Temperature Effects of MD on Municipal Wastewater Treatment in an Integrated Forward Osmosis and Membrane Distillation Process
Abstract
:1. Introduction
2. Materials and Methods
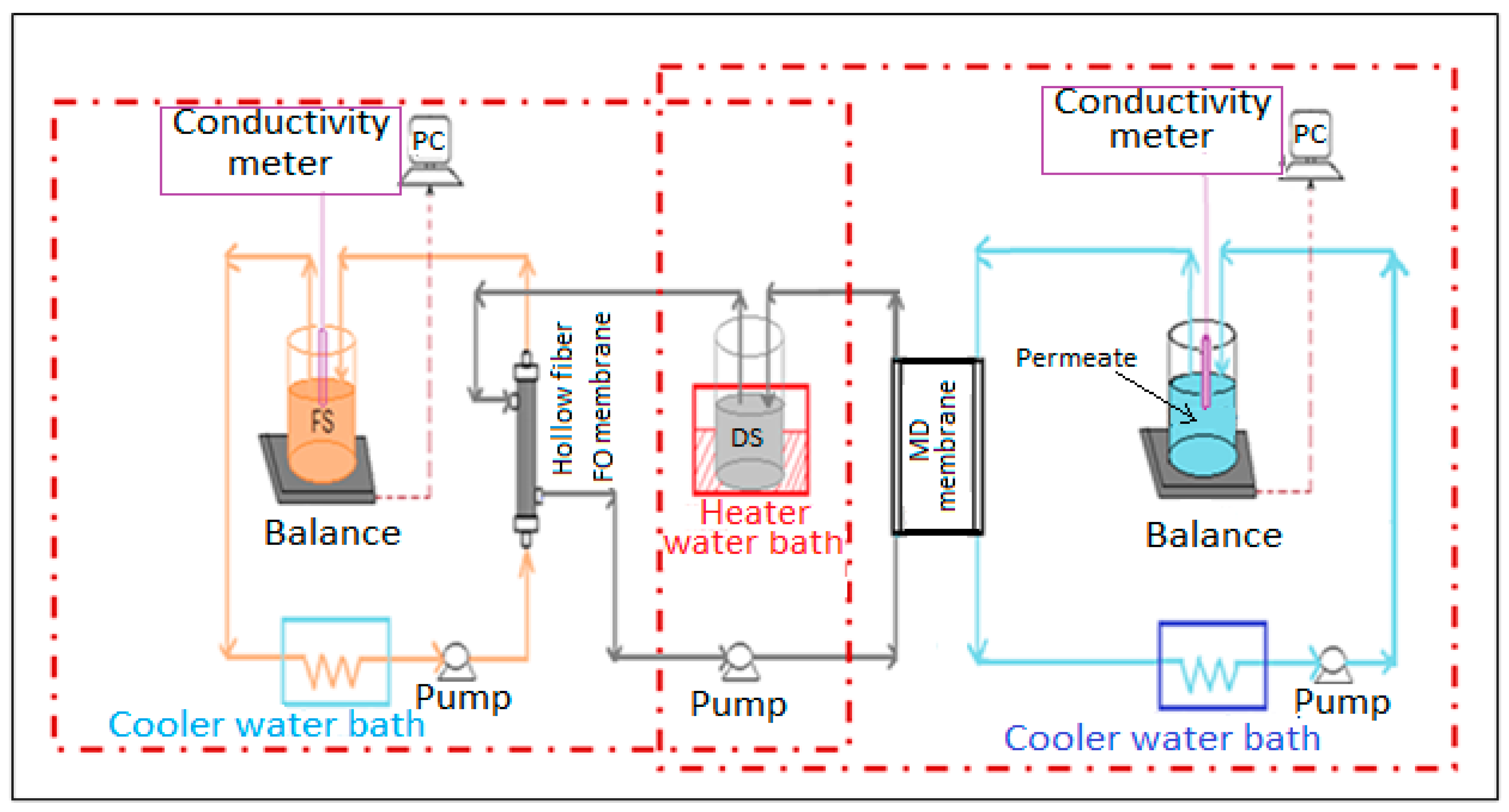
2.1. FO and MD Membranes and the Experimental Setup
2.2. Feed Solution and Draw Solution
2.3. Operation of the Experimental Rigs
2.4. Analytical Methods
2.5. Calculations
2.5.1. Water Flux and Reverse Solute Flux
2.5.2. Concentration Factor and Rejection Rate of Contaminates in FS
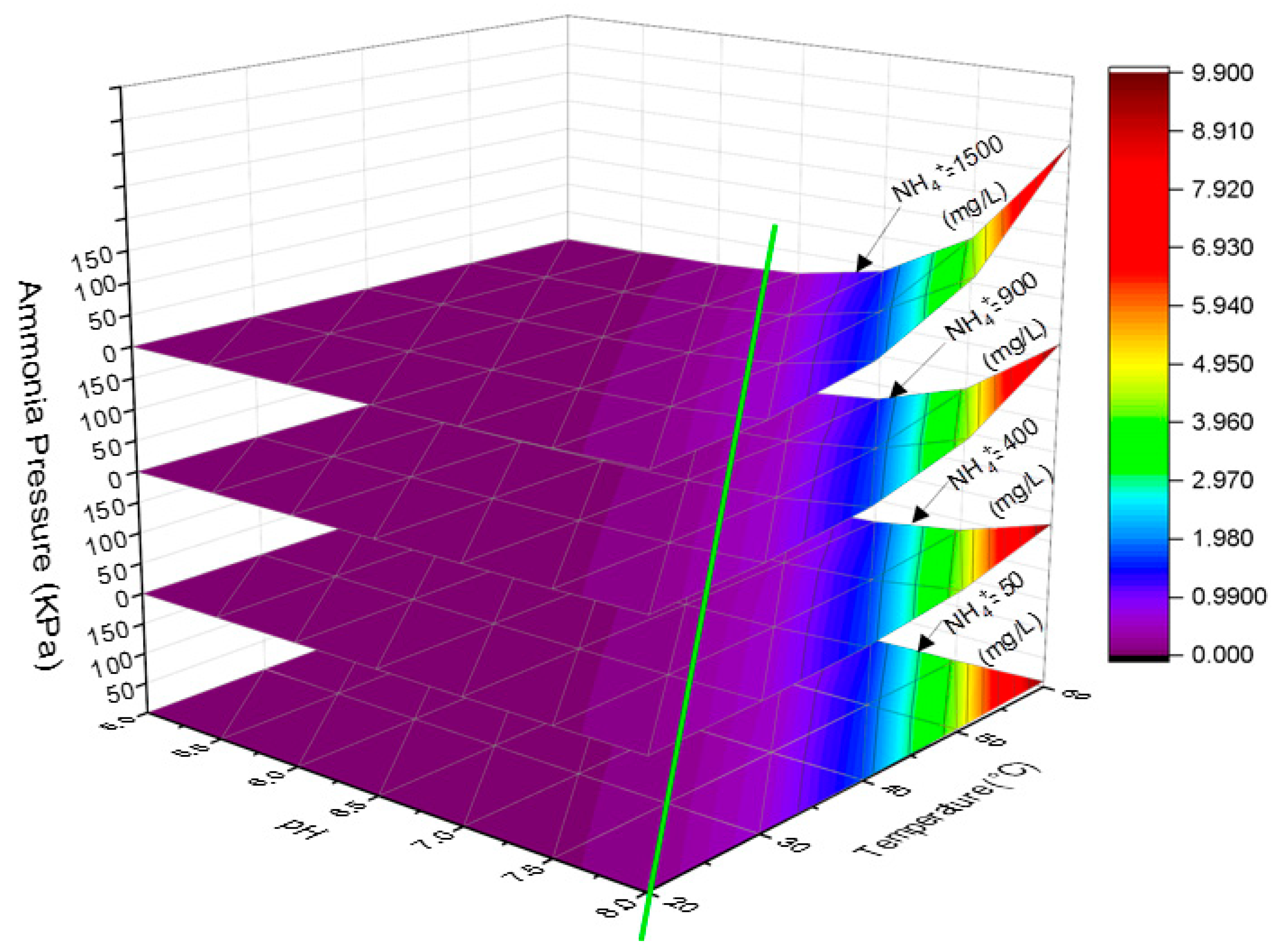
2.5.3. Ammonia Pressure in the MD
2.5.4. Heat Balance and Heat Demand in a FO-MD System without Internal Heat Recovery
3. Results and Discussion
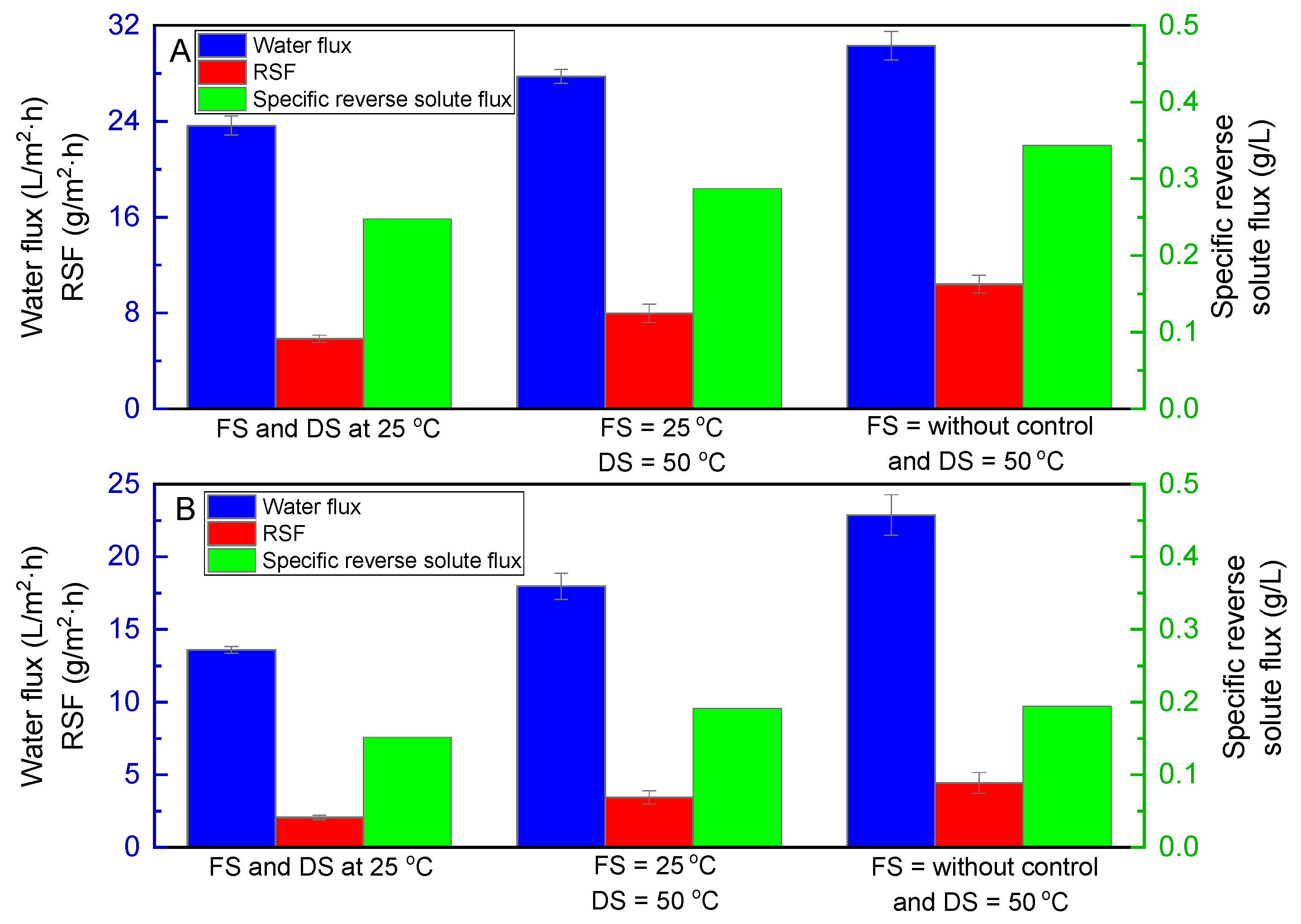
3.1. Effects of Temperature of Feed and Draw Solutions on Water Flux and RSF with NaCl and Glucose as Draw Solutions and DI Water as FS
- The osmotic pressure of DS is proportional to temperature according to Equation (3);
- The diffusivity of both FS and DS increases with the increase in temperature, resulting in high water flux and reverse solute flux across the membrane [31];
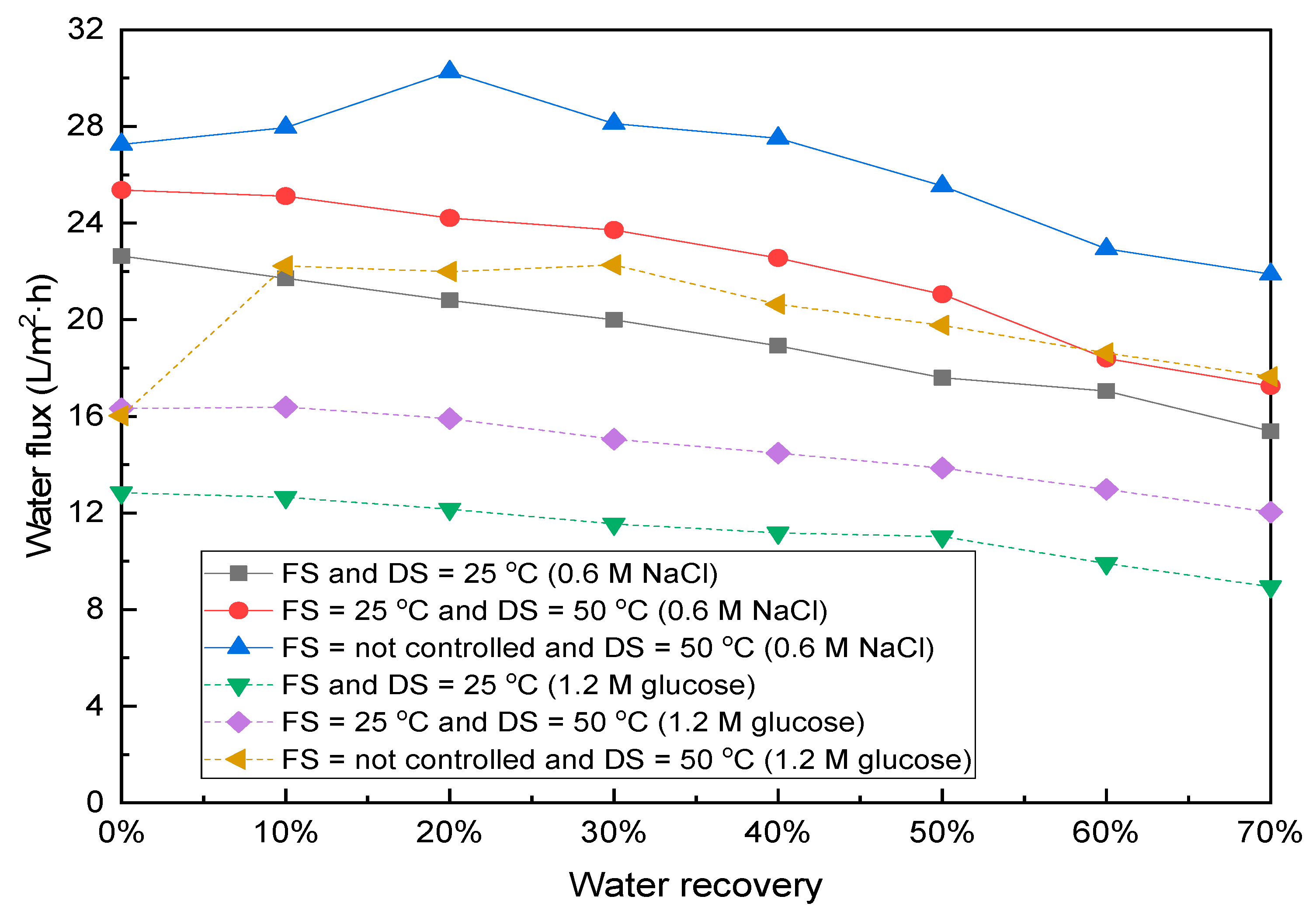
3.2. Effects of Temperature of Feed and Draw Solutions on FO Filtration with Synthetic Wastewater as FS
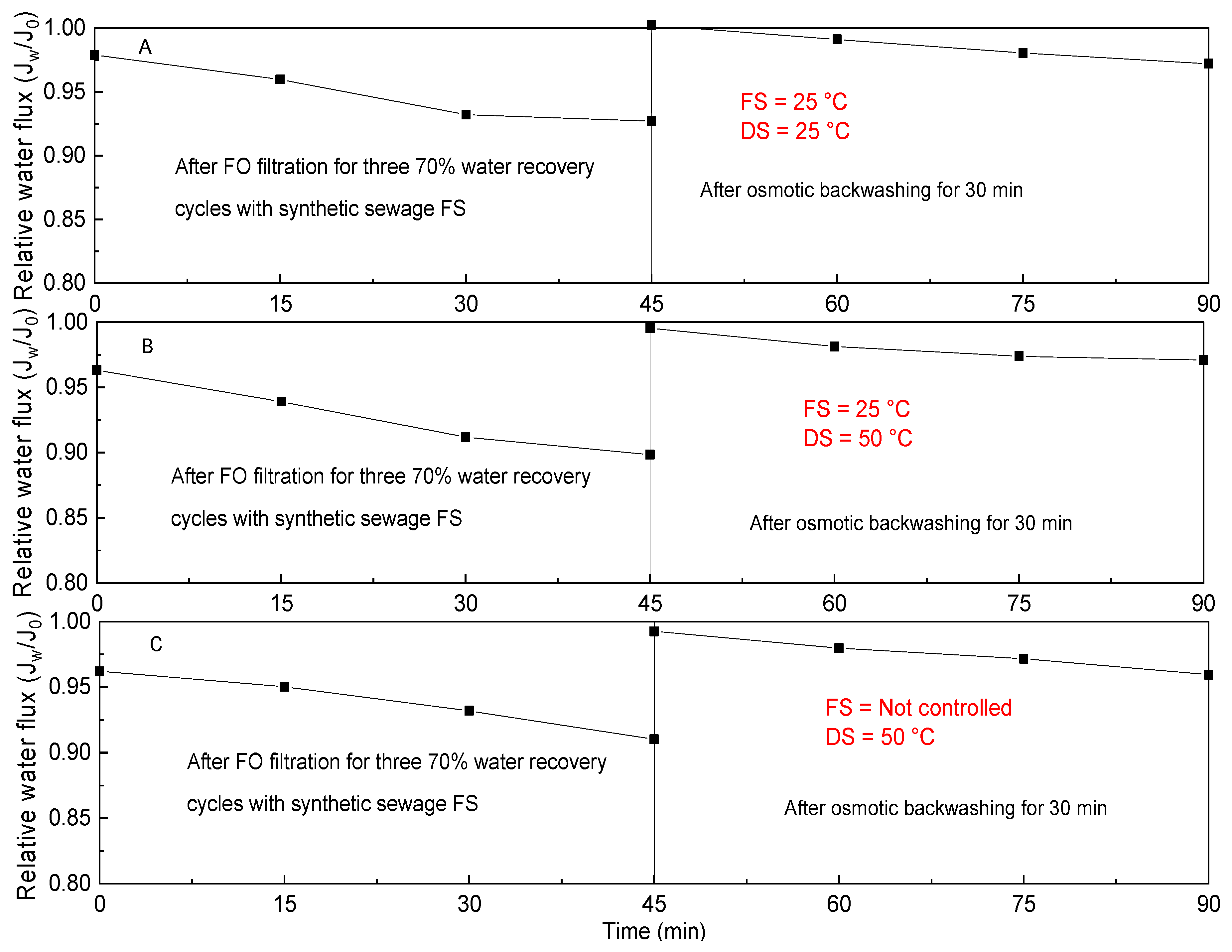
3.3. Membrane Fouling and Cleaning at Different Feed and Draw Solutions Temperatures in the TFC FO Filtration Process with Synthetic Municipal Wastewater
3.4. Ammonia Vaporisation in a Direct Contact MD Membrane Filtration Process for Simultaneous DS Regeneration and Water Recovery
3.5. Heat Evaluation for an Integrated FO-MD Process in Treating Wastewater
