Molecular Simulation Comparison of Two Ultrafine Coal-Based Activated Carbons for the Removal of Methylene Blue from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
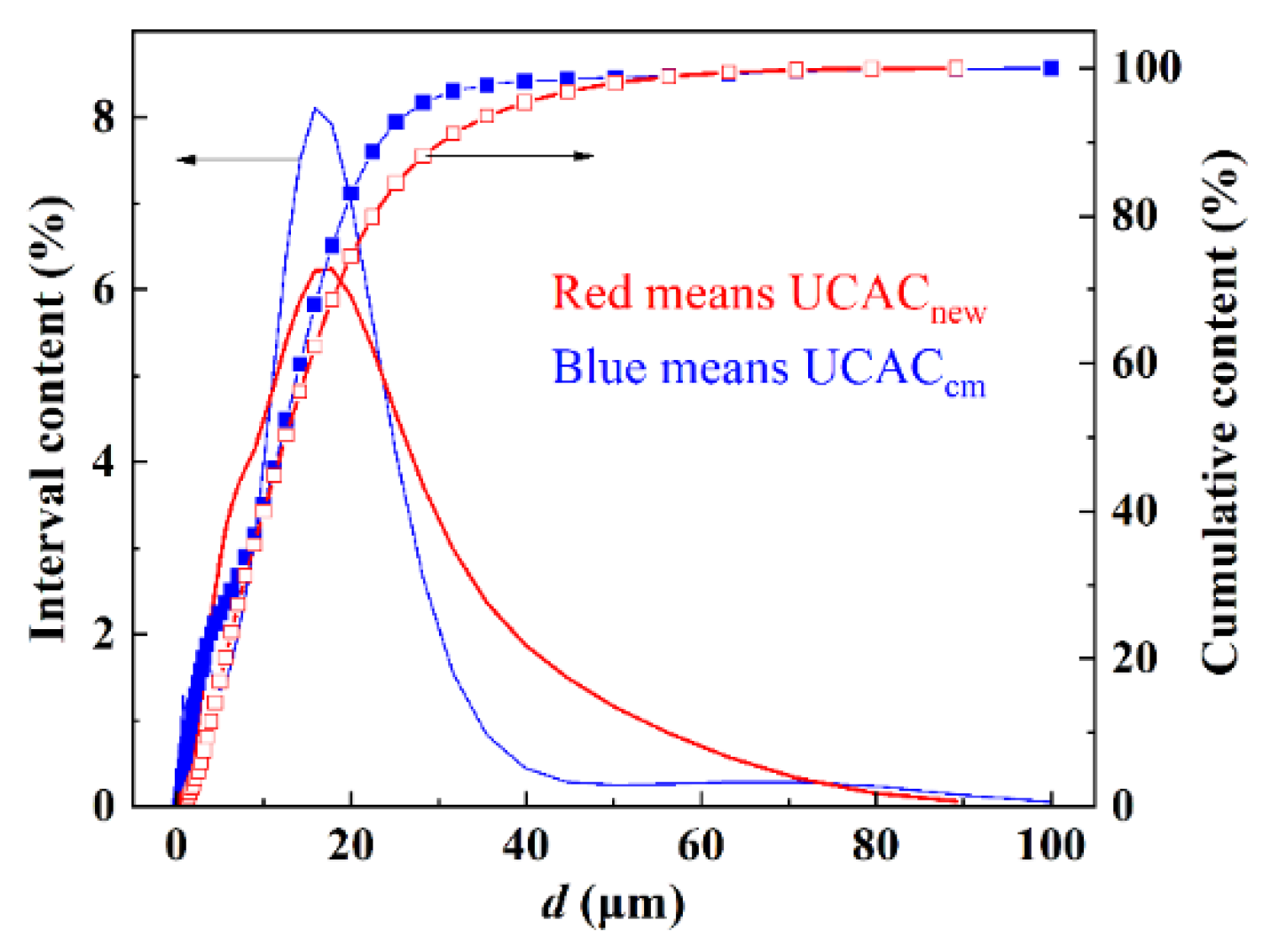
2.2. Particle Size Distribution and Pore Structure Measurement
2.3. Preparation of MB Standard Solution and Adsorption Test
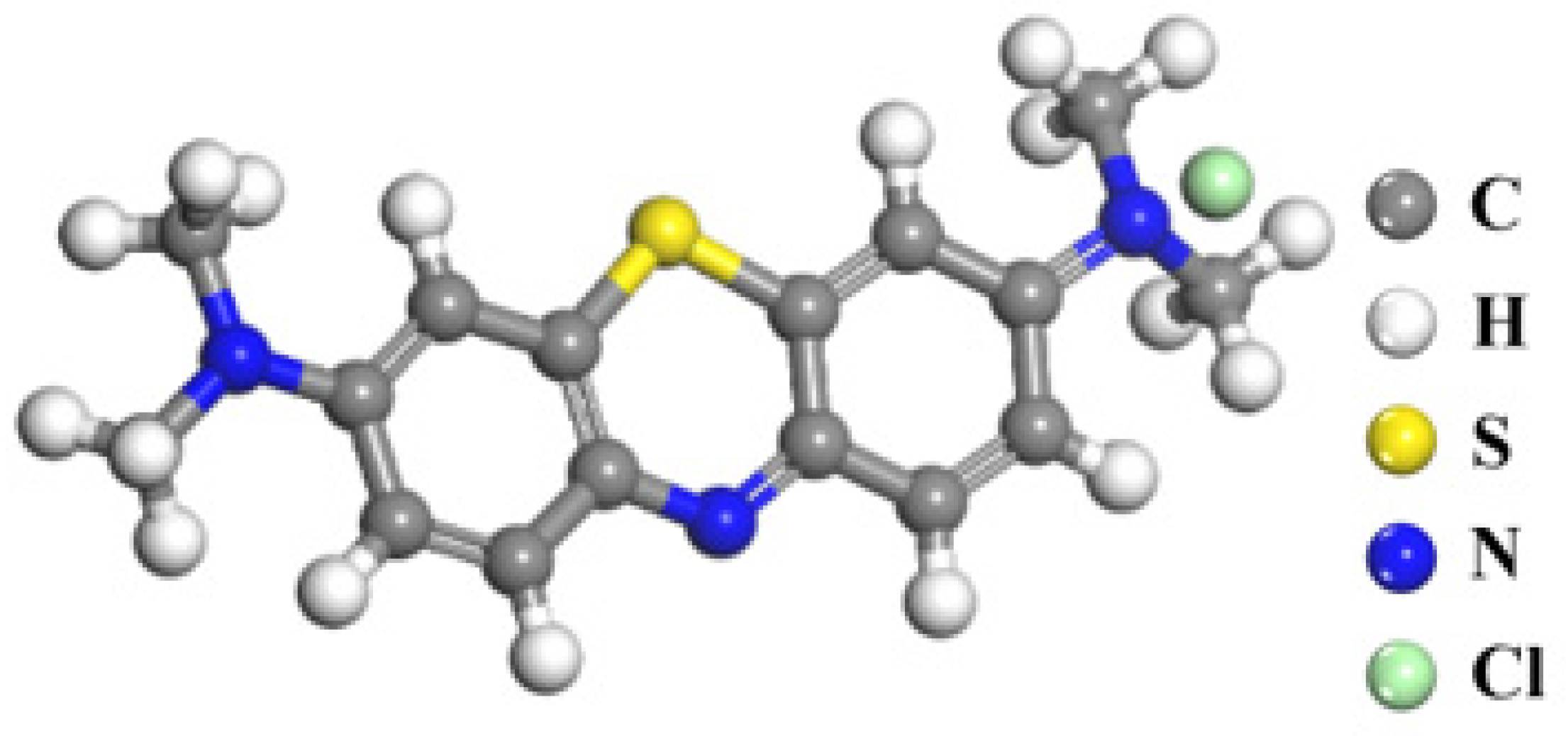
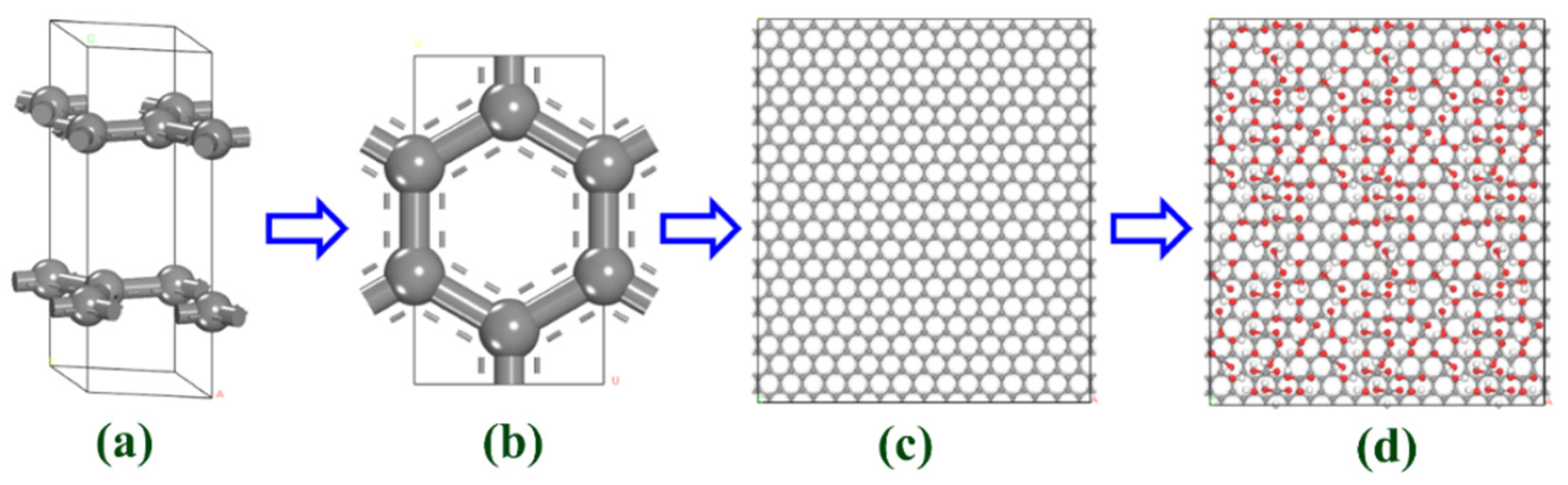
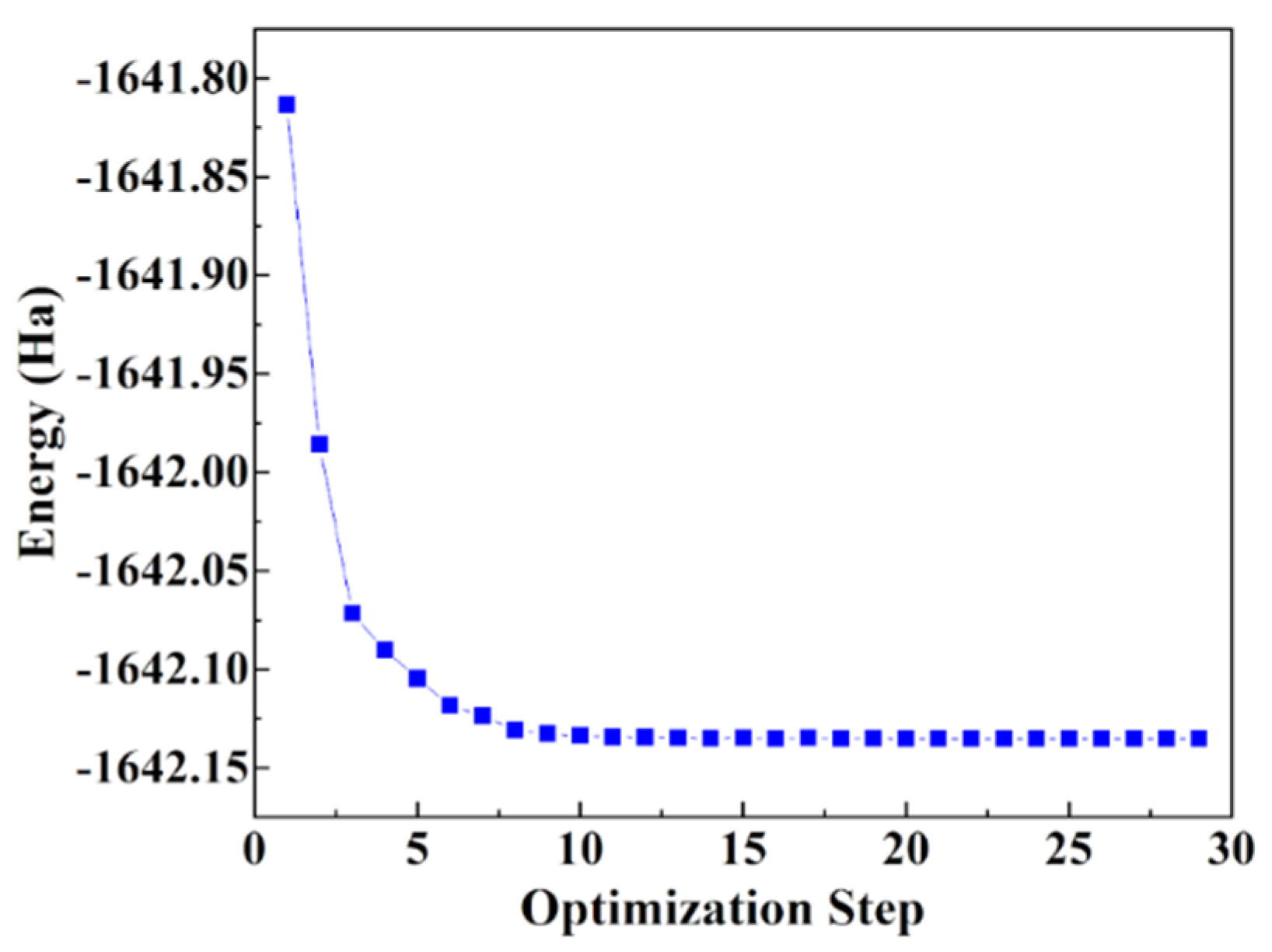
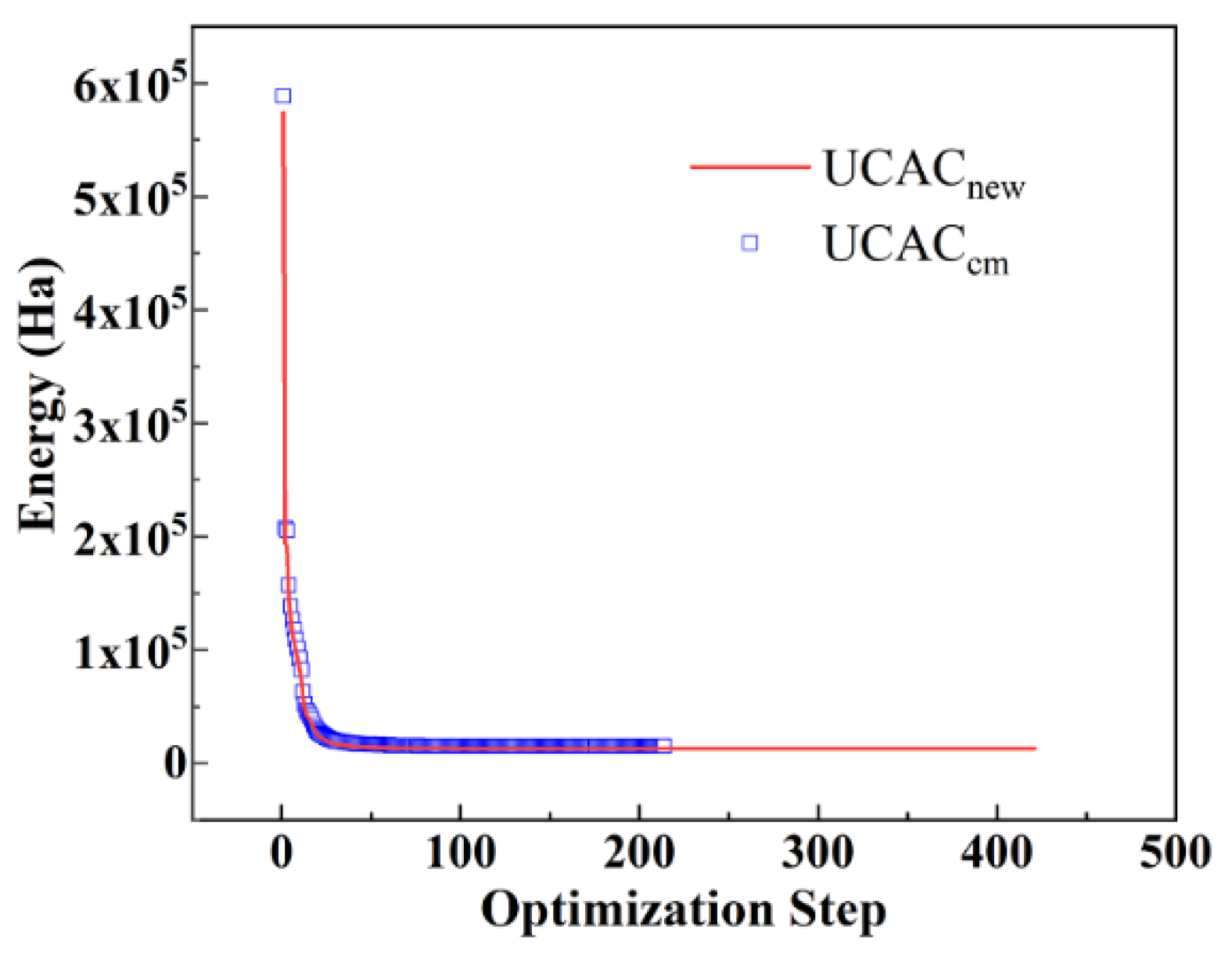
2.4. Molecular Simulation Methods
3. Results and Discussion
3.1. Adsorption Test Results
3.2. PSD and Pore Structure
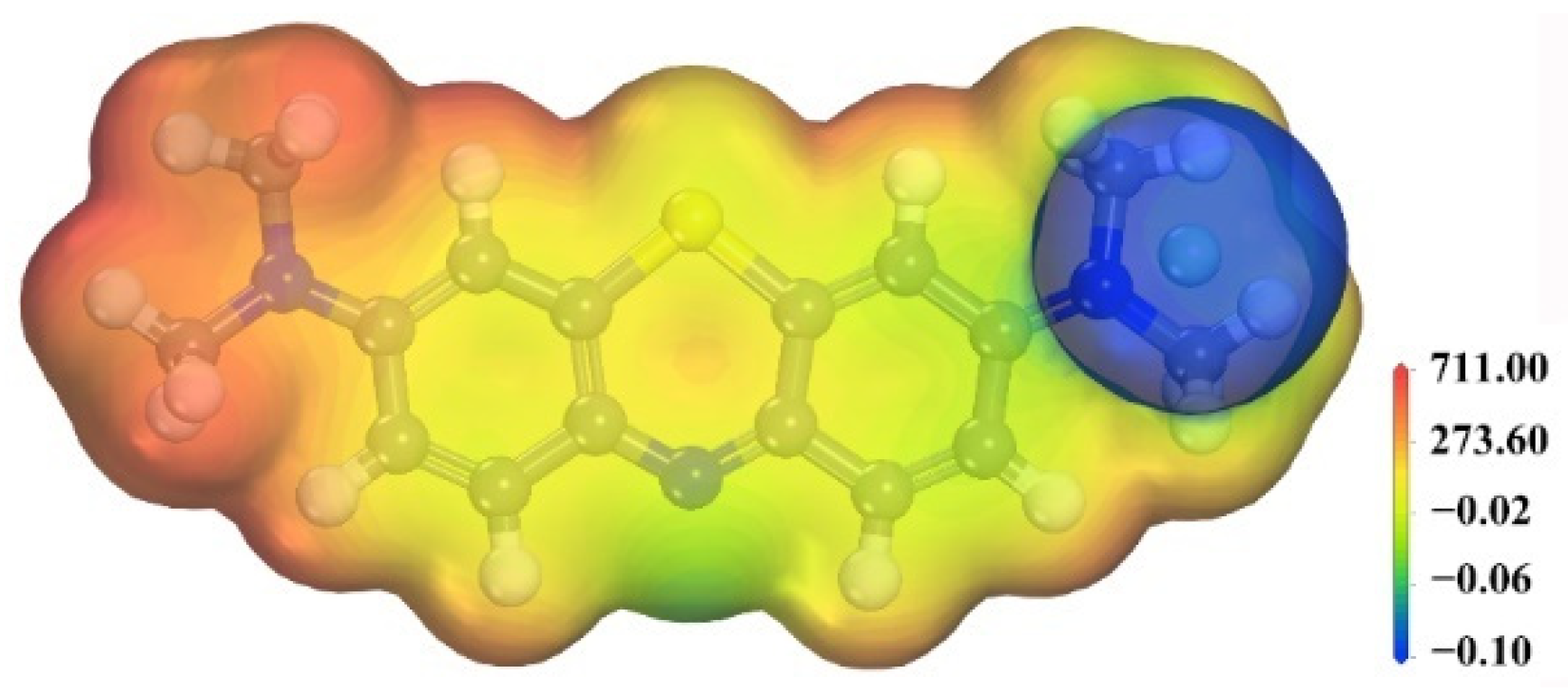
3.3. Simulation Results
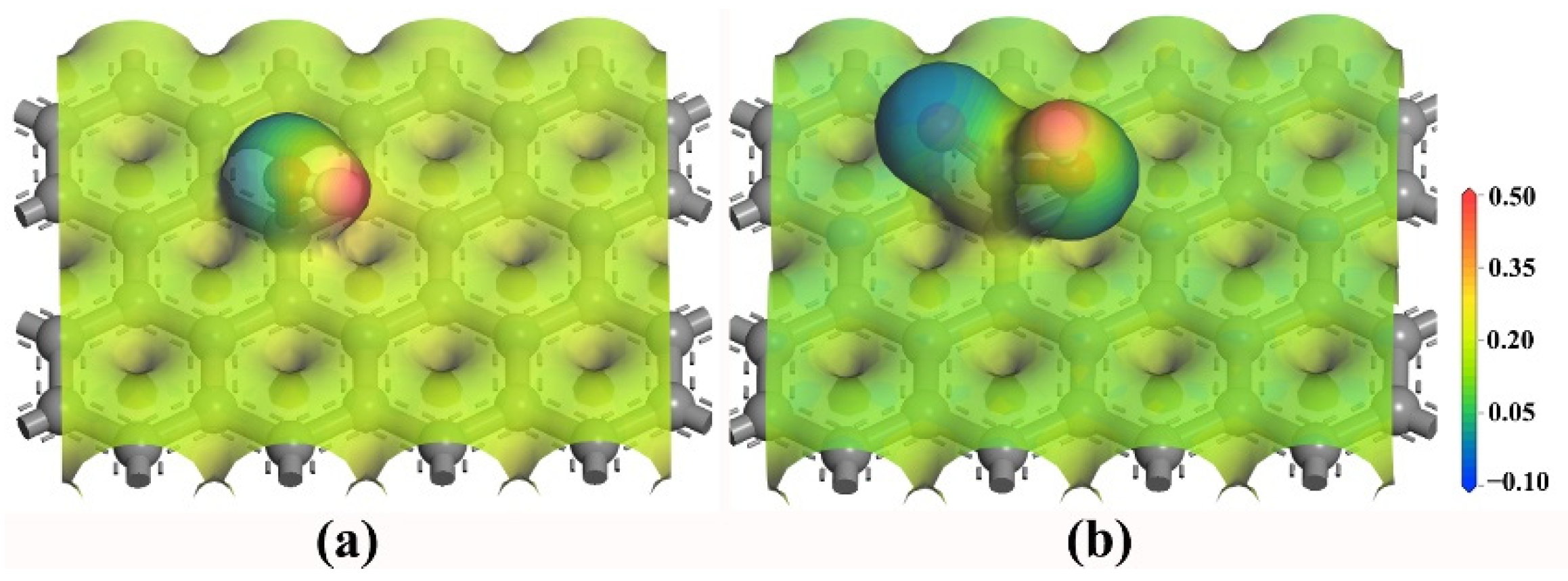
3.4. Effect of Functional Groups
