Investigation of Impulse and Continuous Discharge Characteristics of Large-Capacity Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
- Time charts of LIB voltage at different impulse discharge currents;
- Dependence of open circuit voltage on the state of charge;
- Discharge characteristics at different discharge currents [20].
3. Results
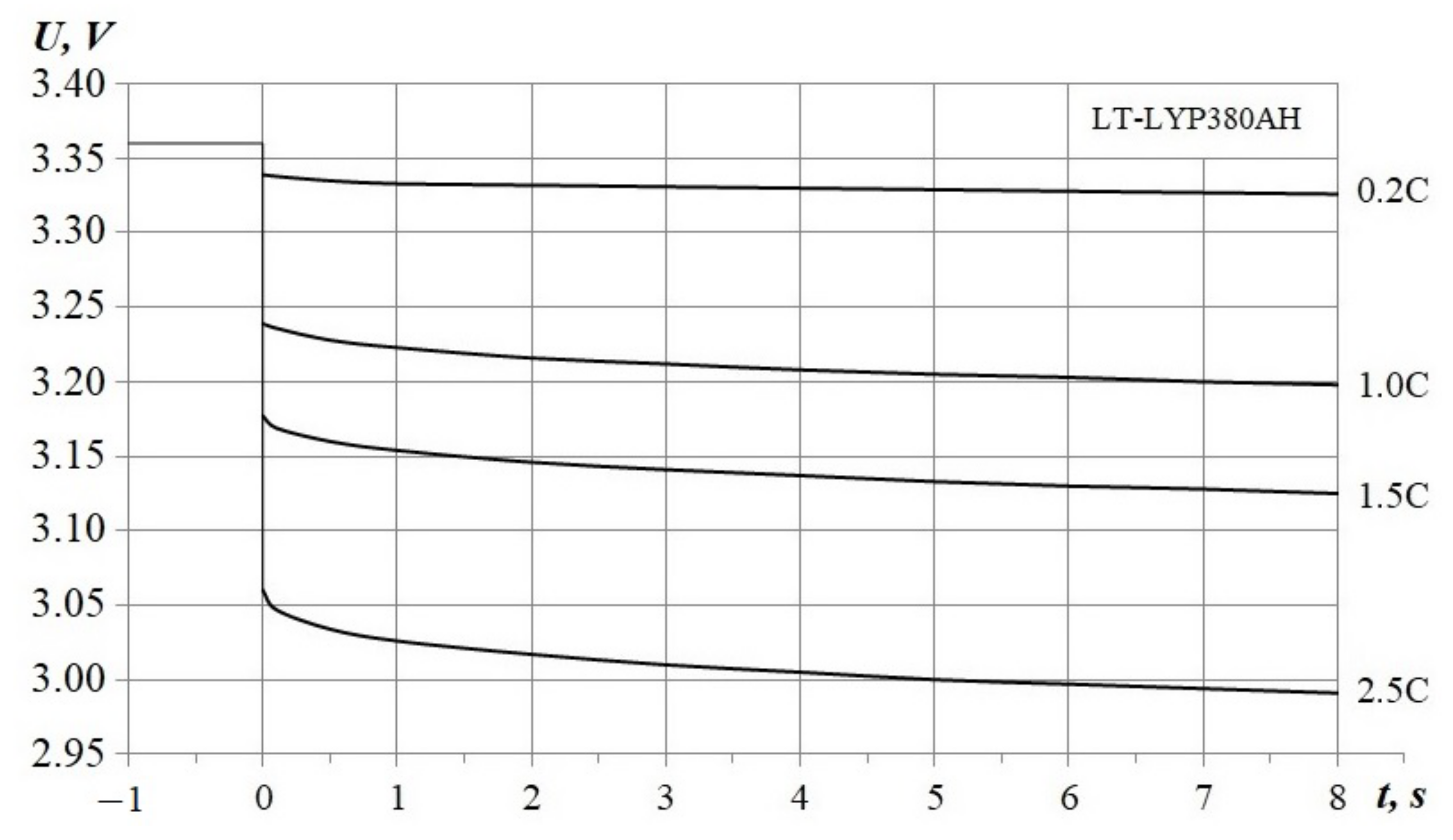
3.1. Impulse Discharges
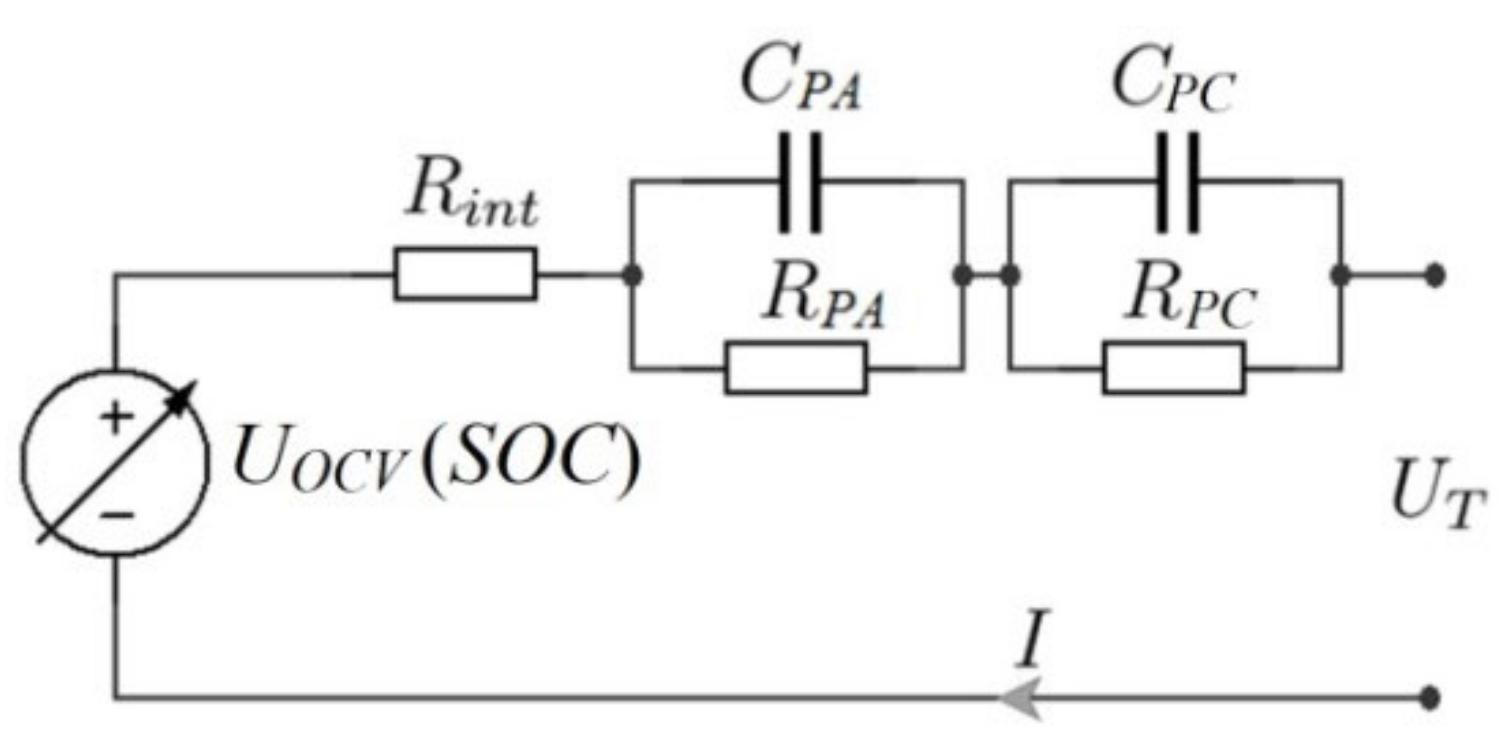