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zohrabian, L.; Hankins, N.P.; Field, R.W. Hybrid forward osmosis-membrane distillation system: Demonstration of technical feasibility. J. Water Process Eng. 2020, 33, 101042. [Google Scholar] [CrossRef]
- Panagopoulos, A. Energetic, economic and environmental assessment of zero liquid discharge (ZLD) brackish water and seawater desalination systems. Energy Convers. Manag. 2021, 235, 113957. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Kyong, H.; Hong, S. Organic fouling mechanisms in forward osmosis membrane process under elevated feed and draw solution temperatures. Desalination 2015, 355, 169–177. [Google Scholar] [CrossRef]
- Ali, S.M.; Kim, J.E.; Phuntsho, S.; Jang, A.; Choi, J.Y.; Shon, H.K. Forward osmosis system analysis for optimum design and operating conditions. Water Res. 2018, 145, 429–441. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Esparra-Alvarado, M.; Wang, X.; Yang, H.; Xie, Y. Effects of pH and temperature on forward osmosis membrane flux using rainwater as the makeup for cooling water dilution. Desalination 2014, 351, 70–76. [Google Scholar] [CrossRef]
- Wang, K.Y.; Teoh, M.M.; Nugroho, A.; Chung, T.S. Integrated forward osmosis-membrane distillation (FO-MD) hybrid system for the concentration of protein solutions. Chem. Eng. Sci. 2011, 66, 2421–2430. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, P.; Fu, X.; Chung, T.S. Sustainable water recovery from oily wastewater via forward osmosis-membrane distillation (FO-MD). Water Res. 2014, 52, 112–121. [Google Scholar] [CrossRef]
- Lu, D.; Liu, Q.; Zhao, Y.; Liu, H.; Ma, J. Treatment and energy utilization of oily water via integrated ultrafiltration-forward osmosis–membrane distillation (UF-FO-MD) system. J. Membr. Sci. 2018, 548, 275–287. [Google Scholar] [CrossRef]
