macroporous open foams were used to increase the paclitaxel release rate from polylactic-co-glycolic acid (PLGA) foams. In this case, the interconnectivity between pores enhanced the water penetration, and the shorter diffusion path in the polymer increased the release rate of paclitaxel compared with its release from a compressed PLGA disk [7]. In another work, Löbmann et al. (2017) reported that the release of indomethacin was tuned from fast- to slow-release mode by changing the hierarchical structure of the cellulose nanofibers from a paper to a closed-cell macrofoam structure. This slow-release rate in the macro closed-cell foam was mainly due to an extended drug diffusion path, because the drug can only diffuse through the cell walls of the foam and not through the voids formed for each foam cell filled with air [8].
Among the different foaming agents proposed to develop polymeric foams, supercritical fluids (SCFs) are considered one of the most promising [9]. A pure component is at the SCF state if its temperature and pressure values are higher than its critical values [10]. Above these conditions, the fluid shows diffusivity values comparable to the gas phase and solvating properties like those in the liquid phase, and both parameters are easily adjustable by changing the temperature or pressure or using co-solvents. These properties of the supercritical fluids have encouraged its use in different fields such as particle generation [11], adsorption of natural extracts [12], extraction [13], and others. In most of these applications, carbon dioxide (CO2) has been the most-used fluid due to its characterization as a green solvent, because it is non-toxic, non-flammable, and it is inexpensive because it is a byproduct in ethanol and ammonia industrial production. CO2 has a critical point that is easy to reach (pressure (P) > critical pressure (Pc) = 7.38 MPa and temperature (T) > critical temperature (Tc) = 304.15 K), allowing one to carry out polymer processing at near-ambient temperature, thus avoiding the thermal degradation of organic compounds such as drugs, antimicrobials, or antioxidants [14,15]. These advantages have encouraged the use of supercritical carbon dioxide (scCO2) to impregnate polymers with active substances for different applications, such as drug delivery [14] and active packaging systems [15]. Polymer foaming is also one of the most prominent applications of scCO2 [16]. Polymer foaming using scCO2 can be carried out either at lab scale (batch foaming) for preliminary studies or at pilot scale using supercritical continuous extrusion foaming. Regardless of the foam processing method, the main stages of the process can be summarized as follows: (1) saturation of the polymer with scCO2; (2) cell nucleation due to the generation of thermodynamic instability (sudden decrease in pressure or temperature); and (3) cell growth as CO2 diffuses from the saturated polymer [17].
Polylactic acid (PLA) foams have recently arisen as the most promising new material alternative for sustainable packaging over the conventional polymer foams based in polyolefins such as polystyrene, due to the inherent renewable carbon content and optional end of life compostability of PLA foams [18]. Nevertheless, a handicap associated with the supercritical fluid foaming of PLA is that their lower melting strength avoids the formation of PLA nanocellular foams [19]. One of the solutions for facing this problem has arisen from nanotechnology thorough the use of nanoclays in order to increase the melting strength of the polymer (superior viscoelastic behavior). Several studies have found that these long aspect ratio platelet-shaped nanoparticles can act as heterogeneous cell nucleating agents, allowing the formation, in most of cases, of stable closed-cell structures [20]. Particularly, PLA presents high affinity toward the organo-modified montmorillonite Cloisite® 30B nanoclay (C30B), which allows for obtaining nanocomposite materials with intercalated or exfoliated structures [21]. This fact allows the modification of the cell size in PLA foams from microcellular to nanocellular sizes in terms of C30B nanoclay loading [18]. Recently, the feasibility of antibacterial biopolymeric foams based on the commercial biodegradable polymer Mater-Bi® (MB) and carvacrol as an antibacterial agent for food packaging applications were investigated [22,23].
This work was focused on the application of scCO2 technology toward the development of PLA nanocomposite foams and their functionalization with Ci. The impregnated PLA nanocomposite foams were obtained by two-step supercritical processing involving the foaming with scCO2 of PLA samples with different concentrations of the organo-modified montmorillonite C30B® and the subsequent CO2-assisted impregnation of these samples with Ci. This work includes the experimental and theoretical description of the release process of Ci from the PLA nanocomposite foams to a fatty food simulant, which was analyzed and explained in terms of the results obtained from the thermal and structural characterization of the PLA nanocomposite foams. To our knowledge, this is the first study on the supercritical foaming of PLA and impregnation with Ci and its kinetic release assessment for application in food packaging.
2. Materials and Chemicals
2.1. Materials and PLA Nanocomposite Preparation
Polylactic acid (PLA) 2003D (specific gravity ¼ 1.24; MFR g/10 min (210 °C, 2.16 kg)) was purchased from Natureworks® Co., (Minnetonka, MN, USA). Absolute ethanol and methanol (99.9% HPLC grade) were supplied by Merck (Darmstadt, Germany). Cinnamaldehyde (Ci) (≥99.5%) was purchased from Aldrich® Chemistry (St. Louis, MO, USA). The commercial organo-modified montmorillonite Cloisite® 30B nanoclay (C30B) (100 meq/100 g) was provided by Southern Clay Products (Austin, TX, USA). The C30B presented a plate-like geometry with typical particle sizes (less than 10%: 2 μm; less than 50%: 6 μm; less than 90%: 13 μm). Carbon dioxide was supplied by Linde (Santiago, Chile).
2.2. Foaming with scCO2 and Impregnation with Ci of PLA Foams
The PLA foams were prepared as follows. First, PLA nanocomposite films without additives and with different concentrations of C30B nanoclay (1, 2, and 3 wt.%) were obtained by means of extrusion using a LabTech LTE20 twin-screw extruder (Samutprakarn, Thailand). Both the PLA powder and C30B were previously dried in a vacuum at 60 °C for 24 h. The temperature profile in the extruder was between 175 and 200 °C with a screw speed of 30 rpm, and the films were collected in a Scientific Labtech LBCR-150 chill roll attachment (Samutprakarn, Thailand) at 1.8 rpm. The nanocomposite films were stored in a desiccator until they were submitted to the supercritical processing. The average thickness of the nanocomposite films was measured by a Mitutoyo ID-C112 digital micrometer.
Figure 1 shows the set-up used for the supercritical foaming of PLA nanocomposites. PLA nanocomposite films (1.5-cm2 film sample, ~600 µm thickness) were placed inside a 100-mL high-pressure cell, and CO2 was loaded into the cell by means of a Teledyne ISCO 500D high-pressure pump, which was operated at a constant pressure regime during supercritical foaming runs. The temperature of the high-pressure cell was controlled using a thermostatic electric resistance around the cell. In this work, the temperature and pressure were kept constant for all the foaming runs at 135 °C and 25 MPa, respectively. The samples were kept under these conditions for the temperature and pressure for 35 min. Subsequently, CO2 was released by depressurization of the system (within 2 s). The resulting foams were stored into a desiccator until their use in the supercritical impregnation process. The average thickness of the PLA nanocomposite foams was measured by a Mitutoyo ID-C112 digital micrometer.
