Bulk Process for Enrichment of Capsinoids from Capsicum Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Fruit Processing
2.3. General Experimental
2.4. Extractions of Dried Fruit
2.5. Steam Distillation of Fresh Fruit for Essential Oil
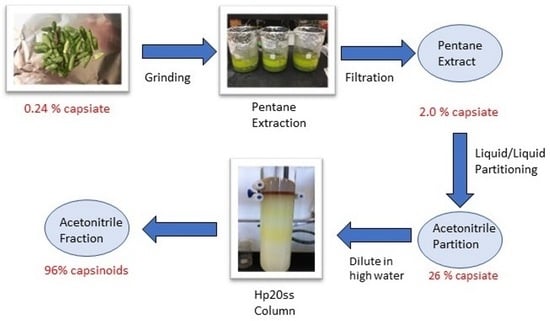
2.6. Bulk Purification—Pentane Extraction and Liquid/Liquid Partitioning Using Pentane/Acetonitrile
2.7. HPLC Quantitative Analysis
2.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kobata, K.; Todo, T.; Yazawa, S.; Iwai, K.; Watanabe, T. Novel capsaicinoid-like substances, capsiate and dihydrocapsiate, from the fruits of a non-pungent cultivar, CH-19 sweet, of pepper (Capsicum annuum L.). J. Agric. Food Chem. 1998, 46, 1695–1697. [Google Scholar] [CrossRef]
- Rosa, A.; Deiana, M.; Casu, V.; Paccagnini, S.; Appendino, G.; Ballero, M.; Dessí, M.A. Antioxidant activity of capsinoids. J. Agric. Food Chem. 2002, 50, 7396–7401. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nolan, N.A.; Brown, K.C.; Miles, S.L.; Akers, A.T.; Colclough, K.W.; Seidler, J.M.; Rimoldi, J.M.; Valentovic, M.A.; Dasgupta, P. Anticancer activity of natural and synthetic capsaicin analogs. J. Pharmacol. Exper. Therapeutics. 2018, 364, 462–473. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. The two faces of capsaicin. Cancer Res. 2019, 71, 2809–2814. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, H.; Hiura, A. QX-314 induces analgesia to nociceptive thermal stimulus by co-application with capsiate or anandamide. Austin Biomark. Diag. 2014, 1, 4. [Google Scholar]
- Onuki, K.; Niwa, S.; Maeda, S.; Inoue, N.; Yazawa, S.; Fushiki, T. Sweet, nonpungent cultivar of sweet pepper increases body temperature and oxygen consumption in humans. Biosci. Biotech. Biochem. 2001, 65, 2033–2036. [Google Scholar] [CrossRef]
- Onuki, K.; Hararmizu, S.; Watanabe, T.; Yazawa, S.; Fushiki, T. Administration of capsiate, a non-pungent capsaicin analog, promotes energy metabolism and suppresses body fat accumulation in mice. Biosci. Biotech. Biochem. 2001, 65, 2375–2380. [Google Scholar] [CrossRef]
- Pyun, B.-J.; Choi, C.; Lee, Y.; Kim, T.-W.; Min, J.-K.; Kim, Y.; Kim, B.D.; Kim, J.H.; Kim, T.Y.; Kim, Y.M.; et al. Capsiate, a non-pungent capsaicin-like compound inhibits angiogenesis and vascular permeability via a direct inhibition of Src kinase activity. Cancer Res. 2008, 68, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Sasahara, I.; Furuhata, Y.; Iwasaki, Y.; Inoue, N.; Sato, H.; Watanabe, T.; Takahashi, M. Assessment of the biological similarity of three capsaicin analogs (capsinoids) found in nonpungent chili pepper (CH-19 Sweet) fruits. Biosci. Biotech. Biochem. 