Abstract
Polycyclic aromatic hydrocarbons (PAHs) are chemically recalcitrant carcinogenic and mutagenic compounds with primarily anthropogenic origin. The investigation of the effects of emissions from energy enterprises on soil microbiomes is of a high priority for modern soil science. In this study, metagenomic profiling of technogenic contaminated soils was carried out based on bioinformatic analysis of shotgun metagenome data with PAH-degrading genes identification. The use of prokaryotic consortia has been often used as one of the bio-remediation approaches to degrade PAHs with different molecular weight. Since the process of PAH degradation predominantly includes non-culturable or yet-to-be cultured species, metagenomic approaches are highly recommended for studying the composition and metabolic abilities of microbial communities. In this study, whole metagenome shotgun sequencing of DNA from two soils with varying PAH levels was performed. In the control site, the total content of 12 priority PAHs was 262 µg kg−1. The background soil levels in the polluted site for PAHs with 3 or more rings exceeded this, at 800 µg kg−1. The abundance of genes and taxa associated with PAH degradation in these two sites were estimated. Despite differences in PAH concentrations up to 1200 µg kg−1, individual and operon-organized PAH degradation genes were almost equally abundant and diverse in pristine and highly contaminated areas. The most numerous taxa in both spots were actinobacteria from Terrabacteria group. In addition to well-known PAH degraders such as Gordonia and Rhodococcus, genes corresponding to the PAH degradation were found in Azoarcus, Burkholderia and Variovorax. The data shows non-specificity and multifunctionality of metabolic pathways encoded in the genes of PAH-degrading microorganisms.
1. Introduction
Polycyclic hydrocarbons (PAHs) are a class of the most toxic and persistent chemically stable compounds. Their danger to living organisms, including humans, increases with increased numbers of rings in their molecular structure [1,2,3]. PAHs are widespread in the soils of industrial enterprises, due to emissions, as well as in agricultural soils in connection with irrigation with sewage [4].
For minimizing the availability or eliminating the PAHs from terrestrial ecosystems, various chemical, physical, and biological approaches have been adopted [5,6]. The availability of PAHs for microbial destruction depends on the size of the soil fraction and the types of PAH [7], as well as the composition of the soil microbial community and the presence of metabolic pathways genes and operons of PAH catabolism [8].
The biochemical pathways of aerobic degradation of PAHs by bacteria have been described in sufficient detail. In short, the initial stage is the PAHs’ oxidation to dihydrodiol. The dihydrodiols can then be degraded via the ortho- or meta-pathway, yielding metabolites such as protocatechoates and catechols, that further undergo transformation to the tricarboxylic acid cycle’ metabolites [9]. Therefore, PAH-degrading bacteria may utilize these molecules as their single carbon source, as well as the energy source. Representatives of such genera as Rhodococcus, Pseudomonas, Sphingomonas, and Arthrobacter are the most prevalent natural PAH destructors.
Use of PAHs as sole energy and carbon source has been repeatedly noticed in Gram-positive bacteria. Mycobacterium strains can grow on pyrene and anthracene, while Rhodococcus strains can only grow on anthracene [10]. Although many Rhodococcus strains may utilize PAHs, particularly naphthalene, the complete biodegradation process has yet to be identified. Some Rhodococcus species use gentisate to transport naphthalene, whereas others use catechol [11,12]. In Rhodococcus, naphthalene and salicylate activate the genes involved in naphthalene metabolism [13].
The breakdown of PAHs is controlled by dioxygenase-encoding genes. The narAa and narAb genes, for example, encode the two subunits of naphthalene-1,2-dioxygenase (NDO), which converts naphthalene to (+)-cis-(1R, 2S)-dihydroxy-1,2-dihydronaphthalene [13,14]. The nar genetic systems were also found in other actinobacteria, in particular, in representatives of the genus Gordonia [15].
In Gram-negative bacteria, particularly Proteobacteria, the organization of naphthalene catabolism genes is well studied. These genes are mainly located on plasmids and are grouped into 2 operons. The upper operon includes the nahA-nahF genes, while the lower one contains the nahG-nahM genes. These operons control the transformation of salicylate from, and salicylate metabolism through, catechol with ring destruction and the incorporation of metabolites into the tricarboxylic acid cycle.
The study of the genetic organization of the PAH destruction metabolic pathways by individual strains makes it possible to further identify these pathways in the general pool of the soil microbial community genes.
Many researchers in their studies on the metabolization of PAHs in soils focused on the degradative abilities of individual isolated strains [7,16]. Nevertheless, isolated bacteria can quickly utilize PAHs in model environments, but lose their properties in soil. Isolates are not fully adapted to soil conditions, namely, to the content of carbon, nitrogen, and phosphorus, the types and bioavailability of PAHs, and interactions with native soil bacteria [17,18]. Currently, a lot of attention is paid to bacterial communities, since, as a rule, representatives of different taxa are involved in multi-stage degradation of the same pollutants [19]. Soil microbial communities are complex systems, the representatives of which are in inextricable interaction with each other [20]. The mechanisms of these interactions cannot be investigated by isolating individual members of the community. To understand the metabolic processes occurring in soils and grounds, the microbiome of such ecosystems should be considered as a single complex of organisms.
Lack of knowledge about the metabolic and adaptive potential of microbial soil communities is a serious fundamental problem of modern systemic ecology. This work aimed to study the PAH destruction genes (abundance and diversity) in pristine and technogenically transformed soils of Southern Russia using shotgun metagenomics methods. Shotgun metagenomics is an untargeted sequencing of all microbial genomes present in a sample [21,22]. This makes it possible to study the genetic and functional potential of microbial communities in a sample. Here, a comparison was done of the taxonomic diversity of microbial communities in contaminated and uncontaminated (control) plots, as well as a comparison of the abundance of genes (abundancy) and operons of PAH catabolism in soil samples. During the study, metagenome-assembled genomes were reconstructed and evaluated in terms of bioremediation of polyaromatics-contaminated soils.
2. Materials and Methods
2.1. Study Sites
To achieve the objective of the study, two monitoring sites were established. The sites were located in the steppe landscapes of the Rostov Region (Figure 1). The selection of sampling sites was based on historical differences in the use of territories. The soil cover of the studied steppe landscapes is represented by Haplic Chernozem soil. Monitoring site A is located at a distance from industrial enterprises. It is located within the specially protected natural area (SPNA) “Persianovskaya Reserve Steppe” and serves as a background area. A distinctive feature of this soil is the complete absence of haymaking and cattle grazing. The soil of monitoring site B is under the continuous influence of airborne industrial emissions from an enterprise of the first hazard class—Novocherkassk Power Station (NPS). This enterprise is the largest supplier of electricity in Russia among coal-fired power plants. Areas nearby the station are constantly exposed to ash coal, soot emissions, sulfur dioxide and other pollutants.
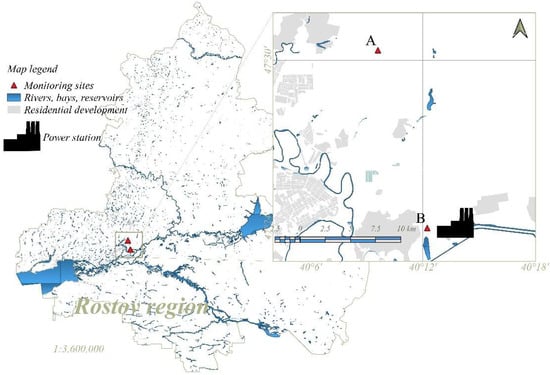
Figure 1.