- Xie, M.; Price, W.E.; Nghiem, L.D.; Elimelech, M. Effects of feed and draw solution temperature and transmembrane temperature difference on the rejection of trace organic contaminants by forward osmosis. J. Membr. Sci. 2013, 438, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Li, Y.; Wang, Y. Treatment of greywater by forward osmosis technology: Role of the operating temperature. Environ. Technol. 2019, 40, 3434. [Google Scholar] [CrossRef]
- Arena, J.T.; Manickam, S.S.; Reimund, K.K.; Freeman, B.D.; Mccutcheon, J.R. Solute and water transport in forward osmosis using polydopamine modified thin film composite membranes. Desalination 2014, 343, 8–16. [Google Scholar] [CrossRef]
- Xue, W.; Tobino, T.; Nakajima, F.; Yamamoto, K. Seawater-driven forward osmosis for enriching nitrogen and phosphorous in treated municipal wastewater: Effect of membrane properties and feed solution chemistry. Water Res. 2015, 69, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hahn, H.H.; Hoffmann, E. Effects of solution conditions on the precipitation of phosphate for recovery A thermodynamic evaluation. Chemosphere 2002, 48, 1029–1034. [Google Scholar] [CrossRef]
- Almoalimi, K.; Liu, Y. Fouling and cleaning of thin film composite forward osmosis membrane treating municipal wastewater for resource recovery. Chemosphere 2022, 288, 132507. [Google Scholar] [CrossRef]
- Metcalf, E. Water Reuse Issues, Technologies, and Applications; McGraw-Hill Professional Publishing: New York, NY, USA, 2007. [Google Scholar]
- Altaee, A.; Mabrouk, A.; Bourouni, K. A novel Forward osmosis membrane pretreatment of seawater for thermal desalination processes. Desalination 2013, 326, 19–29. [Google Scholar] [CrossRef]
