Preparation and Application of Coal-Liquefaction-Residue-Based Carbon Material
Abstract
1. Introduction
2. Experimental
2.1. Instrument and Materials
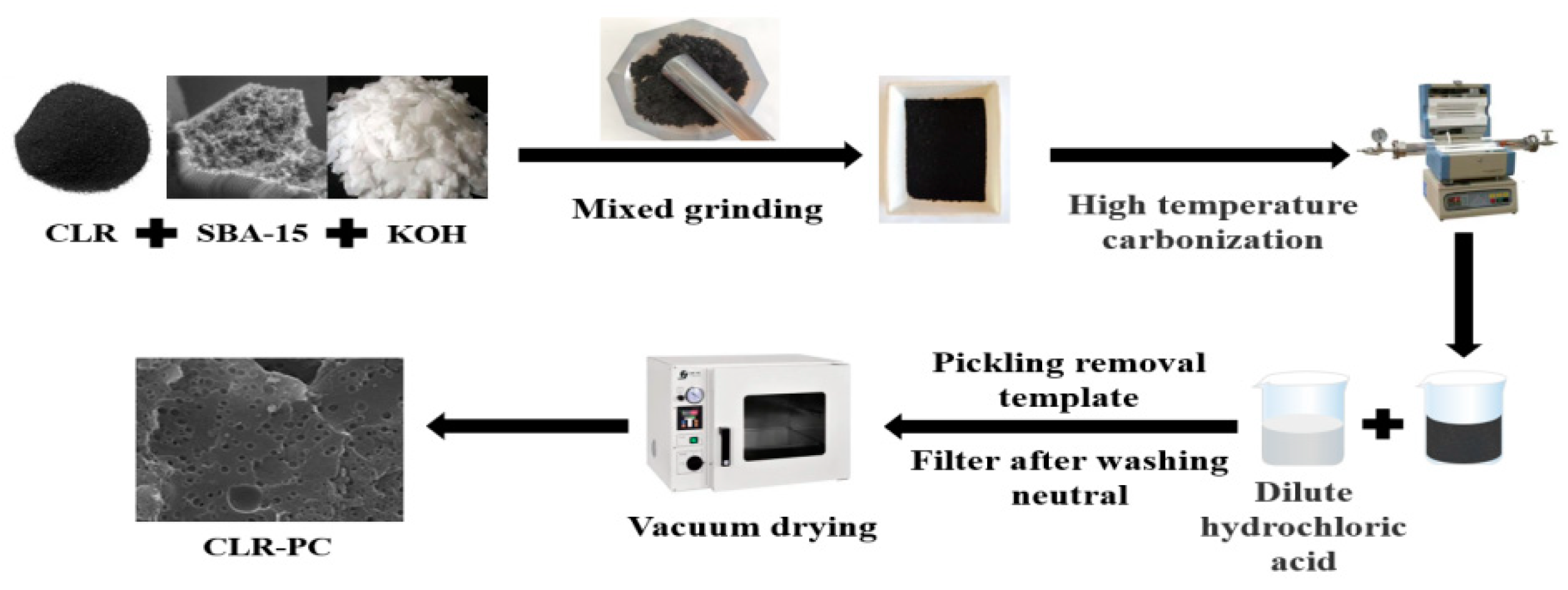
2.2. Preparation of CLR-Based Carbon Materials
2.3. Preparation of ClR-PC/GCE
3. Result and Discussion
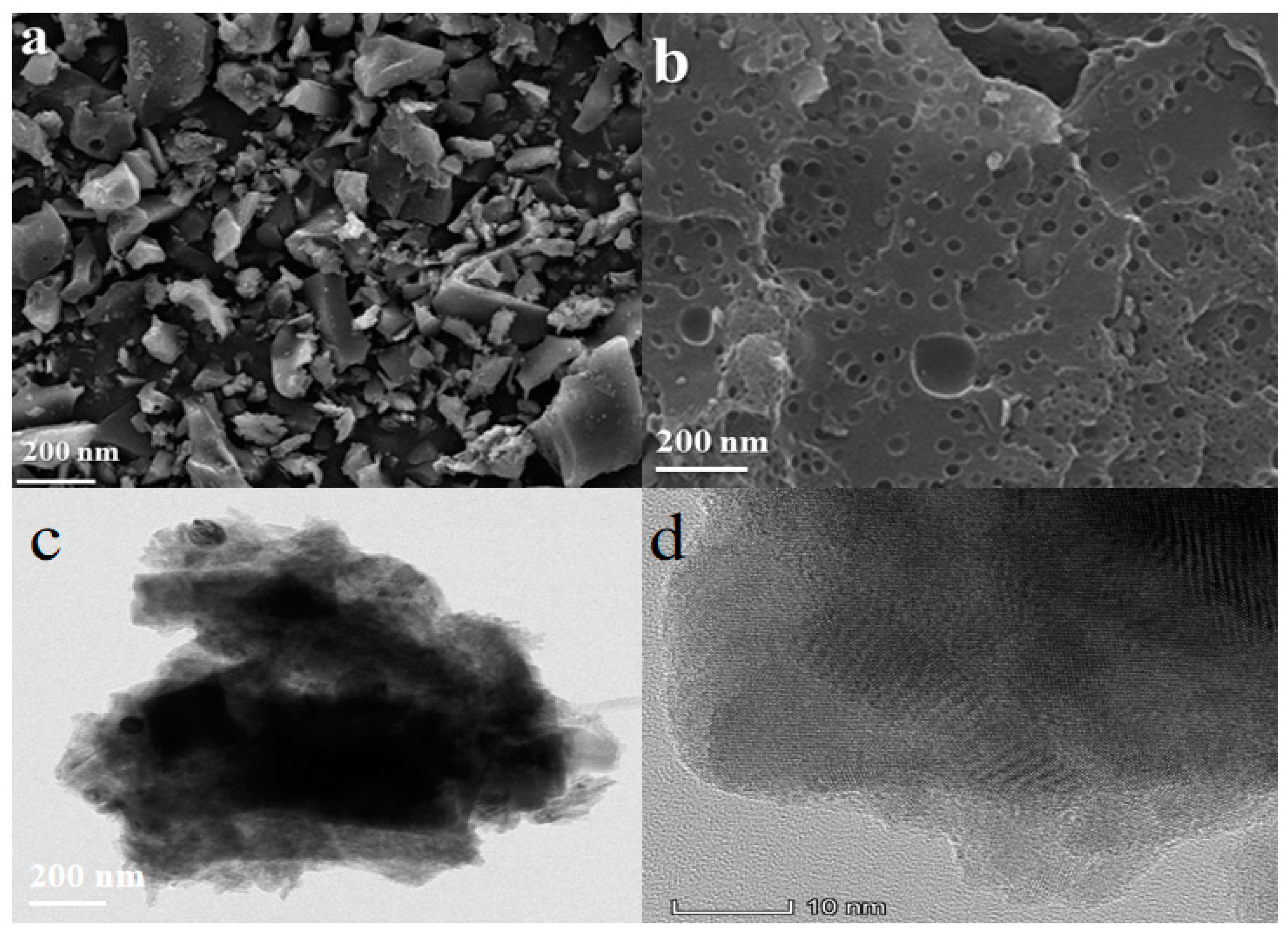
3.1. Characterization of CLR-PC
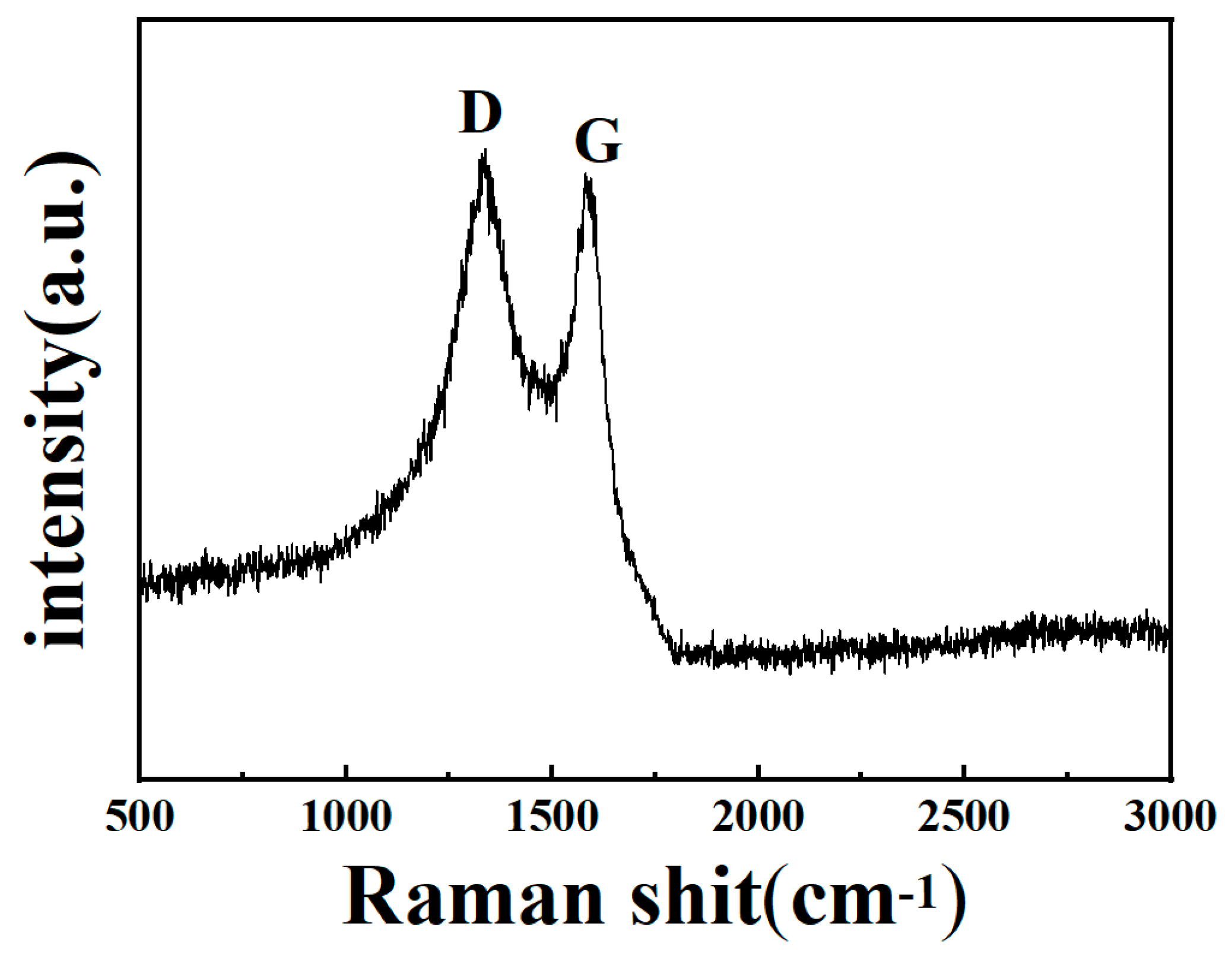
3.2. Raman Spectrum of CLR-PC
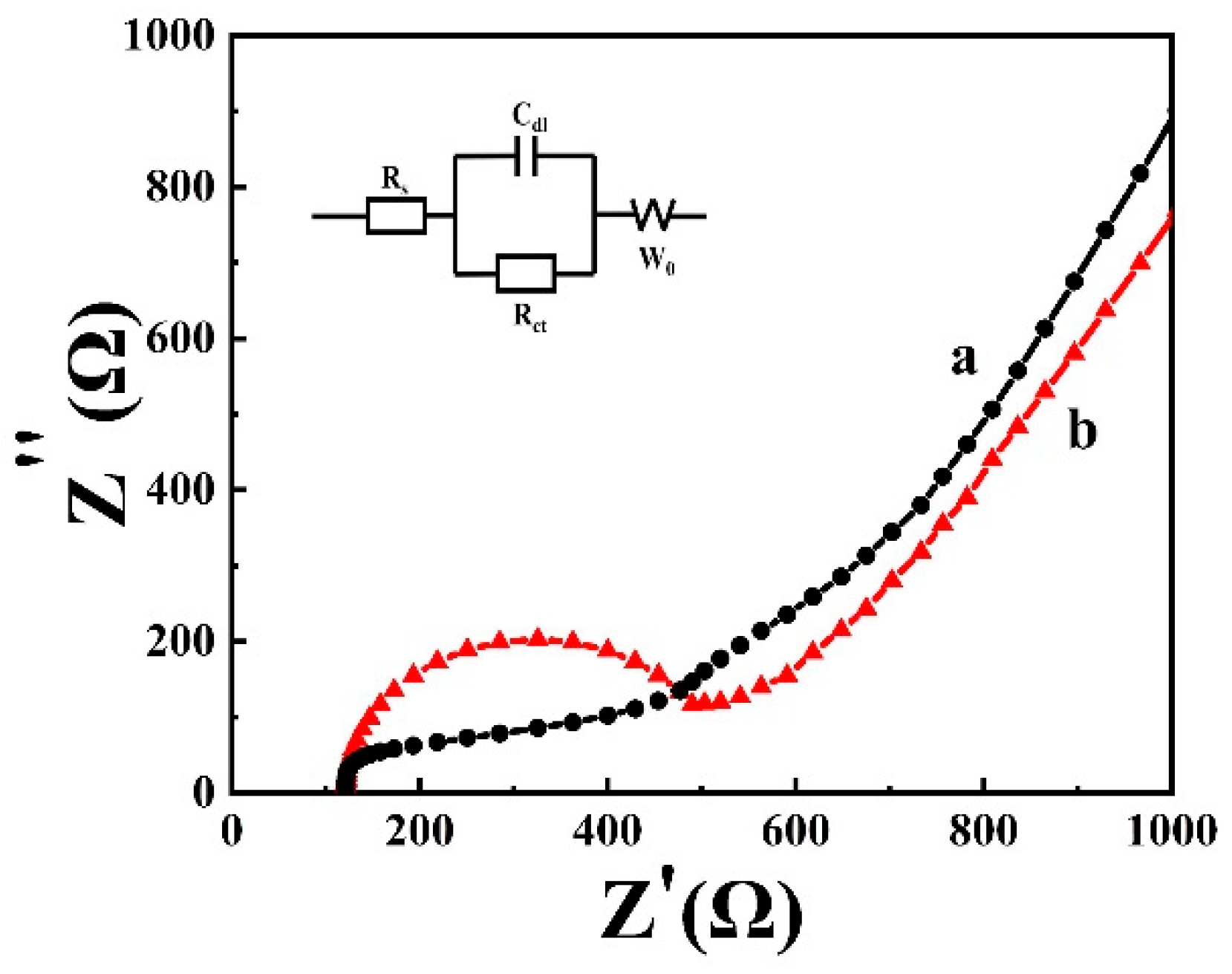
3.3. Electrochemical Impedance of CLR-PC/GCE
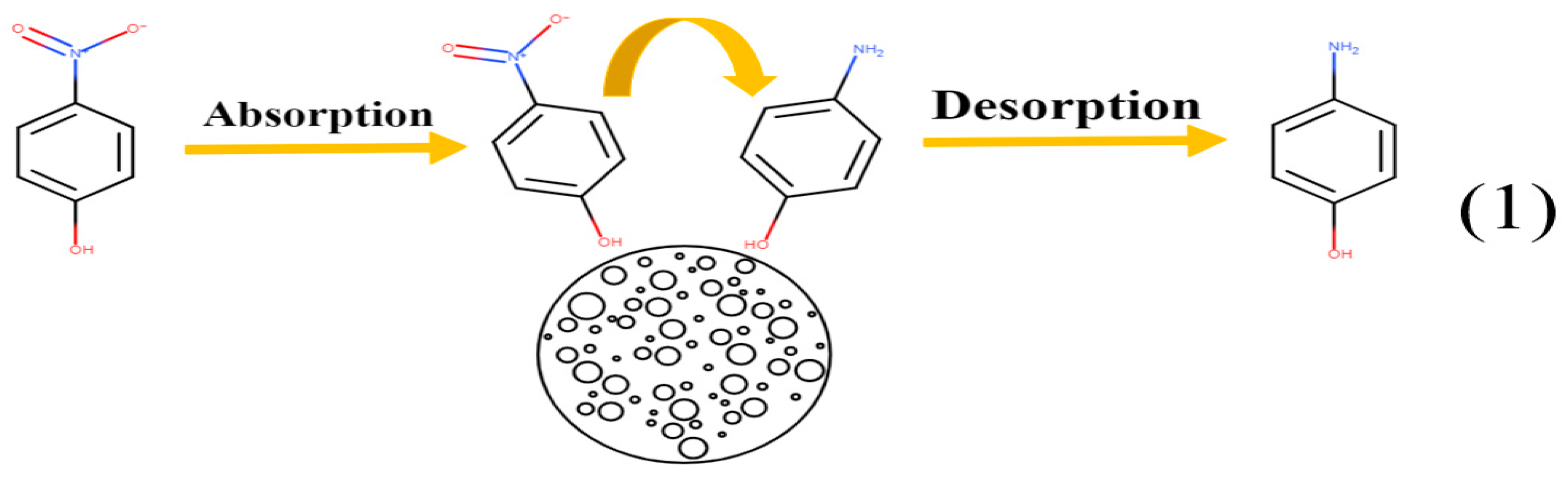
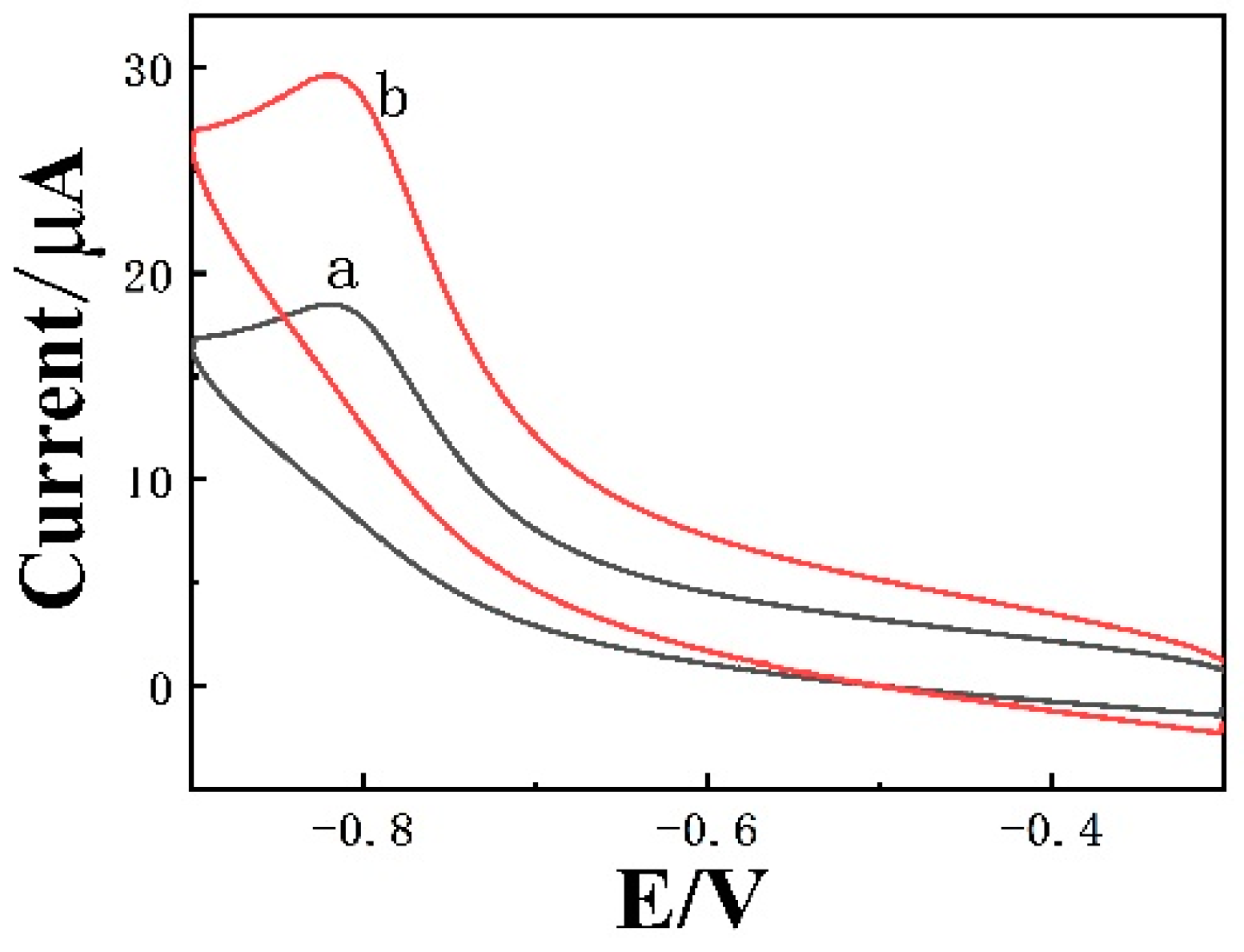
3.4. Electrochemical Behavior of 4-NP on CLR-PC/GCE
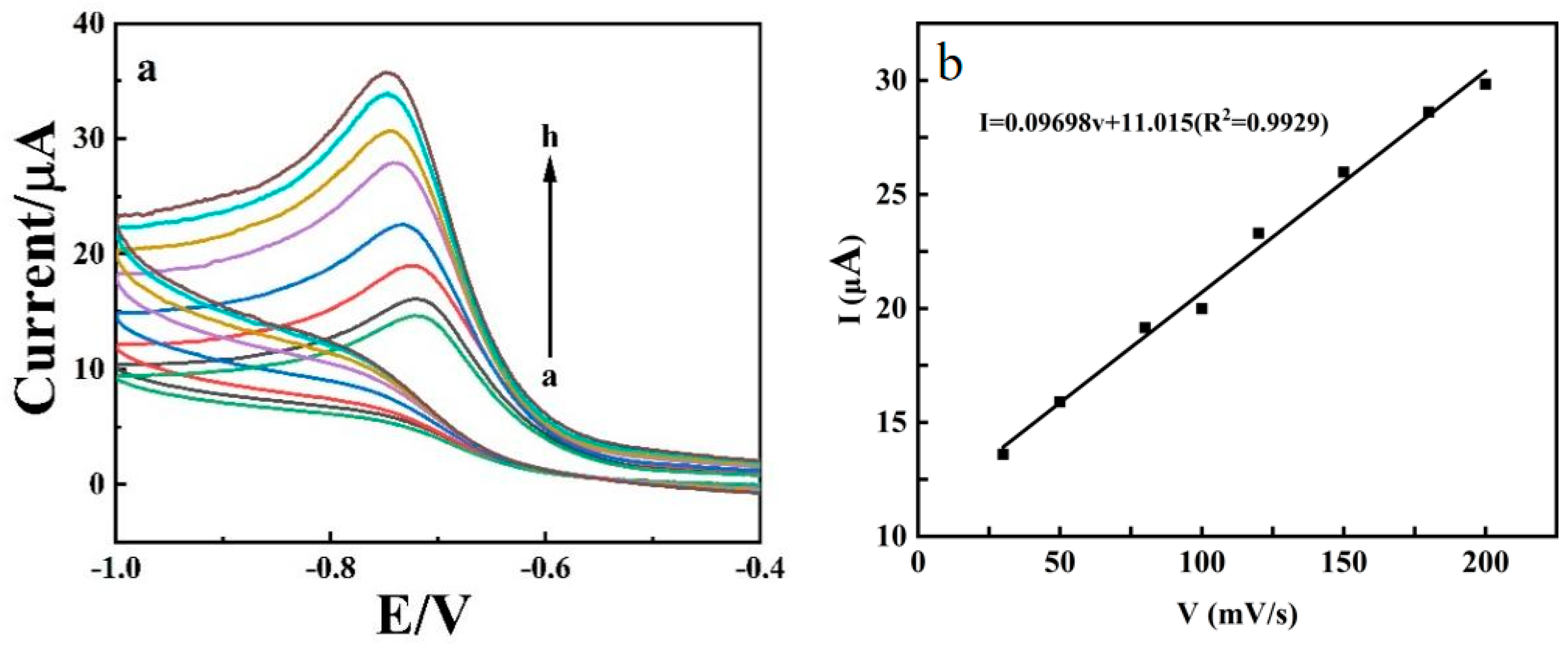
3.5. Effects of Scan Rate
3.6. pH Effect
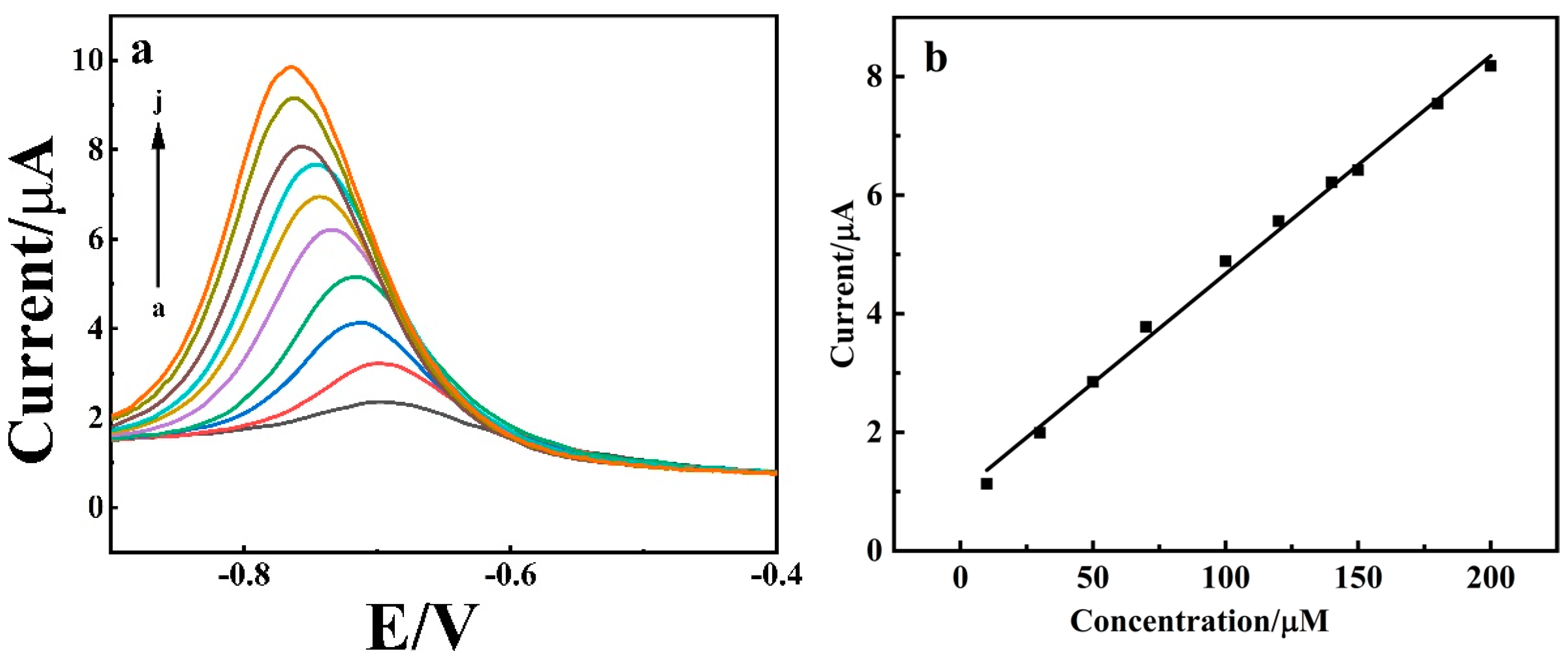
3.7. Electrochemical Determination of 4-NP
3.8. Inferences Study
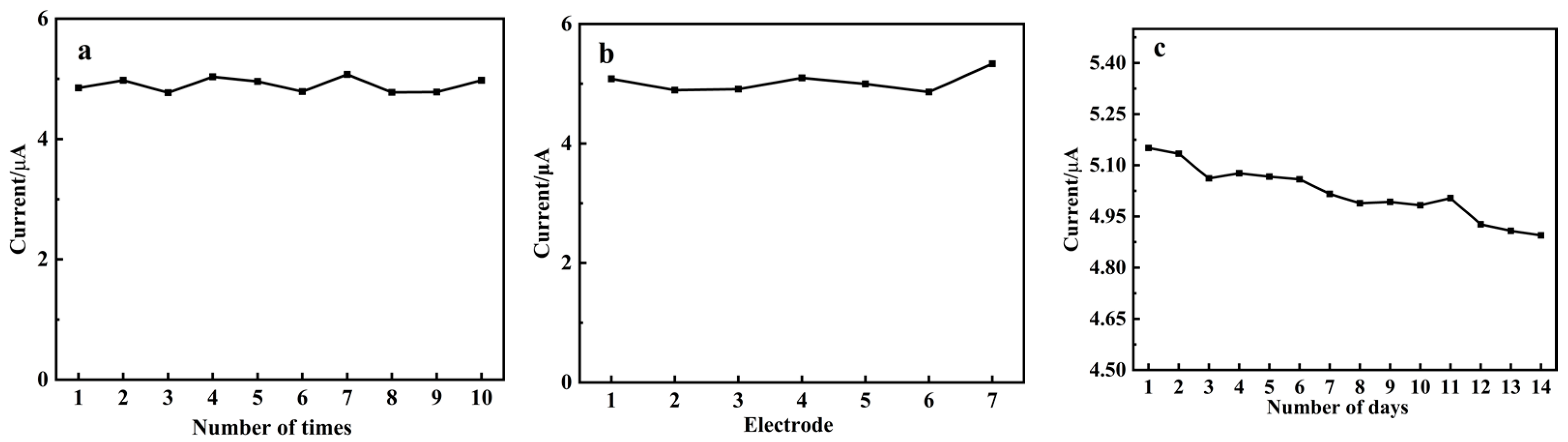
3.9. Repeatability, Reproducibility and Stability of CLR-PC/GCE
3.10. Analytical Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Y.; Kong, L.T.; Yang, R.; Wang, L.; Liu, J.H.; Huang, J.X. Single-walled carbon nanotube/pyrenecyclodextrin nanohybrids for ultrahighly sensitive and selective detection of p-nitrophenol. Langmuir 2011, 27, 10295–10301. