Bioremediation of Crude Oil by Haematococcus Pluvialis: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Growth
Cultivation with Crude Oil
2.2. Pigment Estimation
2.3. Biodegradation Activity
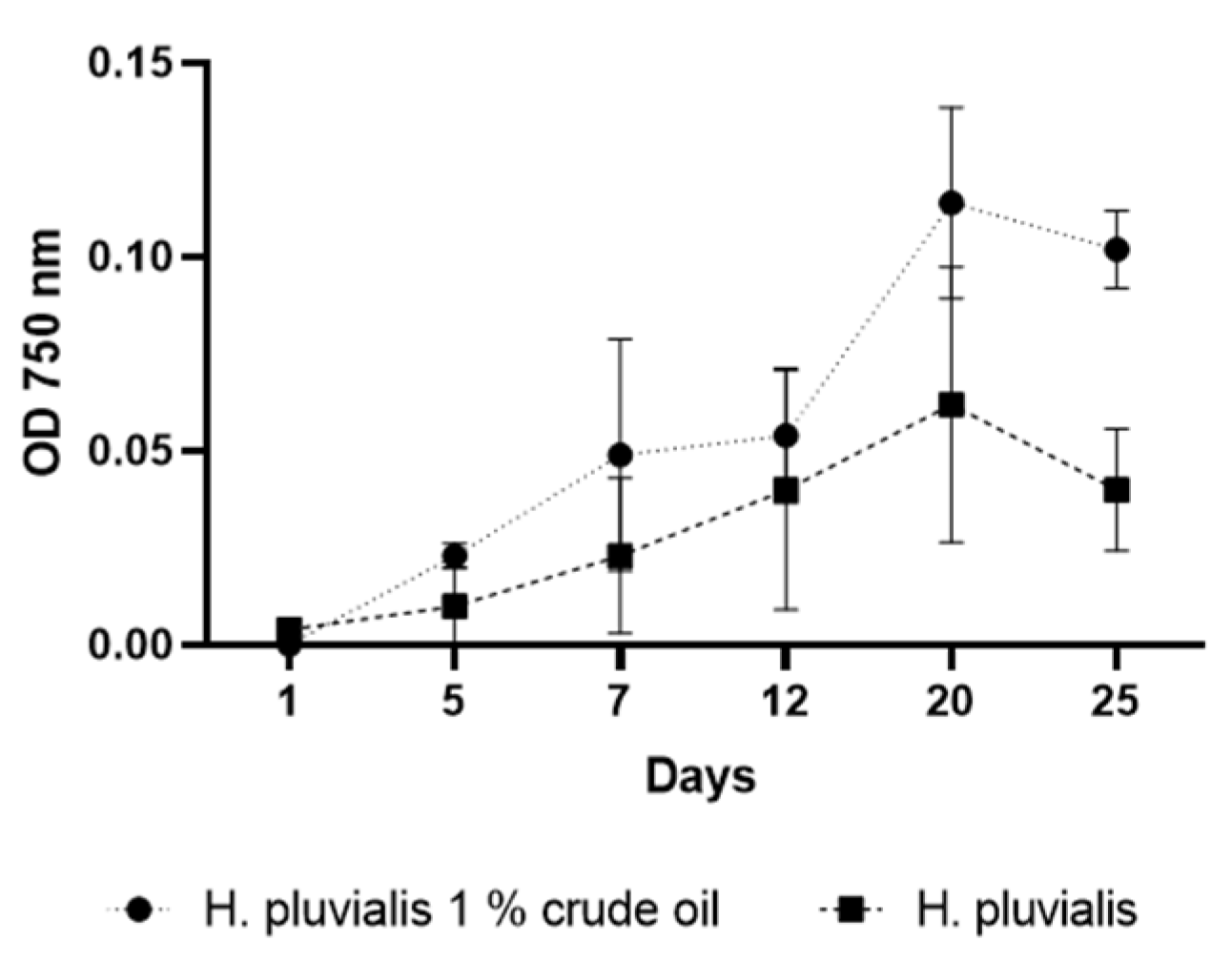
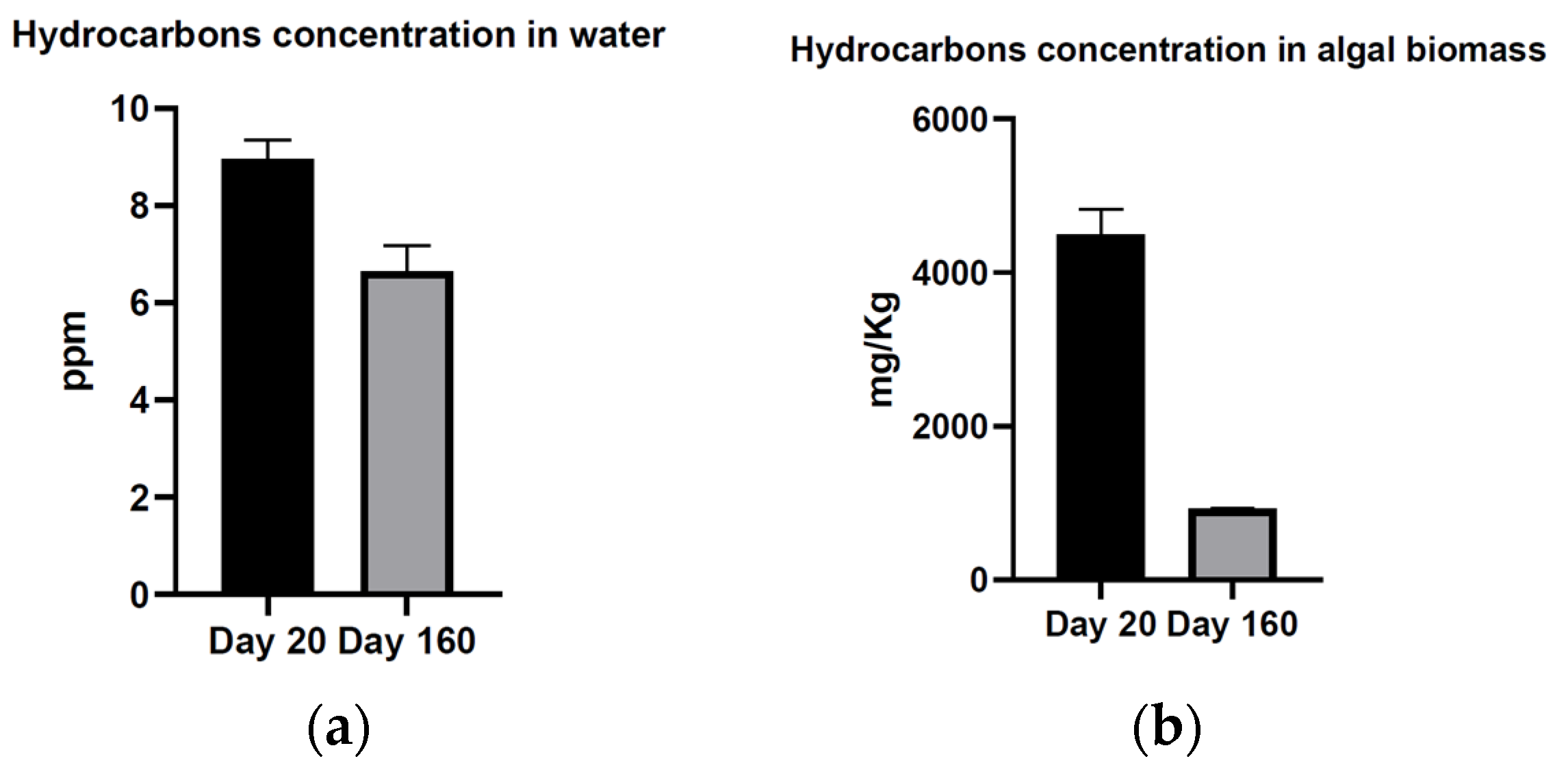
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hassan, A.; Ilyas, S.Z.; Jalil, A.; Ullah, Z. Monetization of the Environmental Damage Caused by Fossil Fuels. Environ. Sci. Pollut. Res. 2021, 28, 21204–21211. [Google Scholar] [CrossRef] [PubMed]
- Chilvers, B.L.; Morgan, K.J.; White, B.J. Sources and Reporting of Oil Spills and Impacts on Wildlife 1970–2018. Environ. Sci. Pollut. Res. 2021, 28, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.E.; Brant, J.L.; Campo, P.; Clark, D.R.; Coulon, F.; Gregson, B.H.; McGenity, T.J.; McKew, B.A. Effects of Dispersants and Biosurfactants on Crude-Oil Biodegradation and Bacterial Community Succession. Microorganisms 2021, 9, 1200. [Google Scholar] [CrossRef] [PubMed]
- Faisal, W.; Almomani, F. A Critical Review of the Development and Demulsification Processes Applied for Oil Recovery from Oil in Water Emulsions. Chemosphere 2022, 291, 133099. [Google Scholar] [CrossRef] [PubMed]
- Dell’ Anno, F.; Rastelli, E.; Sansone, C.; Brunet, C.; Ianora, A.; Dell’ Anno, A. Bacteria, Fungi and Microalgae for the Bioremediation of Marine Sediments Contaminated by Petroleum Hydrocarbons in the Omics Era. Microorganisms 2021, 9, 1695. [Google Scholar] [CrossRef] [PubMed]
- Chernikova, T.N.; Bargiela, R.; Toshchakov, S.V.; Shivaraman, V.; Lunev, E.A.; Yakimov, M.M.; Thomas, D.N.; Golyshin, P.N. Hydrocarbon-Degrading Bacteria Alcanivorax and Marinobacter Associated With Microalgae Pavlova Lutheri and Nannochloropsis Oculata. Front. Microbiol. 2020, 11, 572931. [Google Scholar] [CrossRef]
- French, K.E.; Zhou, Z.; Terry, N. Horizontal ‘Gene Drives’ Harness Indigenous Bacteria for Bioremediation. Sci. Rep. 2020, 10, 15091. [Google Scholar] [CrossRef]
- Yadav, G.; Dash, S.K.; Sen, R. A Biorefinery for Valorization of Industrial Waste-Water and Flue Gas by Microalgae for Waste Mitigation, Carbon-Dioxide Sequestration and Algal Biomass Production. Sci. Total Environ. 2019, 688, 129–135. [Google Scholar] [CrossRef]