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, R.; Liu, G.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ali, M.U. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation-A review. J. Clean. Prod. 2020, 242, 118409. [Google Scholar] [CrossRef]
- Kosek, K.; Luczkiewicz, A.; Fudala-Książek, S.; Jankowska, K.; Szopińska, M.; Svahn, O.; Tränckner, J.; Kaiser, A.; Langas, V.; Björklund, E. Implementation of advanced micropollutants removal technologies in wastewater treatment plants (WWTPs)—Examples and challenges based on selected EU countries. Environ. Sci. Policy 2020, 112, 213–226. [Google Scholar] [CrossRef]
- Rocha, L.S.; Pereira, D.; Sousa, É.; Otero, M.; Esteves, V.I.; Calisto, V. Recent advances on the development and application of magnetic activated carbon and char for the removal of pharmaceutical compounds from waters: A review. Sci. Total Environ. 2020, 718, 137272. [Google Scholar] [CrossRef]
- Mariana, M.; HPS, A.K.; Mistar, E.M.; Yahya, E.B.; Alfatah, T.; Danish, M.; Amayreh, M. Recent advances in activated carbon modification techniques for enhanced heavy metal adsorption. J. Water Process Eng. 2021, 43, 102221. [Google Scholar] [CrossRef]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.M.N. A review on experimental chemically modified activated carbon to enhance dye and heavy metals adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Maheshwari, P.H.; Sundriyal, S. Recent advances in biomass derived activated carbon electrodes for hybrid electrochemical capacitor applications: Challenges and opportunities. Carbon 2020, 170, 1–29. [Google Scholar] [CrossRef]