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Sheikh, T.A.; Asiri, A.M.; Alamry, K.A.; Hasnat, M.A. Fabrication of an ultra-sensitive para-nitrophenol sensor based on facile Zn-doped Er2O3 nanocomposites via an electrochemical approach. Anal. Methods 2020, 12, 3470–3483. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, T.; Mahmood, T.; Ayub, K. Silver-graphene quantum dots based electrochemical sensor for trinitrotoluene and p-nitrophenol. Mol. Liq. 2020, 306, 112878–112890. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Q.; Xia, Z.; Gui, G.F.; Deng, F. Graphdiyne oxides as new modifier for the simultaneous electrochemical detection of phenolic compounds. Electroanal. Chem. 2020, 863, 114058–114088. [Google Scholar] [CrossRef]
- Yu, M.Y.; Liu, J.H.; Liu, C.; Pei, W.Y.; Ma, J.F. Resorcin[4]arene-based microporous metal–organic framework/reduced graphene oxide composite as an electrocatalyst for effective and simultaneous determination of p-nitrophenol and o-nitrophenol isomers. Sens. Actuators B Chem. 2021, 347, 130604–130625. [Google Scholar] [CrossRef]
- Mohanta, D.; Mahanta, A.; Mishra, S.R.; Jasimuddin, S.; Ahmaruzzaman, M. Novel SnO2@ZIF-8/gC3N4 nanohybrids for excellent electrochemical performance towards sensing of p-nitrophenol. Environ. Res. 2021, 197, 111077–111090. