A Review on the Hydrothermal Treatment of Food Waste: Processing and Applications
Abstract
1. Introduction
2. Hydrothermal Process
| Hydrothermal Process | Temperature Range (°C) | Pressure Range | Target Product |
|---|---|---|---|
| Hydrothermal carbonization | 180–260 | 1–8 MPa | Hydrochar |
| Hydrothermal liquefaction | 260–374 | 10–22.1 MPa | Oil |
| Hydrothermal gasification | >374 | >22.1 MPa | Syngas |
3. Factors Affecting the Hydrothermal Process of Food Waste
3.1. Temperature
3.1.1. The Influence of Temperature on Solid Products
3.1.2. The Influence of Temperature on Liquid Products
3.1.3. The Influence of Temperature on Gas Products
3.2. Pyrolysis Time
3.2.1. The Effect of Time on Solid Products
3.2.2. The Effect of Time on Liquid Products
3.2.3. The Effect of Time on Gas Products
3.3. Catalyst
3.3.1. Alkaline Catalysts
3.3.2. Acid Catalysts
3.3.3. Metal-Related Catalysts
3.3.4. Biocatalysts
3.4. Raw Material
4. Hydrothermal Reaction Pathways
5. Applications
5.1. Solid Product
5.2. Liquid Product
5.3. Gas Product
6. Challenges and Development Directions of Hydrothermal Treatment of Food Waste
- Economic analysis of the hydrothermal process of food waste is scarce, so it is necessary to analyze the economic efficiency of hydrothermal treatment to promote industrial applications.
- At present, the main research is intermittent experimental research, and there is almost no research on continuous hydrothermal treatment of food waste. In addition, there is no relevant continuous hydrothermal equipments on the market, so it is necessary to increase the research and development of relative equipment.
- The influence of reaction conditions has been discussed a lot regarding temperature and time, but the specific impact mechanisms of the catalyst are unclear. At the same time, there are few studies on influencing factors such as the aqueous phase cycle, pressure, and heating rate. Thus, the detailed study of hydrothermal experimental parameters should be strengthened in the future.
- In terms of the application of hydrothermal products, there is less research on the utilization of all components of oil, hydrochar and gas products, so it is necessary to strengthen research in this area to improve the high-value utilization of food waste.
- Most of the current reaction mechanisms are derived from model compounds, which is very different from the actual hydrothermal mechanism of food waste. Therefore, it is necessary to study the reaction mechanism of real food waste and explore the interaction mechanism between different components.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ajay, C.M.; Mohan, S.; Dinesha, P. Decentralized energy from portable biogas digesters using domestic kitchen waste: A review. Waste Manag. 2021, 125, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Srivastava, M.; Abd_Allah, E.F.; Singh, R.; Hashem, A.; Gupta, V.K. Biohydrogen production using kitchen waste as the potential substrate: A sustainable approach. Chemosphere 2021, 271, 129537. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, L.; An, Y.; He, W.; Themelis, N.J.; Li, G. Hydrothermal liquefaction of three kinds of starches into reducing sugars. J. Clean. Prod. 2016, 112, 1049–1054. [Google Scholar] [CrossRef]
- Chen, J.; Fan, Y.; Jiaqiang, E.; Cao, W.; Zhang, F.; Gong, J.; Liu, G.; Xu, W. Effects analysis on the gasification kinetic characteristics of food waste in supercritical water. Fuel 2019, 241, 94–104. [Google Scholar] [CrossRef]
- Su, H.; Hantoko, D.; Yan, M.; Cai, Y.; Kanchanatip, E.; Liu, J.; Zhou, X.; Zhang, S. Evaluation of catalytic subcritical water gasification of food waste for hydrogen production: Effect of process conditions and different types of catalyst loading. Int. J. Hydrog. Energy 2019, 44, 21451–21463. [Google Scholar] [CrossRef]