Overview of map showing location of the two sites for sampling.
The chemical and physical properties of soils are summarized in Table 1. These soils are characterized by a heavy particle size distribution with a neutral reaction of the environment, and a high content of organic matter.

Table 1.
Main soil properties at the study sites.
2.2. Methods
2.2.1. Sample Collection and Processing
Sampling was carried out from each monitoring site using the envelope method as prescribed in ISO 10381-1 [23]. The sampling depth was 0–20 cm, which corresponds to the arable soil horizon. In each site, five (5) probes of soil not as far as 1 m from each other were taken for the mixed sample. The samples were delivered to the laboratory in sealed bags with constant refrigeration. The soil was cleaned by removing plant residues and other natural or anthropogenic inclusions.
2.2.2. Soil Analysis
PAHs Determination in the Soil Samples
PAHs were extracted from the soils by the standardized method to remove the co-extracted compounds from the soil extracts using saponification [24,25,26]. Before extracting PAHs, the moisture level in the soil was measured during sampling. A rotary evaporator was used to evaporate 1 g of air-dry soil. The flask was then filled with 20 mL of a 2% KOH solution prepared in ethanol. The extract was then refluxed for three hours in a water bath. Refluxing would saponify soil lipids, increasing PAH recovery and lowering the quantity of coextracted chemicals. The supernatant was separated in the Erlenmeyer flask with the addition of n-hexane (15 mL) and distilled water (5 mL) for improved layer separation. The liquid was shaken for 10 min before being transferred to the dividing funnel. The extracted n-hexane was rinsed with distilled water until the pH was neutral. In the dark, the solution was placed in a glass jar with a lid. The analyzing solution was then dewatered using five grams of anhydrous Na2SO4. After eight hours, the extract was transferred to another flask and evaporated until dry using a rotary evaporator bath at 40 ℃ For further analysis, the residue was redissolved in 1 mL acetonitrile. The PAH content was determined by the standardized external method [27]. The following equation was used to deduce the PAH content:
where Cs and Cst are the PAH concentrations determined in the soil sample and the standard PAH solution, respectively (μg kg−1). Sst and Si correspond to areas under the peak of PAH standard solution and studied sample, respectively. V, k, and m are assigned to the acetonitrile volume used for extraction (mL), the recovery factor for PAH from the sample, and the mass sample (g), respectively. The efficiency of target PAHs extraction from soils was calculated using a spike matrix.
Cs = k Si × Cst × V/(Sst × m),
Samples were analyzed for 16 PAHs (naphthalene; chrysene; phenanthrene; benzo[k]fluoranthene; anthracene; acenaphthylene; pyrene; fluoranthene; biphenyl; benzo[a]anthracene; benzo[b]fluoranthene; acenaphthene; benzo[a]pyrene; dibenzo[a,h]anthracene; fluorine; benzo[g,h,i]perylene) with an Agilent 1260 (Germany) Infinity high-performance chromatography (HPLC) equipped with a fluorescence detector following the ISO 13859:2014 requirements [28]. The HPLC system was coupled to reversed-phase column Hypersil BDS C18 (150 × 4.6 mm, 5 μm) with a mixture of acetonitrile and ultrapure water as the mobile phase. The retention time reported by the appropriate analytical reference samples was used to identify compounds. The analysis included HPLC grade acetonitrile (99.9%, analytical grade), anhydrous Na2SO4, n-hexane (99.9%, analytical grade), ethanol (96.9%, analytical grade), potassium hydrate (98.9%, analytical grade), and NaOH (97.9%, analytical grade). Standards for the abovementioned PAHs in acetonitrile with 200 µg/cm3 concentration (priority pollutant PAHs (in acetonitrile) NIST® SRM® 1647f) were used to prepare standard solutions for HPLC analyses. For every target PAH, an individual standard was used for determination. For the standards, see [17].
According to the Agilent Application Solution (ISO 13877-2005), quality control of every HPLC detection was performed. Individual standard solutions were purchased from Sigma-Aldrich (Merch). The certified reference materials and calibration curves were obtained to calculate the lower detection limits (LODs) and lower quantification limits (LOQs). For the used method, a random component of the measurement error was estimated. The error in the concentration range of 2–200 μg kg−1 ranged between 3.5 and 14%.
DNA Extraction and Sequencing
Total soil DNA was isolated using the FastDNA™ Spin Kit for soil. To prepare the libraries, the NEBNext Ultra II DNA library prep kit (NEB) was utilized according to manufacturer’s instructions. Ultrasonic fragmentation of genomic DNA was performed on a Covaris S220 device. The quality of the obtained libraries was checked on a 2100 Bioanalyzer (Agilent) using the DNA High Sensitivity kit (Agilent). The DNA concentration in the preparations was assessed using a Qubit 2.0 instrument. Sequencing was performed on Illumina NextSeq 500 equipment installed at the Interdisciplinary Center of Shared Use of Kazan Federal University (Kazan, Russia; www.kpfu.ru (accessed on 15 June 2021)).
Metagenome Quality Control, Assembly, and Annotation
Reads corresponding to human DNA were removed using the BMTagger program [29]. Duplicate reads were removed using clumpify from the bbmap package [30]. The FastQC program was used to control the quality of the readings [31]. Adapter sequences, low quality (Q < 10) and short (<40 bp) reads were removed with Trimmomatic ver. 0.38 [32]. The results of the preprocessing are presented in Table 2.

Table 2.
The number of raw readings in samples before and after preprocessing (Quality Control).
Spades ver. was used to assemble metagenomes, version 3.15.2 (k 55) [33]. The filtered read set was mapped to the final assembly and coverage information was generated using bbmap (Version 38.25) using default parameters, except for ambiguous = random.
Assembled metagenomes containing contigs with the length > 500 bp were processed through MG-RAST. Coding DNA sequences (CDSs) were predicted using prodigal version 2.6.3 [34]. Functional annotation was performed with diamond version v2.0.13 [35] and the KEGG database (October 2018 release) [36], and taxonomic assignment was performed using kaiju version 1.7.3 [37]. Data visualization was performed using Krona [38].
Extraction of PAH Catabolic Genes from Shotgun Data
As a result of the annotation, the genera of microorganisms commonly represented in the communities were identified (at least 300 contigs in the assembly of at least one sample corresponded to such genera). A list of 179 genera is presented in Supplementary Table S1.
The Genbank service was used to search for known genes involved in PAH catabolism in the genomes of microorganisms listed in Supplementary Table S1. A database of genes involved in PAH catabolism was compiled, which was later used as a reference to identify reads corresponding to catabolism genes in samples’ PAHs, and determination of their taxonomic affiliation. The database includes genes and operons of PAH catabolism in microorganisms of 54 genera, with a total length of ~175 kb.
To identify reads corresponding to PAH catabolic genes, filtered reads of each sample were mapped to a reference database in sensitive-local mode. Organisms in which such genes were found, as well as the results of mapping, are presented in Supplementary Table S2. Genes whose readings are contained in the samples were identified and their taxonomic affiliation was determined.
Statistical Analysis
Statistical processing was done using the numerical program, STATISTICA 7. The reliability of the difference between the average values of the PAH concentration in the soils of different monitoring sites was assessed with the Student’s test at p-level < 0.05.