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Lichtfouse, E.; Song, J.; Gong, R.; Zhang, J.H.; Wang, S.; Xiao, L.L. In situ electrochemical synthesis of graphene-poly(arginine) composite for p-nitrophenol monitoring. J. Hazard Mater. 2022, 421, 126718–126727. [Google Scholar] [CrossRef]
- Baikeli, Y.; Mamat, X.; Chen, L.; Liu, X.S.; Shen, L.; Luy, Y.; Li, C.H. Ultrasensitive and simultaneous determination of p-Nitrophenol and p-Nitrobenzoic acid by a modified glassy carbon electrode with N-rich nanoporous carbon derived from ZIF-8. Electroanal. Chem. 2021, 899, 115567–115585. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Li, R.; Zhao, Y.J.; Zhe, T.T.; Bu, T.; Liu, Y.N.; Sun, X.Y.; Hu, H.F.; Zhang, M.; Zheng, X.H.; et al. Surface morphology-controllable magnetic covalent organic frameworks: A novel electrocatalyst for simultaneously high-performance detection of p-nitrophenol and o-nitrophenol. Talanta 2020, 219, 121255–121286. [Google Scholar] [CrossRef]
- Wei, W.; Yang, S.; Hu, H.H.; Li, H.; Jiang, Z.F. Hierarchically grown ZnFe2O4-decorated polyaniline-coupled-graphene nanosheets as a novel electrocatalyst for selective detecting p-nitrophenol. Microchem. J. 2021, 160, 105777–106791. