Differences in Physiological Metabolism and Antioxidant System of Different Ecotypes of Miscanthus floridulus under Cu Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Experimental Soil
2.3. The Experimental Design
2.4. Test Index and Method
2.5. Data Processing
3. Results
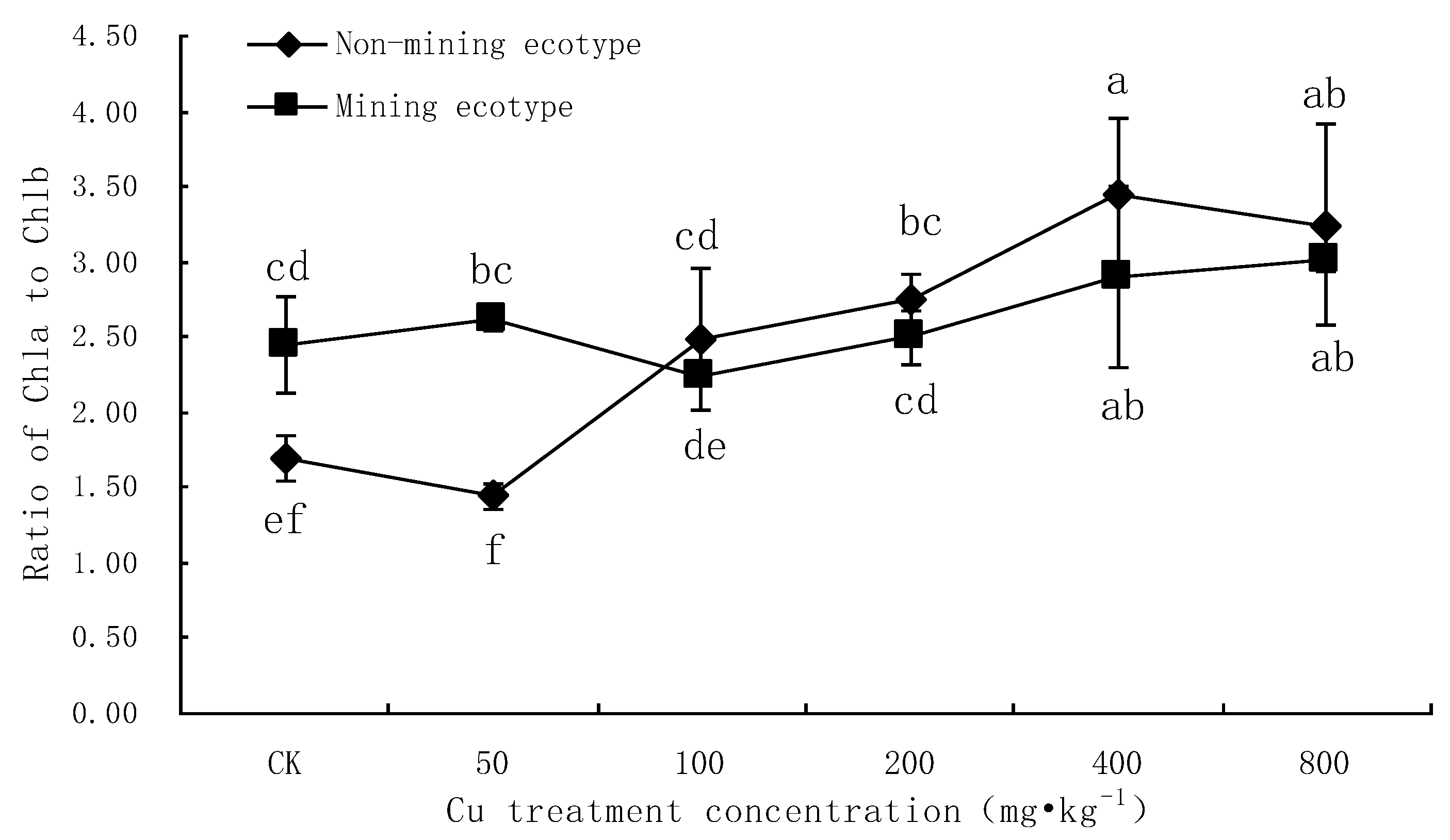
3.1. Effect of Cu Stress on Chlorophyll Content in Leaves of M. floridulus
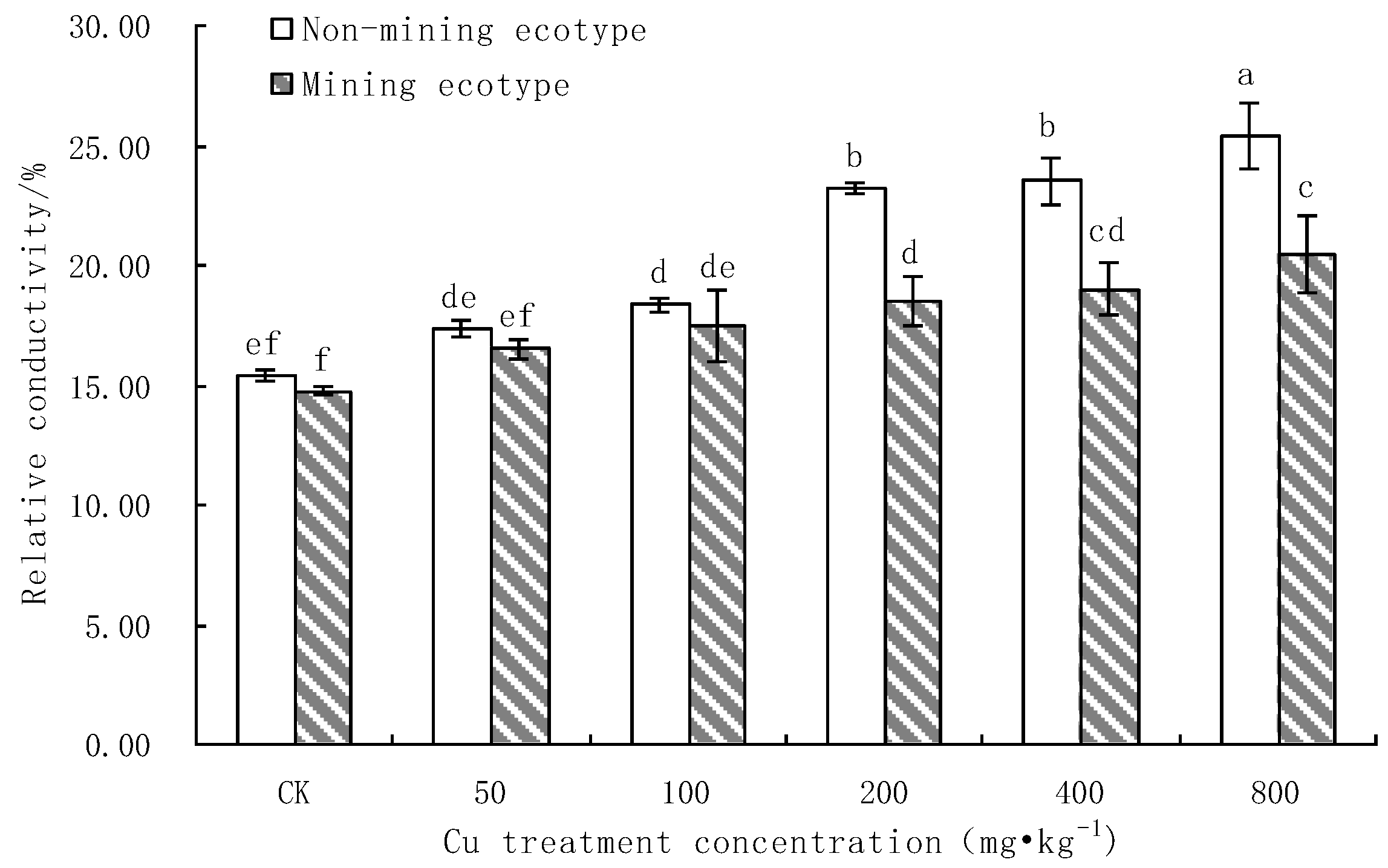
3.2. The Effect of Cu Stress on the Membrane Protection System of M. floridulus