- Altaee, A.; Hilal, N. Dual-stage forward osmosis/pressure retarded osmosis process for hypersaline solutions and fracking wastewater treatment. Desalination 2014, 350, 79–85. [Google Scholar] [CrossRef]
- Cornelissen, E.R.; Harmsen, D.; Korte KFDe Ruiken, C.J.; Qin, J.; Oo, H.; Wessels, L.P. Membrane fouling and process performance of forward osmosis membranes on activated sludge. J. Membr. Sci. 2008, 319, 158–168. [Google Scholar] [CrossRef]
- Corzo, B.; de la Torre, T.; Sans, C.; Ferrero, E.; Malfeito, J.J. Evaluation of draw solutions and commercially available forward osmosis membrane modules for wastewater reclamation at pilot scale. Chem. Eng. J. 2017, 326, 1–8. [Google Scholar] [CrossRef]
- Devia, Y.P.; Imai, T.; Higuchi, T.; Kanno, A.; Yamamoto, K.; Sekine, M.; Van Le, T. Potential of Magnesium Chloride for Nutrient Rejection in Forward Osmosis. J. Water Resour. Prot. 2015, 7, 730. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.S.; Chen, S.S.; Sangeetha, D.; Chang, H.M.; Thanh, C.N.D.; Le, Q.H.; Ku, H.M. Developments in forward osmosis and membrane distillation for desalination of waters. Environ. Chem. Lett. 2018, 16, 1247–1265. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of nitrification by ammonia and nitrous acid. J. Water Pollut. Control. Fed. 1976, 48, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Poullis, M.; Cth, F. Exploring the Boundaries of Perfusion. Left Field: Square Tubes and Current Changes! J. Extracorpor. Technol. 2009, 41, P21. [Google Scholar]
- Dasgupta, P.K.; Dong, S. Solubility of Ammonia in Liquid Water and Generation of Trace Levels of Standard Gaseous Ammonia. Atmos. Environ. 1985, 20, 565–570. [Google Scholar] [CrossRef]