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, J.; Kumar, V.; Tikoo, K.B.; Rana, S.; Kaushik, A.; Singhal, S. Unfolding the electrocatalytic efficacy of highly conducting NiFe2O4-rGO nanocomposites on the road to rapid and sensitive detection of hazardous p-Nitrophenol. Electroanal. Chem. 2021, 887, 115161–115167. [Google Scholar]
- Ata, S.; Feroz, M.; Bibi, I. Investigation of electrochemical reduction and monitoring of p-nitrophenol on imprinted polymer modified electrode. Synth. Met. 2022, 287, 117083–117099. [Google Scholar] [CrossRef]
- Dib, M.; Moutcine, A.; Ouchetto, H. Novel synthesis of α-Fe2O3@Mg/Al-CO3-LDH nanocomposite for rapid electrochemical detection of p-nitrophenol. Inorg. Chem. Commun. 2021, 131, 108788–108800. [Google Scholar] [CrossRef]
- Cheng, X.L.; Xia, X.; Xu, Q.Q.; Wang, J.; Sun, J.C.; Zhang, Y.X.; Li, S.S. Superior conductivity FeSe2 for highly sensitive electrochemical detection of p-nitrophenol and o-nitrophenol based on synergistic effect of adsorption and catalysis. Sens. Actuators B Chem. 2021, 348, 130692–130704. [Google Scholar] [CrossRef]