- Zhou, Y.; Engler, N.; Nelles, M. Symbiotic relationship between hydrothermal carbonization technology and anaerobic digestion for food waste in China. Bioresour. Technol. 2018, 260, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.P.; Kaushik, R.; Parshetti, G.K.; Mahmood, R.; Balasubramanian, R. Food waste-to-energy conversion technologies: Current status and future directions. Waste Manag. 2015, 38, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Aierzhati, A.; Stablein, M.J.; Wu, N.E.; Kuo, C.T.; Si, B.; Kang, X.; Zhang, Y. Experimental and model enhancement of food waste hydrothermal liquefaction with combined effects of biochemical composition and reaction conditions. Bioresour. Technol. 2019, 284, 139–147. [Google Scholar] [CrossRef]
- Déniel, M.; Haarlemmer, G.; Roubaud, A.; Weiss-Hortala, E.; Fages, J. Energy valorisation of food processing residues and model compounds by hydrothermal liquefaction. Renew. Sustain. Energy Rev. 2016, 54, 1632–1652. [Google Scholar] [CrossRef]
- Maag, A.; Paulsen, A.; Amundsen, T.; Yelvington, P.; Tompsett, G.; Timko, M. Catalytic Hydrothermal Liquefaction of Food Waste Using CeZrOx. Energies 2018, 11, 564. [Google Scholar] [CrossRef]
- Fernandez-Sanroman, A.; Lama, G.; Pazos, M.; Rosales, E.; Sanroman, M.A. Bridging the gap to hydrochar production and its application into frameworks of bioenergy, environmental and biocatalysis areas. Bioresour. Technol. 2021, 320 Pt B, 124399. [Google Scholar] [CrossRef]
- Tekin, K.; Karagöz, S.; Bektaş, S. A review of hydrothermal biomass processing. Renew. Sustain. Energy Rev. 2014, 40, 673–687. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, X.; Wang, C.; Zhong, L.; Fu, F.; Zhu, J.; Zhang, Z.; Qin, Y.; Yang, D.; Xu, C.C. Lignin derived carbon materials: Current status and future trends. Carbon Res. 2022, 1, 14. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, T.; Zhu, Y.; Peng, C.; Wang, B.; Li, X.; Li, C.; Zeng, G. Production of fuel pellets via hydrothermal carbonization of food waste using molasses as a binder. Waste Manag. 2018, 77, 185–194. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Hydrothermal conversion of urban food waste to chars for removal of textile dyes from contaminated waters. Bioresour. Technol. 2014, 161, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Song, Y.; Kwon, J.E.; Woo, J.; Kim, H. Characterization of food waste-driven carbon dot focusing on chemical structural, electron relaxation behavior and Fe3+ selective sensing. Data Brief 2019, 25, 104038. [Google Scholar] [CrossRef]
- Shen, Y. A review on hydrothermal carbonization of biomass and plastic wastes to energy products. Biomass Bioenergy 2020, 134, 105479. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, Y.-Y.; Liu, H.-C.; Chen, T.-C.; Hung, C.-H.; Chen, C.-H.; Ong, H.C. A comprehensive analysis of food waste derived liquefaction bio-oil properties for industrial application. Appl. Energy 2019, 237, 283–291. [Google Scholar] [CrossRef]
- Matharu, A.S.; Houghton, J.A.; Lucas-Torres, C.; Moreno, A. Acid-free microwave-assisted hydrothermal extraction of pectin and porous cellulose from mango peel waste—Towards a zero waste mango biorefinery. Green Chem. 2016, 18, 5280–5287. [Google Scholar] [CrossRef]