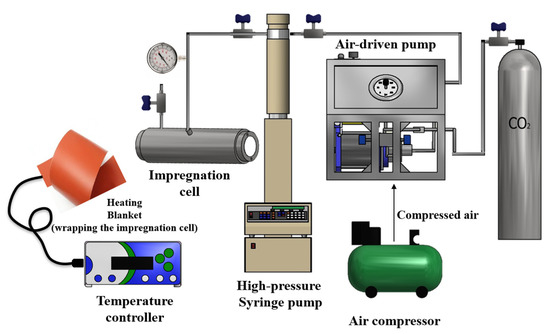
Figure 1.
Outline of the experimental set-up for the foaming with scCO2 and impregnation of the PLA nanocomposite foams [15].
scCO2 fluid impregnation of Ci in the developed PLA nanocomposite foams with different concentrations of C30B (1, 2, and 3 wt.%) was carried out using the apparatus schematically described in Figure 1. This process was carried out using the same 100-mL high-pressure cell. Ci (0.5 mL) was placed at the bottom of the vessel in a glass container. PLA foam and PLA nanocomposite foams (25-cm2 foam sample with a 1477.9 ± 127 µm average thickness) were placed into the high-pressure cell. Next, scCO2 was loaded in the system by means of an ISCO 500D syringe pump operated at a constant pressure rate during the impregnation runs. The impregnation experiments were carried out for 3 h under a constant pressure (12 MPa), depressurization rate (1 MPa min−1), and temperature (40 °C). Previous works developed by our research group have already shown that the conditions used in this work optimized the impregnation yield of cinnamaldehyde in PLA [21,24]. The supercritical batch impregnation process could be divided in three stages. Briefly, in the first stage, the dissolution of the compounds to be impregnated in the polymer takes place. The following stage considers the sorption of the mixture (active compound and CO2) in the polymer. In this stage, the molecular diffusion of the active compound is improved due to the polymer’s swelling and plasticization caused by the high-pressure CO2. The final stage of the process involves the depressurization of the system, which allows for obtaining a solvent-free impregnated polymer [15].
The amount of Ci impregnated into the PLA foam samples was determined by a method of dissolution and precipitation of the polymer and subsequent HPLC quantification. The procedure is explained in detail in Section 2.3.4.
2.3. Characterization of PLA Nanocomposite Foams
2.3.1. Morphological Analysis
The morphologies of the neat PLA foam, PLA nanocomposite foams, and PLA nanocomposite foams impregnated with Ci were studied using a VEGAN3 TESCAN scanning electron microscope (SEM) with the accelerating voltage at 10 kV. The cross-sections of the nitrogen-fractured samples were coated with gold palladium using a Hummer 6.2 sputtering system. Image J image processing software was used to determine the average values of the pore diameter and cell density of the foams using Equations (1) and (2) [25,26]:
where corresponds to the number of pores with a pore size of , and n, M, and A correspond to the number of cells in the SEM image, magnification, and area of the micrograph, respectively.
2.3.2. Structural Analysis through Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy
Fourier-transform infrared (FTIR) spectroscopy was used to characterize the presence of specific chemical groups in the materials. The FTIR spectra were found in attenuated total reflection (ATR) mode with a Bruker IFS 66V spectrometer. The spectra were the results of 64 co-added interferograms at 4 cm−1 and resolutions in the wavenumber range from 4000 to 400 cm−1. The spectra analyses were performed using OPUS Software Version 7.
2.3.3. Thermal Properties
Differential scanning calorimetry (DSC) analyses were carried out with a Mettler-Toledo model STAR 822e (Schwerzenbach, Switzerland) coupled to a HAAKE EK 90/MT cooling unit (Newtington, CT, USA). Samples of 6–8 mg each were subjected to heating from 0 to 250 °C, cooling from 250 to 0 °C, and a second round of heating from 0 to 250 °C at a constant speed of 10 °C min−1 under a nitrogen atmosphere. The parameters reported were the melting temperature (Tm), cold crystallization temperature (Tcc), melting enthalpy (ΔHm), and cold crystallization enthalpy (ΔHcc). The crystallinity was calculated by following Equation (3):
where ΔHm is the specific melting enthalpy of the sample (J g−1), ΔHcc is the specific cold crystallization enthalpy of the sample (J g−1), and is the specific melting enthalpy of a wholly crystalline PLA (93 J g−1) [27].
Thermogravimetric analysis (TGA) was performed using a Mettler Toledo Gas Controller GC20 Stare System TGA/DCS (Schwerzenbach, Switzerland). Approximately 7 mg of each sample was deposited in porcelain capsules which were subjected to a temperature scan from 30 to 600 °C at a 10 °C min−1 heating rate under a nitrogen atmosphere (flow rate: 50 mL min−1). an onset temperature of decomposition (Tonset) corresponding to 2.5 wt.% of mass loss and temperature at the maximum degradation rate (Td) were reported.
2.3.4. Study of the Release Kinetics
The Ci release kinetics were characterized through specific experimental migration assays using an EtOH 50% (v/v) solution as a fatty food simulant in order to describe the mass transfer of Ci from the PLA nanocomposite foams. Migration assays were carried out in duplicate following the European Committee for Standardization guidelines and according to EU regulations [28]. The PLA nanocomposite foams impregnated with Ci (25 cm2) were immersed into glass tubes filled with 50 mL of food simulant. These tubes were placed in an oven at 40 °C for at least 4 days. The detection and quantification of the Ci which migrated to the food simulant was carried out through high-performance liquid chromatography (HPLC). Chromatographic analysis was performed in an HPLC (Hitachi LaChrom Elite, Dallas, TX, USA) equipped with a Hitachi L-2455 diode array detector and a Hitachi L-2200 autosampler. The chromatographic column used was an Inertsil ODS-3 C18 (5 μm, 4.6 × 250 mm). The mobile phase consisted of a mixture of acetonitrile and distilled water (40:60) at a flow rate of 2 mL min−1 with an injection volume of 5 μL. The oven temperature was constant at 40 °C. The detection of Ci was performed at 275 nm. The calibration curve was constructed for the peak area against the Ci concentration of standard solutions from 6 to 340 mg kg−1 (ppm), with three samples for each Ci concentration. The same HPLC method was used to determine the amount of Ci impregnated in the different PLA nanocomposite foams. This analysis was carried out by a dissolution and polymer precipitation method, where 0.1 g of the impregnated foams was dissolved with 20 mL of chloroform at room temperature into a centrifuge tube. Subsequently, 30 mL of methanol was added to produce the precipitation of the polymer. Finally, the phase separation was carried out by centrifugation (4500 rpm for 10 min), and the liquid phase was analyzed by HPCL-UV.
