Effects of Novel Extraction Strategies on the Recovery of Phenolic Compounds and Associated Antioxidant Properties from Buckwheat Hull (Fagopyrum esculentum)
Abstract
:1. Introduction
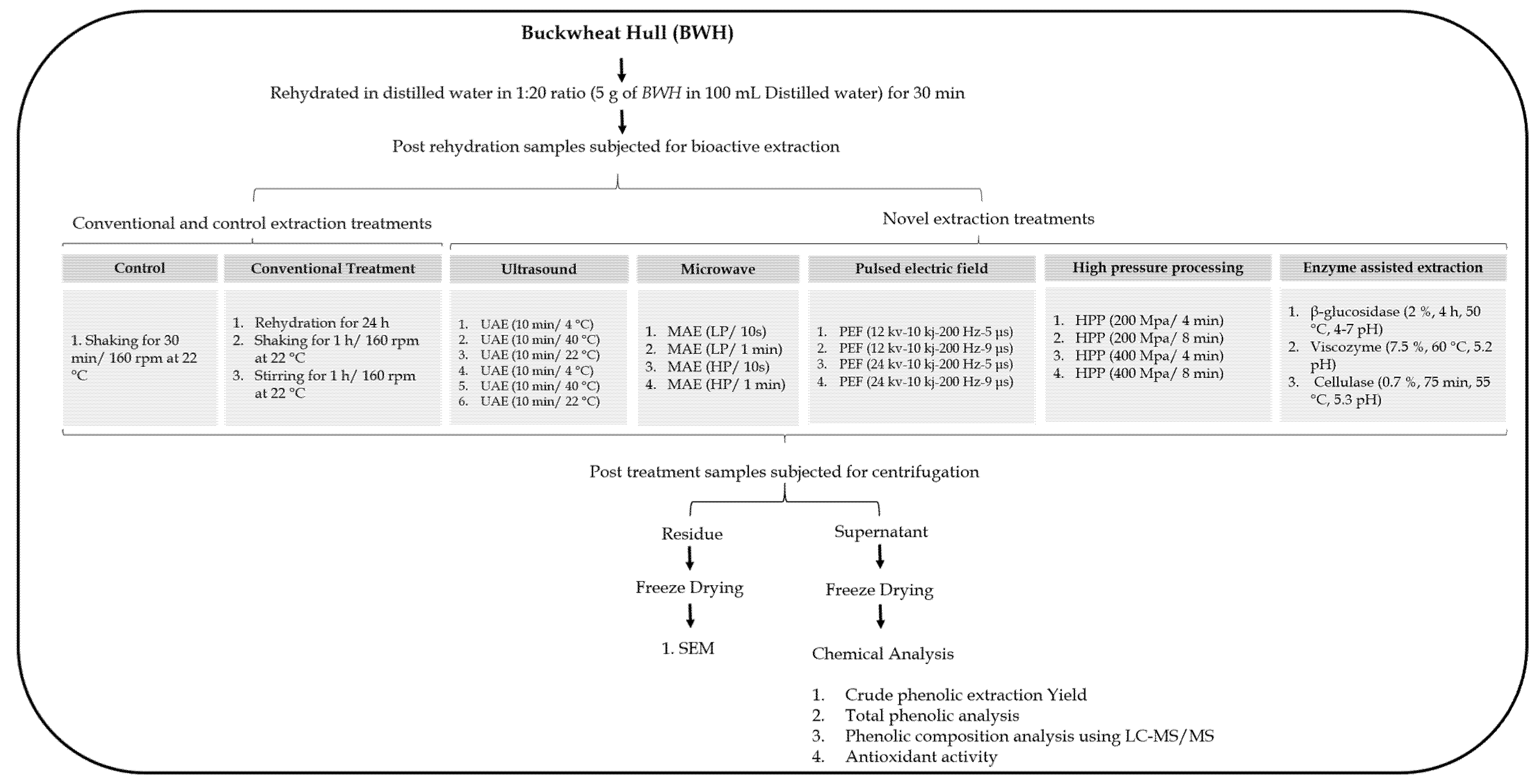
2. Material and Methods
2.1. Buckwheat Hull
2.2. Chemicals and Solvents
2.3. Extraction Procedures
2.3.1. Novel Extraction Procedures
2.3.2. Conventional Extraction Procedures
2.4. Extraction Yield
2.5. Phytochemical Analyses
2.5.1. Total Phenolic Content (TPC)
2.5.2. DPPH Radical-Scavenging Assay
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6. Liquid Chromatography-Mass Spectroscopy Analysis (LC-MS)
2.7. Scanning Electron Microscopy
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effects of Novel Extraction Strategies on Recovery Yields of Buckwheat Extracts
3.2. Total Phenolic Content (TPC)
3.3. Antioxidant Analyses
| Extraction Strategies | TPC (mg GAE/100 mg DW Extract) | FRAP (mM TE/mg DW Extracts) | DPPH (%) | |
|---|---|---|---|---|
| CNE | Direct extraction | 4.92 ± 0.07 MN d | 5.09 ± 0.05 O d | 45.55 ± 0.74 L d |
| CAE | Soaking Overnight | 13.88 ± 0.08 DEF a | 9.36 ± 0.10 K a | 69.68 ± 0.94 EF a |
| stirring | 9.29 ± 0.52 IJ b | 8.01 ± 0.02 L b | 51.65 ± 0.44 K c | |
| shaking | 11.36 ± 0.08 GH b | 7.52 ± 0.14 M c | 57.39 ± 0.52 J b | |
| UAE | US-10 min 4 °C | 15.89 ± 0.08 BC a | 17.00 ± 0.14 E c | 73.77 ± 0.06 C a |
| US-10 min 40 °C | 15.98 ± 0.04 BC a | 17.20 ± 0.29 E c | 62.15 ± 0.09 H d | |
| US-10 min RT | 4.72 ± 0.14 MN c | 14.73 ± 0.05 GH e | 61.88 ± 0.07 H d | |
| US-30 min 4 °C | 16.14 ± 0.06 BC a | 20.34 ± 0.34 D b | 64.62 ± 0.07 G c | |
| US-30 min 40 °C | 6.24 ± 0.83 LM c | 22.18 ± 0.03 C a | 66.15 ± 0.08 G b | |
| US-30 min RT | 13.24 ± 0.96 EF b | 16.16 ± 0.08 F d | 59.45 ± 0.02 I e | |
| MAE | MW-LP-10 s | 4.76 ± 0.10 MN b | 14.34 ± 0.06 H d | 77.80 ± 0.05 B a |
| MW-LP-1 min | 4.88 ± 0.19 MN b | 25.28 ± 0.04 A a | 57.82 ± 0.14 IJ c | |
| MW-HP-10 s | 12.63 ± 0.13 FG a | 16.29 ± 0.00 F c | 71.16 ± 0.93 DE b | |
| MW-HP-1 min | 11.50 ± 0.59 G a | 23.98 ± 0.00 B b | 76.37 ± 0.63 B a | |
| HPP | HPP-200 MPa-4 min | 15.85 ± 0.07 BC bc | 9.13 ± 0.00 K d | 71.68 ± 0.12 D b |
| HPP-200 MPa-8 min | 21.76 ± 0.78 A a | 11.36 ± 0.08 J c | 79.88 ± 0.12 A a | |
| HPP-400 MPa-4 min | 17.36 ± 0.05 B b | 13.31 ± 0.03 I b | 80.91 ± 0.22 A a | |
| HPP-400 MPa-8 min | 14.91 ± 0.30 CD c | 14.95 ± 0.07 G a | 71.14 ± 0.45 DE b | |
| PEF | PEF-12 kv-10 kj-200 Hz-5 μs | 7.73 ± 0.12 KL b | 7.01 ± 0.01 N a | 66.17 ± 0.10 G a |
| PEF-12 kv-10 kj-200 Hz-9 μs | 9.94 ± 0.02 HI a | 4.86 ±0.00 O b | 62.38 ± 0.78 H b | |