- Kato, Y.; Hasunuma, T. Metabolic Engineering for Carotenoid Production Using Eukaryotic Microalgae and Prokaryotic Cyanobacteria. Adv. Exp. Med. Biol. 2021, 1261, 121–135. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; dos Reis Coimbra, J.S.; de Leite, M.O.; Albino, L.F.T.; Martins, M.A. Microalgae Proteins: Production, Separation, Isolation, Quantification, and Application in Food and Feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Plöhn, M.; Spain, O.; Sirin, S.; Silva, M.; Escudero-Oñate, C.; Ferrando-Climent, L.; Allahverdiyeva, Y.; Funk, C. Wastewater Treatment by Microalgae. Physiol. Plant 2021, 173, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Kumar Patel, A.; Tseng, Y.-S.; Rani Singhania, R.; Chen, C.-W.; Chang, J.-S.; di Dong, C. Novel Application of Microalgae Platform for Biodesalination Process: A Review. Bioresour. Technol. 2021, 337, 125343. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Mi, Y.; Zhao, C.; Wei, Q. A Comprehensive Review on Carbon Source Effect of Microalgae Lipid Accumulation for Biofuel Production. Sci. Total Environ. 2022, 806, 151387. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Hamouda, R.A.; Nizam, A.A. Biodegradation of Crude Oil by Scenedesmus Obliquus and Chlorella Vulgaris Growing under Heterotrophic Conditions. Int. Biodeterior. Biodegrad. 2013, 82, 67–72. [Google Scholar] [CrossRef]
- Hamouda, R.A.E.F.; Sorour, N.M.; Yeheia, D.S. Biodegradation of Crude Oil by Anabaena Oryzae, Chlorella Kessleri and Its Consortium under Mixotrophic Conditions. Int. Biodeterior. Biodegrad. 2016, 112, 128–134. [Google Scholar] [CrossRef]
- Kuttiyathil, M.S.; Mohamed, M.M.; Al-Zuhair, S. Using Microalgae for Remediation of Crude Petroleum Oil-Water Emulsion. Biotechnol. Prog. 2020, 37, e3098. [Google Scholar] [CrossRef]
- Znad, H.; al Ketife, A.M.D.; Judd, S.; AlMomani, F.; Vuthaluru, H.B. Bioremediation and Nutrient Removal from Wastewater by Chlorella Vulgaris. Ecol. Eng. 2018, 110, 1–7. [Google Scholar] [CrossRef]