3. Results
In the background soil of the monitoring site of protected area A, the total content of 12 priority PAHs was 262 µg kg−1 (see Figure 2), which does not exceed the content of these pollutants in the soils of background regions of different regions of the world [39,40,41,42]. The content of benzo(a)pyrene (BaP) is lower than the 0.02 mg kg−1, maximum permissible concentration (MPC) (Figure 3) [43]. The composition of PAHs is dominated by low-molecular-weight 2- and 3-ring compounds.
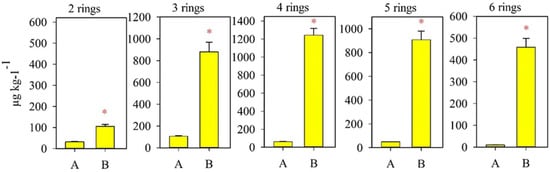
Figure 2.
The content of PAHs with different amounts of benzene rings in the soils of the monitoring sites. Asterisks denote the significance of difference between PAH content in studied sites.
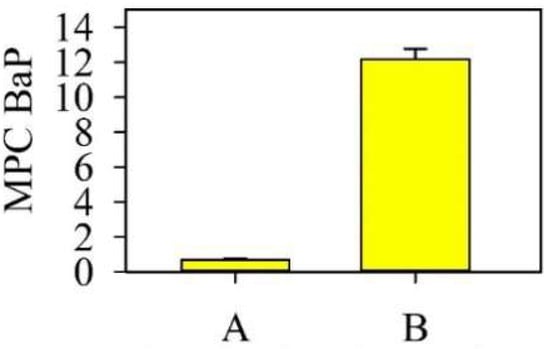
Figure 3.
Levels of benzo(a)pyrene (BaP) in the soils of the monitoring sites.
In the soil of the monitoring site B, located in the zone of influence of the power plant, 4 annular PAHs prevail, which is typical when exposed to the enterprises of the fuel and energy complex [44].
For the soils of both monitoring sites, phenanthrene predominates in the composition of 3 ring-shaped PAH compounds. For the soil of the background plot No. 1, its share of the total content reaches 31%. The composition of 4 rings PAHs is represented mainly by fluoranthene and pyrene. Benzo[b]fluoranthene dominates in the composition of 5 ring PAHs (Figure 4). Thus, the prevailing composition of pollutants, such as PAHs, differs depending on the historical conditions of use of the studied territories, as well as on the genesis of soils.
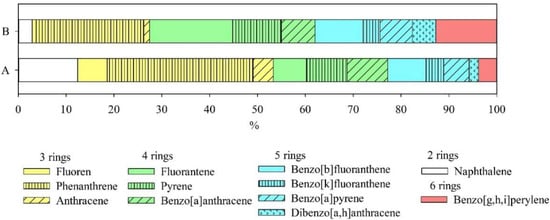
Figure 4.
The share of individual PAH compounds from their total content in soils of the monitoring sites A and B.
3.1. Taxonomic and Functional Data Classification
The relative numbers of bacteria at the class level show that in both samples (uncontaminated and contaminated), the Terrabacteria group dominates (50 and 47% of all bacteria, respectively). The phyla Actinobacteria (84 and 82%, respectively) as well as Chloroflexi (5%) and Firmicutes (7 and 8%) are the most well-represented in the Terrabacteria group. Actinobacteria are well-known soil microflora members, that are known for their metabolic potential to survive in the presence of a wide range of contaminants. In an uncontaminated sample, the Actinomycetia class accounts for 73% of all actinobacteria and 31% of all bacteria, while in a contaminated sample, it accounts for 74% of all actinobacteria and 28% of all bacteria. It is represented by the orders Micrococcales, Corynebacteriales, Propionibacteriales, Streptomycetales, Pseudonocardiaceae, Micromonosporaceae (Figure 5a,b). Firmicutes are represented by the classes Bacilli and Clostridia. The Bacilli class accounts for 50 and 51% of all Firmicutes in uncontaminated and contaminated samples, respectively, while Clostridia accounts for 40 and 39%, respectively.
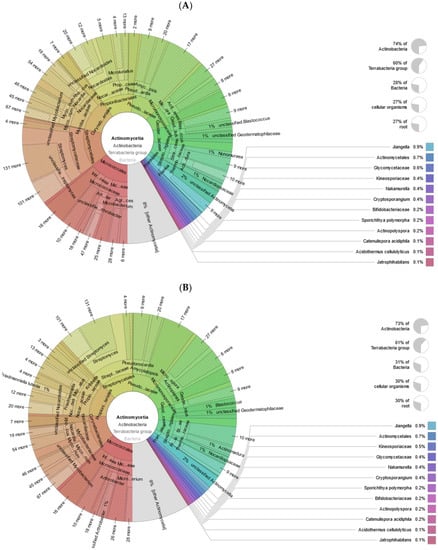
Figure 5.
Krona plots of Actinomycetia detected in metagenomic datasets. (A) non-polluted, (B) polluted soil. Note: Taxonomic nodes are nested sectors arranged from the highest level of the hierarchy at the center and progressing outward. Krona plots simultaneously display relative abundance and hierarchy using a radial space-filling display. Taxonomic groups are separated by color.
3.2. Reconstruction of Metagenome-Assembled Genomes
The raw filtered readings were collected in contigs (Table 3).

Table 3.
Characteristics of assemblies.
Actinobacteria have the highest abundance in the samples. Their share in communities A and B is 50.32% and 48.22%, respectively. The share of proteobacteria is 22.41% and 23.37%, respectively, in A and B (Figure 6).
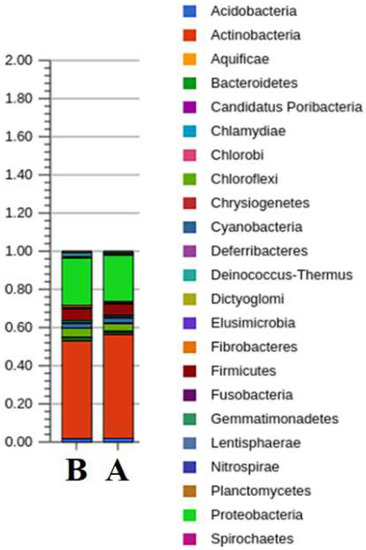
Figure 6.
Stacked bar charts representing relative phylum-level abundances for the most abundant phyla based on the taxonomic affiliation of the annotated proteins within each metagenome.
With the data obtained, we assume that the microbiome of Haplic Chernozem soils in its existence for a long time reaches a certain homeostasis. The soil rich in macro- and microelements makes it possible to maintain a taxonomically diverse community, which has a high potential for adaptive reactions. Due to these properties of the soil, the ingress of complex pollutants into it (in our case, PAHs) does not suppress the microbiome, but allows it to adapt. Based on the calculated values of the alpha-diversity parameter when comparing Shannon’s diversity test (Table 4), we assume that in terms of the abundance of different species and their diversity, the communities of the unpolluted and polluted areas are similar.

Table 4.
Alpha diversity of samples.
Even though archaea are an important part of soil microbiomes, their metabolic potential in comparison with bacteria is extremely poorly understood. However, we can already see that in a contaminated sample containing a mixture of heavy PAHs, the archaeal part of the community is not inhibited. Archaea are dominated by 3 types—Euryarchaeota, Thaumarchaeota and Crenarcheota (Figure 7). The ability to catabolize PAHs in archaea has been repeatedly noted but practically nothing is known about PAH catabolism genes in them [45,46].