| PEF-24 kv-10 kj-200 Hz-5 μs | 7.31 ± 0.26 KL b | 4.21 ± 0.01 P d | 61.75 ± 0.10 H b | |
| PEF-24 kv-10 kj-200 Hz-9 μs | 7.94 ± 0.18 JK b | 4.34 ± 0.01 P c | 62.02 ± 0.18 H b | |
| EAE | b-glucosidase | 4.50 ± 0.21 N c | 4.39 ± 0.00 P a | 68.10 ± 0.95 F b |
| Viscozyme | 14.70 ± 0.18 CDE b | 0.88 ± 0.04 R c | 16.80 ± 0.33 M c | |
| Cellulase | 22.28 ± 0.53 A a | 2.52 ± 0.00 Q b | 72.11 ± 0.11 CD a | |
3.4. Phenolic Profile
3.5. Microstructure Characteristics of Buckwheat Hull
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohnishi, O. Search for the wild ancestor of buckwheat III. The wild ancestor of cultivated common buckwheat, and of tatary buckwheat. Econ. Bot. 1998, 52, 123–133. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, H. Antioxidant activities of barley seeds extracts. Food Chem. 2007, 102, 732–737. [Google Scholar] [CrossRef]
- Lee, L.-S.; Choi, E.-J.; Kim, C.-H.; Sung, J.-M.; Kim, Y.-B.; Seo, D.-H.; Choi, H.-W.; Choi, Y.-S.; Kum, J.-S.; Park, J.-D. Contribution of flavonoids to the antioxidant properties of common and tartary buckwheat. J. Cereal Sci. 2016, 68, 181–186. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Pastucha, E.; Piątkowska, E.; Leszczyńska, T.; Pisulewska, E.; Witkowicz, R.; Francik, R. Basic chemical composition and bioactive compounds content in selected cultivars of buckwheat whole seeds, dehulled seeds and hulls. J. Cereal Sci. 2016, 69, 1–8. [Google Scholar] [CrossRef]
- Zduńczyk, Z.; Flis, M.; Zieliński, H.; Wróblewska, M.; Antoszkiewicz, Z.; Juśkiewicz, J. In vitro antioxidant activities of barley, husked oat, naked oat, triticale, and buckwheat wastes and their influence on the growth and biomarkers of antioxidant status in rats. J. Agric. Food Chem. 2006, 54, 4168–4175. [Google Scholar] [CrossRef]
- Holasova, M.; Fiedlerova, V.; Smrcinova, H.; Orsak, M.; Lachman, J.; Vavreinova, S. Buckwheat—The source of antioxidant activity in functional foods. Food Res. Int. 2002, 35, 207–211. [Google Scholar] [CrossRef]
- Lu, L.; Murphy, K.; Baik, B.K. Genotypic variation in nutritional composition of buckwheat groats and husks. Cereal Chem. 2013, 90, 132–137. [Google Scholar] [CrossRef]
- Sedej, I.; Sakač, M.; Mandić, A.; Mišan, A.; Tumbas, V.; Čanadanović-Brunet, J. Buckwheat (Fagopyrum esculentum Moench) grain and fractions: Antioxidant compounds and activities. J. Food Sci. 2012, 77, C954–C959. [Google Scholar] [CrossRef]
- Mackėla, I.; Andriekus, T.; Venskutonis, P.R. Biorefining of buckwheat (Fagopyrum esculentum) hulls by using supercritical fluid, Soxhlet, pressurized liquid and enzyme-assisted extraction methods. J. Food Eng. 2017, 213, 38–46. [Google Scholar] [CrossRef]