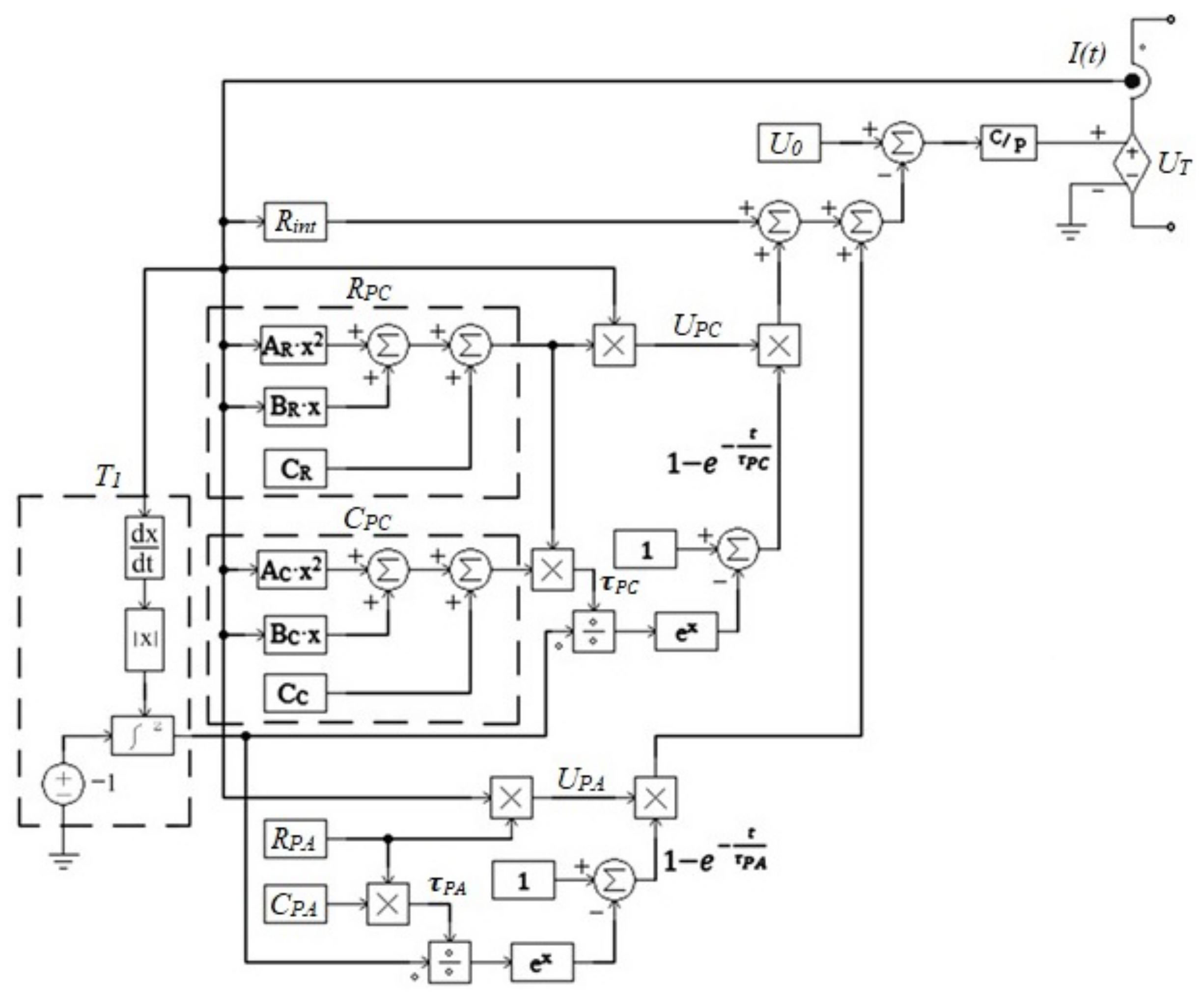
3.1.1. Determination of Circuit Parameters
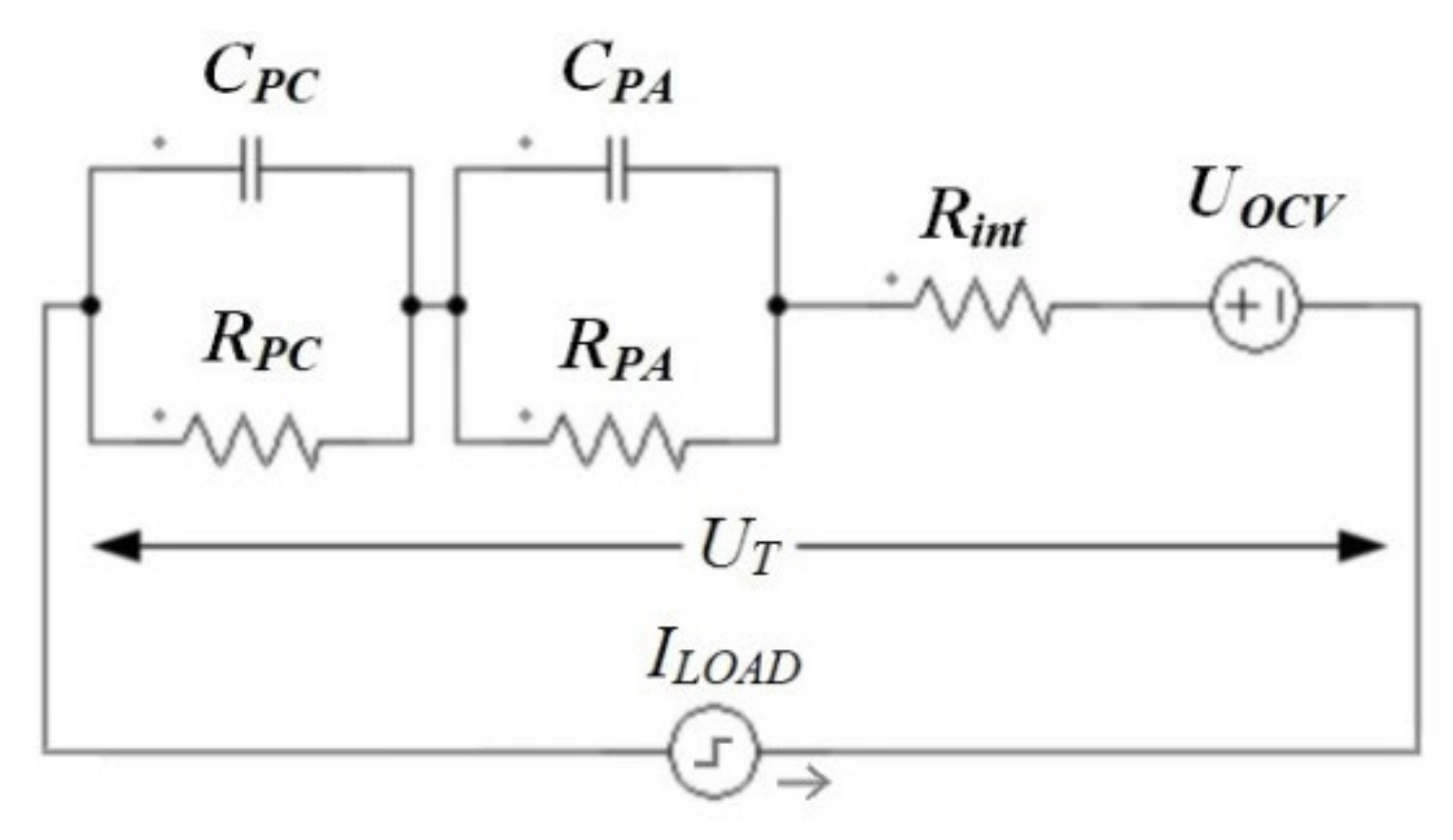
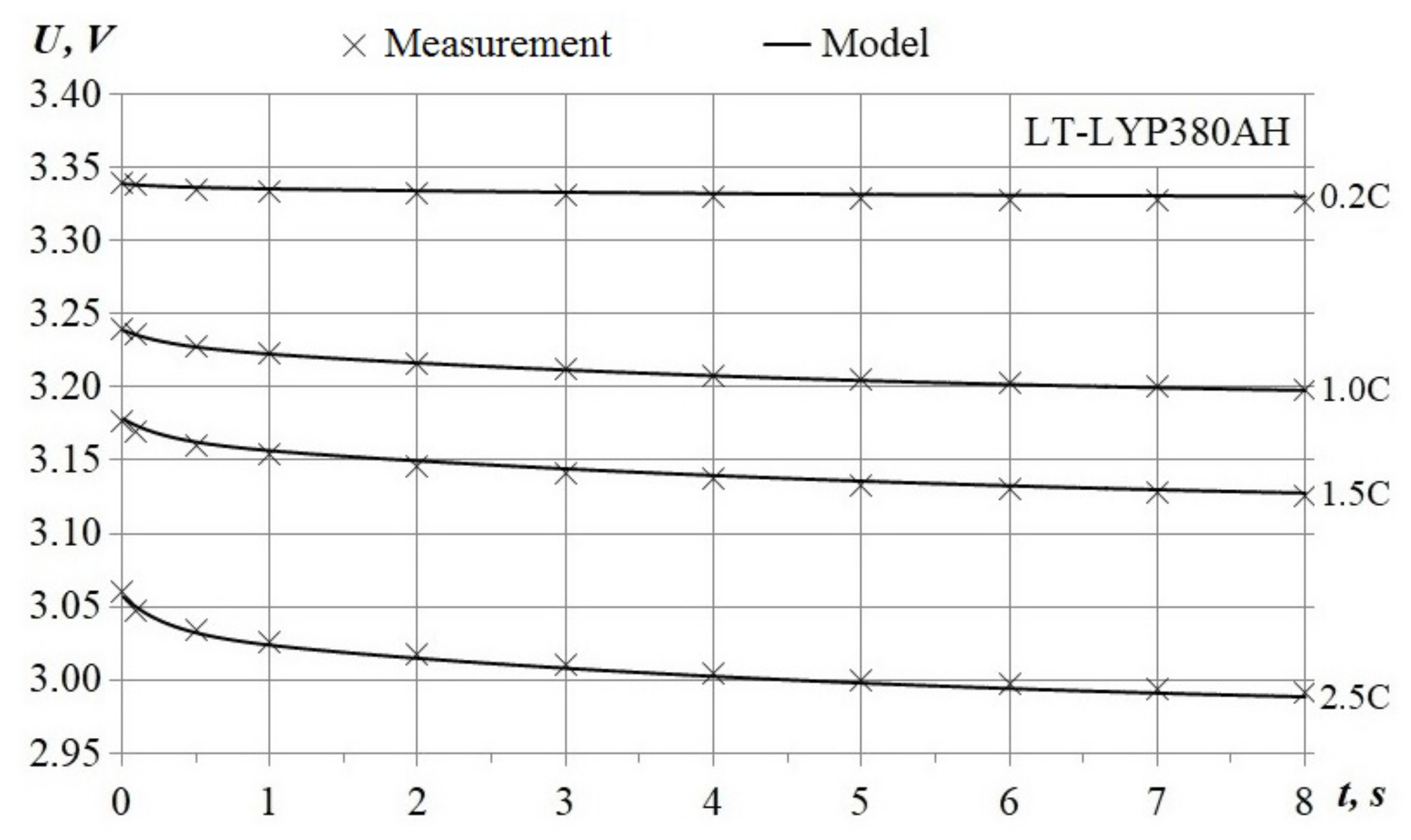
3.1.2. Circuit Simulation