2010, 74, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Tani, Y.; Fujioka, T.; Sumioka, M.; Furuichi, Y.; Hamada, H.; Watanabe, T. Effects of capsinoid on serum and liver lipids in hyperlipidemic rats. J. Nutri. Sci. Vitaminol. 2004, 50, 351–355. [Google Scholar] [CrossRef]
- Zang, Y.; Fan, L.; Chen, J.; Huang, R.; Qin, H. Improvement of lipid and glucose metabolism by capsiate in palmitic acid-treated HepG2 cells via activation of the AMPK/SIRT1 signaling pathway. J. Agric. Food Chem. 2018, 66, 6772–6781. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.-J.; Jeon, M.-Y.; Kim, B.-D.; Kim, J.-H.; Kwon, Y.-G.; Lee, H.; Lee, Y.S.; Yang, J.H.; Kim, T.Y. Capsiate inhibits ultraviolet B-induced skin inflammation by inhibiting Src family kinases and epidermal growth factor receptor signaling. Free Radic. Biol. Med. 2010, 40, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Ludy, M.-J.; Moore, G.E.; Mattes, R.D. The effects of capsaicin and capsiate on energy balance: Critical review and meta-analyses of studies in humans. Chem. Senses 2012, 37, 103–121. [Google Scholar] [CrossRef] [Green Version]
- Zsiboras, C.; Matics, R.; Hegyi, P.; Balasko, M.; Petervari, E.; Szabó, I.; Sarlós, P.; Mikó, A.; Tenk, J.; Rostás, I.; et al. Capsaicin and capsiate could be appropriate agents for treatment of obesity: A meta-analysis of human studies. Crit. Rev. Food Sci. Nutri. 2018, 58, 1419–1427. [Google Scholar] [CrossRef]
- Macho, A.; Lucena, C.; Sancho, R.; Daddario, N.; Minassi, A.; Munoz, E.; Appendino, G.G. Non-pungent capsaicinids from sweet pepper: Synthesis and evaluation of the chemopreventive and anticancer potential. Euro. J. Nutri. 2003, 42, 2–9. [Google Scholar] [CrossRef]
- Castillo, E.; Lopez-Gonzalez, I.; De Regil-Hernandez, R.; Reyes-Duarte, D.; Sanchez-Herrera, D.; López-Munguía, A.; Darszon, A. Enzymatic synthesis of capsaicin analogs and their effect on the T-type Ca2+ channels. Biochem. Biophys. Res. Commun. 2007, 356, 424–430. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hosokawa, M.; Otsu, K.; Watanabe, T.; Yazawa, S. Assessment of capsiconinoid composition, nonpungent capsaicinoid analogues, in Capsicum cultivars. J. Agr. Food Chem. 2009, 57, 5407–5412. [Google Scholar] [CrossRef]
- Singh, S.; Jarret, R.L.; Russo, V.; Majetich, G.; Shimkus, J.; Bushway, R.; Perkins, B. Determination of capsinoids by HPLC-DAD in Capsicum species. J. Agric. Food Chem. 2009, 57, 3452–3457. [Google Scholar] [CrossRef]