- Kim, S.-H.; Cui, C.-B.; Kang, I.-J.; Kim, S.Y.; Ham, S.-S. Cytotoxic effect of buckwheat (Fagopyrum esculentum Moench) hull against cancer cells. J. Med. Food 2007, 10, 232–238. [Google Scholar] [CrossRef]
- Čabarkapa, I.S.; Sedej, I.J.; Sakač, M.B.; Šarić, L.Č.; Plavšić, D.V. Antimicrobial activity of buckwheat (Fagopyrum esculentum Moench) hulls extract. Food Process. Qual. Saf. 2008, 35, 159–163. [Google Scholar]
- Li, T.; Yang, Y.; Wang, X.; Dai, W.; Zhang, L.; Piao, C. Flavonoids derived from buckwheat hull can break advanced glycation end-products and improve diabetic nephropathy. Food Funct. 2021, 12, 7161–7170. [Google Scholar] [CrossRef]
- Li, P.; Tang, Y.; Liu, L.; Wang, D.; Zhang, L.; Piao, C. Therapeutic potential of buckwheat hull flavonoids in db/db mice, a model of type 2 diabetes. J. Funct. Foods 2019, 52, 284–290. [Google Scholar] [CrossRef]
- Zielinska, D.; Szawara-Nowak, D.; Zielinski, H. Antioxidative and anti-glycation activity of buckwheat hull tea infusion. Int. J. Food Prop. 2013, 16, 228–239. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef]
- Yang, X.; Lv, Y.-P. Purification, characterization, and DNA damage protection of active components from tartary buckwheat (Fagopyrum tataricum) hull. Food Sci. Biotechnol. 2015, 24, 1959–1966. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Ravindran, R.; Walsh, O.; O’Doherty, J.; Jaiswal, A.K.; Tiwari, B.K.; Rajauria, G. Evaluation of Ultrasound, Microwave, Ultrasound–Microwave, Hydrothermal and High Pressure Assisted Extraction Technologies for the Recovery of Phytochemicals and Antioxidants from Brown Macroalgae. Mar. Drugs 2021, 19, 309. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Z.; Hinds, L.M.; Sun, D.-W.; Tiwari, B.K. Applications of ultrasound to enhance fluidized bed drying of Ascophyllum Nodosum: Drying kinetics and product quality assessment. Ultrason. Sonochem. 2021, 70, 105298. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, M.; Gomez, L.P.; Senthamaraikannan, R.; Padamati, R.B.; O’Donnell, C.P.; Tiwari, B.K. Investigation of enzyme-assisted methods combined with ultrasonication under a controlled alkali pretreatment for agar extraction from Gelidium sesquipedale. Food Hydrocoll. 2021, 120, 106905. [Google Scholar] [CrossRef]
- Altemimi, A.B.; Al-Hilphy, A.R.; Abedelmaksoud, T.G.; Aboud, S.A.; Badwaik, L.S.; Noore, S.; Pratap-Singh, A. Infrared Radiation Favorably Influences the Quality Characteristics of Key Lime Juice. Appl. Sci. 2021, 11, 2842. [Google Scholar] [CrossRef]