- Nanda, S.; Isen, J.; Dalai, A.K.; Kozinski, J.A. Gasification of fruit wastes and agro-food residues in supercritical water. Energy Convers. Manag. 2016, 110, 296–306. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Akarsu, K.; Duman, G.; Yilmazer, A.; Keskin, T.; Azbar, N.; Yanik, J. Sustainable valorization of food wastes into solid fuel by hydrothermal carbonization. Bioresour. Technol. 2019, 292, 121959. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, J.; Pan, L.; Zhu, X.; Xie, S.; Yu, G.; Wang, Y.; Pan, X.; Zhu, G.; Angelidaki, I. Treatment of digestate residues for energy recovery and biochar production: From lab to pilot-scale verification. J. Clean. Prod. 2020, 265, 121852. [Google Scholar] [CrossRef]
- Saqib, N.U.; Baroutian, S.; Sarmah, A.K. Physicochemical, structural and combustion characterization of food waste hydrochar obtained by hydrothermal carbonization. Bioresour. Technol. 2018, 266, 357–363. [Google Scholar] [CrossRef]
- Ul Saqib, N.; Sarmah, A.K.; Baroutian, S. Effect of temperature on the fuel properties of food waste and coal blend treated under co-hydrothermal carbonization. Waste Manag. 2019, 89, 236–246. [Google Scholar] [CrossRef]
- Fu, M.-M.; Mo, C.-H.; Li, H.; Zhang, Y.-N.; Huang, W.-X.; Wong, M.H. Comparison of physicochemical properties of biochars and hydrochars produced from food wastes. J. Clean. Prod. 2019, 236, 117637. [Google Scholar] [CrossRef]
- Li, L.; Diederick, R.; Flora, J.R.; Berge, N.D. Hydrothermal carbonization of food waste and associated packaging materials for energy source generation. Waste Manag. 2013, 33, 2478–2492. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Influence of temperature on nitrogen fate during hydrothermal carbonization of food waste. Bioresour. Technol. 2018, 247, 182–189. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Peng, X.; Lin, Y.; Yao, Z. Conversion of sweet potato waste to solid fuel via hydrothermal carbonization. Bioresour. Technol. 2018, 249, 900–907. [Google Scholar] [CrossRef]
- Theppitak, S.; Hungwe, D.; Ding, L.; Xin, D.; Yu, G.; Yoshikawa, K. Comparison on solid biofuel production from wet and dry carbonization processes of food wastes. Appl. Energy 2020, 272, 115264. [Google Scholar] [CrossRef]
- Erdogan, E.; Atila, B.; Mumme, J.; Reza, M.T.; Toptas, A.; Elibol, M.; Yanik, J. Characterization of products from hydrothermal carbonization of orange pomace including anaerobic digestibility of process liquor. Bioresour. Technol. 2015, 196, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Zhu, Y.; Gan, X.; Zheng, L.; Peng, C.; Wang, B.; Li, C.; Zeng, G. Evaluation of the clean characteristics and combustion behavior of hydrochar derived from food waste towards solid biofuel production. Bioresour. Technol. 2018, 266, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Song, Y.; Kwon, J.E.; Lee, S.H.; Park, K.S.; Kim, S.; Woo, J.; Kim, H. Food waste-driven N-doped carbon dots: Applications for Fe3+ sensing and cell imaging. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Idowu, I.; Li, L.; Flora, J.R.V.; Pellechia, P.J.; Darko, S.A.; Ro, K.S.; Berge, N.D. Hydrothermal carbonization of food waste for nutrient recovery and reuse. Waste Manag. 2017, 69, 480–491. [Google Scholar] [CrossRef]
- Katakojwala, R.; Kopperi, H.; Kumar, S.; Venkata Mohan, S. Hydrothermal liquefaction of biogenic municipal solid waste under reduced H2 atmosphere in biorefinery format. Bioresour. Technol. 2020, 310, 123369. [Google Scholar] [CrossRef]
- Demirkaya, E.; Dal, O.; Yüksel, A. Liquefaction of waste hazelnut shell by using sub- and supercritical solvents as a reaction medium. J. Supercrit. Fluids 2019, 150, 11–20. [Google Scholar] [CrossRef]
- Posmanik, R.; Labatut, R.A.; Kim, A.H.; Usack, J.G.; Tester, J.W.; Angenent, L.T. Coupling hydrothermal liquefaction and anaerobic digestion for energy valorization from model biomass feedstocks. Bioresour. Technol. 2017, 233, 134–143. [Google Scholar] [CrossRef]