- Hofmann, D.; Hartmann, F.; Herrmann, H. Analysis of nitrophenols in cloud water with a miniaturized light-phase rotary perforator and HPLC-MS. Anal. Bioanal. Chem. 2008, 391, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.; Yang, S. Self-assembled Ti3C2TX MXene/graphene composite for the electrochemical reduction and detection of p-nitrophenol. Microchem. J. 2022, 179, 107473–1007389. [Google Scholar] [CrossRef]
- Karaová, J.; Barek, J.; Schwarzová-Pecková, K. Oxidative and Reductive Detection Modes for Determination of Nitrophenols by High-Performance Liquid Chromatography with Amperometric Detection at a Boron Doped Diamond Electrode. Anal. Lett. 2015, 49, 66–79. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Q.; Du, X. Synthesis of Carbon Nanofibers Film from Coal Liquefaction Residues: Effect of HNO3 Pretreatment. Energy Fuels 2022, 36, 4616–4624. [Google Scholar] [CrossRef]
- Zhang, W.; Wilson, C.R.; Danielson, N.D. Indirect fluorescent determination of selected nitro-aromatic and pharmaceutical compounds via UV-photolysis of 2-phenylbenzimidazole-5-sulfonate. Talanta 2008, 74, 1400–1407. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, G.S.; Dong, J.X. Typical Ecological and Environmental Issues and Countermeasures in Coal Mining in Xinjiang Region. Mining Saf. Environ. Prot. 2017, 44, 106–110. [Google Scholar]