- Yang, S.; Nahar Fatema, K.; Kim, I.J.; Ryu, J.; Eun Shim, S.; Oh, W.C. Novel preparation and high electrical performance effect of Mn-doped ultra-high surface area activated carbon (USAC) as an additive for Ni hybrid capacitors. Adv. Powder Technol. 2021, 32, 1116–1126. [Google Scholar] [CrossRef]
- Czarna-Juszkiewicz, D.; Cader, J.; Wdowin, M. From coal ashes to solid sorbents for hydrogen storage. J. Clean. Prod. 2020, 270, 122355. [Google Scholar] [CrossRef]
- Doğan, M.; Sabaz, P.; Bicil, Z.; Koçer Kizilduman, B.; Turhan, Y. Activated carbon synthesis from tangerine peel and its use in hydrogen storage. J. Energy Inst. 2020, 93, 2176–2185. [Google Scholar] [CrossRef]
- Matos, J.; Poon, P.S.; Montaña, R.; Romero, R.; Gonçalves, G.R.; Schettino, M.A.; Passamani, E.C.; Freitas, J.C.C. Photocatalytic activity of P-Fe/activated carbon nanocomposites under artificial solar irradiation. Catal. Today 2020, 356, 226–240. [Google Scholar] [CrossRef]
- Higai, D.; Lee, C.; Lang, J.; Qian, E.W. Saccharification of cellulose using biomass-derived activated carbon-based solid acid catalysts. Fuel Processing Technol. 2021, 215, 106738. [Google Scholar] [CrossRef]
- Jianyong, X.; Xiaofang, Z.; Lijuan, Z.; Ren, T. Clinical application of nano-carbon particles for radical gastrectomy. Pract. J. Cancer 2018, 33, 852–855. [Google Scholar]
- Jinbiao, W.; Jianchun, J.; Kang, S.; Xinping, X. Research progress on activated carbon as carriers for sustained release of pharmaceuticals. New Chem. Mater. 2014, 42, 4–7. [Google Scholar]
- Sanchez, N.; Fayne, R.; Burroway, B. Charcoal: An ancient material with a new face. Clin. Dermatol. 2020, 38, 262–264. [Google Scholar] [CrossRef]
- Hammani, H.; Laghrib, F.; Farahi, A.; Lahrich, S.; El Ouafy, T.; Aboulkas, A.; El Harfi, K.; El Mhammedi, M.A. Preparation of activated carbon from date stones as a catalyst to the reactivity of hydroquinone: Application in skin whitening cosmetics samples. J. Sci. Adv. Mater. Devices 2019, 4, 451–458. [Google Scholar] [CrossRef]
- Xu, J. Microfiltration Feed Water Pretreatment with Ultrafine Powdered Activated Carbon. Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=1g000gh03k180xa0j26d0pg0bs565049&site=xueshu_se (accessed on 9 January 2022).