Release assays were performed until equilibrium was reached (i.e., when the Ci concentration in the food simulant was maintained as constant over time in at least two continuous consecutive measurements). This thermodynamical equilibrium condition was characterized by the dimensionless distribution coefficient of Ci between the PLA nanocomposite foams and food simulant (KP/FS), represented by the ratio of the Ci concentrations at the interphase between the polymer and food simulant, as Equation (4) shows:
This coefficient was estimated through a mass balance from the results of the Ci release experiments at the equilibrium conditions, where is the concentration of Ci at equilibrium in the PLA nanocomposite foams and corresponds to their values in the PLA nanocomposite foams. Moreover, the analysis of the release mechanism of Ci from the impregnated PLA nanocomposite foams was performed by following the Higuchi and Korsmeyer–Peppas kinetic models, as Equations (5) and (6) represent, respectively [29,30,31]:
where is the amount of Ci released in any time t, is the amount of Ci released in infinite time (initial Ci concentration in the PLA foams), k is the release rate constant, and n is the diffusional exponent which suggests the nature of the release mechanism.
3. Results and Discussion
3.1. Relation between Nanoclay Content, Ci Incorporation, and PLA Nanocomposite Foam Morphology
PLA films with C30B at 1, 2, and 3 wt.% were submitted to foaming with scCO2 as described in Section 2.2. The scCO2 saturation pressure, temperature and time were fixed at 25 MPa, 135 °C, and 35 min, respectively. The expansion ratio of the PLA nanocomposite foams was not drastically affected by the presence of C30B nanoclay or the subsequent impregnation with Ci. Thus, the resulting samples presented a volume approximately 14-fold higher than the non-foamed PLA films, with densities ranging between 0.126 and 0.139 g cm−3. These density values were lower than the reported densities for PLA foams obtained by chemical foaming [32] and by CO2-assisted extrusion foaming [33], which was related to the obtaining of PLA foams with lower pore sizes. Representative scanning electron microscopy (SEM) images of the neat PLA foam, PLA nanocomposite foams with different concentrations of the nanoclay C30B, as well the images of the impregnated PLA nanocomposite foams presented homogeneous microcellular closed-cell structures with average diameters ranging between 20.1 and 23.4 µm (shown in Table 1 and Figure 2). The neat PLA foam presented smooth cells with a pore average diameter (23.4 µm) considerably lower than the value reported for the PLA foams obtained in other studies of foaming with scCO2. Morlin et al. (2021) developed PLA foams by scCO2-assisted extrusion with 88-fold higher pore average diameters (approx. 2060 µm) [33]. This fact could be related to the scCO2 transfer from the mixer cavity into gas bubbles formed inside the PLA during the cell growth stage. This phenomenon increases the mass of scCO2 inside the bubble, promoting the generation of larger pores until the scCO2 concentration inside the bubbles equals that of the outside or the melt is frozen [34]. On the contrary, this phenomenon was avoided in the batch foaming with scCO2 due to the supersaturation with scCO2 of the PLA films during the thermodynamic instability caused by the sudden decrease in pressure upon depressurization to atmospheric pressure.

Table 1.
Pore diameters (d) and cell densities (ρc) of the PLA nanocomposite foams.
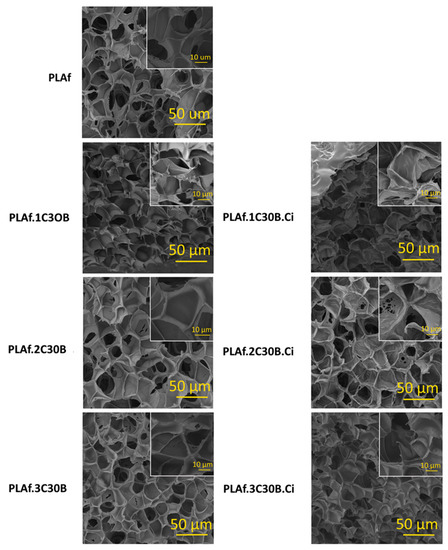
Figure 2.
SEM micrographs of developed PLA foams obtained by foaming with scCO2 and impregnation.
Using batch foaming with scCO2 at 10 MPa and 40 °C, Milovanovic et al. (2019) also reported the production of PLA foams with a higher pore average diameter (68.6 µm) and lower cell density (10.5 × 1012 pores cm−3). These results are very interesting because the sorption of CO2 in PLA at these conditions (~24.50 wt.%) [25] was higher than that expected for our foaming conditions (~17.51 wt.%) [35]. This fact should translate to a higher nucleation rate in PLA. Nevertheless, in our study, the PLA film reached a molten viscous state due to the use of a saturation temperature near the PLA melting temperature (154 °C), which could dramatically decrease the viscosity of PLA and enhance cell nucleation [36,37]. Moreover, the differences in the pore average diameter should mainly be a consequence of the different scCO2 densities and pressure drop used in each study. Particularly, the higher scCO2 density at the conditions used by Milovanovic (0.71776 g mL−1) and lower pressure drop (0.5 MPa min−1) could have resulted in lower scCO2 diffusion rates through the structure of the PLA during the cell growth stage compared with the CO2 diffusion rates established in our system, which probably implied a higher scCO2 residence period in the gas bubbles, promoting the formation of larger pores.
On the other hand, the incorporation of C30B nanoclays did not change the cell morphology but reduced the average pore diameter of the foams slightly as their content increased (Table 1 and Figure 2). This fact could be explained by the improvement of the PLA heat resistance due to the intercalation of C30B nanoclays, which decreased the bubble growth during the free expansion of the polymer [38]. The cell density of the PLA foams also increased with the nanoclay addition, probably due to the promotion of the heterogeneous nucleation of the polymeric structure, which enhanced cell nucleation by decreasing the energy barrier [39]. In this way, the PLA nanocomposite foam containing C30B at 3 wt.% presented the lowest average pore diameter size (21.87 µm) and, consequently, the highest cell density value (173.4 × 10−12 pores cm−3).
Ci was incorporated in the PLA nanocomposite foams by impregnation with scCO2 according to the experimental procedure described in Section 2.2. Impregnation runs were developed while maintaining a constant temperature, pressure, and time (40 °C, 12 MPa, and 3 h). The Ci impregnation yield obtained for the neat PLA foam agreed with the value reported for common PLA films scCO2 impregnated at the same processing conditions (11 wt.%) [21,24]. This result highlighted that although the physical structure of the PLA was modified from a high- (film) to low-density structure (foam), the availability of their functional groups, owing to the interaction with Ci, were not affected. Moreover, as was already expected, the presence of C30B had no impact on the Ci impregnation yield, and all the PLA nanocomposite foams presented an impregnation yield similar to the value obtained for the neat PLA foam (11 wt.%). This effect from C30B on the impregnation yield of Ci has been reported by Villegas et al. (2019) for impregnated PLA films obtained using scCO2 at 12 MPa and 40 °C [21]. SEM images (Figure 2) revealed that the scCO2-assisted Ci incorporation slightly decreased the average pore diameter of the active PLA nanocomposite foams, increasing their cell density. This fact could be attributed to the reduction of the foam viscosity during supercritical impregnation due to the well-known plasticizing effect of Ci on PLA [24], which increased the polymer chain mobility of the foams, allowing the generation of new pores of lower sizes during the depressurization of the system. This phenomenon has been also observed for starch foams loaded with carvacrol [22,23].
3.2. Structural Properties of PLA Nanocomposite Foams
FTIR analysis provides information regarding the chemical interactions in systems with two or more components. In this case, the possible interactions between the polymeric matrix and the active compound and nanoclay were studied. Figure 3 shows the FTIR spectra of the control and active PLA foams containing nanoclays and the active agent in order to observe if the incorporation of Ci and C30B nanoclay entailed the appearance of new peaks or the displacements of some bands.