- Lopes, A.P.; Galuch, M.B.; Petenuci, M.E.; Oliveira, J.H.; Canesin, E.A.; Schneider, V.V.A.; Visentainer, J.V. Quantification of phenolic compounds in ripe and unripe bitter melons (Momordica charantia) and evaluation of the distribution of phenolic compounds in different parts of the fruit by UPLC–MS/MS. Chem. Pap. 2020, 74, 2613–2625. [Google Scholar] [CrossRef]
- Noore, S.; Ramesh, G.; Vendan, S.E.; Nagaraju, V. Persistence and diffusion behaviour of chlorpyrifos in five different species of vegetables: A comparative analysis. Ecotoxicol. Environ. Saf. 2021, 217, 112208. [Google Scholar] [CrossRef]
- Murtey, M.D.; Ramasamy, P. Sample preparations for scanning electron microscopy–life sciences. Mod. Electron Microsc. Phys. Life Sci. 2016, 161–185. [Google Scholar]
- Miron, T.; Herrero, M.; Ibáñez, E. Enrichment of antioxidant compounds from lemon balm (Melissa officinalis) by pressurized liquid extraction and enzyme-assisted extraction. J. Chromatogr. A 2013, 1288, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Fernández, K.; Vega, M.; Aspé, E. An enzymatic extraction of proanthocyanidins from País grape seeds and skins. Food Chem. 2015, 168, 7–13. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Coelho, M.A.Z.; Rosenthal, A.; Gottschalk, L.M.F.; Tonon, R.V. Combination of enzyme-assisted extraction and high hydrostatic pressure for phenolic compounds recovery from grape pomace. J. Food Eng. 2021, 288, 110128. [Google Scholar] [CrossRef]
- Sharif, T.; Bhatti, H.N.; Bull, I.D.; Bilal, M. Recovery of high-value bioactive phytochemicals from agro-waste of mango (Mangifera indica L.) using enzyme-assisted ultrasound pretreated extraction. Biomass Convers. Biorefin. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Martillanes, S.; Ayuso-Yuste, M.C.; Bernalte, M.J.; Gil, M.V.; Delgado-Adámez, J. Cellulase-assisted extraction of phenolic compounds from rice bran (Oryza sativa L.): Process optimization and characterization. J. Food Meas. Charact. 2021, 15, 1719–1726. [Google Scholar] [CrossRef]
- Wang, W.; Gao, Y.-T.; Wei, J.-W.; Chen, Y.-F.; Liu, Q.-L.; Liu, H.-M. Optimization of Ultrasonic Cellulase-Assisted Extraction and Antioxidant Activity of Natural Polyphenols from Passion Fruit. Molecules 2021, 26, 2494. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G.; Pataro, G. Applications of pulsed electric field treatments for the enhancement of mass transfer from vegetable tissue. Food Eng. Rev. 2010, 2, 109–130. [Google Scholar] [CrossRef]