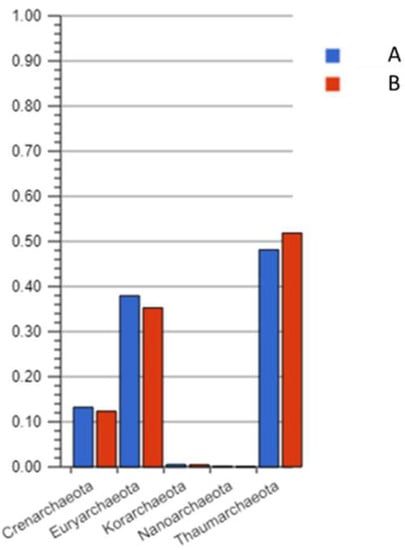
Figure 7.
Archaeal profile of the samples in A (non-polluted), B (polluted) soil.
In the taxonomic classification of reads, we found that among Euryarcheota, the most represented by reads are the Halobacteria and Methanomicrobia (Stenosarchaea group) classes. Among Thaumarchaeota and Crenarcheota, the Nitrososphaeraceae family and the Thermoprotei class (Figure 8A,B), respectively, are most represented by reads.
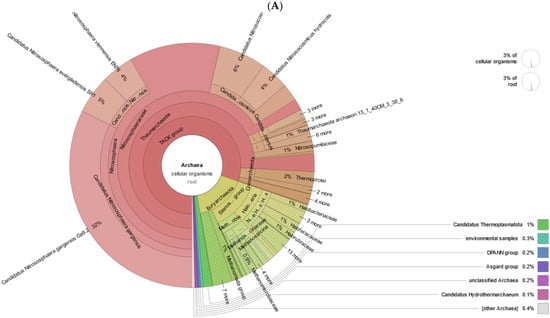
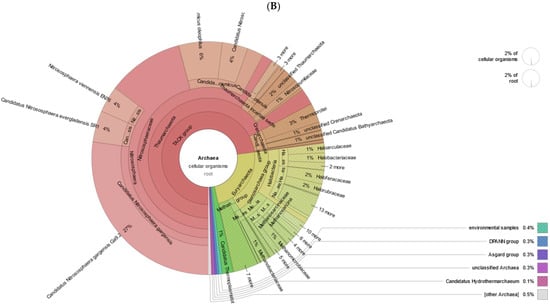
Figure 8.
Krona plots of Archaea detected in metagenomic datasets. (A) non-polluted, (B) polluted soil.
3.3. Identification of Aromatic Hydrocarbon-Degrading Coding DNA Sequences
With the help of the KEGG service, a functional annotation of the collected metagenomes has been summarized (Table 5).

Table 5.
Functional annotation of the collected metagenomes.
In sample B, 316 coding sequences (CDS) were found, and in sample A, 291 CDS were found, that are involved in the catabolism of xenobiotics, including PAHs (Figure 9).
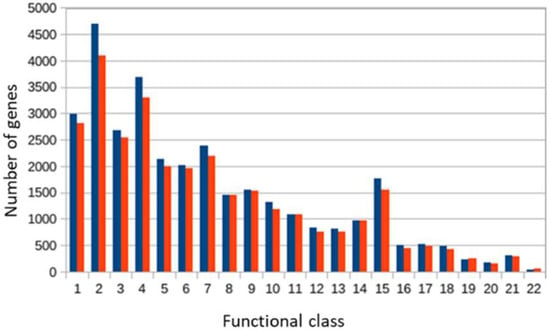
Figure 9.
KEGG function classification genes found in metagenomes of samples B (blue color) and A (red color). 1—Protein families: genetic information process, 2—Carbohydrate metabolism, 3—Protein families: signaling and cellular processes, 4—Genetic Information Processing, 5—Environmental Information Processing, 6—Unclassified: metabolism, 7—Amino acid metabolism, 8—Unclassified, 9—Metabolism of cofactors and vitamins, 10—Nucleotide metabolism, 11—Unclassified: genetic information processing, 12—Cellular processes, 13—Lipid metabolism, 14—Protein families: metabolism, 15—Energy metabolism, 16—Unclassified: signaling and cellular processes, 17—Glycan biosynthesis and metabolism, 18—Metabolism of other amino acids, 19—Metabolism of terpenoids and polyketides, 20—Organismal systems, 21—Biodegradation of xenobiotics, 22—Secondary metabolites’ biosynthesis.
The breakdown of aromatic hydrocarbons occurs through a series of reactions including oxidation, hydroxylation, dehydrogenation, and ring breakdown. To study the representation of PAH catabolism genes in the metagenome of soil communities, we selected 179 bacterial genera that can be classified as common in communities, and using the Genbank NCBI database, we compiled a set of sequences of PAH catabolism genes and operons in representatives of these genera. Since many bacteria do not have information on such genes in the Genbank NCBI, the final version of our database includes information on the genes and operons of PAH catabolism in 55 bacterial genera (Supplementary Table S2).
Complete operons of PAH catabolism are known in such genera as Burkholderia, Pseudomonas, Rhodococcus, Marinobacter, Azoarcus, Gordonia. There is also information on gene clusters of PAH catabolism in Variovorax, Bradyrhizobium, Albidiferax, Sphingobium, Ralstonia. In our samples of Haplic Chernozem (both in unpolluted and contaminated PAHs), readings of the PAH catabolism operons were found in Azoarcus, Bradyrhizobium, Burkholderia, Gordonia, Rhodococcus, Variovorax.
The genetic system of naphthalene catabolism in actinobacteria is quite universal. It is represented by a gene cluster that includes genes encoding rubredoxin (Rub1), regulator proteins NarR1 and NarR2, Naphthalene dioxygenase subunits NarAa and NarAb, Naphthalene dihydrodiol dehydrogenase NarB and Hydratase—aldolase NarC [15].
In the results of sequencing of soil metagenomes of both contaminated and uncontaminated samples, we found readings corresponding to the genes of the catabolism operons of naphthalene for Gordonia (128 and 192 reads, respectively) and Rhodococcus (195 and 264 reads, respectively).
The ability of representatives of Azoarcus to utilize PAHs is periodically mentioned in the literature [47,48]. However, not much is known about the pathway of PAH metabolism in this genus. We analyzed the genomes of known Azoarcus, placed in the Genbank database, and identified 11 genes related to the destruction of naphthalene. Among them are naphthalene dioxygenase in large (nagAa) and small (nagAb) subunits, salicylate-5-hydroxylase (nagG and nagH subunits), nagI dioxygenase gentisate, as well as two regulators-nahR1 and nahR2 and the nahY protein, which is responsible for chemotaxis towards the carbon source. The genes are organized into 2 operons, similar to the structure characteristic of pseudomonads, and the chemotaxis protein is separate.
For Variovorax, we found readings corresponding to two naphthalene dioxygenase subunits and two salicylate hydroxylase subunits. The total length of genes for naphthalene catabolism in Variovorax is about 7000 bp. (Supplementary Table S2). However, it should be noted that these genes in Variovorax are currently poorly understood.
Normalized gene counts of the main PAH catabolic genes were identified in the samples (Table 6). It is worth noting that the number of genes is comparable in a contaminated and uncontaminated sample. Therefore, it can be assumed that both the number of these genes and the number of organisms carrying them do not depend on the presence of the pollutant in the soil.

Table 6.
Normalized gene counts of major PAH catabolic genes in samples.