- (1)
- Effects of Cu stress on MDA content in leaves of M. floridulus
- (2)
- Effect of Cu Stress on Cell Membrane Permeability in Leaves of M. floridulus
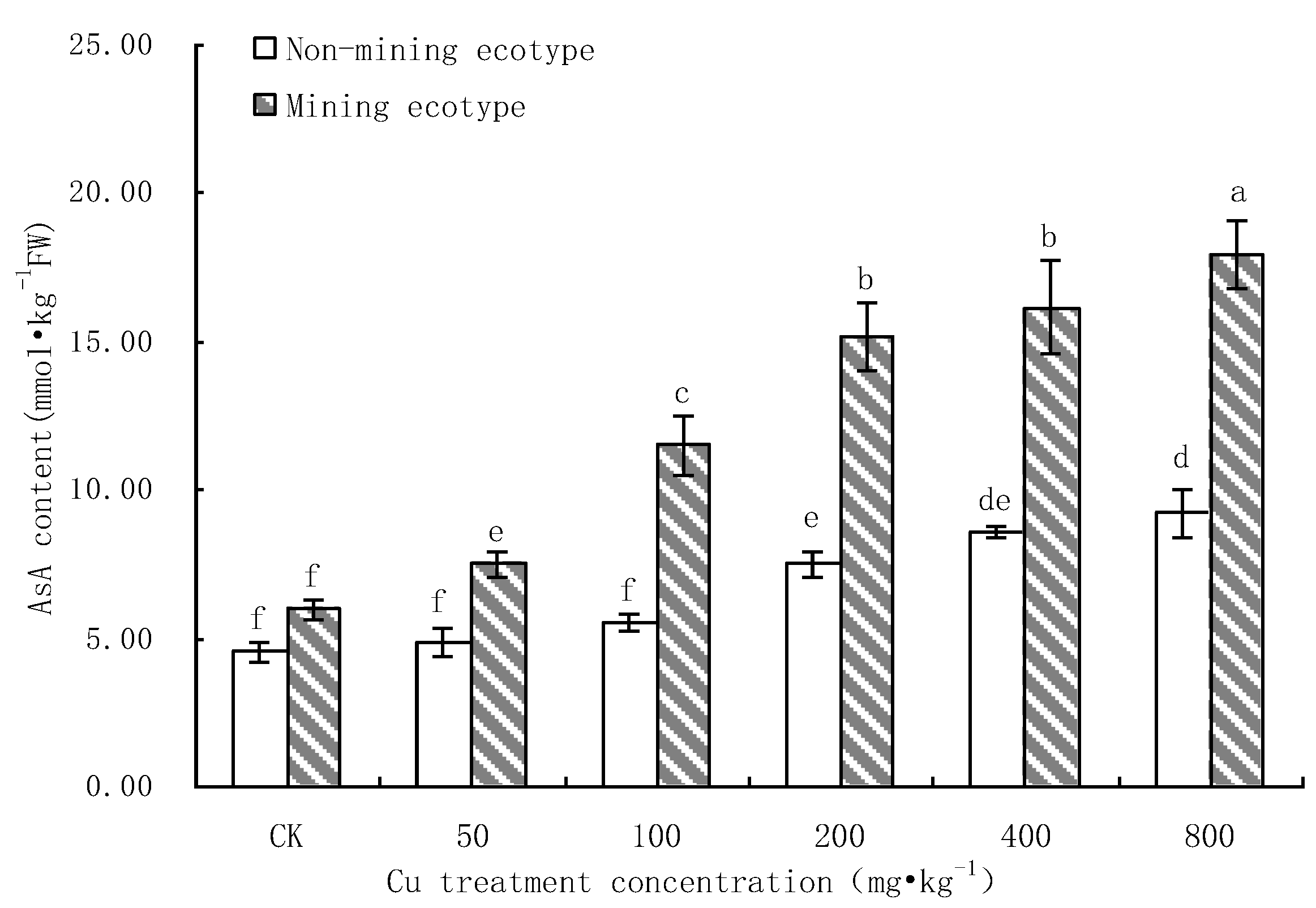
3.3. Effect of Cu Stress on AsA Content of M. floridulus
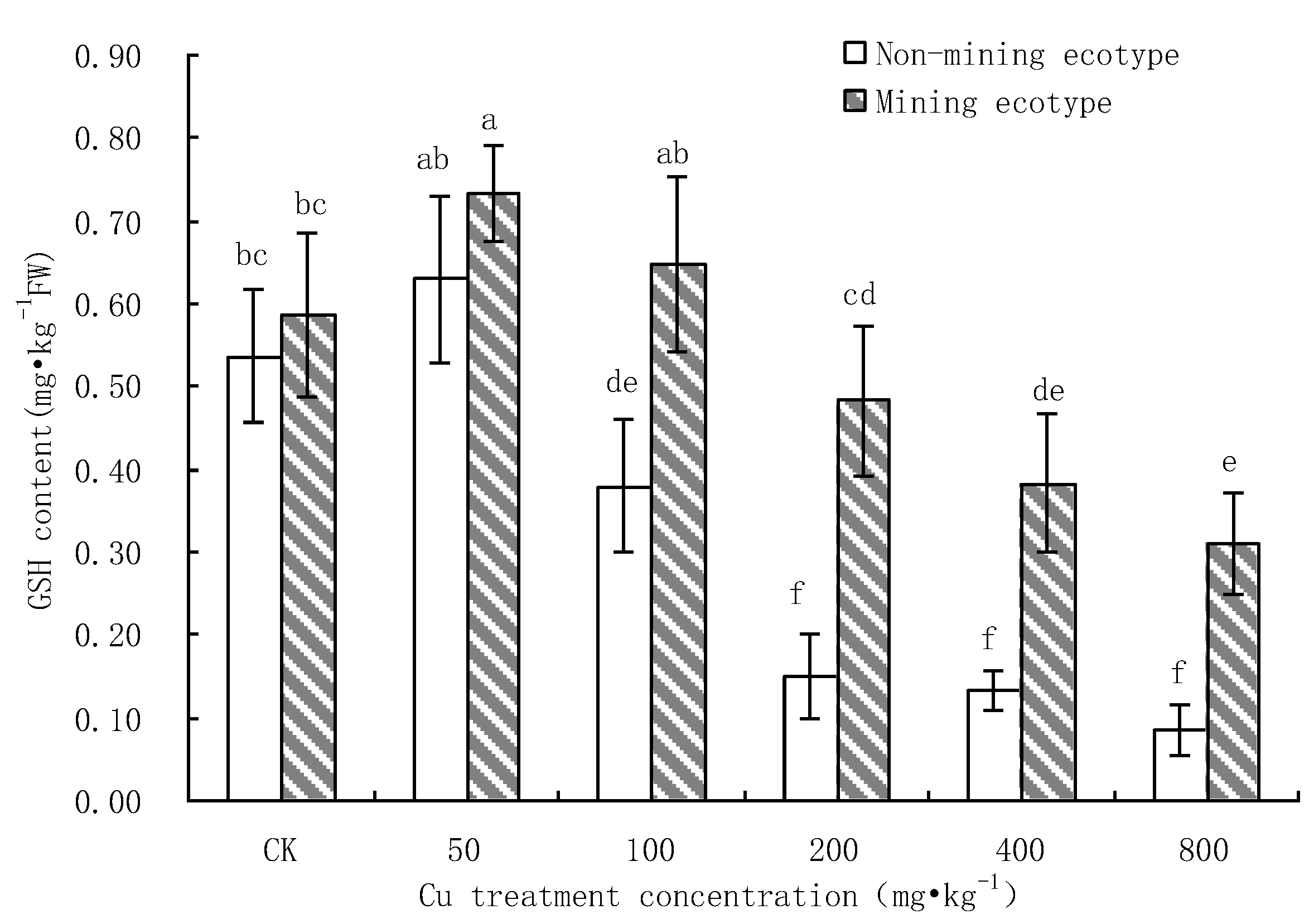
3.4. Effect of Cu Stress on the Content of GSH in M. floridulus
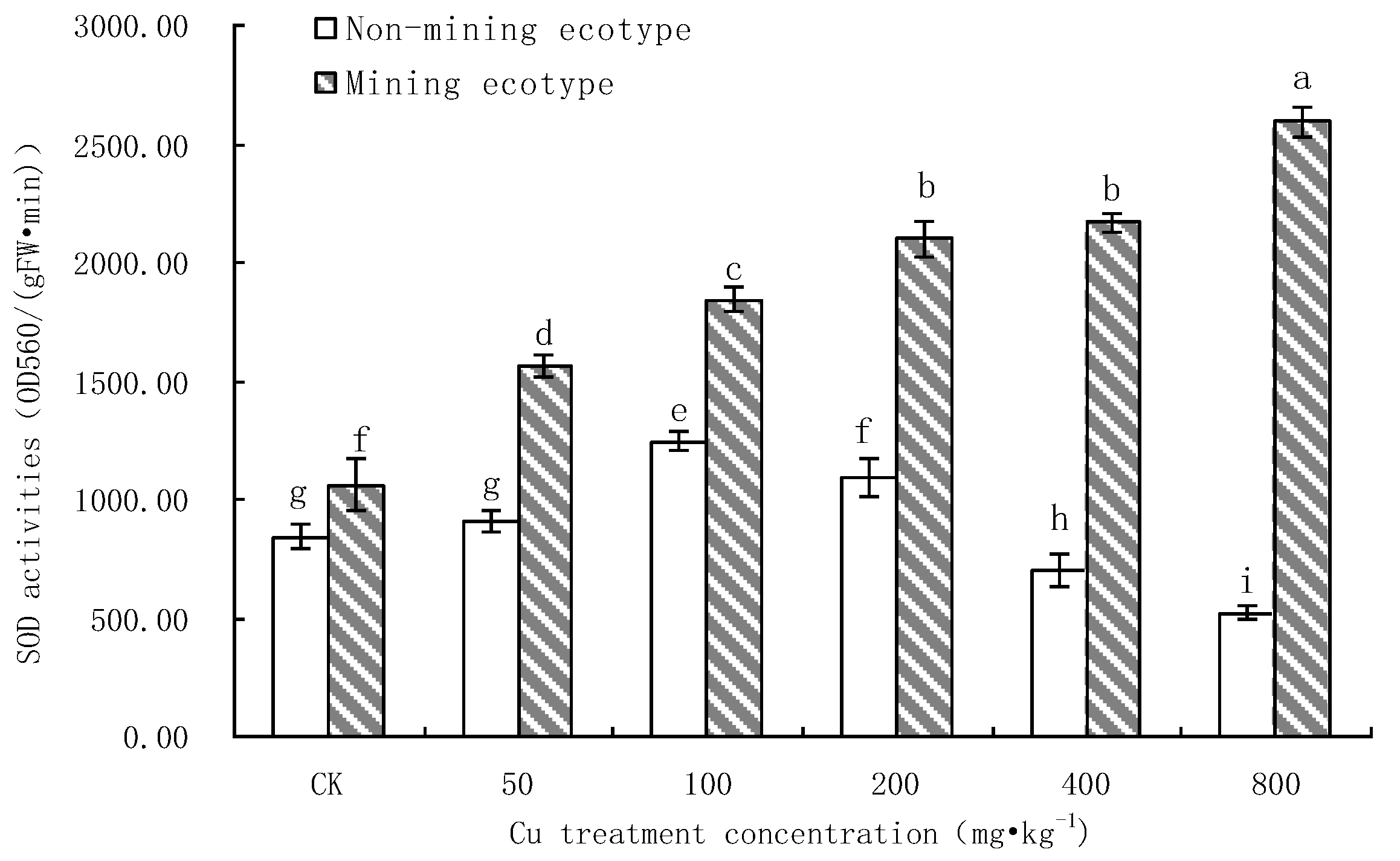
3.5. Effect of Cu Stress on SOD Activity of M. floridulus