3.1.3. Simulation of the Model with Current Feedback
3.2. Open Circuit Voltage Dependence on Depth of Discharge
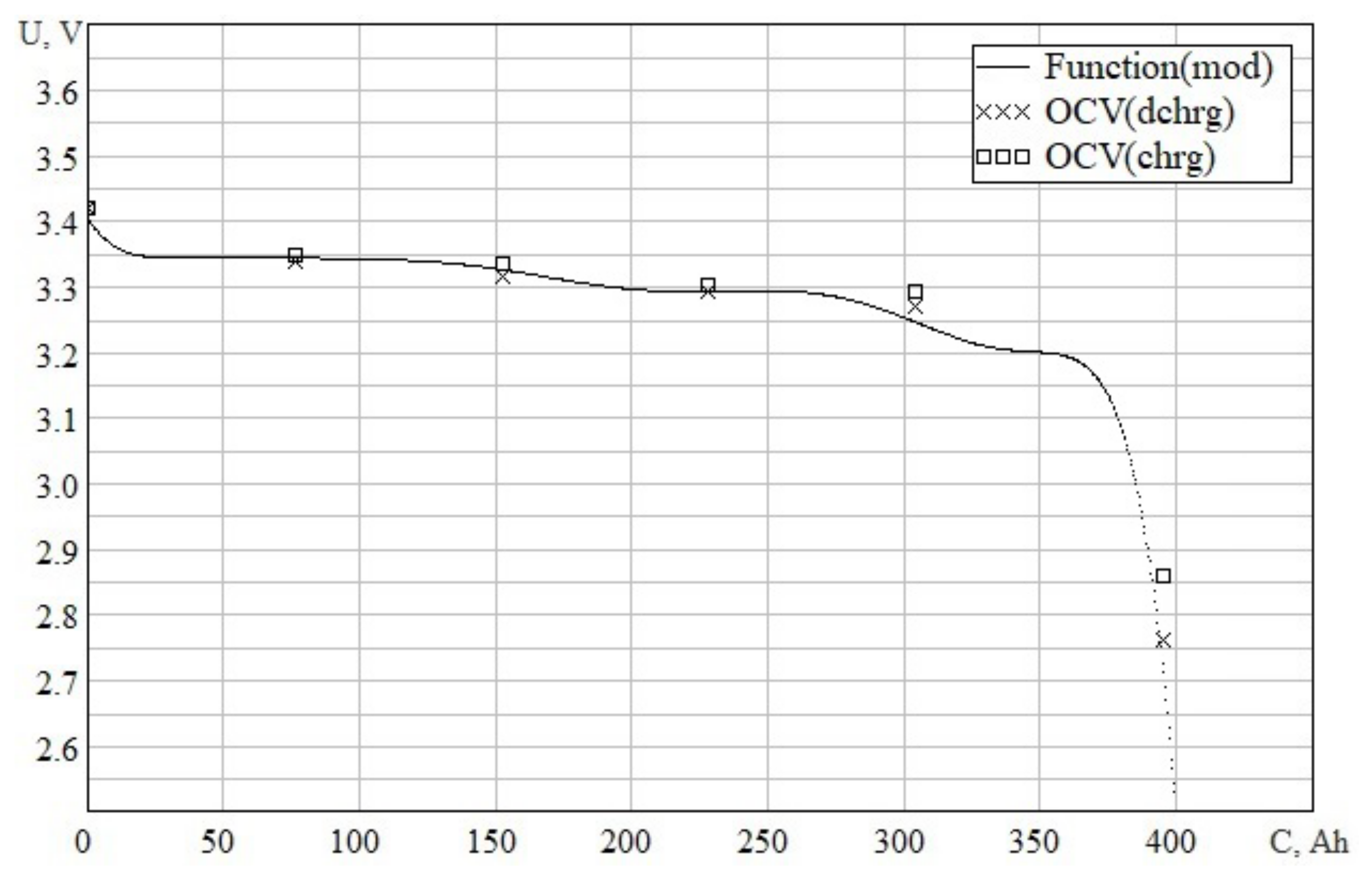
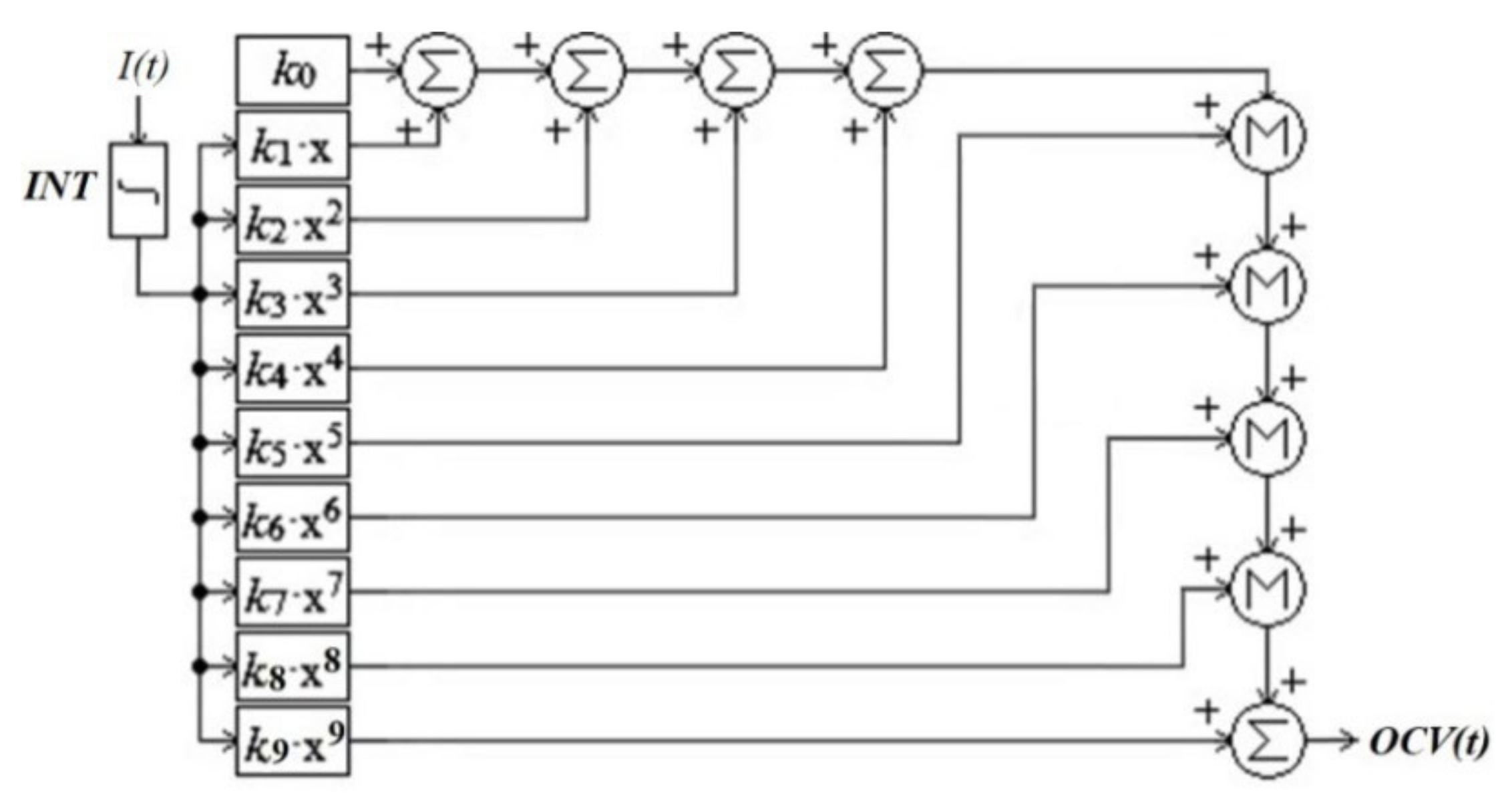
3.2.1. Approximation of the Open Circuit Voltage Dependence