- Zhang, Y.; Minaret, J.; Yuan, Z.; Dutta, A.; Xu, C. Mild Hydrothermal liquefaction of high water content agricultural residue for bio-crude oil production: A parametric study. Energies 2018, 11, 3129. [Google Scholar] [CrossRef]
- Mahmood, R.; Parshetti, G.K.; Balasubramanian, R. Energy, exergy and techno-economic analyses of hydrothermal oxidation of food waste to produce hydro-char and bio-oil. Energy 2016, 102, 187–198. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, Y.-Y.; Liu, H.-C.; Baroutian, S. Optimization of food waste hydrothermal liquefaction by a two-step process in association with a double analysis. Energy 2020, 199, 117438. [Google Scholar] [CrossRef]
- Wang, L.; Chi, Y.; Shu, D.; Weiss-Hortala, E.; Nzihou, A.; Choi, S. Experimental studies of hydrothermal liquefaction of kitchen waste with H+, OH− and Fe3+ additives for bio-oil upgrading. Waste Manag. Res. 2021, 39, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Duman, G.; Akarsu, K.; Yilmazer, A.; Keskin Gundogdu, T.; Azbar, N.; Yanik, J. Sustainable hydrogen production options from food wastes. Int. J. Hydrogen Energy 2018, 43, 10595–10604. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, J.; Qiao, D.; Yue, L.; Li, Y.Y.; Zhou, J.; Cen, K. Investigating hydrothermal pretreatment of food waste for two-stage fermentative hydrogen and methane co-production. Bioresour. Technol. 2017, 241, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Zieminski, K.; Romanowska, I.; Kowalska-Wentel, M.; Cyran, M. Effects of hydrothermal pretreatment of sugar beet pulp for methane production. Bioresour. Technol. 2014, 166, 187–193. [Google Scholar] [CrossRef]
- Li, M.; Xia, T.; Zhu, C.; Xi, B.; Jia, X.; Wei, Z.; Zhu, J. Effect of short-time hydrothermal pretreatment of kitchen waste on biohydrogen production: Fluorescence spectroscopy coupled with parallel factor analysis. Bioresour. Technol. 2014, 172, 382–390. [Google Scholar] [CrossRef]
- Cheng, J.; Yue, L.; Hua, J.; Dong, H.; Zhou, J.; Li, Y.Y. Hydrothermal alkali pretreatment contributes to fermentative methane production of a typical lipid from food waste through co-production of hydrogen with methane. Bioresour. Technol. 2020, 306, 123164. [Google Scholar] [CrossRef]
- Han, D.; Yeom, K.; Park, S.; Cho, O.; Baek, Y. A Study on the Manufacture of Bio-SRF from the Food Waste by Hydrothermal Carbonization (HTC) Process. Trans. Korean Hydrog. New Energy Soc. 2017, 28, 426–432. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, J.; He, Z.; Chen, H.; Kandasamy, S. Hydrothermal liquefaction of fresh lemon-peel: Parameter optimisation and product chemistry. Renew. Energy 2019, 143, 512–519. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Y.; Zhou, Y.; Guo, W.; Jiang, H.; Xu, Q. Characterization of hydrothermal carbonization products (hydrochars and spent liquor) and their biomethane production performance. Bioresour. Technol. 2018, 267, 9–16. [Google Scholar] [CrossRef]
- Cantero-Tubilla, B.; Cantero, D.A.; Martinez, C.M.; Tester, J.W.; Walker, L.P.; Posmanik, R. Characterization of the solid products from hydrothermal liquefaction of waste feedstocks from food and agricultural industries. J. Supercrit. Fluids 2018, 133, 665–673. [Google Scholar] [CrossRef]
- Zhou, Y.; Engler, N.; Li, Y.; Nelles, M. The influence of hydrothermal operation on the surface properties of kitchen waste-derived hydrochar: Biogas upgrading. J. Clean. Prod. 2020, 259, 121020. [Google Scholar] [CrossRef]
- Gallifuoco, A.; Taglieri, L.; Papa, A.A. Hydrothermal carbonization of waste biomass to fuel: A novel technique for analyzing experimental data. Renew. Energy 2020, 149, 1254–1260. [Google Scholar] [CrossRef]