- Balasa, A.; Toepfl, S.; Knorr, D. Impact of pulsed electric field treatment on polyphenolic content of grapes. In Control Applications in Post-Harvest and Processing Technology (CAPPT 2006); Leibniz Institute of Agricultural Engineering and Bio-economy e.V.: Potsdam, Germany, 2006; p. 169. [Google Scholar]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Chudoba, T.; Lojkowski, W.; Witrowa-Rajchert, D. The impact of pulsed electric field treatment on selected bioactive compound content and color of plant tissue. Innov. Food Sci. Emerg. Technol. 2015, 30, 69–78. [Google Scholar] [CrossRef]
- Rajha, H.N.; Abi-Khattar, A.-M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of aqueous extraction efficiency and biological activities of polyphenols from pomegranate peels assisted by infrared, ultrasound, pulsed electric fields and high-voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212. [Google Scholar] [CrossRef]
- Zderic, A.; Zondervan, E.; Meuldijk, J. Breakage of cellular tissue by pulsed electric field: Extraction of polyphenols from fresh tea leaves. Chem. Eng. Trans. 2013, 32, 1795–1800. [Google Scholar]
- Einarsdóttir, R.; Þórarinsdóttir, K.A.; Aðalbjörnsson, B.V.; Guðmundsson, M.; Marteinsdóttir, G.; Kristbergsson, K. The effect of pulsed electric field-assisted treatment parameters on crude aqueous extraction of Laminaria digitata. J. Appl. Phycol. 2021, 33, 3287–3296. [Google Scholar] [CrossRef]
- Knorr, D. Effects of high-hydrostatic-pressure processes on food safety and quality. Food Technol. 1993, 47, 156–161. [Google Scholar]
- Altuner, E.M.; Işlek, C.; Çeter, T.; Alpas, H. High hydrostatic pressure extraction of phenolic compounds from Maclura pomifera fruits. Afr. J. Biotechnol. 2012, 11, 930–937. [Google Scholar]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Strati, I.F.; Gogou, E.; Oreopoulou, V. Enzyme and high pressure assisted extraction of carotenoids from tomato waste. Food Bioprod. Process. 2015, 94, 668–674. [Google Scholar] [CrossRef]
- Noore, S.; Rastogi, N.K.; O’Donnell, C.; Tiwari, B. Novel bioactive extraction and nano-encapsulation. Encyclopedia 2021, 1, 632–664. [Google Scholar] [CrossRef]
- Younas, U.; Iqbal, S.; Bashir, R.; Sajjad, N.; Saeed, Z.; Pervaiz, M.; Hassan, F.; Ali, F.; Ibrahim, S.; Batool, F. An Eco-Friendly Approach for the Extraction of Antioxidant Components from Artemisia Annua Leaves Using Response Surface Methodology. Pol. J. Environ. Stud. 2021, 30, 4827–4833. [Google Scholar] [CrossRef]
- Guo, X.-D.; Wu, C.-S.; Ma, Y.-J.; Parry, J.; Xu, Y.-Y.; Liu, H.; Wang, M. Comparison of milling fractions of tartary buckwheat for their phenolics and antioxidant properties. Food Res. Int. 2012, 49, 53–59. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Liu, H.-M.; Yu, Y.-W.; Lin, S.-D.; Mau, J.-L. Quality and antioxidant property of buckwheat enhanced wheat bread. Food Chem. 2009, 112, 987–991. [Google Scholar] [CrossRef]