3.2.2. Simulation of the Open Circuit Voltage Dependence
- –
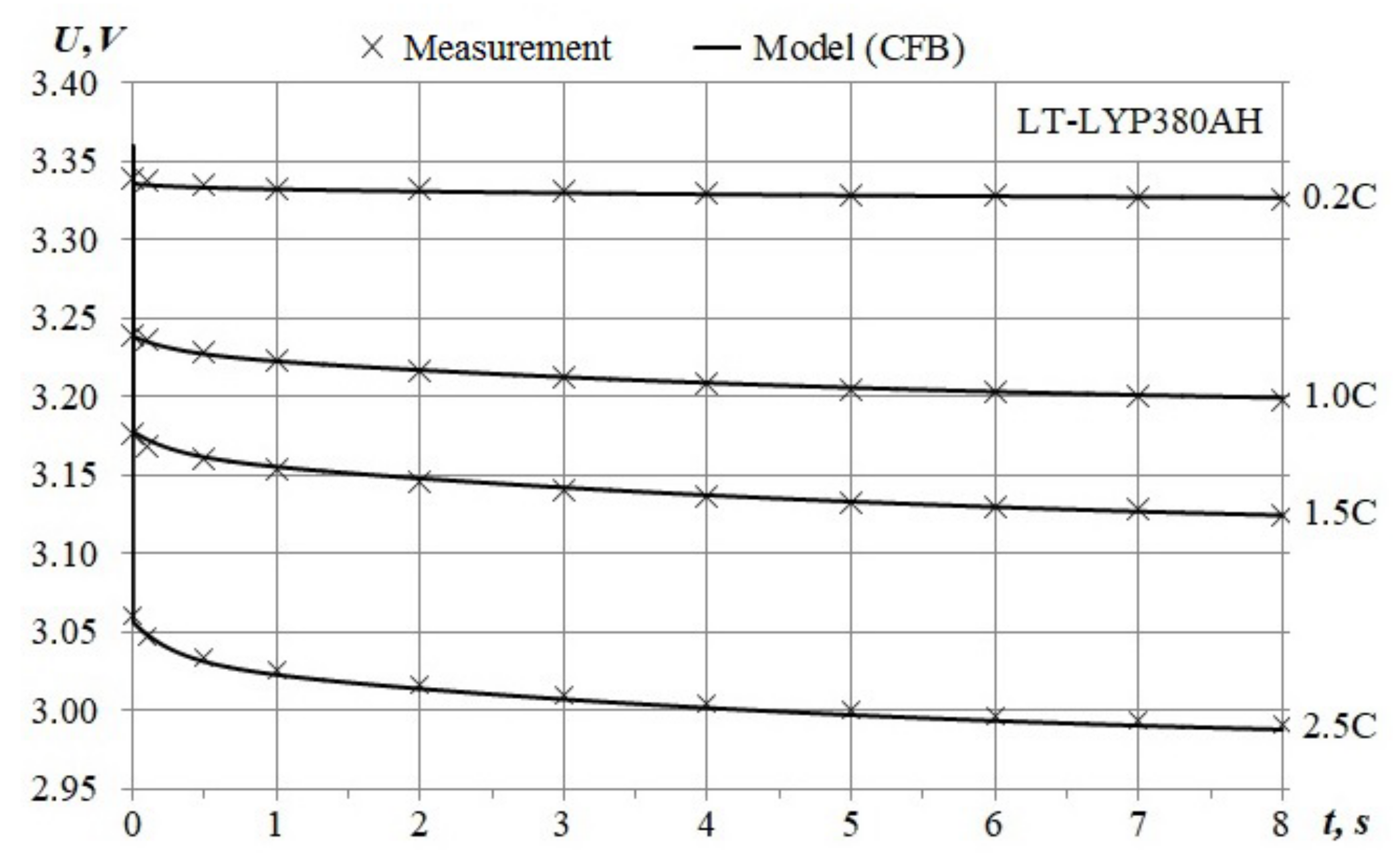
- Maximum average voltage error for the 375 Ah range corresponds to the 0.2 C chart, the error average value is less than 1.7% of the nominal voltage;
- –
- An 8% difference between maximal discharge capacity for the 2.5 C-chart;
- –
- The end-state-of-charge spread is less by 3.5% of the nominal capacity.