- Jarret, R.L.; Bolton, J.; Perkins, L.B. 509-45-1: A Capsicum annuum pepper germplasm containing high concentrations of capsinoids. HortScience 2014, 49, 107–108. [Google Scholar] [CrossRef] [Green Version]
- Lang, Y.; Kisaka, H.; Sugiyama, R.; Nomura, K.; Morita, A.; Watanabe, T.; Tanaka, Y.; Yazawa, S.; Miwa, T. Functional loss of pAMT results in biosynthesis of capsinoids, capsaicinoid analogs, in Capsicum annuum cv. CH-19. Plant J. 2009, 59, 953–961. [Google Scholar] [CrossRef]
- Kobata, K.; Kawamura, M.; Toyoshima, M.; Tamura, Y.; Ogawa, S.; Watanabe, T. Lipase-catalyzed synthesis of capsaicin analogs by amidation of vanillylamine with fatty acid derivatives. Biotechnol. Lett. 1998, 20, 451–453. [Google Scholar] [CrossRef]
- Kobata, K.; Kobayashi, M.; Tamura, T.; Miyoshi, S.; Ogawa, S.; Watanabe, T. Lipase-catalyzed synthesis of capsaicin analogs by transacylation of capsaicin with natural oils or fatty acid derivatives in n-hexane. Biotechnol. Lett. 1999, 21, 547–550. [Google Scholar] [CrossRef]
- Kurosawa, W.; Nakano, T.; Amino, Y. Practical large-scale production of dihydrocapsiate, a nonpungent capsaicinoid-like substance. Biosci. Biotechnol. Biochem. 2017, 81, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantrell, C.L.; Jarret, B. Methods for Extracting and Purifying Capsinoids Such as Capsiate and Dihydrocapsiate from sp. fruit. U.S. Patent 10,414,715 B1, 17 September 2019. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, AKA Least-Squares Means. R Package Version 1.7.0. 2021. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 8 November 2021).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- CRC. Handbook of Chemistry and Physics, 102th ed.; Rumble, J., Ed.; CRC: Boca Raton, FL, USA, 2021; ISBN 9780367712600. [Google Scholar]
- Matsuo, Y.; Fujita, Y.; Ohnishi, S.; Tanaka, T.; Hirabaru, H.; Kai, T.; Sakaida, H.; Nishizono, S.; Kouno, I. Chemical constituents of the leaves of rabbiteye blueberry (Vaccinium ashei) and characterisation of polymeric proanthocyanidins containing phenylpropanoid units and A-type linkages. Food Chem. 2010, 121, 1073–1079. [Google Scholar] [CrossRef] [Green Version]

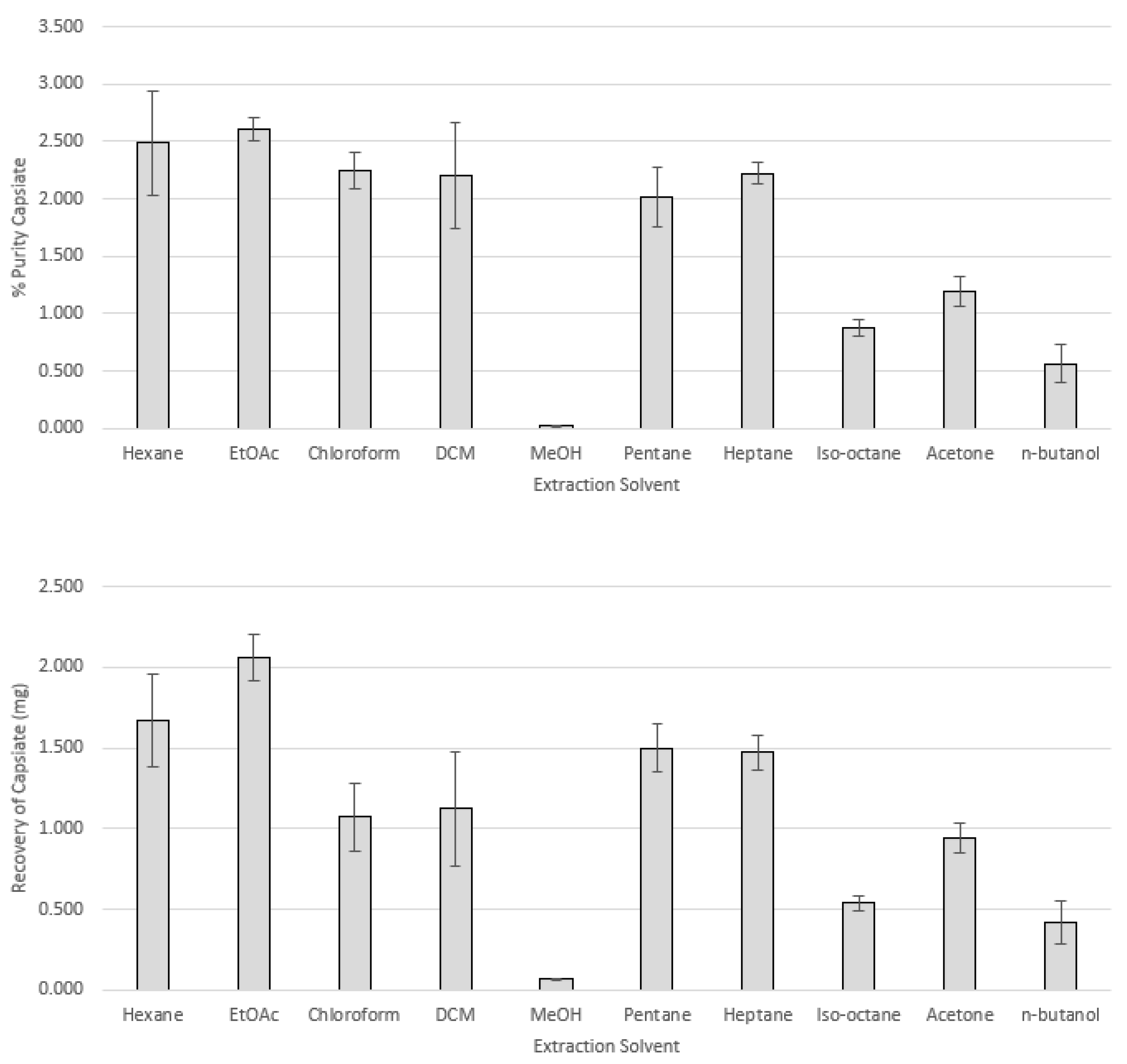

| Extraction Solvent | Yield of Crude Extract (% ± SD, wt/wt) * | Purity in Crude Extract (% ± SD, wt/wt) * | Recovery (mg/1 g Peppers) * | ||
|---|---|---|---|---|---|
| Capsiate | Dihydrocapsiate | Capsiate | Dihydrocapsiate | ||
| hexane | 6.717 ± 0.093 b | 2.487 ± 0.456 a | 1.968 ± 0.325 a | 1.667 ± 0.285 a,b,c | 1.32 ± 0.201 b,c |