- Papa, A.A.; Taglieri, L.; Gallifuoco, A. Hydrothermal carbonization of waste biomass: An experimental comparison between process layouts. Waste Manag. 2020, 114, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Mahajani, S.M.; Garg, A. Investigation on hydrochar and macromolecules recovery opportunities from food waste after hydrothermal carbonization. Sci. Total Environ. 2020, 749, 142294. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-C.; Tsou, H.-K.; Hsu, H.-C.; Hsu, S.-K.; Liou, S.-P.; Ho, W.-F. A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite. Ceram. Int. 2013, 39, 8183–8188. [Google Scholar] [CrossRef]
- Lama-Munoz, A.; Rodriguez-Gutierrez, G.; Rubio-Senent, F.; Gomez-Carretero, A.; Fernandez-Bolanos, J. New hydrothermal treatment of alperujo enhances the content of bioactive minor components in crude pomace olive oil. J. Agric. Food Chem. 2011, 59, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Muangrat, R.; Onwudili, J.A.; Williams, P.T. Alkali-promoted hydrothermal gasification of biomass food processing waste: A parametric study. Int. J. Hydrogen Energy 2010, 35, 7405–7415. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Yu, C.; Jiang, J.; Guo, M.; Hantoko, D.; Yan, M. A two-step process for energy-efficient conversion of food waste via supercritical water gasification: Process design, products analysis, and electricity evaluation. Sci. Total Environ. 2021, 752, 142331. [Google Scholar] [CrossRef]
- Mackintosh, A.F.; Shin, T.; Yang, H.; Choe, K. Hydrothermal polymerization catalytic process effect of various organic wastes on reaction time, yield, and temperature. Processes 2020, 8, 303. [Google Scholar] [CrossRef]
- Yin, K.; Li, L.; Giannis, A.; Weerachanchai, P.; Ng, B.J.H.; Wang, J.-Y. High-quality fuel from food waste-investigation of a stepwise process from the perspective of technology development. Environ. Technol. 2017, 38, 1735–1741. [Google Scholar] [CrossRef]
- Xu, C.; Lad, N. Production of heavy oils with high caloric values by direct liquefaction of woody biomass in sub/near-critical water. Energy Fuels 2008, 22, 635–642. [Google Scholar] [CrossRef]
- Malins, K. The potential of K3PO4, K2CO3, Na3PO4 and Na2CO3 as reusable alkaline catalysts for practical application in biodiesel production. Fuel Process. Technol. 2018, 179, 302–312. [Google Scholar] [CrossRef]
- Muangrat, R.; Onwudili, J.A.; Williams, P.T. Influence of alkali catalysts on the production of hydrogen-rich gas from the hydrothermal gasification of food processing waste. Appl. Catal. B 2010, 100, 440–449. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, L.; Xi, G.; Sun, R.; Zhao, X. Catalytic valorization of expired fructan-rich food into the biofuel 5-ethoxymethylfurfural via a restaurant food waste-derived carbonaceous solid acid. Waste Biomass Valorization 2019, 11, 6223–6233. [Google Scholar] [CrossRef]
- Posmanik, R.; Martinez, C.M.; Cantero-Tubilla, B.; Cantero, D.A.; Sills, D.L.; Cocero, M.J.; Tester, J.W. Acid and alkali catalyzed hydrothermal liquefaction of dairy manure digestate and food waste. ACS Sustain. Chem. Eng. 2017, 6, 2724–2732. [Google Scholar] [CrossRef]
- Shen, D.; Wang, K.; Yin, J.; Chen, T.; Yu, X. Effect of phosphoric acid as a catalyst on the hydrothermal pretreatment and acidogenic fermentation of food waste. Waste Manag. 2016, 51, 65–71. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, M.-R.; Jeon, Y.J.; Kim, S.-K.; Hong, Y.-K.; Jeong, G.-T. Valorization of chitosan as food waste of aquatic organisms into 5-hydroxymethylfurfural by sulfamic acid-catalyzed conversion process. Energy Technol. 2018, 6, 1747–1754. [Google Scholar] [CrossRef]
- Puangubol, S.; Utistham, T.; Wetwatana, U. Production of bio-oil by hydrothermal pyrolysis of food waste over ceria catalyst. Curr. Opin. Biotechnol. 2011, 22, S49. [Google Scholar] [CrossRef]