4. Discussion
Basically, the aerobic catabolism of PAHs by bacteria is studied using the example of a model two-ring compound, naphthalene [49]. However, the process of destruction of PAHs with 3 or more rings has also repeatedly attracted the attention of many scientific groups [50,51]. When analyzing soils subjected to technogenic stress (a sample from the NPS), we found that there are only 3 times more 2-ring PAHs in this sample than in the control one. As for PAHs with 3–6 rings, their content in the contaminated sample was at least 10 times (Figure 3) higher than the amount in the uncontaminated sample. Such compounds, denoted by the general term HMW-PAH (high molecular weight), are extremely resistant to bacterial degradation [52,53]. In this regard, we focused on studying the genetic potential of the microbial community, which makes it possible to utilize these compounds.
The ability to biodegrade three-ring PAHs has been found in many microorganisms [54,55]. We identified genes of the phenanthrene operon Novosphingobium, as well as genes for catabolism of fluorene and protocatechuic acid, in the assemblies of complete metagenomes. In Sphingomonas, genes for catabolism of fluorene and protocatechuic acid are included in the fld operon. According to Pinyakong et al., Sphingomonas can convert fluorene to fluorenone and then through phthalate and protocatechuic acid. The fld cluster of genes has been described in detail [56]. The readings corresponding to the representatives of Sphingomonodaceae in our data (uncontaminated and contaminated samples, respectively) constitute 1 and 0.7% of reads of all bacteria, 4 and 6% of all proteobacteria.
It is also interesting that the pathway of fluorene catabolism in rhodococci is assumed to be different [57]. According to the results of Wang et al., the destruction of fluorene begins with hydroxylation and cleavage of one of the outer rings, similar to the destruction of dibenzothiophene along the Kodama pathway [58]. However, unlike the Kodama pathway, the putative pathway for the cleavage of fluorene by rhodococci is not a dead end but leads to the formation of salicylate. We did not find information in the literature or in the Genbank database on specific genes that control this pathway. Hence, at the moment, we assume that the degradation of fluorene by rhodococci is controlled by the nar gene system, the main reaction of which is hydroxylation of naphthalene in one of the rings.
Among the genes for anthracene catabolism, we noted in our data the specific gene Anthrone monooxygenase. The transformation of anthracene through anthrone is typical for fungi, especially for Penicillium simplicissimum. According to Jove et al., P. simplicissimum oxidizes anthracene to anthrone, which is then transformed into anthraquinone and phthalate [59]. We found an anthrone monooxygenase belonging to Streptomyces. This gene is included in the anthracene catabolic operon, which is specific for streptomycetes.
Addition of rings and increase in molecular wight does not imply the need to use a different type of reaction for their destruction, in addition to those listed above. HMW-PAHs are cleaved by bacteria through sequential hydroxylation and further ring opening, as shown, for example, for bacilli [60]. These reactions are carried out by the same enzyme systems as the catabolism of the lighter PAHs. We found an extensive pool of PAH catabolism genes in the microbial communities of Haplic Chernozem. Genes have different taxonomic affiliations. We got similar results for contaminated and uncontaminated samples. This allows us to assume the following:
Biodegradation of polyaromatic hydrocarbons, as a rule, does not imply highly specific reactions requiring specialized enzymatic systems or unique taxa. As described in detail above, PAHs undergo sequential multistage conversion by oxygenation, hydroxylation, modification of functional groups and cycles to facilitate steric access to them by enzymes, as well as ring opening. Moreover, in the overwhelming majority of cases, enzymes of different microorganisms are simultaneously involved in this process, and the microorganisms themselves are taxonomically quite distant from each other (for example, streptomycetes and pseudomonads). This is especially typical for HMW-PAH due to physicochemical reasons: poor-water solubility and high recalcitrance due to sorption on soil matter lead to very low rates of mobilization and transport of these molecules or their derivatives into the cells of microorganisms, rates that are not compatible with the metabolic needs of individual cells [61]. For this reason, we must talk about the degradation of complex PAHs by the enzymatic system of a consortium of microorganisms, and not by its individual representatives [19].
This state of enzymatic systems for the biodegradation of PAHs as a not-one-genome phenomenon, but dependent on the existence at the level of the metagenome of the entire microbial community, inevitably leads to low specificity, but at the same time multifunctionality of enzymes that catabolize the transformation of PAHs. Thus, many oxygenases have a very wide range of substrates and low specificity [62,63]. For example, oxygenases of the P450 family isolated from fungi have been shown to oxidize both PAHs and lignin, while naphthalene dioxeganases have been shown to be capable of enzymatic conversion of humic substances [64]. Several laccases have been shown to oxidize PAHs, which is true for both fungi and bacteria [65]. All this indicates the ability to transform and metabolize a wide range of complex organic substrates, especially those with a similar structure, which is especially important in the context of the Haplic Chernozem soils in this study.
Haplic Chernozems are characterized by a high content of total and dissolved organic matter, which includes supramolecular organic complexes of varying complexity—humin, humic and fulvic acids, as well as their polymer precursors at different stages of transformation—degradation and condensation products of cellulose, chitin, lignin and proteins. It seems highly probable that at least some of the enzymes involved in PAH catabolism can be used to convert other (poly) aromatic compounds in the soil organic matter of Haplic Chernozem.
This is not the only reason why the found soils exhibit a similar abundance and diversity of PAH destruction genes. Although PAHs present in ecosystems are mainly of petrogenic and pyrogenic origin, polyaromatic hydrocarbons entering soils can also be of non-anthropogenic, namely of biogenic and/or diagenetic nature [66,67]. Non-biogenic diagenetic processes that introduce PAHs into soils are fires, volcanic activity, kerogen diagenesis, and erosion of ancient sedimentary rocks [68]. As for biogenic PAHs, perylene and retene are typical examples [69,70]; naphthalene, phenanthrene, and chrysene derivatives are less frequently mentioned [70,71,72]. Such PAHs are most often formed extracellularly by diagenesis of various biogenic molecules, such as plant waxes, terpenoids, and hopanoids from prokaryotic membranes [67,70,73]. The object without anthropogenic load in this study was represented by the soils of the protected area, where agricultural activities have never been carried out. Since the sorption of PAHs by soil matter increases with the time of the presence of PAHs in the soil, we can assume the accumulation of biogenic polycyclic hydrocarbons in these soils, and hence the selection pressure in favor of the preservation of the potential for PAH destruction by the microbial community.
Finally, a significant part of the soil community capable of PAH degradation does not have to be metabolically active and be constantly presented to PAHs. High concentrations of PAHs in soils are still oligotrophic concentrations, and in addition to PAHs, microorganisms capable of utilizing them have to be able to use other carbon sources. This also implies the previously discussed low specificity and multifunctionality of enzymatic systems for PAH degradation. At the same time, some, mostly oligotrophic, microorganisms may exist most of the time in a dormant state as dormant cells or viable, but non-culturable cells, or in a quasi-stationary state, when the population maintains a relatively constant number of cells [61]. Thus, the processes and causes listed above, most likely, provide a similar representation and diversity of PAH destruction genes in Haplic Chernozem with radically different levels of anthropogenic load.