3.6. Effect of Cu Stress on POD Activity of M. floridulus
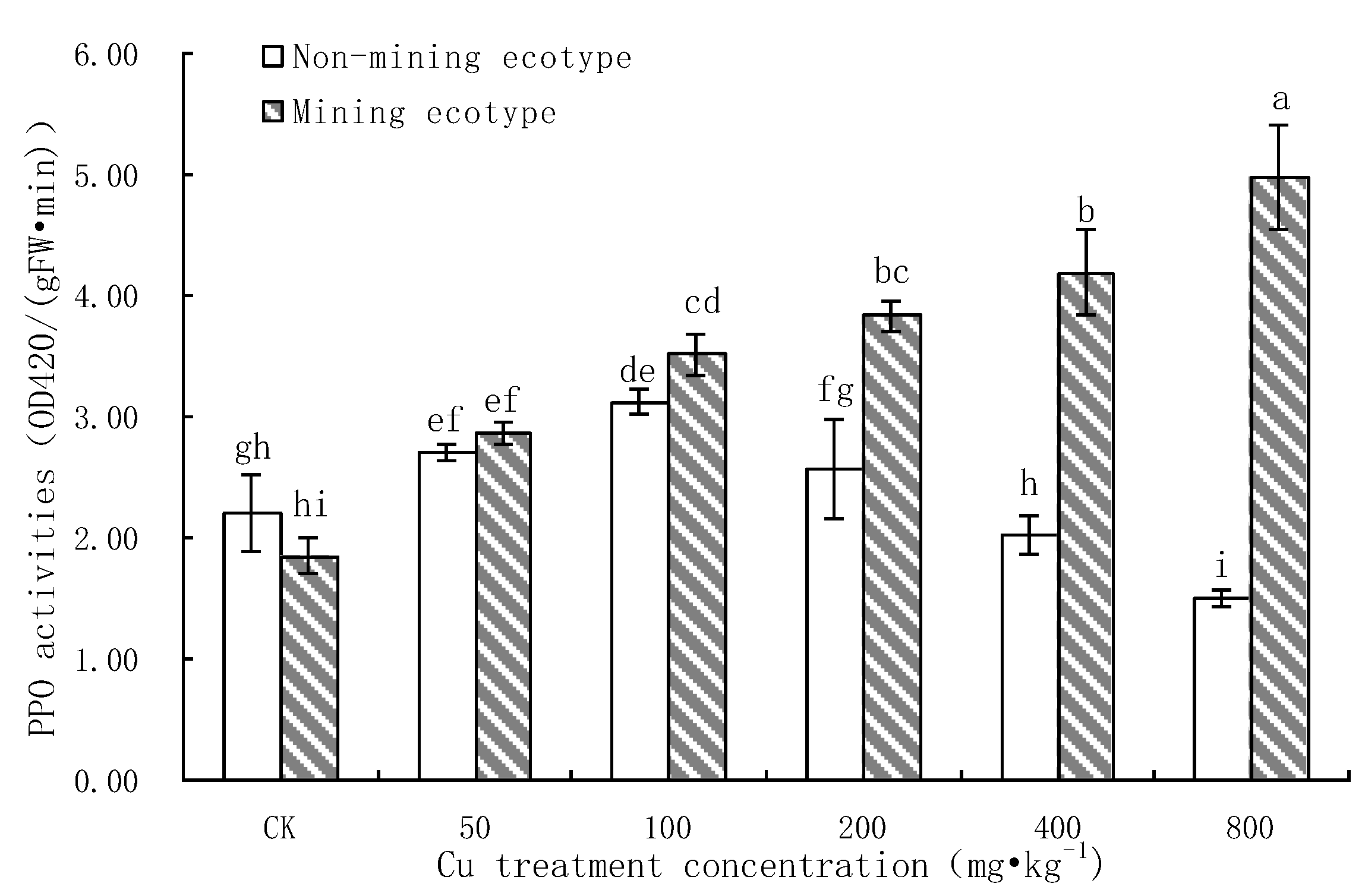
3.7. Effect of Pb Stress on PPO Activity of M. floridulus
3.8. The Correlation between the Physiological Indexes and the Mass Fraction of Cu
4. Discussion
4.1. Analysis of the Effect of Cu Stress on Chlorophyll Content of M. floridulus
4.2. Effects of Cd Stress on Physiological Metabolism of M. floridulus
4.3. Effects of Cu Stress on the Antioxidant Enzyme System of M. floridulus
5. Conclusions
- (1)
- Chlorophyll a, chlorophyll b and total chlorophyll in the leaves of two ecotypes of M. floridulus were negatively correlated with Cu stress concentration (p < 0.01), but the extent of decrease for the ecotypes in the mining area was lower than that for ecotypes in the non-mining area. The values of chlorophyll a/b for both ecotypes increased with increasing Cu treatment concentration, indicating that Cu is more harmful to chlorophyll b than to chlorophyll a for M. floridulus.