- Criscuoli, A. Thermal Performance of Integrated Direct Contact and Vacuum Membrane Distillation Units. Engergies 2021, 14, 7405. [Google Scholar] [CrossRef]
- Swaminathan, J.; Lienhard, J.H. Design and operation of membrane distillation with feed recirculation for high recovery brine concentration. Desalination 2018, 445, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Phuntsho, S.; Vigneswaran, S.; Kandasamy, J.; Hong, S.; Lee, S.; Shon, H.K. Influence of temperature and temperature difference in the performance of forward osmosis desalination process. J. Membr. Sci. 2012, 415–416, 734–744. [Google Scholar] [CrossRef]
- Zhao, S.; Zou, L. Effects of working temperature on separation performance, membrane scaling and cleaning in forward osmosis desalination. Desalination 2011, 278, 157–164. [Google Scholar] [CrossRef]
- Jalab, R.; Awad, A.M.; Nasser, M.S.; Minier-matar, J.; Adham, S. Pilot-scale investigation of flowrate and temperature influence on the performance of hollow fiber forward osmosis membrane in osmotic concentration process. J. Environ. Chem. Eng. 2020, 8, 104494. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; Mccutcheon, R. Elucidating the impact of temperature gradients across membranes during forward osmosis: Coupling heat and mass transfer models for better prediction of real osmotic systems. J. Membr. Sci. 2018, 553, 189–199. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Verliefde, A.R.D.; Roest, K.; Rietveld, L.C.; Cornelissen, E.R. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Guo, W.; Ngo, H.H.; Price, W.E.; Nghiem, L.D. Selection of forward osmosis draw solutes for subsequent integration with anaerobic treatment to facilitate resource recovery from wastewater. Bioresour. Technol. 