- Hemery, Y.M.; Anson, N.M.; Havenaar, R.; Haenen, G.R.; Noort, M.W.; Rouau, X. Dry-fractionation of wheat bran increases the bioaccessibility of phenolic acids in breads made from processed bran fractions. Food Res. Int. 2010, 43, 1429–1438. [Google Scholar] [CrossRef]
- Steadman, K.J.; Burgoon, M.S.; Lewis, B.A.; Edwardson, S.E.; Obendorf, R.L. Minerals, phytic acid, tannin and rutin in buckwheat seed milling fractions. J. Sci. Food Agric. 2001, 81, 1094–1100. [Google Scholar] [CrossRef]
- Verardo, V.; Arráez-Román, D.; Segura-Carretero, A.; Marconi, E.; Fernandez-Gutierrez, A.; Caboni, M.F. Determination of free and bound phenolic compounds in buckwheat spaghetti by RP-HPLC-ESI-TOF-MS: Effect of thermal processing from farm to fork. J. Agric. Food Chem. 2011, 59, 7700–7707. [Google Scholar] [CrossRef]
- Kim, S.-J.; Zaidul, I.; Suzuki, T.; Mukasa, Y.; Hashimoto, N.; Takigawa, S.; Noda, T.; Matsuura-Endo, C.; Yamauchi, H. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem. 2008, 110, 814–820. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Liu, Q.; Bao, J.; Liu, Q. Identification and quantification of polyphenols in hull, bran and endosperm of common buckwheat (Fagopyrum esculentum) seeds. J. Funct. Foods 2017, 38, 363–369. [Google Scholar] [CrossRef]
- Przybylski, R.; Lee, Y.; Eskin, N. Antioxidant and radical-scavenging activities of buckwheat seed components. J. Am. Oil Chem. Soc. 1998, 75, 1595. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Dang, Y.; Wei, H.; Wang, Q. Removal of Cr (VI) from aqueous solutions using buckwheat (Fagopyrum esculentum Moench) hull through adsorption-reduction: Affecting factors, isotherm, and mechanisms. CLEAN–Soil Air Water 2014, 42, 1549–1557. [Google Scholar] [CrossRef]
- Kulokas, M.; Praspaliauskas, M.; Pedišius, N. Investigation of buckwheat hulls as additives in the production of solid biomass fuel from straw. Energies 2021, 14, 265. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, Z.; Liang, J.; Liang, C. Natural biomass-derived porous carbons from buckwheat hulls used as anode for lithium-ion batteries. Diam. Relat. Mater. 2021, 119, 108553. [Google Scholar] [CrossRef]


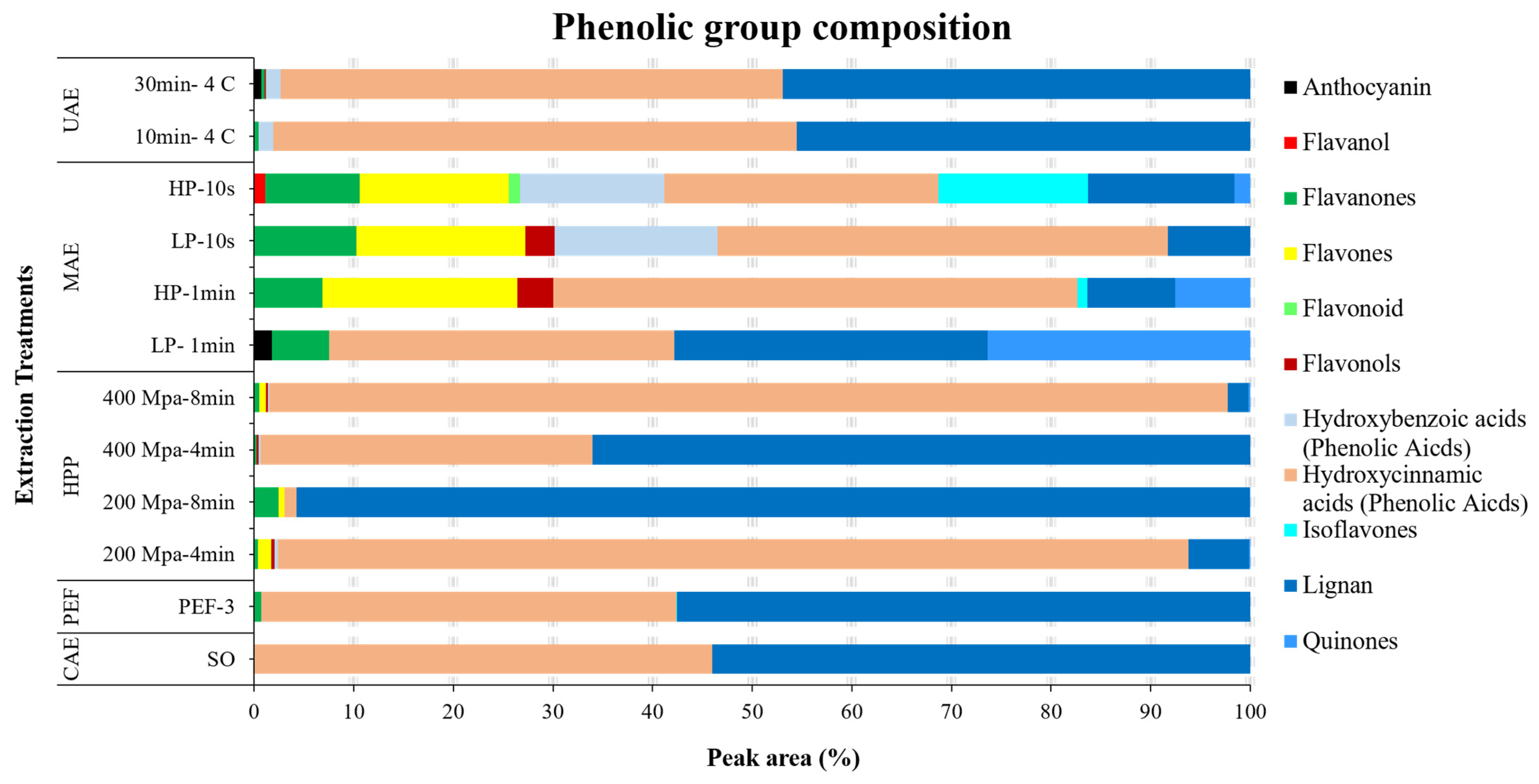
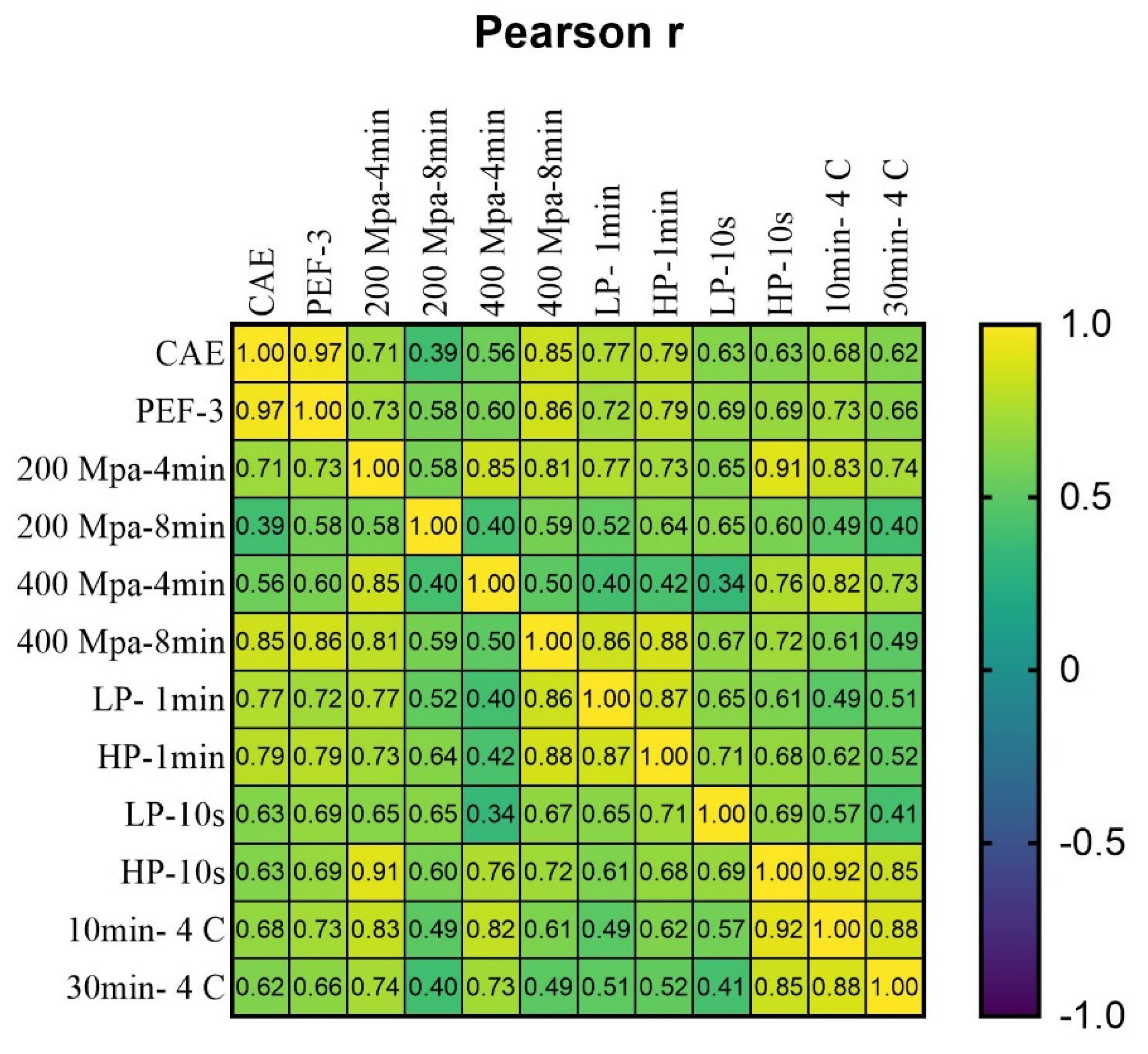
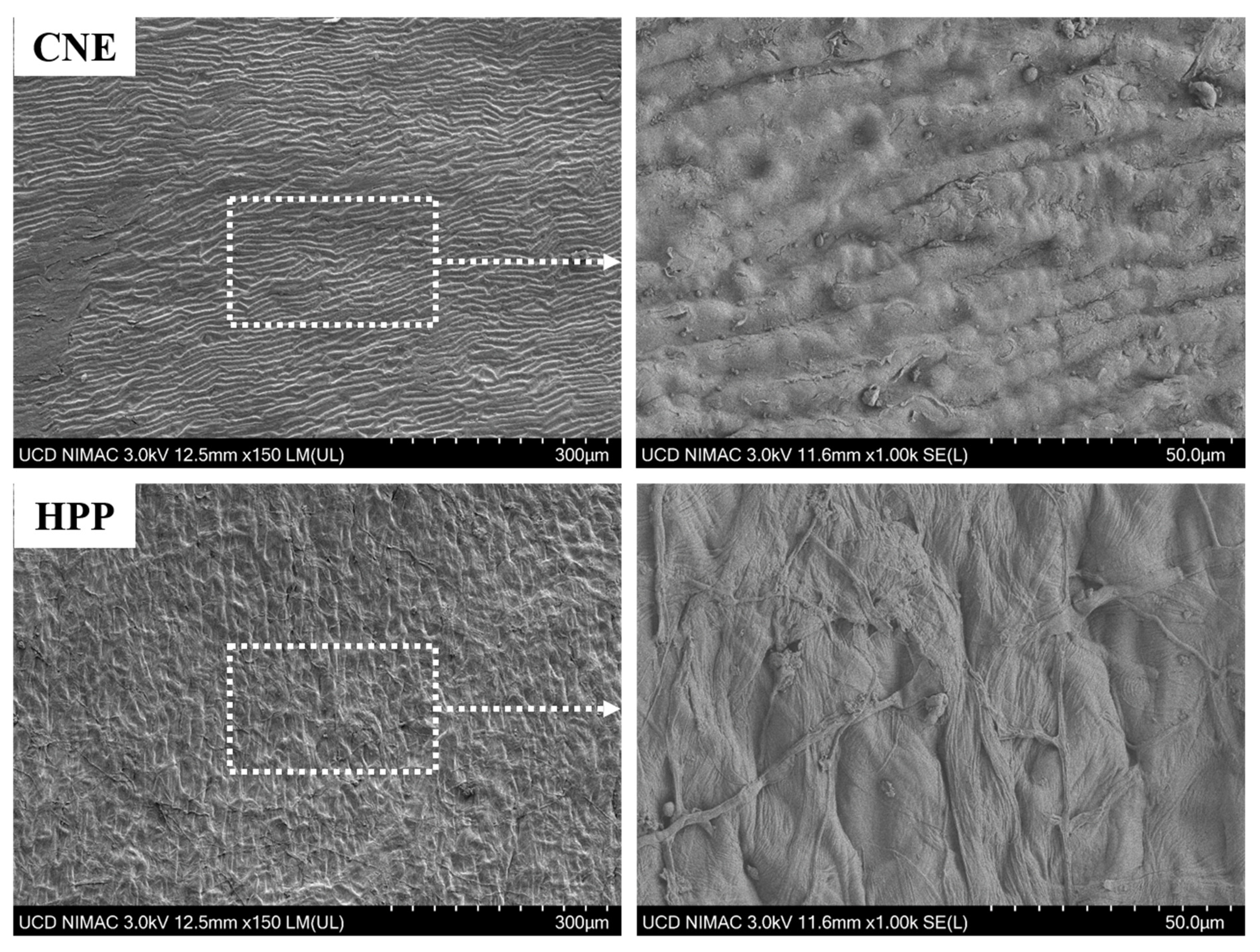
| Phenolic Profile | Relative Concentration (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Compound Name | RT (min) | CAE | PEF | HPP | MAE | UAE | |||||||
| SO | PEF-3 | 200 Mpa-4 min | 200 Mpa-8 min | 400 Mpa-4 min | 400 Mpa-8 min | LP-1 min | HP-1 min | LP-10 s | HP-10 s | 10 min- 4 °C | 30 min- 4 °C | |||