4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menniti, D.; Pinnarelli, A.; Sorrentino, N.; Vizza, P.; Burgio, A.; Brusco, G.; Motta, M. A Real-Life Application of an Efficient Energy Management Method for a Local Energy System in Presence of Energy Storage Systems. In Proceedings of the 2018 IEEE International Conference on Environment and Electrical Engineering and 2018 IEEE Industrial and Commercial Power Systems Europe (EEEIC/I&CPS Europe), Palermo, Italy, 12–15 June 2018; pp. 1–6. [Google Scholar]
- Smith, S.C.; Sen, P.K.; Kroposki, B.; Malmedal, K. Renewable Energy and Energy Storage Systems in Rural Electrical Power Systems: Issues, Challenges and Application Guidelines. In Proceedings of the Rural Electric Power Conference (REPC), Orlando, FL, USA, 16–19 May 2010; p. B4-B4-7. [Google Scholar]
- Chen, Y.; Jia, K.; Liu, B.; Yan, G.; Wang, D.; Li, C. Energy Management Method Applying in Integrated Energy System. In Proceedings of the 2015 5th International Conference on Electric Utility Deregulation and Restructuring and Power Technologies (DRPT), Changsha, China, 26–29 November 2015; pp. 1565–1569. [Google Scholar]
- Keshan, H.; Thornburg, J.; Ustun, T.S. Comparison of lead-acid and lithium ion batteries for stationary storage in off-grid energy systems. In Proceedings of the 4th IET Clean Energy and Technology Conference (CEAT 2016), Kuala Lumpur, Malaysia, 14–15 November 2016; pp. 1–7. [Google Scholar]
- Lyu, N.; Jin, Y.; Xiong, R.; Miao, S.; Gao, J. Real-time overcharge warning and early thermal runaway prediction of Li-ion battery by online impedance measurement. IEEE Trans. Ind. Electron. 2022, 69, 1929–1936. [Google Scholar] [CrossRef]
- Feng, Y.; Xue, C.; Han, Q.L.; Han, F.; Du, J. Robust estimation for state-of-charge and state-of-health of lithium-ion batteries using integraltype terminal sliding-mode observers. IEEE Trans. Ind. Electron. 2020, 67, 4013–4023. [Google Scholar] [CrossRef]
- Deshpande, V.S.; Talele, M.N. Nanotechnology Enabled Hybrid Power System Suitable for Batteries in Hybrid Electric Vehicle. In Proceedings of the 2017 Third International Conference on Advances in Electrical, Electronics, Information, Communication and Bio-Informatics (AEEICB), Chennai, India, 27–28 February 2017; pp. 33–35. [Google Scholar]
- Starostin, I.; Khalyutin, S.; Davidov, A.; Lyovin, A.; Trubachev, A. The Development of a Mathematical Model of Lithium-Ion Battery Discharge Characteristics. In Proceedings of the 2019 International Conference on Electrotechnical Complexes and Systems (ICOECS), Ufa, Russia, 21–25 October 2019. [Google Scholar]
- Qu, J.; Wang, C. Detail Modeling Research and Experimental Verification of Wound Pouch Lithium-Ion Battery. In Proceedings of the 2021 IEEE 4th Advanced Information Management, Communicates, Electronic and Automation Control Conference (IMCEC), Chongqing, China, 18–20 June 2021; pp. 1831–1834. [Google Scholar]
- Xia, Z.; Abu Qahouq, J.A. Evaluation of Parameter Variations of Equivalent Circuit Model of Lithium-ion Battery under Different SOH Conditions. In Proceedings of the 2020 IEEE Energy Conversion Congress and Exposition (ECCE), Detroit, MI, USA, 11–15 October 2020; pp. 1519–1523. [Google Scholar]
- Han, X.; Guo, D.; Feng, X.; Lu, L.; Ouyang, M. Equivalence of Time and Frequency Domain Modeling for Lithium Ion Batteries. In Proceedings of the 2021 IEEE 30th International Symposium on Industrial Electronics (ISIE), Kyoto, Japan, 20–23 June 2021. [Google Scholar]
- Wu, M.; Qin, L.; Wu, G.; Shi, C. State of Charge Estimation of Lithium ion Battery Based on a New Linear Online Model. In Proceedings of the 39th Chinese Control Conference, Shenyang, China, 27–29 July 2020; pp. 5282–5286. [Google Scholar]