5. Conclusions
Currently, shotgun metagenomics of soil communities is amongst the focusses of research in science. The main difficulty in analyzing data on the complete genomes of soil microbial communities is the absence of references for assembly, such as with the metagenomes of the bacterial microflora of humans and animals. We used sequencing data for the complete genomes of microbial communities in Haplic Chernozem to obtain information on the taxonomic diversity of soil bacteria, as well as genes involved in PAH catabolism. A wide variety of such genes has been found, both as individual genes and as operons. Readings of the PAH catabolism operons were found in taxa known for the ability to degrade PAHs, such as Gordonia and Rhodococcus, but also in taxa where this connection is not clear: Azoarcus, Bradyrhizobium, Burkholderia, Variovorax. The revealed abundance of PAH-degraders or their genes in both sites also indicated the unspecificity and multifunctionality of metabolic pathways encoded in the genes of PAH-degrading microorganisms. In our search, we were limited to the information available in the databases on the genes of naphthalene catabolism in bacteria of different genera. Most likely, information on the genes of bacteria that are not widespread soil degraders of PAHs might not have been included in the material presented in this work, since information on these genes is not available in the databases. Nevertheless, the data obtained on the taxonomic and genetic diversity of soil microbial communities make it possible to evaluate the potential of PAH degrading bacteria in Haplic Chernozem.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr10122555/s1, Table S1: Genera, that were represented by 300 or more contigs at least in one sample; Table S2: Dominating taxa for which Genbank search revealed presence of PAH catabolism genes. Column «cluster length» provides information on length of genes or operons associated with PAH catabolism.
Author Contributions
Conceptualization, Y.D.; methodology, S.S., A.G. and K.D.; software, Y.K.; formal analysis, T.D. and I.Z.; investigation, A.B.; data curation, T.M.; writing—original draft preparation, Y.D. and A.F.; writing—review and editing, T.M., S.S., A.M., V.D.R. and T.G.; visualization, K.D. and T.D.; supervision, S.S. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
The study was carried out in the laboratory «Soil Health» of the Southern Federal University with the financial support of the Ministry of Science and Higher Education of the Russian Federation, agreement no. 075-15-2022-1122. The research was supported by the Strategic Academic Leadership Program of the Southern Federal University (“Priority 2030”).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available as the data also form part of an ongoing study.
Acknowledgments
We thank the centers for collective use of Southern Federal University ‘‘Modern Microscopy’’ and ‘‘High Technology’’ for performing chemical-analytical experiments and the center for collective use of Kazan Federal University ‘‘Interdisciplinary Center of Shared Facilities’’ for performing metagenomics analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grimmer, G. PAH—Their contribution to the carcinogenicity of various emissions. Toxicol. Environ. Chem. 1985, 10, 171–181. [Google Scholar] [CrossRef]
- Hodson, P.V. The toxicity to fish embryos of PAH in crude and refined oils. Arch. Environ. Contam. Toxicol. 2017, 73, 12–18. [Google Scholar] [CrossRef]
- Chang, Y.; Siddens, L.K.; Heine, L.K.; Sampson, D.A.; Yu, Z.; Fischer, K.A.; Löhr, C.V.; Tilton, S.C. Comparative mechanisms of PAH toxicity by benzo[a]pyrene and dibenzo [def, p] chrysene in primary human bronchial epithelial cells cultured at air-liquid interface. Toxicol. Appl. Pharmacol. 2019, 379, 114644. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Luqueño, F.; Valenzuela-Encinas, C.; Marsch, R.; Martínez-Suárez, C.; Vázquez-Núñez, E.; Dendooven, L. Microbial communities to mitigate contamination of PAHs in soil—Possibilities and challenges: A review. Environ. Sci. Pollut. Res. 2011, 18, 12–30. [Google Scholar] [CrossRef]
- Sushkova, S.; Minkina, T.; Dudnikova, T.; Barbashev, A.; Mazarji, M.; Chernikova, N.; Lobzenko, I.; Deryabkina, I.; Kizilkaya, R. Influence of carbon-containing and mineral sorbents on the toxicity of soil contaminated with benzo[a]pyrene during phytotesting. Environ. Geochem. Health 2021, 44, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Minkina, T.; Vasilyeva, G.; Popileshko, Y.; Bauer, T.; Sushkova, S.; Fedorenko, A.; Antonenko, E.; Pinskii, D.; Mazarji, M.; Ferreira, C.S.S. Sorption of benzo [a] pyrene by Chernozem and carbonaceous sorbents: Comparison of kinetics and interaction mechanisms. Environ. Geochem. Health 2021, 44, 133–148. [Google Scholar] [CrossRef] [PubMed]
- DeBruyn, J.M.; Mead, T.J.; Sayler, G.S. Horizontal transfer of PAH catabolism genes in Mycobacterium: Evidence from comparative genomics and isolated pyrene-degrading bacteria. Environ. Sci. Technol. 2012, 46, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Minkina, T.; Fedorenko, A.; Hassan, T.; Fedorenko, G.; Chaplygin, V.; Chernikova, N.; Nevidomskaya, D.; Sushkova, S. Morphological Studies of Tyhpa Australis under Stress Environmental Factor; IOP Publishing: Bristol, UK, 2021; p. 012210. [Google Scholar]
- Haritash, A.K. A comprehensive review of metabolic and genomic aspects of PAH-degradation. Arch. Microbiol. 2020, 202, 2033–2058. [Google Scholar]
- Tongpim, S.; Pickard, M.A. Growth of Rhodococcus S1 on anthracene. Can. J. Microbiol. 1996, 42, 289–294. [Google Scholar] [CrossRef]
- Liu, T.-T.; Xu, Y.; Liu, H.; Luo, S.; Yin, Y.-J.; Liu, S.-J.; Zhou, N.-Y. Functional characterization of a gene cluster involved in gentisate catabolism in Rhodococcus sp. strain NCIMB 12038. Appl. Microbiol. Biotechnol. 2011, 90, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Kulakova, A.N.; Reid, K.A.; Larkin, M.J.; Allen, C.C.; Kulakov, L.A. Isolation of Rhodococcus rhodochrous NCIMB13064 derivatives with new biodegradative abilities. FEMS Microbiol. Lett. 1996, 145, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Kulakov, L.A.; Chen, S.; Allen, C.C.; Larkin, M.J. Web-type evolution of Rhodococcus gene clusters associated with utilization of naphthalene. Appl. Environ. Microbiol. 2005, 71, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Kulakov, L.A.; Allen, C.C.; Lipscomb, D.A.; Larkin, M.J. Cloning and characterization of a novel cis-naphthalene dihydrodiol dehydrogenase gene (narB) from Rhodococcus sp. NCIMB12038. FEMS Microbiol. Lett. 2000, 182, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Shen, F.-T.; Tan, C.-C.; Huang, C.-C.; Chen, B.-Y.; Arun, A.; Young, C.-C. Characterization of Gordonia sp. strain CC-NAPH129-6 capable of naphthalene degradation. Microbiol. Res. 2012, 167, 395–404. [Google Scholar] [CrossRef]