- Ashwaniy, V.R.V.; Perumalsamy, M.; Pandian, S. Enhancing the Synergistic Interaction of Microalgae and Bacteria for the Reduction of Organic Compounds in Petroleum Refinery Effluent. Environ. Technol. Innov. 2020, 19, 100926. [Google Scholar] [CrossRef]
- Bauer, A.; Minceva, M. Examination of Photo-, Mixo-, and Heterotrophic Cultivation Conditions on Haematococcus Pluvialis Cyst Cell Germination. Appl. Sci. 2021, 16, 7201. [Google Scholar] [CrossRef]
- Radice, R.P.; Limongi, A.R.; Viviano, E.; Padula, M.C.; Martelli, G.; Bermano, G. Effects of Astaxanthin in Animal Models of Obesity-Associated Diseases: A Systematic Review and Meta-Analysis. Free Radic. Biol. Med. 2021, 171, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xu, N.; Liu, K.; Lv, R.; Shi, J.; Liu, J.; Sun, X.; Hu, C. Increasing Production and Bio-Accessibility of Natural Astaxanthin in Haematococcus Pluvialis by Screening and Culturing Red Motile Cells under High Light Condition. Bioresour. Technol. 2022, 364, 128067. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus Pluvialis: From Single Cell to High Value Commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Nishshanka, G.K.S.H.; Liyanaarachchi, V.C.; Nimarshana, P.H.V.; Ariyadasa, T.U.; Chang, J.-S. Haematococcus Pluvialis: A Potential Feedstock for Multiple-Product Biorefining. J. Clean Prod. 2022, 344, 131103. [Google Scholar] [CrossRef]
- SureshKumar, P.; Thomas, J.; Poornima, V. Structural Insights on Bioremediation of Polycyclic Aromatic Hydrocarbons Using Microalgae: A Modelling-Based Computational Study. Environ. Monit. Assess 2018, 190, 92. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Yamaguchi, K.; Nishio, N.; Nagai, S. Growth and Astaxanthin Formation of Haematococcus Pluvialis in Heterotrophic and Mixotrophic Conditions. J. Ferment. Bioeng. 1992, 74, 17–20. [Google Scholar] [CrossRef]
- Do, C.V.T.; Pham, M.H.T.; Pham, T.Y.T.; Dinh, C.T.; Bui, T.U.T.; Tran, T.D.; Nguyen, V.T. Microalgae and Bioremediation of Domestic Wastewater. Curr. Opin. Green Sustain. Chem. 2022, 34, 100595. [Google Scholar] [CrossRef]