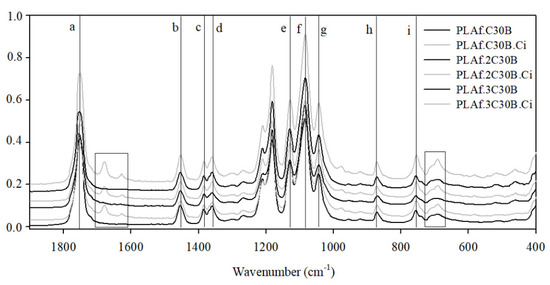
Figure 3.
FTIR spectra of foams, including nanoclays, control foams (dark lines), and foams containing cinnamaldehyde (bright lines). From bottom to top: PLAf.1C30B, PLAf.1C30.Ci, PLAf.2C30B, PLAf.2C30.Ci, PLAf.3C30B, and PLAf.3C30.Ci.
The FTIR spectra clearly evidenced that all foam systems presented the characteristic peaks of PLA without displacements, where peaks at 1747, 1080, 1127, and 1180 cm−1 (peaks a,e–g) were attributed to carbonyl stretching and C=O and C-O symmetric and asymmetric stretching, respectively. Bands at 1450, 1381, and 1360 cm−1 (peaks b–d) were assigned to the bending of CH3 and symmetric and asymmetric vibrational deformations of the CH bond present in CH2, and finally, bands at 867 and 754 cm−1 (peaks h and i) were assigned to the PLA amorphous and crystalline phases, respectively [40,41].
As Figure 3 shows, the incorporation of Ci in the active PLA foams was also clearly evidenced. The active PLA foams presented new characteristic bands near 1600–1700 cm-1 associated with the vibrations of the aromatic ring and the aldehyde group of Ci, and a new peak at 690 cm−1 was attributed to the phenyl group of this active compound, specifically the CH=CH bending out of plane in alkenes [42,43]. The FTIR spectra of all active PLA nanocomposites confirmed that the incorporation of the active compound in the polymeric matrix occurred at similar concentrations, which was explained in Section 3.1.
3.3. Thermal Characterization of the PLA Nanocomposite Foams
The DSC parameters of the PLA nanocomposite foams and the neat PLA foam are shown in Table 2. The neat PLA foam was highly crystalline (56%) with a single melting temperature (Tm) at 154 °C, similar to the Tm value reported for the highly porous PLA foams obtained by scCO2-assisted extrusion [44]. However, the neat PLA foam had a higher crystallinity than that reported for the extruded neat PLA film (4.4%) due to the strain-induced crystallization (biaxial stretching) that took place in the foam expansion process of the PLA, considering that a slight plasticizer effect of the sCO2 on the non-foamed neat PLA has been reported [24]. That aside, the PLA crystals formed by foaming with scCO2 melted at a similar temperature (154 °C) to the crystals formed by cast extrusion PLA (152 °C) [24], indicating that the crystalline structures formed by both processes had similar thermodynamical stability.
The incorporation of Ci in PLA reduced the crystallinity of the polymer due to the well-known plasticizing effect of the active compound [24,45,46], and therefore, the cold crystallization at 115 °C was promoted as well as the formation of two types of crystals which melted at lower temperatures (143 and 148 °C) than the crystalline structures in the neat PLA foam. This melting behavior regarding the formation of two types of crystals—low ordered α’ crystals, and α crystals that are thermodynamically more stable—has been previously reported for PLA foams [44]. The plasticizer effect of essential oils has also been observed in foams of Mater-Bi biodegradable polymer/sodium bicarbonate/carvacrol as a viscosity reduction [22], ductility increase [23], and as a Tg decrease in poly lactic-co-glycolic acid/thymol foams [25].

Table 2.
DSC and TGA parameters of PLA nanocomposite foams impregnated with cinnamaldehyde.
Table 2.
DSC and TGA parameters of PLA nanocomposite foams impregnated with cinnamaldehyde.
| Sample | Tcc (°C) | ΔHcc (J g−1) | Tm1 (°C) | Tm2 (°C) | ΔHm (J g1) | Xc (%) | Tonset (°C) | Td1 (°C) | Td2 (°C) |
|---|---|---|---|---|---|---|---|---|---|
| PLAf | - | - | 154 | - | 52.4 | 56 | 326 | - | 367 |
| PLAf.Ci | 115 | 8.4 | 143 | 148 | 20.7 | 15 | 167 | 173 | 366 |
| PLAf.1C30B | - | - | 154 | - | 44.4 | 48 | 330 | - | 366 |
| PLAf.2C30B | - | - | 151 | 161 | 35.2 | 39 | 323 | - | 365 |
| PLAf.3C30B | - | - | 150 | 162 | 39.4 | 44 | 328 | - | 365 |
| PLAf.1C30B.Ci | 117 | 6.3 | 142 | 150 | 27.7 | 27 | 148 | 153 | 366 |
| PLAf.2C30B.Ci | - | - | 143 | 153 | 17.1 | 21 | 154 | 187 | 366 |
| PLAf.3C30B.Ci | - | - | 143 | 149 | 23.8 | 30 | 165 | 172 | 366 |
Here, - means the sample did not present a thermal transition or peak related to the parameter reported in the corresponding column.
On the other hand, the addition of C30B to PLA promoted the formation of foam structures with less crystallinity than the neat PLA foam. This fact could be related to a nanoclay restriction effect on the mobility of the polymer chains due to the PLA nanoparticle chemical interactions and the intercalation of the polymer chains between the clay platelets. This effect of the nanoclays seem to prevail over their reported nucleating effect in the polymeric structure and tend to be more significant as the clay concentration increased [47]. The chemical interactions could occur between the functional groups of PLA and C30B, as has been reported for PLA nanocomposites containing C30B nanoclays [21,47]. Mainly, chemical interactions occur between the carbonyl and hydroxyl terminal groups of PLA and the hydroxyl groups of the organic modifier of C30B (two OH groups per C30B molecule) [48]. Moreover, two melting peaks associated with the formation of two types of crystals in the PLA nanocomposite foams with C30B at 2 and 3 wt.% were observed. For these PLA nanocomposite foams, crystals with lower chain ordering that melted at 150 °C and other more stable crystals than those observed in PLAf.1C30B that melted near 160 °C were formed due to the nucleating properties of C30B. This phenomenon was also evidenced by Di et al. (2005) for PLA foams with C30B at 5 wt.% prepared by melt mixing and using a compressed mix of CO2 and N2 as a blowing agent [47]. Meanwhile, the PLA nanocomposite foams impregnated with Ci had higher crystallinity and slightly higher Tm2 values than the PLAf.Ci foam, which could be associated with the nucleating effect of C30B. Nonetheless, when the impregnated PLA nanocomposite foams were compared with those without Ci, a decrease in the melting temperatures and crystallinity was observed due to the plasticizing effect of Ci on PLA [21] without a clear trend regarding the clay concentration. Aside from that, a cold crystallization peak was detected only for the PLAf.1C30B.Ci sample because nucleation and molecular mobility during heating were favored at lower concentrations of nanofiller.