| ethyl acetate | 7.91 ± 0.243 b | 2.603 ± 0.099 a | 2.056 ± 0.033 a | 2.061 ± 0.142 a | 1.627 ± 0.076 a,b |
| chloroform | 4.797 ± 1.024 d | 2.241 ± 0.158 a | 1.803 ± 0.162 a | 1.072 ± 0.209 b,c | 0.866 ± 0.196 c,d |
| methylene chloride | 5.027 ± 0.599 cd | 2.202 ± 0.461 a | 1.699 ± 0.35 a | 1.123 ± 0.355 b,c | 0.867 ± 0.271 c,d |
| methanol | 26.447 ± 1.135 a | 0.027 ± 0.001 e | 0.067 ± 0.004 e | 0.07 ± 0.002 f | 0.178 ± 0.016 f |
| pentane | 7.433 ± 0.429 b | 2.02 ± 0.258 a | 1.607 ± 0.203 a | 1.497 ± 0.148 a,b,c | 1.19 ± 0.105 b,c |
| heptane | 6.613 ± 0.227 b | 2.221 ± 0.093 a | 1.843 ± 0.08 a | 1.47 ± 0.111 a,b,c | 1.22 ± 0.094 b,c |
| iso-octane | 6.16 ± 0.02b b | 0.878 ± 0.075 c | 0.953 ± 0.075 c | 0.541 ± 0.045 d,e | 0.587 ± 0.046 d,e |
| acetone | 7.953 ± 0.117 b | 1.191 ± 0.126 b | 1.069 ± 0.162 b | 0.946 ± 0.093 c,d | 0.849 ± 0.12 c,d |
| n-butanol | 7.41 ± 0.269 b | 0.564 ± 0.164 c,d | 0.611 ± 0.118 c,d | 0.42 ± 0.137 e | 0.455 ± 0.103 e |
| Concentration | Partitions | % Purity (wt/wt) in Partition | Total % Purity of Capsinoids | |
|---|---|---|---|---|
| capsiate | dihydrocapsiate | |||
| 20 mg/mL | pentane | 0.026 ± 0 | 0.03 ± 0.005 | 0.056 |
| acetonitrile | 26.331 ± 2.579 | 19.358 ± 1.182 | 45.689 | |
| Fraction | Solids Yield (mg) | Elution Solvents | % Purity (wt/wt) in Partition | Total % Purity of Capsinoids | |||
|---|---|---|---|---|---|---|---|
| % water | % acetonitrile | % methylene chloride | capsiate | dihydro-capsiate | |||
| A | 0 | 100 | 0 | 0 | n.d. | n.d. | - |
| B | 0 | 75 | 25 | 0 | n.d. | n.d. | - |
| C | 0 | 50 | 50 | 0 | n.d. | n.d. | - |
| D | 5 | 25 | 75 | 0 | n.d. | n.d. | - |
| E | 494.6 | 0 | 100 | 0 | 55.464 ± 4.431 | 41.15 ± 3.817 | 96.614 |
| F | 633.5 * | 0 | 0 | 100 | 0.106 ± 0.003 | n.d. | 0.106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantrell, C.L.; Jarret, R.L. Bulk Process for Enrichment of Capsinoids from Capsicum Fruit. Processes 2022, 10, 305. https://doi.org/10.3390/pr10020305
Cantrell CL, Jarret RL. Bulk Process for Enrichment of Capsinoids from Capsicum Fruit. Processes. 2022; 10(2):305. https://doi.org/10.3390/pr10020305
Chicago/Turabian StyleCantrell, Charles L., and Robert L. Jarret. 2022. "Bulk Process for Enrichment of Capsinoids from Capsicum Fruit" Processes 10, no. 2: 305. https://doi.org/10.3390/pr10020305
APA StyleCantrell, C. L., & Jarret, R. L. (2022). Bulk Process for Enrichment of Capsinoids from Capsicum Fruit. Processes, 10(2), 305. https://doi.org/10.3390/pr10020305