| Anthocyanin | Pelargonidin | 0.33 | - | - | - | - | - | - | - | - | - | - | - | 0.74 |
| Pelargonidin 3-O-(6″-succinyl-glucoside) | 9.47 | - | - | - | - | - | - | 1.82 | - | - | - | - | - | |
| Flavanol | (+)-Catechin 3-O-gallate | 0.39 | - | - | - | - | - | - | - | - | 1.14 | - | - | - |
| (−)-Epicatechin 3-O-gallate | 0.39 | - | - | - | - | - | - | - | - | 1.14 | - | - | - | |
| Flavanones | Apigenin 6-C-glucoside | 0.39 | - | - | 1.3 | 0.61 | - | - | - | 19.6 | 14.96 | 16.94 | - | - |
| Pongamoside B | 9.45 | - | - | - | 1.42 | - | - | - | - | - | - | - | - | |
| O-Methylovaliflavanone C | 15.55 | - | - | - | 0.48 | - | - | - | - | - | - | - | - | |
| 6-Geranylnaringenin | 15.57 | - | 0.72 | 0.41 | 0.54 | 0.25 | 0.54 | 5.74 | 6.86 | 9.45 | 10.27 | 0.49 | 0.31 | |
| Flavones | Dihydroquercetin 3-O-rhamnoside | 0.4 | - | - | - | - | - | - | - | 3.59 | - | - | - | - |
| Apigenin-6-glucoside | 0.4 | - | - | - | - | - | 0.62 | - | - | - | - | - | - | |
| Flavonols | Kaempferol 3-O-glucuronid | 0.38 | - | - | 0.31 | - | - | - | - | - | - | - | - | - |
| Pyrogallol | 0.39 | - | 0.39 | 0.18 | 0.18 | 0.39 | ||||||||
| Syringetin-3-glucuronide | 0.4 | - | - | - | - | - | - | - | - | - | - | - | 0.13 | |
| Quercetine 3-O-acetyl-rhamnoside | 0.42 | - | - | - | - | 0.21 | 0.21 | - | - | - | - | - | - | |
| Quercetine-3-O-xylosyl | 15.3 | - | - | - | - | - | - | - | - | - | 2.95 | - | - | |
| Hydroxybenzoic acids (Phenolic acid) | Gallic acid | 0.39 | - | - | - | - | - | - | - | - | 14.5 | 16.36 | 1.03 | 1.45 |
| 6-Gingerol | 12.8 | - | - | - | - | - | - | - | 9.64 | - | - | - | - | |
| Hydroxycinnamic acids (Phenolic Acids) | Oleuropein-aglycone | 0.38 | - | 0.06 | - | - | - | - | - | - | - | - | - | - |
| Ferulic acid | 0.39 | - | - | 0.45 | - | - | - | 5.17 | - | - | - | - | - | |
| Caffeic acid | 0.39 | - | - | 0.88 | - | - | - | 5.55 | - | - | - | - | - | |
| 5–8′-Dehydrodiferulic acid | 0.41 | - | - | - | - | - | 0.55 | - | 19.1 | 13.1 | 16.39 | - | - | |
| 5–8′-Dehydroferulic acid | 0.41 | - | - | 1.28 | 0.61 | - | - | - | - | - | - | - | - | |
| p-Coumaric acid 4-O-glucoside | 0.41 | - | - | - | - | - | - | - | 17.83 | - | - | - | - | |
| o-Coumaric acid | 0.44 | - | - | - | - | - | - | 4.65 | - | 7.52 | 2.85 | - | - | |
| Daidzin | 6.3 | - | - | - | - | - | - | - | 0.98 | - | - | - | - | |
| 4-Vinylsyringol | 8.72 | 1.25 | 0.96 | 1.29 | 0.58 | 1.71 | 1.3 | 19.25 | 6.05 | 6.9 | 23.12 | 1.2 | 1.75 | |
| Carnosol | 10.44 | - | - | - | - | - | - | - | - | - | 2.81 | - | - | |
| isoacitrein | 11.58 | - | - | - | - | - | - | - | - | - | - | 51.32 | - | |