Table 2 also shows the TGA parameters of the PLA nanocomposite foams and the neat PLA foam. The Tonset and Td of the neat PLA foams were 326 and 367 °C, respectively [49,50]. Meanwhile, two temperatures at the maximum degradation rate—173 and 366 °C—were recorded for the PLA foam impregnated with Ci associated with the weight loss stages of Ci and PLA, respectively. These temperatures agreed with the thermal degradation of the extruded PLA films impregnated with Ci [24]. The thermal degradation of PLA could be related to hydrolysis, lactide reformation, oxidative main chain cleavage, and inter- or intramolecular transesterification reactions [51]. Meanwhile, Ci could lose weight by evaporation of water and its volatilization from 125 °C with a maximum rate of weight loss temperature around 200 °C [46,52,53,54].
Finally, C30B’s addition to PLA did not cause significant changes in the Tonset or in the Td regarding the values obtained for the neat PLA foam, even considering that the pristine C30B initiated its decomposition at 267 °C and presented two temperatures for the maximum degradation rate (299 and 404 °C), according to the report by Velásquez et al. (2021) [55]. These thermal results and the DSC evidence suggested that chemical interactions between C30B, PLA, and its degradation products formed a thermally stable network similar to that observed for fibrillar microcomposites containing PLA, PCL, and C30B [48]. Meanwhile, the PLA nanocomposite foams impregnated with Ci showed two temperatures for the maximum degradation rate Td1 and Td2, corresponding to the degradation stages of Ci and the nanocomposite, respectively. The differences between Tonset and Td1 for the PLA nanocomposite foams impregnated with Ci could be related to differences in the degree of Ci confinement between the polymer chains and clay platelets.
3.4. Study of the Cinnamaldehyde Release Kinetics
Ci’s release from the PLA nanocomposite foams was studied following the standard specific migration procedure described in Section 2.3.4. Ci’s migration into EtOH 50% was determined through HPCL and expressed as a Ci percentage release regarding the initial amount of Ci in the PLA foam samples. The equilibrium condition was expressed in terms of the partition coefficient of Ci (KP/FS), which is a thermodynamic parameter that corresponds to the ratio between the concentration of the Ci in the polymer (P) and the food simulant (FS). Meanwhile, the Ci release mechanism in the PLA nanocomposite foams was investigated by mathematical modeling using the Korsmeyer–Peppas and Higuchi kinetic models. The experimental partition coefficients of Ci, regression coefficients (R2), release rate constants k, and n parameter of the Korsmeyer–Peppas and Higuchi kinetic models are shown in Table 3.

Table 3.
Distribution coefficient (KP/FS), regression coefficient (R2), release rate constant (k), and the parameter n of the Korsmeyer–Peppas and Higuchi kinetic models.
Figure 4a shows the Ci release profiles from the different PLA nanocomposite foams. The regression coefficient (R2) values for the Higuchi and Korsmeyer–Peppas models were obtained by plotting the cumulative amount of Ci released into the food simulant as a function of the square root of time (Figure 4b) and the log of time (Figure 4c), respectively. The R2 values obtained by the correlation of the experimental Ci release data with the Korsmeyer–Peppas model were higher than those obtained by the Higuchi model. Thus, the release mechanism involved in the delivery of Ci from the PLA foams was unveiled, obtaining the “n” parameter of the Korsmeyer–Peppas equation, which corresponded to the diffusional exponent that classified the release mechanism as follows: Fickian diffusion (n = 0.5), anomalous transport (n > 0.5), and quasi-Fickian diffusion (n < 0.5) [56,57,58]. The values of the “n” parameter were calculated from the slopes of the plots shown in Figure 4c and ranged between 0.20 and 0.27. In this way, the release of Ci from the PLA foams could be considered a quasi-Fickian diffusion process, indicating that the relaxation of the polymeric matrix in contact with the food simulant was not coupled to the Ci release process. The quasi-Fickian diffusion mechanism has been also reported for other compounds in porous polymeric matrices, such as oregano essential oil polyphenols in starch foams, with the “n” values of the Korsmeyer–Peppas model ranging between 0.18 and 0.49, and chloramphenicol in chitosan-based foams, with diffusional exponent (n) values in the range of 0.14–0.34 [59].
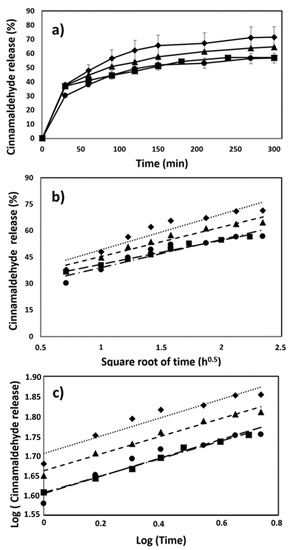
Figure 4.
(a) Cinnamaldehyde release profiles in 50% EtOH at 40 °C and correlation of the cinnamaldehyde release process (dashed lines) from the PLA foams using (b) Higuchi and (c) Korsmeyer–Peppas kinetic models. Figures represent the experimental cinnamaldehyde release values from the following: ■ = PLAf.Ci; ◆ = PLAf.1C30B.Ci; ● = PLAf.2C30B.Ci and ▲ = PLAf.3C30B.Ci.
Table 3 summarizes the distribution coefficient (KP/FS) and the constant rate of diffusion (k) that characterize the thermodynamical equilibrium and the kinetic process of Ci from the developed PLA nanocomposite foams. Both parameters were highly influenced by changing the hierarchical structure of PLA from a high- (film) to low-density structure (foam). The porous structuration of PLA seemed to be the main factor related to the 2.4-fold decrease in the Ci KP/FS values (Table 3) when compared with the values previously reported for the release of Ci from extruded PLA films using the same release conditions (EtOH 50% and 40 °C) [60]. In particular, the high porosity of the PLA samples prepared in our study was related to their low densities, ranging from 126 to 139 kg m−3, probably favoring the higher accessibility of food simulant throughout the porous structure of PLA compared with that obtained in the dense PLA film (1200 kg m−3), which probably negatively affected the chemical interaction between the Ci and foamed PLA, contributing to a higher Ci migration potential. This factor could also be related with the notable decrease in the time required to reach the thermodynamical equilibrium regarding the values reported by Villegas et al. (2021). The resulting k parameters were similar for all the foam samples, with values ranging between 0.38 and 0.49. This trend was expected due to the different PLA foam samples developed in this study presenting similar pore average diameters (Table 1), and this parameter has been included among the main factors that influences the release of active compounds from polymer foam structures [31,61,62].