- Wang, Y.; Rao, G.Y.; Hu, J.Y. Adsorption of EDCs/PPCPs from drinking water by submicron-sized powdered activated carbon. Water Sci. Technol. Water Supply 2011, 11, 711–718. [Google Scholar] [CrossRef]
- Yoo, J.; Winogradoff, D.; Aksimentiev, A. Molecular dynamics simulations of DNA–DNA and DNA–protein interactions. Curr. Opin. Struct. Biol. 2020, 64, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, I.; Kotia, A.; Ghosh, S.K.; Ali, M.K.A. Recent advances of molecular dynamics simulations in nanotribology. J. Mol. Liq. 2021, 335, 116154. [Google Scholar] [CrossRef]
- Yener, J.; Kopac, T.; Dogu, G.; Dogu, T. Dynamic analysis of sorption of Methylene Blue dye on granular and powdered activated carbon. Chem. Eng. J. 2008, 144, 400–406. [Google Scholar] [CrossRef]
- Li, W.-G.; Gong, X.-J.; Wang, K.; Zhang, X.-R.; Fan, W.-B. Adsorption characteristics of arsenic from micro-polluted water by an innovative coal-based mesoporous activated carbon. Bioresour. Technol. 2014, 165, 166–173. [Google Scholar] [CrossRef]
- Sohraby, F.; Aryapour, H. Rational drug repurposing for cancer by inclusion of the unbiased molecular dynamics simulation in the structure-based virtual screening approach: Challenges and breakthroughs. Semin. Cancer Biol. 2021, 68, 249–257. [Google Scholar] [CrossRef]
- Nian, B.; Xu, Y.J.; Liu, Y. Molecular dynamics simulation for mechanism revelation of the safety and nutrition of lipids and derivatives in food: State of the art. Food Res. Int. 2021, 145, 110399. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, Z.; Zhang, R.; Xing, Y.; Gui, X. Enhancement of the surface hydrophobicity of low-rank coal by adsorbing DTAB: An experimental and molecular dynamics simulation study. Fuel 2019, 239, 145–152. [Google Scholar] [CrossRef]
- Mirzaee, S.A.; Bayati, B.; Valizadeh, M.R.; Gomes, H.T.; Noorimotlagh, Z. Adsorption of diclofenac on mesoporous activated carbons: Physical and chemical activation, modeling with genetic programming and molecular dynamic simulation. Chem. Eng. Res. Des. 2021, 167, 116–128. [Google Scholar] [CrossRef]
- Yikun, Z.; Xiaoyi, L.; Junjie, C.; Yuexing, L. The dynamics simulation of acetone adsorption in the modified activated carbon pore. J. At. Mol. Phys. 2018, 35, 147–153. [Google Scholar]
- Huang, B.; Zhao, R.; Xu, H.; Deng, J.; Li, W.; Wang, J.; Yang, H.; Zhang, L. Adsorption of Methylene Blue on Bituminous Coal: Adsorption Mechanism and Molecular Simulation. ACS Omega 2019, 4, 14032–14039. [Google Scholar] [CrossRef] [Green Version]
- Bergaoui, M.; Nakhli, A.; Benguerba, Y.; Khalfaoui, M.; Erto, A.; Soetaredjo, F.E.; Ismadji, S.; Ernst, B. Novel insights into the adsorption mechanism of methylene blue onto organo-bentonite: Adsorption isotherms modeling and molecular simulation. J. Mol. Liq. 2018, 272, 697–707. [Google Scholar] [CrossRef]
- An, Y.; Fu, Q.; Zhang, D.; Wang, Y.; Tang, Z. Performance evaluation of activated carbon with different pore sizes and functional groups for VOC adsorption by molecular simulation. Chemosphere 2019, 227, 9–16. [Google Scholar] [CrossRef]
- Fu, Q.; Tanaka, H.; Miyahara, M.T.; Qin, Y.; Shen, Y.; Zhang, D. CHF3–CHClF2 Binary Competitive Adsorption Equilibria in Graphitic Slit Pores: Monte Carlo Simulations and Breakthrough Curve Experiments. Ind. Eng. Chem. Res. 2018, 57, 6440–6450. [Google Scholar] [CrossRef]
- Chen, T.; Liu, H.; Bie, R. Temperature rise characteristics of coal-KOH adduct under microwave heating and the properties of resultant activated carbon for catalytic methane decomposition. J. Anal. Appl. Pyrolysis 2020, 145, 104739. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, W.; Yuan, P.; Yu, W.; Tan, D.; Liu, H.; He, H. Physical activation of diatomite-templated carbons and its effect on the adsorption of methylene blue (MB). Appl. Surf. Sci. 2013, 282, 838–843. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Li, M.; Zheng, H.; Du, Q.; Li, H.; Wang, Y.; Wang, D.; Wang, C.; Sui, K.; et al. Removal of methylene blue from aqueous solution using high performance calcium alginate/activated carbon membrane. Int. J. Cloth. Sci. Technol. 2020, 32, 307–321. [Google Scholar] [CrossRef]
- Mazzone, A.M.; Morandi, V. Solution of the time-dependent, multi-particle Schrödinger equation using Monte Carlo and numerical integration. Comput. Mater. Sci. 2006, 38, 231–239. [Google Scholar] [CrossRef]
- Li, S.; Huang, L.; Zhang, H.; Huang, Z.; Jia, Q.; Zhang, S. Adsorption mechanism of methylene blue on oxygen-containing functional groups modified graphitic carbon spheres: Experiment and DFT study. Appl. Surf. Sci. 2021, 540, 148386. [Google Scholar] [CrossRef]
- Niknam, P.; Jamehbozorgi, S.; Rezvani, M.; Izadkhah, V. Understanding delivery and adsorption of Flutamide drug with ZnONS based on: Dispersion-corrected DFT calculations and MD simulations. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 135, 114937. [Google Scholar] [CrossRef]