- Savanth, P.; Shailesh, K.R. Reduction of Parameters in a Lithium Ion Cell Model by Experimental Validation of Relationship between OCV and SOC. In Proceedings of the 2016 Online International Conference on Green Engineering and Technologies (IC-GET), Coimbatore, India, 19 November 2016. [Google Scholar]
- Zhang, H.; Na, W.; Kim, J. State-of-Charge Estimation of the Lithium-Ion Battery Using Neural Network Based on an Improved Thevenin Circuit Model. In Proceedings of the 2018 IEEE Transportation Electrification Conference and Expo (ITEC), Long Beach, CA, USA, 13–15 June 2018; pp. 342–346. [Google Scholar]
- Huang, M.; Wang, C.; Zhao, J. State of Charge Estimation of Lithium-Ion Battery Based on Second-order Extended Kalman Filter. In Proceedings of the 2019 IEEE 4th Advanced Information Technology, Electronic and Automation Control Conference (IAEAC 2019), Chengdu, China, 20–22 December 2019; pp. 335–338. [Google Scholar]
- Ceylan, M.; Sarıkurt, T.; Balıkçı, A. A Novel Lithium-Ion-Polymer Battery Model for Hybrid/Electric Vehicles. In Proceedings of the 2014 IEEE 23rd International Symposium on Industrial Electronics (ISIE), Istanbul, Turkey, 1–4 June 2014; pp. 366–369. [Google Scholar]
- Wang, S.; Takyi-Aninakwa, P.; Jin, S.; Yu, C.; Fernandez, C.; Stroe, D.-I. An improved feedforward-long short-term memory modeling method for the whole-life-cycle state of charge prediction of lithium-ion batteries considering current–voltagetemperature variation. Energy 2022, 254, 124224. [Google Scholar] [CrossRef]
- Nacu, R.C.; Fodorean, D. Lithium-Ion Cell Characterization, Using Hybrid Current Pulses, for Subsequent Battery Simulation in Mobility Applications. Processes 2022, 10, 2108. [Google Scholar] [CrossRef]
- Xiaokang, L.; Wang, Q.; Yang, Y.; Kang, J. Correlation between Capacity Loss and Measurable Parameters of Lithium-Ion Batteries. Int. J. Electr. Power Energy Syst. 2019, 110, 819–826. [Google Scholar]
- Kuchak, S.V.; Voroshilov, A.N.; Chudinov, E.A. Discharge Characteristics of Lithium-Ion Accumulators under Different Currents. In Proceedings of the 18th International Conference of Young Specialists on Micro/Nanotechnologies and Electron Devices EDM 2017, Erlagol, Russia, 29 June 2017–3 July 2017; pp. 435–438. [Google Scholar]

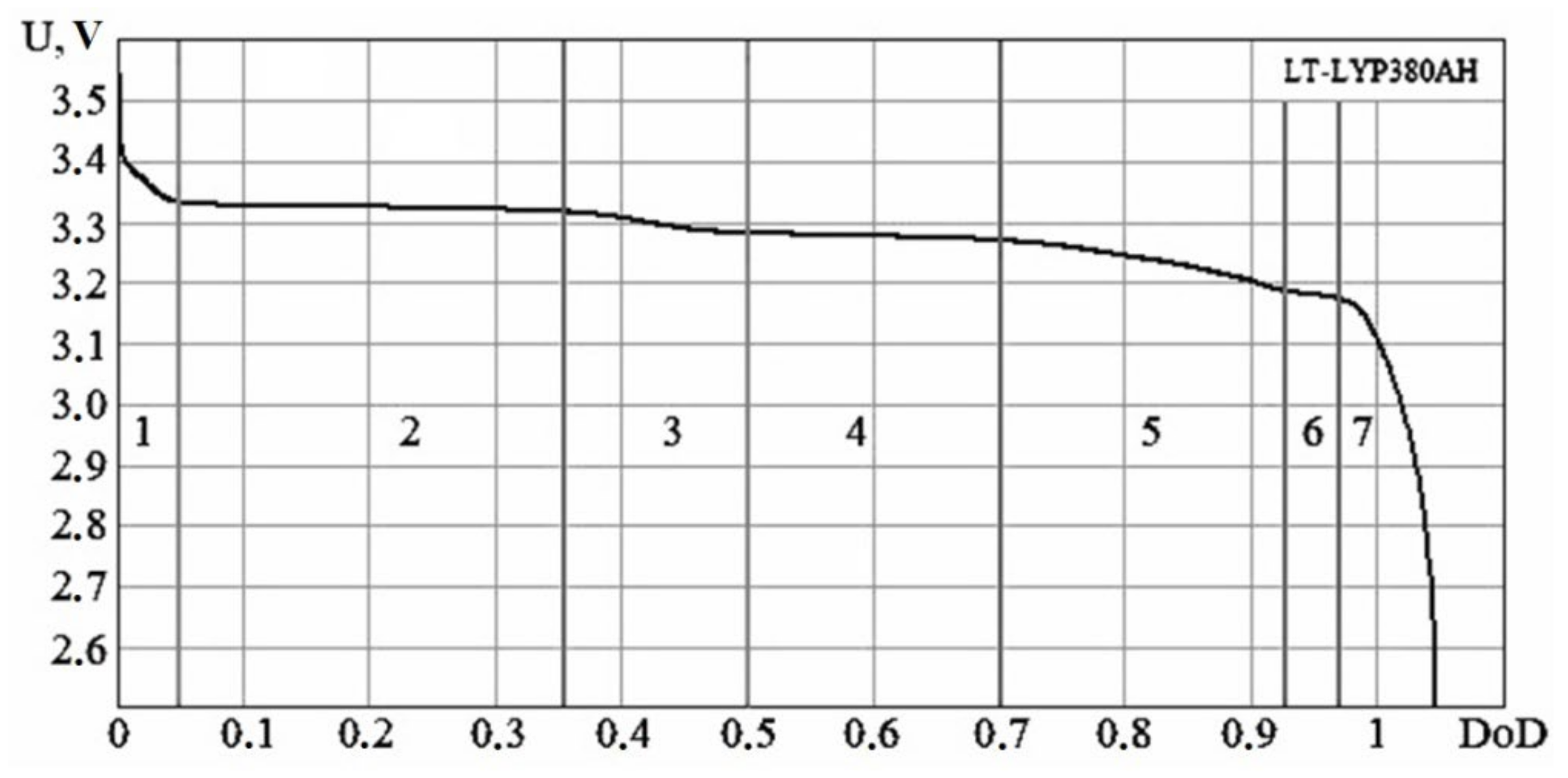


| № | DoD Range | Separate Sections | Voltage Range, V |
|---|---|---|---|
| 1 | 0–0.05 | Sharp decline | 3.55–3.33 |
| 2 | 0.05–0.35 | Linear, horizontal | 3.33–3.32 |
| 3 | 0.35–0.50 | Smooth decline | 3.32–3.28 |
| 4 | 0.50–0.70 | Linear, horizontal | 3.28–3.27 |
| 5 | 0.70–0.93 | Smooth decline | 3.27–3.18 |
| 6 | 0.93–0.97 | Linear, horizontal | 3.18–3.17 |
| 7 | 0.97–1.05 | Sharp decline | 3.17–2.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuchak, S.V.; Brovanov, S.V. Investigation of Impulse and Continuous Discharge Characteristics of Large-Capacity Lithium-Ion Batteries. Processes 2022, 10, 2473. https://doi.org/10.3390/pr10122473
Kuchak SV, Brovanov SV. Investigation of Impulse and Continuous Discharge Characteristics of Large-Capacity Lithium-Ion Batteries. Processes. 2022; 10(12):2473. https://doi.org/10.3390/pr10122473
Chicago/Turabian StyleKuchak, Sergey V., and Sergey V. Brovanov. 2022. "Investigation of Impulse and Continuous Discharge Characteristics of Large-Capacity Lithium-Ion Batteries" Processes 10, no. 12: 2473. https://doi.org/10.3390/pr10122473
APA StyleKuchak, S. V., & Brovanov, S. V. (2022). Investigation of Impulse and Continuous Discharge Characteristics of Large-Capacity Lithium-Ion Batteries. Processes, 10(12), 2473. https://doi.org/10.3390/pr10122473