4. Conclusions
PLA nanocomposite foams functionalized with Ci were successfully developed by two-step supercritical foaming and impregnation processing. The presence of C30B in the PLA foams had no impact on the Ci incorporation, and all the samples presented a similar impregnation yield (11 wt.%), which was confirmed by the FTIR results. The developed PLA foam samples presented homogenous microcellular closed-cell structures with average diameters ranging between 20.1 and 23.4 µm. C30B’s presence in PLA and Ci incorporation reduced the average pore diameter slightly and increased the cell density of the foams. The effect of C30B on these properties was related to the improvement in PLA heat resistance as its content increased due to the intercalation of the PLA chains on the C30B structure. Meanwhile, the effect of Ci was related to the reduction in the foam viscosity during the supercritical impregnation process due to its well-known plasticizing effect on PLA. These factors also explained the lower crystallinities of the different nanocomposite (from 39 to 48%) and PLA foams impregnated with Ci (from 15 to 30%) with respect to the values obtained for the neat PLA foam (56%).
Experimental and theoretical characterization of Ci’s release from the neat PLA foam and PLA nanocomposite foams with different C30B contents (1, 2, and 3 wt.%) in EtOH 50% was analyzed. The R2 values obtained using the Korsmeyer–Peppas model were higher than those obtained by the Higuchi model. The “n” values of the Korsmeyer–Peppas model were observed to be in the range of 0.20–0.27. Thus, the release of cinnamaldehyde from the different PLA foams corresponded to a quasi-Fickian diffusion process. Meanwhile, the distribution coefficient (KP/FS) and the rate constant of diffusion (k) for cinnamaldehyde in the different samples were observed to be between 46 and 57 and 0.38 and 0.49, respectively, showing that the release process of Ci was drastically influenced by the change of the hierarchical structure of PLA from a high- (film) to low-density structure (foam). In particular, a higher potential of migration and a higher kinetic release were observed for Ci in the PLA foam samples developed in this study compared with the results reported in common PLA films. This fact could be related to the higher porosity of the foams, which probably favored a higher accessibility of the food simulant throughout the porous structure, which probably negatively affected the chemical interaction between Ci and the matrix of PLA, also increasing Ci’s diffusion through the structure. Finally, this work shows that biopolymeric foams could potentially be used in active food packaging applications to improve the mass transfer of active compounds with low migration potential in order to stimulate their preferential partitions toward the food instead of the polymer phase improving their biological activity in the food product.
Author Contributions
Conceptualization, M.J.G. and A.R.; methodology, A.T., C.V. and A.R.; validation, M.J.G. and A.R.; investigation, S.F. and P.R.; resources, M.J.G., A.G. and J.R.; writing—original draft preparation, A.R., C.L.d.D. and E.V.; writing—review and editing, A.R. and C.L.d.D.; project administration, M.J.G.; funding acquisition, M.J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded through the FONDECYT regular project N° 1201301 and the “Programa de Financiamiento Basal para Centros Científicos y Tecnológicos de Excelencia” (Project AFB180001), with the support of the University of Santiago de Chile. A.R. thanks the support of CONICYT Fondecyt Postdoctoral Grant 3190929. C.L.d.D. thanks the support of Tecniospring Industry ACE003/20/000066 by Acció (Generalitat de Catalunya) and Marie Sklodowska-Curie grant agreement No. 801342. A.T. thanks the support of the FONDECYT regular project N° 1190302.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Perumal, A.B.; Huang, L.; Nambiar, R.B.; He, Y.; Li, X.; Sellamuthu, P.S. Application of Essential Oils in Packaging Films for the Preservation of Fruits and Vegetables: A Review. Food Chem. 2022, 375, 131810. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Ghaderi, J.; Gómez-Guillén, M.C. Tailoring Physico-Mechanical and Antimicrobial/Antioxidant Properties of Biopolymeric Films by Cinnamaldehyde-Loaded Chitosan Nanoparticles and Their Application in Packaging of Fresh Rainbow Trout Fillets. Food Hydrocoll. 2022, 124, 107249. [Google Scholar] [CrossRef]
- Thongsrikhem, N.; Taokaew, S.; Sriariyanun, M.; Kirdponpattara, S. Antibacterial Activity in Gelatin-Bacterial Cellulose Composite Film by Thermally Crosslinking with Cinnamaldehyde towards Food Packaging Application. Food Packag. Shelf Life 2022, 31, 100766. [Google Scholar] [CrossRef]
- Okolieocha, C.; Raps, D.; Subramaniam, K.; Altstädt, V. Microcellular to Nanocellular Polymer Foams: Progress (2004–2015) and Future Directions—A Review. Eur. Polym. J. 2015, 73, 500–519. [Google Scholar] [CrossRef]
- Hoc, D.; Haznar-Garbacz, D. Foams as Unique Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2021, 167, 73–82. [Google Scholar] [CrossRef]
- Lee, L.Y.; Ranganath, S.H.; Fu, Y.; Zheng, J.L.; Lee, H.S.; Wang, C.H.; Smith, K.A. Paclitaxel Release from Micro-Porous PLGA Disks. Chem. Eng. Sci. 2009, 64, 4341–4349. [Google Scholar] [CrossRef]
- Löbmann, K.; Svagan, A.J. Cellulose Nanofibers as Excipient for the Delivery of Poorly Soluble Drugs. Int. J. Pharm. 2017, 533, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Mensitieri, G. Foams and Their Applications. Supercrit. Fluid Sci. Technol. 2021, 9, 1–20. [Google Scholar] [CrossRef]
- Clifford, A.A.; Williams, J.R. Introduction to Supercritical Fluids and Their Applications. In Supercritical Fluid Methods and Protocols; Humana Press: Totowa, NJ, USA, 2000; pp. 1–16. [Google Scholar]
- Kumar, R.; Thakur, A.K.; Banerjee, N.; Chaudhari, P. A Critical Review on the Particle Generation and Other Applications of Rapid Expansion of Supercritical Solution. Int. J. Pharm. 2021, 608, 121089. [Google Scholar] [CrossRef]