- (2)
- Under Cu stress, the content of antioxidant substances (GSH, AsA) in the mining ecotype was significantly higher than that in the non-mining ecotype. The membrane permeability increased for both ecotypes at high concentrations of copper treatment, and the MDA content of the non-mining ecotype was significantly higher than that of the mining ecotype. The experimental data obtained showed that, under copper stress, the non-mining ecotype M. floridulus suffered more severe peroxidation damage than the mining ecotype. The endogenous GSH and AsA of M. floridulus play an important role in scavenging free radical accumulation caused by excess copper.
- (3)
- The SOD activity in the leaves of the non-mining ecotype was inhibited by Cu stress, and the POD activity was increased by Cu stress, but the increase for the mining ecotype was larger than that for the non-mining ecotype. At the highest Cu concentrations, both enzyme activities were significantly higher in the mining ecotype plants than in the non-mining ecotype plants. The results suggest that, in the long-term adaptation process, the mining ecotype M. floridulus becomes a resistant ecotype, and that the non-enzymatic system plays an important role in raising the level of resistance.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; He, J.Z.; Ma, Y.H.; Zhu, Y.G.; Zhang, L. Molecular mechanism of biological copper tolerance and biological joint remediation of copper polluted environment. Acta Ecol. Sin. 2007, 6, 2615–2626. [Google Scholar]
- Li, D.L.; Chen, J.W.; Zhang, H.; Li, J.J. Effects of copper pollution on soil bacterial community structure and heavy metal resistance genes. J. Environ. Sci. 2021, 41, 1082–1090. [Google Scholar]
- Hector, A.; Bagchi, R. Biodiversity and ecosystem multifunctionality. Nature 2007, 448, 188–190. [Google Scholar] [CrossRef]
- Song, J.; Shen, Q.; Wang, L.; Qiu, G.; Shi, J.; Xu, J.; Philip, C.B.; Liu, X. Effects of Cd, Cu, Zn and their combined action on microbial biomass and bacterial community structure. Environ. Pollut. 2018, 243, 510–518. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Li, J.; Liu, Y.; Shi, W.; Hu, H. The toxic factor of copper should be adjusted during the ecological risk assessment for soil bacterial community. Ecol. Indic. 2020, 111, 106072. [Google Scholar] [CrossRef]
- Zheng, B.J.; Xu, S.C.; Tu, Y.H.; Zou, F.; Liang, D.D.; Wang, H.; Mo, Y.W. Research progress on copper toxicity to crops. J. Shaoxing Univ. Arts Sci. 2022, 42, 58–64. [Google Scholar]
- Lin, M.Z.; Jin, M.F.; Chen, Y.; Zeng, Q.L. Photosynthetic characteristics and mechanism of wedelia chinensis under soil Cu pollution stress. J. Anhui Agric. Univ. 2018, 45, 657–663. [Google Scholar]
- Yang, K.; Huang, C.; Lu, S.; Peng, G.Y.; Huang, C.G. Selection of reference genes in real-time quantitative PCR of root tissue of Commelina purpurea under copper stress. Acta Phytophysiol. Sin. 2021, 57, 195–204. [Google Scholar]
- Qin, J.Q.; Zhao, H.R.; Dai, M.; Zhao, P.; Chen, X.; Liu, H.; Lu, B.Z. Speciation Distribution and Influencing Factors of Heavy Metals in Rhizosphere Soil of Miscanthus Floridulus in the Tailing Reservoir Area of Dabaoshan Iron Polymetallic Mine in Northern Guangdong. Processes 2022, 10, 1217. [Google Scholar] [CrossRef]
- Wu, B.H.; Luo, S.H.; Luo, H.Y.; Huang, H.Y.; Xu, F.; Feng, S.; Xu, H. Improved phytoremediation of heavy metal contaminated soils by Miscanthus floridulus under a varied rhizosphere ecological characteristic. Sci. Total Environ. 2021, 808, 151995. [Google Scholar] [CrossRef]
- Nie, G.; Zhong, M.Y.; Cai, J.B.; Yang, X.Y.; Zhou, J.; Appiah, C.; Tang, M.Y.; Wang, X.; Feng, G.Y.; Huang, L.K.; et al. Transcriptome characterization of candidate genes related to chromium uptake, transport and accumulation in Miscanthus sinensis. Ecotoxicol. Environ. Saf. 2021, 221, 112445. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.F.; Du, W.B.; Li, Z.A.; Wang, Z.F.; Peng, S.L. Heavy metals accumulation in mining area’s Miscanthus sinensis populations and its relationship with soil characters. Chin. J. Ecol. 2006, 25, 255–258. [Google Scholar]
- Liao, B.; Deng, D.M.; Yang, B.; Shu, W.S.; Lin, L.; Lan, C.Y. Study on copper tolerance and accumulation of Commelina communis. J. Environ. Sci. 2003, 6, 797–801. [Google Scholar]
- Wen, C.H.; Duan, C.Q.; Chang, X.X. Differentiation in Datura stramonium L. populations exposed to heavy-metal pollution at different durations: RAPD analysis. Acta Ecol. Sin. 2001, 21, 1239–1245. [Google Scholar]
- Peng, S.L.; Du, W.B.; Li, Z.A. A review of heavy metal accumulation and tolerance by plants of different ecotype. J. Jishou Univ. 2004, 25, 19–26. [Google Scholar]