2015, 191, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Valladares Linares, R.; Li, Z.; Yangali-Quintanilla, V.; Ghaffour, N.; Amy, G.; Leiknes, T.; Vrouwenvelder, J.S. Life cycle cost of a hybrid forward osmosis-low pressure reverse osmosis system for seawater desalination and wastewater recovery. Water Res. 2016, 88, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Boo, C.; Ma, J.; Elimelech, M. Bidirectional diffusion of ammonium and sodium cations in forward osmosis: Role of membrane active layer surface chemistry and charge. Environ. Sci. Technol. 2014, 48, 14369–14376. [Google Scholar] [CrossRef]
- Cath, T.Y. Solute Coupled Diffusion in Osmotically Driven Membrane Processes. Environ. Sci. Technol. 2009, 43, 6769–6775. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Drewes, J.E.; Nghiem, L.D. Forward osmosis as a platform for resource recovery from municipal wastewater—A critical assessment of the literature. J. Membr. Sci. 2017, 529, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Matin, A.; Laoui, T.; Falath, W.; Farooque, M. Fouling control in reverse osmosis for water desalination & reuse: Current practices & emerging environment-friendly technologies. Sci. Total Environ. 2021, 765, 142721. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, P.; Wan, C.; Chung, T.S. Polyelectrolyte-promoted Forward Osmosis-Membrane Distillation (FO-MD) hybrid process for dye wastewater treatment. Environ. Sci. Technol. 2012, 46, 6236–6243. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Chen, S.S.; Jain, S.; Nguyen, H.T.; Ray, S.S.; Ngo, H.H.; Guo, W.; Lam, N.T.; Duong, H.C. Exploration of an innovative draw solution for a forward osmosis-membrane distillation desalination process. Environ. Sci. Pollut. Res. 2018, 25, 5203–5211. [Google Scholar] [CrossRef]