- Yang, L.; Feng, Y.; Cao, M.; Yao, J. Two-step preparation of hierarchical porous carbon from KOH-activated wood sawdust for supercapacitor. Mater. Chem. Phys. 2019, 238, 121956. [Google Scholar] [CrossRef]
- Guan, L.; Pan, L.; Peng, T.; Gao, C.; Wu, M. Synthesis of biomass-derived nitrogen-doped porous carbon nanosheests for high-performance supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 8405–8412. [Google Scholar] [CrossRef]
- Tan, X.; Zhao, G.; Zhou, X.; Li, T.; Lei, H.; Du, G.; Yang, L. Electrochemical recognition of nitrophenol isomers by assembly of pillar[5]arenes mutifilms. Anal. Chim. Acta 2018, 1036, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Du, J.; Qiu, C.; Huang, L.; Ma, H.; Shen, D.; Ding, Y. Electrochemical sensor for detection of p-nitrophenol based on nanoporous gold. Electrochem. Commun. 2009, 11, 1365–1368. [Google Scholar] [CrossRef]
- Madhu, R.; Karuppiah, C.; Chen, S.M.; Veerakumar, P.; Liu, S.B. Electrochemical detection of 4-nitrophenol based on biomass derived activated carbons. Anal. Methods 2014, 6, 5274–5280. [Google Scholar] [CrossRef]
- Wang, H.B.; Li, Y.; Xu, M.T.; Dong, G.L.; Bai, H.Y. Porous Carbon Nanospheres for 4-aminophenol Detection based on Porous Carbon Nanospheres. Xinyang Norm. Univ. 2017, 30, 596–599. [Google Scholar]