- Yu, H.; Zheng, X.; Weng, W.; Yan, X.; Chen, P.; Liu, X.; Peng, T.; Zhong, Q.; Xu, K.; Wang, C.; et al. Synergistic effects of antimony and arsenic contaminations on bacterial, archaeal and fungal communities in the rhizosphere of Miscanthus sinensis: Insights for nitrification and carbon mineralization. J. Hazard. Mater. 2021, 411, 125094. [Google Scholar] [CrossRef]
- Barbosa, B.; Boléo, S.; Sidella, S.; Costa, J.; Duarte, M.P.; Mendes, B.; Cosentino, S.L.; Fernando, A.L. Phytoremediation of heavy metal-contaminated soils using the perennial energy crops Miscanthus spp. and Arundo donax L. Bioenerg. Res. 2015, 8, 1500–1511. [Google Scholar] [CrossRef]
- Zadel, U.; Nesme, J.; Michalke, B.; Vestergaard, G.; Plaza, G.A.; Schroder, P.; Radl, V.; Schloter, M. Changes induced by heavy metals in the plant-associated microbiome of Miscanthus × Giganteus. Sci. Total Environ. 2020, 711, 134433. [Google Scholar] [CrossRef]
- Sun, J.; Tie, B.Q.; Qin, P.F. Investigation of contaminated soil and plants by heavy metals in Pb-Zn mining area. J. Plant Resour. Environ. 2006, 15, 63–67. [Google Scholar]
- Zhu, Y.G. Microinterface processes in soil-plant systems and their eco-environmental effects. J. Environ. Sci. 2003, 23, 205–210. [Google Scholar]
- Wang, H.B.; Shu, W.S.; Lan, C. Ecology for heavy metal pollution: Recent advances and future prospects. Acta Ecol. Sin. 2005, 25, 596–605. [Google Scholar]
- Zhou, S.; Deng, R.; Hursthouse, A. Risk Assessment of Potentially Toxic Elements Pollution from Mineral Processing Steps at Xikuangshan Antimony Plant, Hunan, China. Processes 2020, 8, 29. [Google Scholar] [CrossRef]
- Fu, S.; Zhou, Y.; Zhao, Y.; Gao, Q.Z.; Peng, X.Z.; Dang, Z.; Zhang, C.B.; Yang, X.Q.; Yang, Z.J.; Dou, L.; et al. Study on heavy metals in soils contaminated by acid mine drainage from Dabaoshan Mine, Guangdong. Environ. Sci. 2007, 28, 805–812. [Google Scholar]
- Zhao, H.R.; Wang, D.; Zhu, B.T.; Jin, X. Evaluation of heavy metal pollution of soil in the Dabaoshan mining area based on the information diffusion theory. J. Agro-Environ.Sci. 2019, 38, 79–86. [Google Scholar]
- Chen, J.Y.; Liu, G.B.; Cui, J.L.; Xiao, T.F. Migration process and risk assessment of heavy metals in soil plant system of Dabaoshan mining area in Guangdong Province. Environ. Sci. 2019, 40, 5629–5639. [Google Scholar]
- Qin, J.Q.; Xia, B.C.; Hu, M.; Zhao, P.; Zhao, H.R.; Lin, X.F. Analysis of the vegetation succession of tailing wasteland of Dabaoshan Mine, Guangdong Province. J. Agro-Environ. Sci. 2009, 28, 2085–2091. [Google Scholar]
- Qin, J.Q.; Zhao, H.R.; Liu, H.; Dai, M.; Zhao, P.; Chen, X.; Wu, X.G. The Difference of Lead Accumulation and Transport in Different Ecotypes of Miscanthus floridulus. Processes 2022, 10, 2219. [Google Scholar] [CrossRef]
- Zhao, H.R.; Xia, B.C.; Qin, J.Q.; Zhang, J. Hydrogeochemical and mineralogical characteristics related to heavy metal attenuation in a stream polluted by acid mine drainage: A case study in Dabaoshan mine, China. J. Environ. Sci. 2012, 24, 979–989. [Google Scholar] [CrossRef]
- Zhao, H.R.; Xia, B.C.; Fan, C.; Zhao, P.; Shen, S. Human health risk from soil heavy metal contamination under different land uses near Dabaoshan mine, southern China. Sci. Total Environ. 2012, 417, 45–54. [Google Scholar] [CrossRef]
- Cui, J.L.; Luo, C.L.; Tang, C.W.; Chang, T.; Li, X. Speciation and leaching of trace metal contaminants from e-waste contaminated soils. J. Hazard. Mater. 2017, 329, 150–158. [Google Scholar] [CrossRef]
- Lu, R. Methods of Soil and Agricultural Chemistry; Beijing Science and Technology Press: Beijing, China, 1999; pp. 235–285. [Google Scholar]
- Li, H.S.; Sui, Q.; Zhao, S.J.; Zhang, W.H. Experimental Principle and Technology of Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2000; pp. 134–138. [Google Scholar]
- Zhang, Z.L.; Qu, W.J. A Guide Book of Plant Physiology Experiment; Higher Education Press: Beijing, China, 2004. [Google Scholar]
- Chen, J.X.; Wang, X.F. Experimental Instruction in Plant Physiology; South China University of Technology Press: Guangzhou, China, 2002; pp. 124–125. [Google Scholar]
- Brun, L.A.; Maillet, J.; Hinsinge, P.; Pepin, M. Evalution of copper availability to plants in copper contaminated vineyard soils. Environ. Pollut. 2001, 111, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.F.; Liu, Y.G.; Zeng, G.M. Effects of cadrnium stress on active oxygen generation, lipid peroxidation and antioxidant enzyme actives in radish seedlings. J. Plant Physiol. Mol. Biol. 2004, 30, 469–474. [Google Scholar]
- Zou, C.F.; Wu, G.R.; Shi, G.X. The role of antioxidant systems in Cu2+ stress resistance in Alternanthera philoxeroides. Acta Bot. Sin. 2001, 43, 389–494. [Google Scholar]
- Nagalakshmi, N.; Prasad, M.N.V. Copper-induced oxidative stress in Scenedesmus bijugatus: Protective role of free radical scavengers. Bull. Environ. Contam. Toxicol. 1998, 61, 623–628. [Google Scholar] [CrossRef]