- Fedorenko, A.G.; Chernikova, N.; Minkina, T.; Sushkova, S.; Dudnikova, T.; Antonenko, E.; Fedorenko, G.; Bauer, T.; Mandzhieva, S.; Barbashev, A. Effects of benzo[a]pyrene toxicity on morphology and ultrastructure of Hordeum sativum. Environ. Geochem. Health 2021, 43, 1551–1562. [Google Scholar] [CrossRef]
- Gorovtsov, A.; Demin, K.; Sushkova, S.; Minkina, T.; Grigoryeva, T.; Dudnikova, T.; Barbashev, A.; Semenkov, I.; Romanova, V.; Laikov, A. The effect of combined pollution by PAHs and heavy metals on the topsoil microbial communities of Spolic Technosols of the lake Atamanskoe, Southern Russia. Environ. Geochem. Health 2021, 44, 1299–1315. [Google Scholar] [CrossRef]
- Minkina, T.; Konstantinova, E.; Bauer, T.; Mandzhieva, S.; Sushkova, S.; Chaplygin, V.; Burachevskaya, M.; Nazarenko, O.; Kizilkaya, R.; Gülser, C. Environmental and human health risk assessment of potentially toxic elements in soils around the largest coal-fired power station in Southern Russia. Environ. Geochem. Health 2021, 43, 2285–2300. [Google Scholar] [CrossRef]
- Mishra, S.; Lin, Z.; Pang, S.; Zhang, W.; Bhatt, P.; Chen, S. Recent advanced technologies for the characterization of xenobiotic-degrading microorganisms and microbial communities. Front. Bioeng. Biotechnol. 2021, 9, 31. [Google Scholar] [CrossRef]
- Sushkova, S.N.; Yakovleva, E.V.; Minkina, T.M.; Gabov, D.N.; Antonenko, E.M.; Dudnikova, T.S.; Rajput, V.D. Accumulation of benzo[a]pyrene in plants of different species and organogenic horizon of soils of steppe phytocenosis under technogenic pollution. [НАКОПЛЕНИЕ БЕНЗ[А]ПИРЕНА В РАСТЕНИЯХ РАЗНЫХ ВИДОВ И ОРГАНОГЕННОМ ГОРИЗОНТЕ ПОЧВ СТЕПНЫХ ФИТОЦЕНОЗОВ ПРИ ТЕХНОГЕННОМ ЗАГРЯЗНЕНИИ]. Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2021, 331, 200–214. [Google Scholar] [CrossRef]
- Quince, C.; Walker, A.W.; Simpson, J.T.; Loman, N.J.; Segata, N. Shotgun metagenomics, from sampling to analysis. Nat. Biotechnol. 2017, 35, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Gorovtsov, A.V.; Fedorenko, G.M.; Minkina, T.M.; Fedorenko, A.G.; Lysenko, V.S.; Sushkova, S.S.; Mandzhieva, S.S.; Elinson, M.A. The influence of application of biochar and metal-tolerant bacteria in polluted soil on morpho-physiological and anatomical parameters of spring barley. Environ. Geochem. Health 2021, 43, 1477–1489. [Google Scholar] [CrossRef] [PubMed]
- ISO 10381-1; Soil Quality—Sampling. Part 1: Guidance on the Design of Sampling Programmes. International Organization for Standardization: Geneva, Switzerland, 2002.
- Directive document 52.10.556-95. Methodical Instructions. In Definition of Polluting Substances in Sediments and Suspension; Roshydromet: Moscow, Russia, 2002. (In Russian) [Google Scholar]
- Sushkova, S.; Minkina, T.; Turina, I.; Mandzhieva, S.; Bauer, T.; Kizilkaya, R.; Zamulina, I. Monitoring of benzo[a]pyrene content in soils under the effect of long-term technogenic poluttion. J. Geochem. Explor. 2017, 174, 100–106. [Google Scholar] [CrossRef]
- Sushkova, S.; Minkina, T.; Tarigholizadeh, S.; Antonenko, E.; Konstantinova, E.; Gülser, C.; Dudnikova, T.; Barbashev, A.; Kizilkaya, R. PAHs accumulation in soil-plant system of Phragmites australis Cav. in soil under long-term chemical contamination. Eurasian J. Soil Sci. 2020, 9, 242–253. [Google Scholar] [CrossRef]
- Procedure of Measurements Benz(a)pyrene Content in Soils, Sediments, and Sludges by Highly Effective Liquid Chromatography Method; Certificate 27-08; Rosstandart: Moscow, Russia, 2008; p. 27. (In Russian)
- ISO 13859-2014; Soil Quality. Determination of Polycyclic Aromatic Hydrocarbons (PAH) by Gas Chromatography (GC) and High Performance Liquid Chromatography (HPLC). ISO: Geneva, Switzerland, 2014.
- Rotmistrovsky, K.; Agarwala, R. BMTagger: Best Match Tagger for Removing Human Reads from Metagenomics Datasets. 2011. Available online: ftp://ftp.ncbi.nlm.nih.gov/pub/agarwala/bmtagger/ (accessed on 8 June 2021).
- Sourceforge.net. Available online: https://sourceforge.net/projects/bbmap/ (accessed on 20 June 2021).
- Bioinformatics.babraham.ac.uk. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 14 May 2021).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.; Korobeynikov, A.; Lapidus, A.; Prjibelsky, A.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling genomes and mini-metagenomes from highly chimeric reads. In Annual International Conference on Research in Computational Molecular Biology; Springer: Berlin, Heidelberg, 2013; pp. 158–170. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.L.; LoCascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 1–11. [Google Scholar] [CrossRef]
- Buchfink, B.; Huson, D.H.; Xie, C. MetaScope-Fast and accurate identification of microbes in metagenomic sequencing data. arXiv 2015, arXiv:1511.08753. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Menzel, P.; Ng, K.L.; Krogh, A. Fast and sensitive taxonomic classification for metagenomics with Kaiju. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 1–10. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y. Polycyclic aromatic hydrocarbons contamination in surface soil of China: A review. Sci. Total Environ. 2017, 605, 1011–1020. [Google Scholar] [CrossRef]
- Sosa, D.; Hilber, I.; Faure, R.; Bartolomé, N.; Fonseca, O.; Keller, A.; Schwab, P.; Escobar, A.; Bucheli, T.D. Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in soils of Mayabeque, Cuba. Environ. Sci. Pollut. Res. 2017, 24, 12860–12870. [Google Scholar] [CrossRef] [PubMed]
- Gabov, D.; Yakovleva, E.; Vasilevich, R. Vertical distribution of PAHs during the evolution of permafrost peatlands of the European arctic zone. Appl. Geochem. 2020, 123, 104790. [Google Scholar] [CrossRef]
- Pikovskii, Y.I.; Smirnova, M.; Gennadiev, A.; Zavgorodnyaya, Y.A.; Zhidkin, A.; Kovach, R.; Koshovskii, T. Parameters of the native hydrocarbon status of soils in different bioclimatic zones. Eurasian Soil Sci. 2019, 52, 1333–1346. [Google Scholar] [CrossRef]
- GN 2.1.7.2041-06; Maximum permissible concentration (MPC) of chemicals in soils. Rospotrebnadzor: Moscow, Russia, 2006; p. 15. (In Russian)
- Soukarieh, B.; El Hawari, K.; El Husseini, M.; Budzinski, H.; Jaber, F. Impact of Lebanese practices in industry, agriculture and urbanization on soil toxicity. Eval. Polycycl. Aromat. Hydrocarb. (PAHs) Levels Soil. Chemosphere 2018, 210, 85–92. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Xie, S. Molecular characterization of phenanthrene-degrading methanogenic communities in leachate-contaminated aquifer sediment. Int. J. Environ. Sci. Technol. 2012, 9, 705–712. [Google Scholar] [CrossRef]