- Husnain, T.; Liu, Y.; Riffat, R.; Mi, B. Integration of forward osmosis and membrane distillation for sustainable wastewater reuse. Sep. Purif. Technol. 2015, 156, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Husnain, T.; Mi, B.; Riffat, R. A Combined Forward Osmosis and Membrane Distillation System for Sidestream Treatment. J. Water Resour. Prot. 2015, 7, 1111–1120. [Google Scholar] [CrossRef] [Green Version]


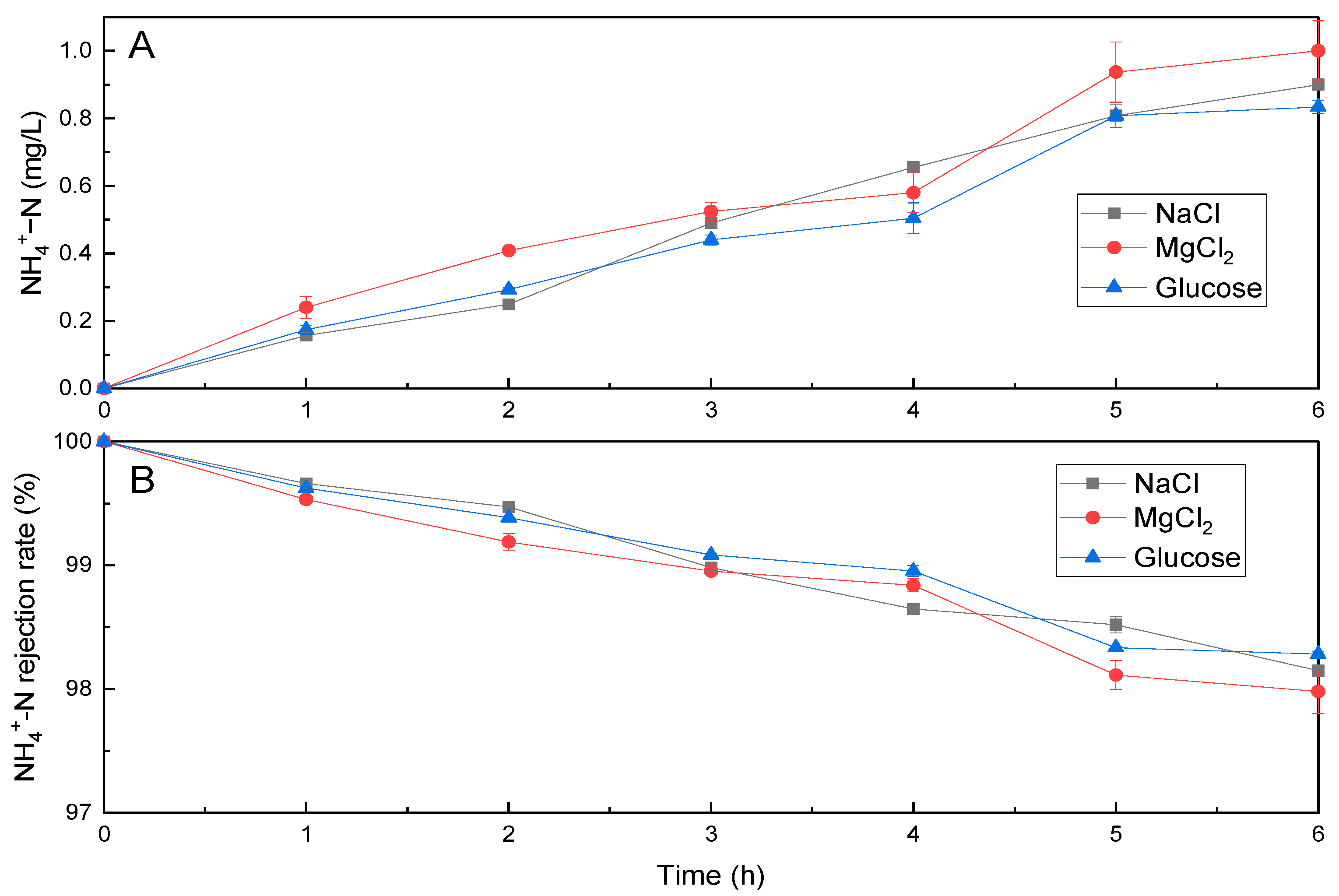

| COD | PO43−-P | NH4+-N | Ca2+ | Mg2+ | K+ | Salinity | pH | |||
|---|---|---|---|---|---|---|---|---|---|---|
| mg/L | ||||||||||
| Initial | 460.66 ± 32.54 | 7.90 ± 0.15 | 53.96 ± 0.66 | 37.25 ± 0.73 | 6.28 ± 0.35 | 20.27 ± 1.36 | - | 6.98 ± 0.02 | ||
| NaCl | FS and DS = 25 °C | Final (FS) | 1427.55 ± 16.97 | 23.33 ± 0.06 | 34.66 ± 0.23 | 101.04 ± 1.77 | 19.97 ± 0.50 | 34.66 ± 0.93 | 1724.44 ± 77.84 | 7.10 |
| CF Final (DS) | 3.10 - | 2.95 Not detected | −1.56 25.29 ± 0.43 | 2.71 - | 3.17 - | 1.71 - | - - | - - | ||
| FS = 25 °C and DS = 50 °C | Final (FS) | 1415.56 ± 101.79 | 22.71 ± 0.03 | 26.89 ± 0.26 | 100.45 ± 1.81 | 19.67 ± 0.34 | 29.24 ± 1.46 | 1953.33 ± 23.09 | 7.16 | |
| CF Final (DS) | 3.07 - | 2.88 Not detected | −2.01 26.89 ± 0.37 | 2.70 - | 3.13 - | 1.44 - | - - | - - | ||
| FS = Not controlled and DS = 50 °C | Final (FS) | 1439.55 ± 67.86 | 22.14 ± 0.15 | 18.21 ± 0.10 | 97.06 ± 1.36 | 18.89 ± 0.76 | 23.33 ± 0.48 | 2400 ± 72.11 | 7.19 | |
| CF Final (DS) | 3.12 - | 2.80 Not detected | −2.96 27.06 ± 0.49 | 2.61 - | 3.00 - | 1.15 - | - - | - - | ||
| Glucose | FS and DS = 25 °C | Final (FS) | 1736.68 ± 33.93 | 23.63 ± 0.30 | 164.34 ± 1 | 110.16 ± 1.03 | 20.35 ± 1.04 | 63.44 ± 0.98 | - | 7.08 |