- Ouzounidou, G. Copper-induced changes on growth metal conent and phyotosynthetic function of Alyssummon tanum plants. Environ. Exp. Bot. 1994, 34, 165–172. [Google Scholar] [CrossRef]
- Huang, Y.S.; Qiu, G.H. Physiological differences of Festuca rubra copper tolerant merlin and sensitive S59 to copper treatment at their early developmental stages. Chin. J. Appl. Environ. Biol. 1998, 4, 126–131. (In Chinese) [Google Scholar]
- Prasad, M.N.V.; Malec, P. Physiological Responses of Lemna trisulca L. (duckweed) to Cadmium and Copper Bioaccumulation. Plant Sci. 2001, 161, 881–889. [Google Scholar] [CrossRef]
- Kupper, H.; Kupper, F.; Spiller, M. Environmental Relevance of Heavy Metal Substituted Chlorophylls Using the Example of Water Plants. J. Exp. Bot. 1996, 47, 259–266. [Google Scholar] [CrossRef]
- Ren, Q.Q.; Huang, Z.R.; Huang, W.L.; Huang, W.T.; Chen, H.H.; Yang, L.T.; Ye, X.; Chen, L.S. Physiological and molecular adaptations of Citrus grandis roots to long-term copper excess revealed by physiology, metabolome and transcriptome. Environ. Exp. Bot. 2022, 203, 105049. [Google Scholar] [CrossRef]
- Huang, H.; Li, S.; Guo, J.L. Effects of cadmium stress on antioxidant system and photosynthesis of maize seedlings. J. Agro-Environ. Sci. 2010, 29, 211–215. [Google Scholar]
- Sujkowska-Rybkowska, M.; Muszyñska, E.; Labudda, M. Structural Adaptation and Physiological Mechanisms in the Leaves of Anthyllis vulneraria L. from Metallicolous and Non-Metallicolous Populations. Plants 2020, 9, 662. [Google Scholar] [PubMed]
- Xie, M.J.; Ke, W.S.; Wang, W.X. MDA accumulation and antioxidation capacity of two Elsholtzia splendens populations under copper stress. Chin. J. Ecol. 2005, 24, 935–938. [Google Scholar]
- Li, X.Y.; Wang, X.; Lu, L.-f; Yin, B.; Zhang, M.; Cui, X.-m. Effects of exogenous NO on ascorbic acid glutathione cycle in tomato seedling roots under copper stress. Chin. J. Appl. Ecol. 2013, 24, 1023–1030. [Google Scholar]
- Du, Y.L.; Zhao, Q.; Chen, L.W.; Yao, X.D.; Zhang, W.; Zhang, B.; Xie, F.T. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Han, Y.Y.; Zhou, S.; Chen, Y.H.; Kong, X.; Xu, Y.; Wang, W. The involvement of expansins in responses to phosphorus availability in wheat, and its potentials in improving phosphorus efficiency of plants. Plant Physiol. Biochem. 2014, 78, 53–62. [Google Scholar] [CrossRef]
- Li, J.Y.; Zheng, B.H.; He, Y.Z.; Zhou, Y.Y.; Chen, X.; Ruan, S.; Yang, Y.; Dai, C.H.; Tang, L. Antimony contamination, consequences and removal techniques: A review. Ecotoxicol. Environ. Saf. 2018, 156, 125–134. [Google Scholar] [CrossRef]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Ambrosini, V.G.; Rosa, D.J.; de Melo, G.W.B.; Zalamena, J.; Cella, C.; Simão, D.G.; da Silva, L.S.; dos Santos, H.P.; Toselli, M.; Tiecher, T.L.; et al. High copper content in vineyard soils promotes modifications in photosynthetic parameters and morphological changes in the root system of ‘Red Niagara’ plantlets. Plant Physiol. Biochem. 2018, 128, 89–98. [Google Scholar] [CrossRef]
- Pietrini, F.; Di Baccio, D.; Iori, V.; Veliksar, S.; Lemanova, N.; Juškaitė, L.; Maruška, A.; Zacchini, M. Investigation on metal tolerance and phytoremoval activity in the poplar hybrid clone “Monviso” under Cu-spiked water: Potential use for wastewater treatment. Sci. Total Environ. 2017, 592, 412–418. [Google Scholar] [CrossRef]
- Oustriere, N.; Marchand, L.; Lottier, N.; Motelica, M.; Mench, M. Long-term Cu stabilization and biomass yields of Giant reed and poplar after adding a biochar, alone or with iron grit, into a contaminated soil from a wood preservation site. Sci. Total Environ. 2017, 579, 620–627. [Google Scholar] [CrossRef]
- Li, C.; Xiao, B.; Wang, Q.H.; Yao, S.H.; Wu, J.Y. Phytoremediation of Zn- and Cr-contaminated soil using two promising energy grasses. Water Air Soil Pollut. 2014, 225, 2027. [Google Scholar] [CrossRef]
- Feigl, G.; Kumar, D.; Lehotai, N.; Tugyi, N.; Molnár, Á.; Ördög, A.; Szepesi, Á.; Gémes, K.; Laskay, G.; Erdei, L.; et al. Physiological and morphological responses of the root system of Indian mustard (Brassica juncea L. Czern.) and rapeseed (Brassica napus L.) to copper stress. Ecotoxicol. Environ. Saf. 2013, 94, 179–189. [Google Scholar] [CrossRef]
- Marzilli, M.; Di Santo, P.; Palumbo, G.; Maiuro, L.; Paura, B.; Tognetti, R.; Cocozza, C. Cd and Cu accumulation, translocation and tolerance in Populus alba clone (Villafranca) in autotrophic in vitro screening. Environ. Sci. Pollut. Res. 2018, 25, 10058–10068. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Luo, J.; Ma, C.; Li, S.; Qu, L.; Gai, Y.; Jiang, X.; Janz, D.; Polle, A. A transcriptomic network underlies microstructural and physiological responses to cadmium in Populus canescens. Plant Physiol. 2013, 162, 424–439. [Google Scholar] [CrossRef]