- González-Jiménez, R.; Nikolakopoulos, A.; Jachowicz, N.; Udías, J.M. Nuclear effects in electron-nucleus and neutrino-nucleus scattering within a relativistic quantum mechanical framework. Phys. Rev. C 2019, 100, 45501. [Google Scholar] [CrossRef]
- Trucano, P.; Chen, R. Structure of graphite by neutron diffraction. Nature 1975, 258, 136–137. [Google Scholar] [CrossRef]
- Li, S.; Song, K.; Zhao, D.; Rugarabamu, J.R.; Diao, R.; Gu, Y. Molecular simulation of benzene adsorption on different activated carbon under different temperatures. Microporous Mesoporous Mater. 2020, 302, 110220. [Google Scholar] [CrossRef]
- Ou, X.; Han, Q.; Dai, H.H.; Wang, J. Molecular dynamic simulations of the water absorbency of hydrogels. J. Mol. Modeling 2015, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Qifang, Q.; Zhou, J.; Li, L. Accuracy prediction on glass transition temperature of polystyrene with molecular dynamics simulation and experimental studies. Polym. Bull. 2020, 258, 66–72. [Google Scholar]
- IUPAC. Manual of symbols and terminology: Appendix 2. Part I: Colloid and surface chemistry. Pure Appl. Chem. 1972, 31, 578. [Google Scholar]
- Qiang, X.; Xianglan, Z.; Dingcheng, L.; Junya, C.; Jinchang, L. Directional preparation of coal-based activated carbon principles, approaches and applications. Coal Sci. Technol. 2020, 48, 1–28. [Google Scholar]
- Pelekani, C.; Snoeyink, V.L. Competitive adsorption between atrazine and methylene blue on activated carbon: The importance of pore size distribution. Carbon 2000, 38, 1423–1436. [Google Scholar] [CrossRef]
- Christina, S.; Myroslav, M.; Ihor, B.; Fedir, I.; Anatoliy, B.; Yuriy, K. Structural, magnetic and adsorption characteristics of magnetically susceptible carbon sorbents based on natural raw materials. J. Water Land Dev. 2020, 47, 160–168. [Google Scholar] [CrossRef]
- Mariana, M.; Mistar, E.M.; Alfatah, T.; Supardan, M.D. High-porous activated carbon derived from Myristica fragrans shell using one-step KOH activation for methylene blue adsorption. Bioresour. Technol. Rep. 2021, 16, 100845. [Google Scholar] [CrossRef]








| Parameters | Parameter Value |
|---|---|
| XC functional | GGA-PBE |
| Core treatment | DSPP |
| Basis set | DNP |
| SCF convergence accuracy (Ha) | 1.0 × 10−6 |
| Energy convergence accuracy (Ha) | 1 × 10−5 |
| Maximum force convergence accuracy (Ha/Å) | 0.002 |
| Maximum displacement convergence accuracy (Å) | 5 × 10−3 |
| Calculated Parameters | Setting Result |
|---|---|
| Force field | COMPASS II |
| Charge calculation method | Forcefield assigned |
| Van der Waals force calculation method | Atom based |
| Van der Waals force calculation cut-off radius (Å) | 12.5 |
| Calculation method of electrostatic force | Ewald |
| Calculation accuracy of electrostatic force (kcal/mol) | 1 × 10−3 |
| Sample Name | UCACnew | UCACcm |
|---|---|---|
| SSA (m2/g) | 1225.36 | 713.76 |
| Vt (cm3/g) | 0.99 | 0.51 |
| VSF (cm3/g) | 0.52 | 0.32 |
| P (nm) | 3.24 | 2.77 |
| PBJH (nm) | 2.28 | 2.10 |
| PSF (nm) | 0.75 | 0.71 |
| Model Name | Average Number of Adsorbed MB Molecules | MB Molecular Simulated Adsorption Capacity (mmol/g) | |
|---|---|---|---|
| Simulated Value | Experimental Value | ||
| UCACcm model | 34.94 | 2.73 | 2.04 |
| UCACnew model | 57.00 | 4.46 | 2.34 |
| Model Name | Adsorption Energy (kcal/mol) | Standard Deviation (kcal/mol) | Contribution of Electrostatic Force | Contribution of Van der Waals’ Force |
|---|---|---|---|---|
| Hydroxyl model | −43.64 | 1.85 | 24.84% | 75.16% |
| Carboxyl model | −49.77 | 2.15 | 36.31% | 63.69% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Shen, L.; Liu, Y.; Wang, C.; Zhu, H.; Shao, H.; Zhu, J. Molecular Simulation Comparison of Two Ultrafine Coal-Based Activated Carbons for the Removal of Methylene Blue from Water. Processes 2022, 10, 290. https://doi.org/10.3390/pr10020290
Zhu Z, Shen L, Liu Y, Wang C, Zhu H, Shao H, Zhu J. Molecular Simulation Comparison of Two Ultrafine Coal-Based Activated Carbons for the Removal of Methylene Blue from Water. Processes. 2022; 10(2):290. https://doi.org/10.3390/pr10020290
Chicago/Turabian StyleZhu, Zaisheng, Liang Shen, Yin Liu, Chuanzhen Wang, Hongzheng Zhu, Huaizhi Shao, and Jinbo Zhu. 2022. "Molecular Simulation Comparison of Two Ultrafine Coal-Based Activated Carbons for the Removal of Methylene Blue from Water" Processes 10, no. 2: 290. https://doi.org/10.3390/pr10020290
APA StyleZhu, Z., Shen, L., Liu, Y., Wang, C., Zhu, H., Shao, H., & Zhu, J. (2022). Molecular Simulation Comparison of Two Ultrafine Coal-Based Activated Carbons for the Removal of Methylene Blue from Water. Processes, 10(2), 290. https://doi.org/10.3390/pr10020290