- Bhatt, N.C.; Panwar, A.; Bisht, T.S.; Tamta, S. Coupling of Algal Biofuel Production with Wastewater. Sci. World J. 2014, 2014, 210504. [Google Scholar] [CrossRef]
- Das, P.; AbdulQuadir, M.; Thaher, M.; Khan, S.; Chaudhary, A.K.; Alghasal, G.; Al-Jabri, H.M.S.J. Microalgal Bioremediation of Petroleum-Derived Low Salinity and Low PH Produced Water. J. Appl. Phycol. 2019, 31, 435–444. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Fei, X.; Wright, D.A.; Spalding, M.H. Expression Activation and Functional Analysis of HLA3, a Putative Inorganic Carbon Transporter in Chlamydomonas Reinhardtii. Plant J. 2015, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Q. Microalgae-Based Nitrogen Bioremediation. Algal Res. 2020, 46, 101775. [Google Scholar] [CrossRef]
- Su, Y. Revisiting Carbon, Nitrogen, and Phosphorus Metabolisms in Microalgae for Wastewater Treatment. Sci. Total Environ. 2021, 762, 144590. [Google Scholar] [CrossRef] [PubMed]




| TOC | Nitrates | Fluorides | Chlorides | Sulfates | Phosphates | |
|---|---|---|---|---|---|---|
| DAY 0 | 23.90 ± 0.78 | 0.95 ± 0.18 | 0.40 ± 0.14 | 10.75 ± 0.18 | 11.50 ± 1.06 | 70.20 ± 1.27 |
| DAY 10 | 44.50 ± 1.06 | 0.24 ± 0.04 | 0.20 ± 0.07 | 12.50 ± 0.35 | 9.00 ± 0.85 | 53.50 ± 1.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radice, R.P.; Sansone, M.; D’Arienzo, G.; Scopa, A.; Martelli, G. Bioremediation of Crude Oil by Haematococcus Pluvialis: A Preliminary Study. Processes 2022, 10, 2472. https://doi.org/10.3390/pr10122472
Radice RP, Sansone M, D’Arienzo G, Scopa A, Martelli G. Bioremediation of Crude Oil by Haematococcus Pluvialis: A Preliminary Study. Processes. 2022; 10(12):2472. https://doi.org/10.3390/pr10122472
Chicago/Turabian StyleRadice, Rosa Paola, Maria Sansone, Gabriele D’Arienzo, Antonio Scopa, and Giuseppe Martelli. 2022. "Bioremediation of Crude Oil by Haematococcus Pluvialis: A Preliminary Study" Processes 10, no. 12: 2472. https://doi.org/10.3390/pr10122472
APA StyleRadice, R. P., Sansone, M., D’Arienzo, G., Scopa, A., & Martelli, G. (2022). Bioremediation of Crude Oil by Haematococcus Pluvialis: A Preliminary Study. Processes, 10(12), 2472. https://doi.org/10.3390/pr10122472