- Zhu, H.; Teng, Y.; Wang, X.; Zhao, L.; Ren, W.; Luo, Y.; Christie, P. Changes in clover rhizosphere microbial community and diazotrophs in mercury-contaminated soils. Sci. Total Environ. 2021, 767, 145473. [Google Scholar] [CrossRef]
- Rehman, M.; Maqbool, Z.; Peng, D.; Liu, L. Morpho-physiological traits, antioxidant capacity and phytoextraction of copper by ramie (Boehmeria nivea L.) grown as fodder in copper-contaminated soil. Environ. Sci. Pollut. Res. 2019, 26, 5851–5861. [Google Scholar] [CrossRef]


| Soil Sample Point | pH | Organic C (g·kg−1) | Available P (mg·kg−1) | Available N (mg·kg−1) | Heavy Metal Content (mg·kg−1) | |||
|---|---|---|---|---|---|---|---|---|
| Zn | Pb | Cu | Cd | |||||
| Dabaoshan Mining Area | 6.2 | 14.7 ± 0.9 b | 32.2 ± 2.0 b | 30.2 ± 1.9 b | 1768.7 ± 91.1 a | 1253.3 ± 71.3 a | 1701.3 ± 77.5 a | 9.1 ± 0.9 a |
| Boluo County | 6.6 | 13.8 ± 0.9 b | 26.6 ± 1.8 b | 28.4 ± 2.7 b | 135.2 ± 13.1 b | 242.6 ± 44.1 b | 48.4 ± 9.5 b | 1.1 ± 0.2 b |
| Soil samples tested | 6.5 | 28.4 ± 1.8 a | 61.5 ± 9.9 a | 55.5 ± 9.1 a | 80.2 ± 8.9 b | 8.2 ± 7.2 c | 1.5 ± 0.7 c | 0.30 ± 0.1 b |
| Cu Treatment Concentration (mg·kg−1) | 0 | 50 | 100 | 200 | 400 | 800 | |
|---|---|---|---|---|---|---|---|
| Chl a (mg·g−1FW) | Non-mining ecotype | 1.30 ± 0.02 | 1.32 ± 0.01 | 1.21 ± 0.07 | 1.19 ± 0.03 | 1.01 ± 0.14 | 1.12 ± 0.11 |
| Mining ecotype | 1.27 ± 0.02 | 1.27 ± 0.01 | 1.28 ± 0.01 | 1.21 ± 0.04 | 1.17 ± 0.12 | 1.14 ± 0.02 | |
| Chl b (mg·g−1FW) | Non-mining ecotype | 0.77 ± 0.06 | 0.91 ± 0.07 | 0.50 ± 0.13 | 0.43 ± 0.04 | 0.30 ± 0.08 | 0.35 ± 0.10 |
| Mining ecotype | 0.52 ± 0.08 | 0.47 ± 0.01 | 0.56 ± 0.01 | 0.48 ± 0.05 | 0.41 ± 0.12 | 0.38 ± 0.01 | |
| Carotenoid (mg·g−1FW) | Non-mining ecotype | 0.42 ± 0.01 | 0.43 ± 0.01 | 0.37 ± 0.03 | 0.38 ± 0.02 | 0.33 ± 0.02 | 0.37 ± 0.04 |
| Mining ecotype | 0.38 ± 0.01 | 0.37 ± 0.01 | 0.39 ± 0.01 | 0.37 ± 0.01 | 0.35 ± 0.05 | 0.34 ± 0.01 | |
| Total chlorophyll (mg·g−1FW) | Non-mining ecotype | 2.07 ± 0.05 | 2.23 ± 0.07 | 1.71 ± 0.11 | 1.62 ± 0.05 | 1.31 ± 0.12 | 1.47 ± 0.20 |
| Mining ecotype | 1.80 ± 0.09 | 1.74 ± 0.02 | 1.84 ± 0.02 | 1.70 ± 0.10 | 1.59 ± 0.21 | 1.52 ± 0.02 | |
| Chl a/b | Non-mining ecotype | 1.70 ± 0.16 | 1.44 ± 0.09 | 2.49 ± 0.47 | 2.74 ± 0.18 | 3.44 ± 0.52 | 3.25 ± 0.66 |
| Mining ecotype | 2.44 ± 0.32 | 2.61 ± 0.06 | 2.25 ± 0.06 | 2.50 ± 0.18 | 2.90 ± 0.60 | 3.00 ± 0.07 | |
| Type | Item | Cu | Chl | MDA | RPP | AsA | GSH | SOD | POD | PPO |
|---|---|---|---|---|---|---|---|---|---|---|
| Non-mining ecotype | Cu | 1 | ||||||||
| Chl | −0.671 ** | 1 | ||||||||
| MDA | 0.972 ** | −0.717 ** | 1 | |||||||
| RPP | 0.855 ** | −0.757 ** | 0.902 ** | 1 | ||||||
| AsA | 0.884 ** | −0.810 ** | 0.891 ** | 0.936 ** | 1 | |||||
| GSH | −0.763 ** | 0.842 ** | −0.818 ** | −0.903 ** | −0.883 ** | 1 | ||||
| SOD | −0.713 ** | 0.310 | −0.560 * | −0.437 | −0.530 * | 0.316 | 1 | |||
| POD | −0.465 | 0.086 | −0.296 | −0.080 | −0.203 | −0.008 | 0.852 ** | 1 | ||
| PPO | −0.747 ** | 0.354 | −0.635 ** | −0.548* | −0.649 ** | 0.478 * | 0.853 ** | 0.650 ** | 1 | |
| Mining ecotype | Cu | 1 | ||||||||
| Chl | −0.667 ** | 1 | ||||||||
| MDA | 0.920 ** | −0.698 ** | 1 | |||||||
| RPP | 0.797 ** | −0.466 | 0.909 ** | 1 | ||||||
| AsA | 0.831 ** | −0.622 ** | 0.932 ** | 0.852 ** | 1 | |||||
| GSH | −0.794 ** | 0.424 | −0.718 ** | −0.738 ** | −0.762 ** | 1 | ||||
| SOD | 0.868 ** | −0.543 * | 0.955 ** | 0.883 ** | 0.939 ** | −0.686 ** | 1 | |||
| POD | 0.875 ** | −0.583 * | 0.952 ** | 0.873 ** | 0.963 ** | −0.740 ** | 0.970 ** | 1 | ||
| PPO | 0.861 ** | −0.501 * | 0.922 ** | 0.855 ** | 0.909 ** | −0.702 ** | 0.976 ** | 0.959 ** | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Yan, Z.; Jiang, X.; Zhao, H.; Liu, S.; Dai, M.; Xiong, D.; Chen, X. Differences in Physiological Metabolism and Antioxidant System of Different Ecotypes of Miscanthus floridulus under Cu Stress. Processes 2022, 10, 2712. https://doi.org/10.3390/pr10122712
Qin J, Yan Z, Jiang X, Zhao H, Liu S, Dai M, Xiong D, Chen X. Differences in Physiological Metabolism and Antioxidant System of Different Ecotypes of Miscanthus floridulus under Cu Stress. Processes. 2022; 10(12):2712. https://doi.org/10.3390/pr10122712
Chicago/Turabian StyleQin, Jianqiao, Zhiqiang Yan, Xueding Jiang, Huarong Zhao, Shasha Liu, Min Dai, Dexin Xiong, and Xi Chen. 2022. "Differences in Physiological Metabolism and Antioxidant System of Different Ecotypes of Miscanthus floridulus under Cu Stress" Processes 10, no. 12: 2712. https://doi.org/10.3390/pr10122712
APA StyleQin, J., Yan, Z., Jiang, X., Zhao, H., Liu, S., Dai, M., Xiong, D., & Chen, X. (2022). Differences in Physiological Metabolism and Antioxidant System of Different Ecotypes of Miscanthus floridulus under Cu Stress. Processes, 10(12), 2712. https://doi.org/10.3390/pr10122712