| CF Final (DS) | 3.77 - | 2.99 Not detected | 3.05 Not detected | 2.96 - | 3.24 - | 3.13 - | - - | - - | ||
| FS = 25 °C and DS = 50 °C | Final (FS) | 1847.25 ± 16.97 | 22.91 ± 0.49 | 160.70 ± 0.50 | 107.22 ± 2.08 | 19.89 ± 0.76 | 63.65 ± 2.11 | - | 7.15 | |
| CF Final (DS) | 4.01 - | 2.90 Not detected | 2.98 Not detected | 2.88 - | 3.17 - | 3.14 - | - - | - - | ||
| FS = Not controlled and DS = 50 °C | Final (FS) | 1870.28 ± 33.93 | 22.68 ± 0.08 | 151.11 ± 0.37 | 104.30 ± 0.98 | 19.18 ± 0.23 | 62 ± 1.07 | - | 7.16 | |
| CF Final (DS) | 4.06 - | 2.87 Not detected | 2.80 Not detected | 2.80 - | 3.05 - | 3.06 - | - - | - - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almoalimi, K.; Liu, Y.-Q.; Booth, A.; Heo, S. Temperature Effects of MD on Municipal Wastewater Treatment in an Integrated Forward Osmosis and Membrane Distillation Process. Processes 2022, 10, 355. https://doi.org/10.3390/pr10020355
Almoalimi K, Liu Y-Q, Booth A, Heo S. Temperature Effects of MD on Municipal Wastewater Treatment in an Integrated Forward Osmosis and Membrane Distillation Process. Processes. 2022; 10(2):355. https://doi.org/10.3390/pr10020355
Chicago/Turabian StyleAlmoalimi, Khaled, Yong-Qiang Liu, Alexander Booth, and Seongbong Heo. 2022. "Temperature Effects of MD on Municipal Wastewater Treatment in an Integrated Forward Osmosis and Membrane Distillation Process" Processes 10, no. 2: 355. https://doi.org/10.3390/pr10020355
APA StyleAlmoalimi, K., Liu, Y.-Q., Booth, A., & Heo, S. (2022). Temperature Effects of MD on Municipal Wastewater Treatment in an Integrated Forward Osmosis and Membrane Distillation Process. Processes, 10(2), 355. https://doi.org/10.3390/pr10020355