- Arvinte, A.; Mahosenaho, M.; Pinteala, M.; Sesay, A.; Virtanen, V. Electrochemical oxidation of p-nitrophenol using graphene-modified electrodes, and a comparison to the performance of MWNT -based electrodes. Microchim Acta 2011, 174, 337–343. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Zhao, J.; Li, S.H.; Guo, M.J.; Fan, Z. Electrochemical Sensor for Detection of p-Nitrophenol Based on Cyclodextrin Decorated Gold Nanoparticles-Mesoporous Carbon Hybrid. Analyst 2019, 144, 4395–4399. [Google Scholar] [CrossRef]
- Saadati, F.; Ghahramani, F.; Shayani-jam, H.; Piriet, F.; Yaftian, M.R. Synthesis and characterization of nanostructure molecularly imprinted polyaniline/graphene oxide composite as highly selective electrochemical sensor for detection of p -nitrophenol. J. Taiwan Inst. Chem. Eng. 2018, 86, 213–221. [Google Scholar] [CrossRef]
- Li, C.; Wu, Z.; Yang, H.; Deng, L.; Chen, X. Reduced graphene oxide-cyclodextrin-chitosan electrochemical sensor: Effective and simultaneous determination of o- and p-nitrophenols. Sens. Actuators 2017, b251, 446–454. [Google Scholar] [CrossRef]


| Electrode Material | Linear Range (μmol·L−1) | Limit of Detection (μmol·L−1) | Reference |
|---|---|---|---|
| MWNT-Nafion/GCE 1 | 10–620 | 1.3 | [27] |
| AcSCD-AuNPs-MC 2 | 0.1–350 | 26.1 | [28] |