- Carvalho, V.S.; Dias, A.L.B.; Rodrigues, K.P.; Hatami, T.; Mei, L.H.I.; Martínez, J.; Viganó, J. Supercritical Fluid Adsorption of Natural Extracts: Technical, Practical, and Theoretical Aspects. J. CO2 Util. 2022, 56, 101865. [Google Scholar] [CrossRef]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical Fluid Extraction of Seed Oils—A Short Review of Current Trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jérôme, C. Drug Loading of Polymer Implants by Supercritical CO2 Assisted Impregnation: A Review. J. Control. Release 2015, 209, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Rojas, A.; Torres, A.; Galotto, M.J.; Guarda, A.; Julio, R. Supercritical Impregnation for Food Applications: A Review of the Effect of the Operational Variables on the Active Compound Loading. Crit. Rev. Food Sci. Nutr. 2020, 60, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Sarver, J.A.; Kiran, E. Foaming of Polymers with Carbon Dioxide—The Year-in-Review—2019. J. Supercrit. Fluids 2021, 173, 105166. [Google Scholar] [CrossRef]
- Di Maio, E.; Kiran, E. Foaming of Polymers with Supercritical Fluids and Perspectives on the Current Knowledge Gaps and Challenges. J. Supercrit. Fluids 2018, 134, 157–166. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly (Lactic Acid) Foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Han, Z.Z.; Zhang, Y.C.; Yang, W.M.; Xie, P.C. Advances in Microcellular Foam Processing of PLA. Key Eng. Mater. 2016, 717, 68–72. [Google Scholar] [CrossRef]
- Tsimpliaraki, A.; Tsivintzelis, I.; Marras, S.I.; Zuburtikudis, I.; Panayiotou, C. The Effect of Surface Chemistry and Nanoclay Loading on the Microcellular Structure of Porous Poly(d,l Lactic Acid) Nanocomposites. J. Supercrit. Fluids 2011, 57, 278–287. [Google Scholar] [CrossRef]
- Villegas, C.; Arrieta, M.P.; Rojas, A.; Torres, A.; Faba, S.; Toledo, M.J.; Gutierrez, M.A.; Zavalla, E.; Romero, J.; Galotto, M.J.; et al. PLA/Organoclay Bionanocomposites Impregnated with Thymol and Cinnamaldehyde by Supercritical Impregnation for Active and Sustainable Food Packaging. Compos. Part B Eng. 2019, 176, 107336. [Google Scholar] [CrossRef]
- Lopresti, F.; Botta, L.; Scaffaro, R.; Bilello, V.; Settanni, L.; Gaglio, R. Antibacterial Biopolymeric Foams: Structure–Property Relationship and Carvacrol Release Kinetics. Eur. Polym. J. 2019, 121, 109298. [Google Scholar] [CrossRef]
- Gaglio, R.; Botta, L.; Garofalo, G.; Miceli, A.; Settanni, L.; Lopresti, F. Carvacrol Activated Biopolymeric Foam: An Effective Packaging System to Control the Development of Spoilage and Pathogenic Bacteria on Sliced Pumpkin and Melon. Food Packag. Shelf Life 2021, 28, 100633. [Google Scholar] [CrossRef]
- Villegas, C.; Torres, A.; Rios, M.; Rojas, A.; Romero, J.; de Dicastillo, C.L.; Valenzuela, X.; Galotto, M.J.; Guarda, A. Supercritical Impregnation of Cinnamaldehyde into Polylactic Acid as a Route to Develop Antibacterial Food Packaging Materials. Food Res. Int. 2017, 99, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, S.; Markovic, D.; Mrakovic, A.; Kuska, R.; Zizovic, I.; Frerich, S.; Ivanovic, J. Supercritical CO2—Assisted Production of PLA and PLGA Foams for Controlled Thymol Release. Mater. Sci. Eng. C 2019, 99, 394–404. [Google Scholar] [CrossRef]
- Xu, L.-Q.; Huang, H.-X. Foaming of Poly(Lactic Acid) Using Supercritical Carbon Dioxide as Foaming Agent: Influence of Crystallinity and Spherulite Size on Cell Structure and Expansion Ratio. Ind. Eng. Chem. Res. 2014, 53, 2277–2286. [Google Scholar] [CrossRef]
- Ambrosio-Martín, J.; Fabra, M.J.; Lopez-Rubio, A.; Lagaron, J.M. Melt Polycondensation to Improve the Dispersion of Bacterial Cellulose into Polylactide via Melt Compounding: Enhanced Barrier and Mechanical Properties. Cellulose 2015, 22, 1201–1226. [Google Scholar] [CrossRef]
- Galotto, M.J.; Torres, A.; Guarda, A.; Moraga, N.; Romero, J. Experimental and Theoretical Study of LDPE versus Different Concentrations of Irganox 1076 and Different Thickness. Food Res. Int. 2011, 44, 566–574. [Google Scholar] [CrossRef]
- Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. [CrossRef]
- Milovanovic, S.; Djuris, J.; Dapčević, A.; Medarevic, D.; Ibric, S.; Zizovic, I. Soluplus®, Eudragit®, HPMC-AS Foams and Solid Dispersions for Enhancement of Carvedilol Dissolution Rate Prepared by a Supercritical CO2 Process. Polym. Test. 2019, 76, 54–64. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Ardao, I.; Goimil, L.; Gomez-Amoza, J.L.; García-González, C.A. Solvent-Free Processing of Drug-Loaded Poly(ε-Caprolactone) Scaffolds with Tunable Macroporosity by Combination of Supercritical Foaming and Thermal Porogen Leaching. Polymers 2021, 13, 159. [Google Scholar] [CrossRef]
- Litauszki, K.; Kmetty, Á. Characterization of Chemically Foamed Poly(Lactic Acid). IOP Conf. Ser. Mater. Sci. Eng. 2020, 903, 012018. [Google Scholar] [CrossRef]
- Morlin, B.; Litauszki, K.; Petrény, R.; Kmetty, Á.; Mészáros, L. Characterization of Polylactic Acid-Based Nanocomposite Foams with Supercritical CO2. Measurement 2021, 178, 109385. [Google Scholar] [CrossRef]
- Guanghong, H.; Yue, W. Microcellular Foam Injection Molding Process. In Some Critical Issues for Injection Molding; Wang, J., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0297-7. [Google Scholar]
- Aionicesei, E.; Škerget, M.; Knez, Ž. Measurement of CO2 Solubility and Diffusivity in Poly(l-Lactide) and Poly(d,l-Lactide-Co-Glycolide) by Magnetic Suspension Balance. J. Supercrit. Fluids 2008, 47, 296–301. [Google Scholar] [CrossRef]
- Tomasko, D.L.; Li, H.; Liu, D.; Han, X.; Wingert, M.J.; Lee, L.J.; Koelling, K.W. A Review of CO2 Applications in the Processing of Polymers. Ind. Eng. Chem. Res. 2003, 42, 6431–6456. [Google Scholar] [CrossRef]
- Ivanovic, J.; Knauer, S.; Fanovich, A.; Milovanovic, S.; Stamenic, M.; Jaeger, P.; Zizovic, I.; Eggers, R. Supercritical CO2 Sorption Kinetics and Thymol Impregnation of PCL and PCL-HA. J. Supercrit. Fluids 2016, 107, 486–498. [Google Scholar] [CrossRef]
- Keshtkar, M.; Nofar, M.; Park, C.B.; Carreau, P.J. Extruded PLA/Clay Nanocomposite Foams Blown with Supercritical CO2. Polymer 2014, 55, 4077–4090. [Google Scholar] [CrossRef]