| Isoacitretin | 11.95 | 44.74 | 40.62 | 87.44 | 31.6 | 94.35 | - | - | - | - | - | 48.65 | ||
| Isoflavones | Genistin | 0.41 | - | - | - | - | - | - | - | - | 14.96 | - | - | - |
| Broussonin C | 9.47 | 52.55 | 55.86 | 4.01 | 94.83 | 64.28 | 0.47 | - | - | - | 0.79 | 44.34 | 45.16 | |
| Glycitin | 15.33 | - | 0.07 | - | - | - | - | - | - | - | - | - | - | |
| Lignan | Pterostilbene | 8.73 | 1.27 | 0.98 | 1.32 | 0.59 | 1.77 | 1.3 | 19.19 | 6.27 | 7.13 | 1.22 | 1.8 | |
| Todolactol A | 8.87 | - | 0.17 | - | - | - | - | - | - | - | - | - | - | |
| 9-Azabicyclo [1.3.3]nonane,1H-indazole-3-carboxamide deriv. | 9.43 | - | - | - | - | - | - | - | 5.64 | - | - | - | - | |
| Conidendrin | 9.57 | - | - | - | - | - | - | - | 1.17 | - | - | - | - | |
| Secoisolariciresinol | 10.11 | 0.19 | 0.57 | 0.74 | 0.26 | - | 0.29 | 3.97 | 1.4 | 7.57 | 7.51 | - | - | |
| Estra-1,3,5(10),16-tetran-3-ol benzoate | 15.15 | - | - | - | - | - | - | 8.3 | - | - | - | - | - | |
| Quinones | N-(p-Hydroxyphenethyl) actinidine | 9.47 | - | - | - | - | - | - | 24.49 | - | - | - | - | - |
| O-Desmethylquinidine | 12.81 | - | - | - | - | - | - | - | - | 1.62 | - | - | - | |
| Sinapine | 15.13 | - | - | 0.16 | 0.08 | - | 0.19 | 1.88 | 1.88 | - | - | - | - | |
| The total number of compounds detected | 5 | 9 | 13 | 10 | 7 | 11 | 11 | 13 | 12 | 10 | 7 | 8 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noore, S.; Joshi, A.; Kumari, B.; Zhao, M.; O’Donnell, C.; Tiwari, B.K. Effects of Novel Extraction Strategies on the Recovery of Phenolic Compounds and Associated Antioxidant Properties from Buckwheat Hull (Fagopyrum esculentum). Processes 2022, 10, 365. https://doi.org/10.3390/pr10020365
Noore S, Joshi A, Kumari B, Zhao M, O’Donnell C, Tiwari BK. Effects of Novel Extraction Strategies on the Recovery of Phenolic Compounds and Associated Antioxidant Properties from Buckwheat Hull (Fagopyrum esculentum). Processes. 2022; 10(2):365. https://doi.org/10.3390/pr10020365
Chicago/Turabian StyleNoore, Shaba, Akanksha Joshi, Bibha Kumari, Ming Zhao, Colm O’Donnell, and Brijesh Kumar Tiwari. 2022. "Effects of Novel Extraction Strategies on the Recovery of Phenolic Compounds and Associated Antioxidant Properties from Buckwheat Hull (Fagopyrum esculentum)" Processes 10, no. 2: 365. https://doi.org/10.3390/pr10020365
APA StyleNoore, S., Joshi, A., Kumari, B., Zhao, M., O’Donnell, C., & Tiwari, B. K. (2022). Effects of Novel Extraction Strategies on the Recovery of Phenolic Compounds and Associated Antioxidant Properties from Buckwheat Hull (Fagopyrum esculentum). Processes, 10(2), 365. https://doi.org/10.3390/pr10020365