- Krzmarzick, M.J.; Taylor, D.K.; Fu, X.; McCutchan, A.L. Diversity and niche of archaea in bioremediation. Archaea 2018, 2018, 3194108. [Google Scholar] [CrossRef]
- Rochman, F.F.; Sheremet, A.; Tamas, I.; Saidi-Mehrabad, A.; Kim, J.-J.; Dong, X.; Sensen, C.W.; Gieg, L.M.; Dunfield, P.F. Benzene and naphthalene degrading bacterial communities in an oil sands tailings pond. Front. Microbiol. 2017, 8, 1845. [Google Scholar] [CrossRef]
- Benedek, T.; Szentgyörgyi, F.; Szabó, I.; Farkas, M.; Duran, R.; Kriszt, B.; Táncsics, A. Aerobic and oxygen-limited naphthalene-amended enrichments induced the dominance of Pseudomonas spp. from a groundwater bacterial biofilm. Appl. Microbiol. Biotechnol. 2020, 104, 6023–6043. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, Y.; Jeon, C.O. Biodegradation of naphthalene, BTEX, and aliphatic hydrocarbons by Paraburkholderia aromaticivorans BN5 isolated from petroleum-contaminated soil. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Weissenfels, W.D.; Beyer, M.; Klein, J. Degradation of phenanthrene, fluorene and fluoranthene by pure bacterial cultures. Appl. Microbiol. Biotechnol. 1990, 32, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, P.A.; Arvin, E. Kinetics of degradation of surfactant-solubilized fluoranthene by a Sphingomonas paucimobilis. Environ. Sci. Technol. 1999, 33, 2571–2578. [Google Scholar] [CrossRef]
- Reddy, P.V.; Karegoudar, T.; Monisha, T.; Mukram, I.; Nayak, A.S. Biodegradation of fluoranthene by Paenibacillus sp. strain PRNK-6: A pathway for complete mineralization. Arch. Microbiol. 2018, 200, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Luan, T.; Lin, L.; Liu, H.; Tam, N.F. Production of metabolites in the biodegradation of phenanthrene, fluoranthene and pyrene by the mixed culture of Mycobacterium sp. and Sphingomonas sp. Bioresour. Technol. 2011, 102, 2965–2972. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-x.; Hu, X.; Cao, Y.; Pang, W.-j.; Huang, J.-y.; Guo, P.; Huang, L. Biodegradation of phenanthrene and heavy metal removal by acid-tolerant Burkholderia fungorum FM-2. Front. Microbiol. 2019, 10, 408. [Google Scholar] [CrossRef]
- Bankole, P.O.; Semple, K.T.; Jeon, B.-H.; Govindwar, S.P. Biodegradation of fluorene by the newly isolated marine-derived fungus, Mucor irregularis strain bpo1 using response surface methodology. Ecotoxicol. Environ. Saf. 2021, 208, 111619. [Google Scholar] [CrossRef]
- Pinyakong, O.; Habe, H.; Omori, T. The unique aromatic catabolic genes in sphingomonads degrading polycyclic aromatic hydrocarbons (PAHs). J. Gen. Appl. Microbiol. 2003, 49, 1–19. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Y.-S. Fluorene degradation by Rhodococcus sp. A2-3 isolated from hydrocarbon contaminated sediment of the Pearl River estuary, China. Ecotoxicology 2021, 30, 929–935. [Google Scholar] [CrossRef]
- Wang, L.; Ji, G.; Huang, S. Contribution of the Kodama and 4S pathways to the dibenzothiophene biodegradation in different coastal wetlands under different C/N ratios. Res. J. Environ. Sci. 2019, 76, 217–226. [Google Scholar] [CrossRef]
- Jové, P.; Olivella, M.À.; Camarero, S.; Caixach, J.; Planas, C.; Cano, L.; De Las Heras, F.X. Fungal biodegradation of anthracene-polluted cork: A comparative study. J. Environ. Sci. Health A 2016, 51, 70–77. [Google Scholar] [CrossRef]
- Bhatt, K.K.; Lily, M.K.; Joshi, G.; Dangwal, K. Benzo[a]pyrene degradation pathway in Bacillus subtilis BMT4i (MTCC 9447). Turk. J. Biochem. 2018, 43, 693–701. [Google Scholar] [CrossRef]
- Johnsen, A.R.; Wick, L.Y.; Harms, H. Principles of microbial PAH-degradation in soil. Environ. Pollut. 2005, 133, 71–84. [Google Scholar] [CrossRef]
- Cochrane, R.V.; Vederas, J.C. Highly selective but multifunctional oxygenases in secondary metabolism. Acc. Chem. Res. 2014, 47, 3148–3161. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, M.A.; Loviso, C.L.; Lozada, M.; Ferreira, F.V.; Dionisi, H.M. Substrate specificities of aromatic ring-hydroxylating oxygenases of an uncultured gammaproteobacterium from chronically-polluted subantarctic sediments. Int. Biodeterior. Biodegrad. 2019, 137, 127–136. [Google Scholar] [CrossRef]
- AbuBakr, S.; Macmil, S.L.; Nanny, M.A.; Duncan, K.E. Enzymatic transformation of humic substances by NDO. Soil Biol. Biochem. 2008, 40, 2055–2062. [Google Scholar] [CrossRef]
- Wu, Y.; Teng, Y.; Li, Z.; Liao, X.; Luo, Y. Potential role of polycyclic aromatic hydrocarbons (PAHs) oxidation by fungal laccase in the remediation of an aged contaminated soil. Soil Biol. Biochem. 2008, 40, 789–796. [Google Scholar] [CrossRef]
- Kumar, A.; Schimmelmann, A.; Sauer, P.E.; Brassell, S.C. Distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in laminated Santa Barbara Basin sediments. Org. Geochem. 2017, 113, 303–314. [Google Scholar] [CrossRef]
- Holman, A.I.; Grice, K. δ13C of aromatic compounds in sediments, oils and atmospheric emissions: A review. Org. Geochem. 2018, 123, 27–37. [Google Scholar] [CrossRef]
- Gao, P.; Li, H.; Wilson, C.P.; Townsend, T.G.; Xiang, P.; Liu, Y.; Ma, L.Q. Source identification of PAHs in soils based on stable carbon isotopic signatures. Crit. Rev. Environ. Sci. Technol. 2018, 48, 923–948. [Google Scholar] [CrossRef]
- Bakhtiari, A.R.; Zakaria, M.P.; Yaziz, M.I.; Lajis, M.N.H.; Bi, X.; Abd Rahim, M.C. Vertical distribution and source identification of polycyclic aromatic hydrocarbons in anoxic sediment cores of Chini Lake, Malaysia: Perylene as indicator of land plant-derived hydrocarbons. Appl. Geochem. 2009, 24, 1777–1787. [Google Scholar] [CrossRef]
- Wakeham, S.G.; Canuel, E.A. Biogenic polycyclic aromatic hydrocarbons in sediments of the San Joaquin River in California (USA), and current paradigms on their formation. Environ. Sci. Pollut. Res. 2016, 23, 10426–10442. [Google Scholar] [CrossRef] [PubMed]
- Bixiong, Y.; Zhihuan, Z.; Ting, M. Pollution sources identification of polycyclic aromatic hydrocarbons of soils in Tianjin area, China. Chemosphere 2006, 64, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.A.; Neves, P.A.; Patchineelam, S.R.; da Silva, J.F.B.; Takiyama, L.R.; Martins, C.C.; Lourenço, R.A.; Taniguchi, S.; Elias, V.O.; Bícego, M.C. Anthropogenic and natural inputs of polycyclic aromatic hydrocarbons in the sediment of three coastal systems of the Brazilian Amazon. Environ. Sci. Pollut. Res. 2021, 28, 19485–19496. [Google Scholar] [CrossRef] [PubMed]
- Cabrerizo, A.; Tejedo, P.; Dachs, J.; Benayas, J. Anthropogenic and biogenic hydrocarbons in soils and vegetation from the South Shetland Islands (Antarctica). Sci. Total Environ. 2016, 569, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).