| p -NP-MIP-PANI/GO 3 | 60–140 | 20 | [29] |
| CLR-PC/GCE | 10~200 | 1.169 | This work |
| Sample | Injection (μmol·L−1) | Detection (μmol·L−1) | RSD (%, n = 3) | Recovery Efficiency (%) |
|---|---|---|---|---|
| 10 | 9.006 | 2.67% | 90.06% | |
| Tap water | 30 | 28.183 | 1.904% | 94.94% |
| 70 | 66.622 | 2.17% | 95.17% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Lu, Y.; Yalikun, N.; Shi, C.; Wang, H.; Xu, Y.; Liu, J. Preparation and Application of Coal-Liquefaction-Residue-Based Carbon Material. Processes 2022, 10, 2455. https://doi.org/10.3390/pr10112455
Xu L, Lu Y, Yalikun N, Shi C, Wang H, Xu Y, Liu J. Preparation and Application of Coal-Liquefaction-Residue-Based Carbon Material. Processes. 2022; 10(11):2455. https://doi.org/10.3390/pr10112455
Chicago/Turabian StyleXu, Liang, Yizhe Lu, Nuerbiya Yalikun, Congchao Shi, Haoyang Wang, Yueyuan Xu, and Jie Liu. 2022. "Preparation and Application of Coal-Liquefaction-Residue-Based Carbon Material" Processes 10, no. 11: 2455. https://doi.org/10.3390/pr10112455
APA StyleXu, L., Lu, Y., Yalikun, N., Shi, C., Wang, H., Xu, Y., & Liu, J. (2022). Preparation and Application of Coal-Liquefaction-Residue-Based Carbon Material. Processes, 10(11), 2455. https://doi.org/10.3390/pr10112455