- Colton, J.S.; Suh, N.P. The Nucleation of Microcellular Thermoplastic Foam with Additives: Part I: Theoretical Considerations. Polym. Eng. Sci. 1987, 27, 485–492. [Google Scholar] [CrossRef]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of Processing Conditions on the Physical, Chemical and Transport Properties of Polylactic Acid Films Containing Thymol Incorporated by Supercritical Impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Bruna, J.; Torres, A.; Alvarado, N.; Guarda, A.; Galotto, M.J. A Traditional Aboriginal Condiment as an Antioxidant Agent in the Development of Biodegradable Active Packaging. J. Appl. Polym. Sci. 2017, 134, 44692. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Villegas, C.; Garrido, L.; Roa, K.; Torres, A.; Galotto, M.; Rojas, A.; Romero, J. Modifying an Active Compound’s Release Kinetic Using a Supercritical Impregnation Process to Incorporate an Active Agent into PLA Electrospun Mats. Polymers 2018, 10, 479. [Google Scholar] [CrossRef] [Green Version]
- De Souza, A.C.; Dias, A.M.A.; Sousa, H.C.; Tadini, C.C. Impregnation of Cinnamaldehyde into Cassava Starch Biocomposite Films Using Supercritical Fluid Technology for the Development of Food Active Packaging. Carbohydr. Polym. 2014, 102, 830–837. [Google Scholar] [CrossRef] [Green Version]
- Bocz, K.; Tábi, T.; Vadas, D.; Sauceau, M.; Fages, J.; Marosi, G. Characterisation of Natural Fibre Reinforced PLA Foams Prepared by Supercritical CO2 Assisted Extrusion. Express Polym. Lett. 2016, 10, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Cerro, D.; Bustos, G.; Villegas, C.; Buendia, N.; Truffa, G.; Godoy, M.P.; Rodríguez, F.; Rojas, A.; Galotto, M.J.; Constandil, L.; et al. Effect of Supercritical Incorporation of Cinnamaldehyde on Physical-Chemical Properties, Disintegration and Toxicity Studies of PLA/Lignin Nanocomposites. Int. J. Biol. Macromol. 2021, 167, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Akgün, M.; Başaran, İ.; Suner, S.C.; Oral, A. Geraniol and Cinnamaldehyde as Natural Antibacterial Additives for Poly(Lactic Acid) and Their Plasticizing Effects. J. Polym. Eng. 2020, 40, 38–48. [Google Scholar] [CrossRef]
- Di, Y.; Iannace, S.; di Maio, E.; Nicolais, L. Poly(Lactic Acid)/Organoclay Nanocomposites: Thermal, Rheological Properties and Foam Processing. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 689–698. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Kelnar, I.; Luyt, A.S. Effect of Layered Silicates on the Thermal Stability of PCL/PLA Microfibrillar Composites. Polym. Test. 2016, 50, 9–14. [Google Scholar] [CrossRef]
- Huang, A.; Yu, P.; Jing, X.; Mi, H.-Y.; Geng, L.-H.; Chen, B.-Y.; Peng, X.-F. The Effect of Talc on the Mechanical, Crystallization and Foaming Properties of Poly(Lactic Acid). J. Macromol. Sci. Part B 2016, 55, 908–924. [Google Scholar] [CrossRef]
- Jeong, E.J.; Park, C.K.; Kim, S.H. Fabrication of Microcellular Polylactide/Modified Silica Nanocomposite Foams. J. Appl. Polym. Sci. 2020, 137, 48616. [Google Scholar] [CrossRef]
- Borkotoky, S.S.; Dhar, P.; Katiyar, V. Biodegradable Poly (Lactic Acid)/Cellulose Nanocrystals (CNCs) Composite Microcellular Foam: Effect of Nanofillers on Foam Cellular Morphology, Thermal and Wettability Behavior. Int. J. Biol. Macromol. 2018, 106, 433–446. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Cai, J.; Zhang, X.; Duhoranimana, E.; Su, J. Gelatin and Pectin Complex Coacervates as Carriers for Cinnamaldehyde: Effect of Pectin Esterification Degree on Coacervate Formation, and Enhanced Thermal Stability. Food Hydrocoll. 2019, 87, 712–722. [Google Scholar] [CrossRef]
- Canales, D.; Montoille, L.; Rivas, L.M.; Ortiz, J.A.; Yañez-S, M.; Rabagliati, F.M.; Ulloa, M.T.; Alvarez, E.; Zapata, P.A. Fungicides Films of Low-Density Polyethylene (LDPE)/Inclusion Complexes (Carvacrol and Cinnamaldehyde) Against Botrytis Cinerea. Coatings 2019, 9, 795. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Cui, H.; Muhoza, B.; Duhoranimana, E.; Xia, S.; Hayat, K.; Hussain, S.; Tahir, M.U.; Zhang, X. Fabrication of Low Environment-Sensitive Nanoparticles for Cinnamaldehyde Encapsulation by Heat-Induced Gelation Method. Food Hydrocoll. 2020, 105, 105789. [Google Scholar] [CrossRef]
- Velásquez, E.; Espinoza, S.; Valenzuela, X.; Garrido, L.; Galotto, M.J.; Guarda, A.; López de Dicastillo, C. Effect of Organic Modifier Types on the Physical–Mechanical Properties and Overall Migration of Post-Consumer Polypropylene/Clay Nanocomposites for Food Packaging. Polymers 2021, 13, 1502. [Google Scholar] [CrossRef]
- Trongchuen, K.; Ounkaew, A.; Kasemsiri, P.; Hiziroglu, S.; Mongkolthanaruk, W.; Wannasutta, R.; Pongsa, U.; Chindaprasirt, P. Bioactive Starch Foam Composite Enriched with Natural Antioxidants from Spent Coffee Ground and Essential Oil. Starch Stärke 2018, 70, 1700238. [Google Scholar] [CrossRef]
- Olejnik, A.; Kapuscinska, A.; Schroeder, G.; Nowak, I. Physico-Chemical Characterization of Formulations Containing Endomorphin-2 Derivatives. Amino Acids 2017, 49, 1719–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moodley, T.; Singh, M. Polymeric Mesoporous Silica Nanoparticles for Combination Drug Delivery In Vitro. Biointerface Res. Appl. Chem. 2020, 11, 11905–11919. [Google Scholar] [CrossRef]
- Michailidou, G.; Christodoulou, E.; Nanaki, S.; Barmpalexis, P.; Karavas, E.; Vergkizi-Nikolakaki, S.; Bikiaris, D.N. Super-Hydrophilic and High Strength Polymeric Foam Dressings of Modified Chitosan Blends for Topical Wound Delivery of Chloramphenicol. Carbohydr. Polym. 2019, 208, 1–13. [Google Scholar] [CrossRef]
- Villegas, C.; Torres, A.; Bruna, J.; Bustos, M.I.; Díaz-Barrera, A.; Romero, J.; Rojas, A.; Guarda, A. Obtaining Active Polylactide (PLA) and Polyhydroxybutyrate (PHB) Blends Based Bionanocomposites Modified with Graphene Oxide and Supercritical Carbon Dioxide (ScCO2)-Assisted Cinnamaldehyde: Effect on Thermal-Mechanical, Disintegration and Mass Transport Properties. Polymers 2021, 13, 3968. [Google Scholar] [CrossRef]
- Tezcaner, A.; Keskin, D. Bioactive Agent Delivery in Bone Tissue Regeneration. In Active Implants and Scaffolds for Tissue Regeneration; Springer: Berlin/Heidelberg, Germany, 2010; pp. 193–223. [Google Scholar]
- Goimil, L.; Braga, M.E.M.; Dias, A.M.A.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; de Sousa, H.C.; García-González, C.A. Supercritical Processing of Starch Aerogels and Aerogel-Loaded Poly(ε-Caprolactone) Scaffolds for Sustained Release of Ketoprofen for Bone Regeneration. J. CO2 Util. 2017, 18, 237–249. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).