- Wang, C.; Xie, S.; Zhong, M. Effect of hydrothermal pretreatment on kitchen waste for biodiesel production using alkaline catalyst. Waste Biomass Valorization 2016, 8, 369–377. [Google Scholar] [CrossRef]
- Cheng, F.; Tompsett, G.A.; Murphy, C.M.; Maag, A.R.; Carabillo, N.; Bailey, M.; Hemingway, J.J.; Romo, C.I.; Paulsen, A.D.; Yelvington, P.E.; et al. Synergistic effects of inexpensive mixed metal oxides for catalytic hydrothermal liquefaction of food wastes. ACS Sustain. Chem. Eng. 2020, 8, 6877–6886. [Google Scholar] [CrossRef]
- Ishida, Y.; Kumabe, K.; Hata, K.; Tanifuji, K.; Hasegawa, T.; Kitagawa, K.; Isu, N.; Funahashi, Y.; Asai, T. Selective hydrogen generation from real biomass through hydrothermal reaction at relatively low temperatures. Biomass Bioenergy 2009, 33, 8–13. [Google Scholar] [CrossRef]
- Tran, T.T.; Kaiprommarat, S.; Kongparakul, S.; Reubroycharoen, P.; Guan, G.; Nguyen, M.H.; Samart, C. Green biodiesel production from waste cooking oil using an environmentally benign acid catalyst. Waste Manag. 2016, 52, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Arun, J.; Gopinath, K.P.; SundarRajan, P.; Malolan, R.; Adithya, S.; Sai Jayaraman, R.; Srinivaasan Ajay, P. Hydrothermal liquefaction of Scenedesmus obliquus using a novel catalyst derived from clam shells: Solid residue as catalyst for hydrogen production. Bioresour. Technol. 2020, 310, 123443. [Google Scholar] [CrossRef] [PubMed]
- Chinglenthoiba, C.; Das, A.; Vandana, S. Enhanced biodiesel production from waste cooking palm oil, with NaOH-loaded Calcined fish bones as the catalyst. Environ. Sci. Pollut. Res. Int. 2020, 27, 15925–15930. [Google Scholar] [CrossRef]
- Chen, S.S.; Yu, I.K.M.; Cho, D.-W.; Song, H.; Tsang, D.C.W.; Tessonnier, J.-P.; Ok, Y.S.; Poon, C.S. Selective glucose isomerization to fructose via a nitrogen-doped solid base catalyst derived from spent coffee grounds. ACS Sustain. Chem. Eng. 2018, 6, 16113–16120. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, H.; Li, Y.; Yi, L.; Zhang, X.; Hu, H.; Yao, H. Correlations between hydrochar properties and chemical constitution of orange peel waste during hydrothermal carbonization. Bioresour. Technol. 2018, 265, 432–436. [Google Scholar] [CrossRef]
- Shen, D.; Yin, J.; Yu, X.; Wang, M.; Long, Y.; Shentu, J.; Chen, T. Acidogenic fermentation characteristics of different types of protein-rich substrates in food waste to produce volatile fatty acids. Bioresour. Technol. 2017, 227, 125–132. [Google Scholar] [CrossRef]
- Gollakota, A.; Savage, P.E. Hydrothermal liquefaction of model food waste biomolecules and ternary mixtures under isothermal and fast conditions. ACS Sustain. Chem. Eng. 2018, 6, 9018–9027. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Xiao, K.; Jin, M.; Xiao, H.; Yao, H. Combustion and pyrolysis characteristics of hydrochar prepared by hydrothermal carbonization of typical food waste: Influence of carbohydrates, proteins, and lipids. Energy Fuels 2019, 34, 430–439. [Google Scholar] [CrossRef]
- Kostyukevich, Y.; Vlaskin, M.; Borisova, L.; Zherebker, A.; Perminova, I.; Kononikhin, A.; Popov, I.; Nikolaev, E. Investigation of bio-oil produced by hydrothermal liquefaction of food waste using ultrahigh resolution Fourier transform ion cyclotron resonance mass spectrometry. Eur. J. Mass Spectrom. 2018, 24, 116–123. [Google Scholar] [CrossRef]
- Posmanik, R.; Cantero, D.A.; Malkani, A.; Sills, D.L.; Tester, J.W. Biomass conversion to bio-oil using sub-critical water: Study of model compounds for food processing waste. J. Supercrit. Fluids 2017, 119, 26–35. [Google Scholar] [CrossRef]
- Tasca, A.L.; Puccini, M.; Gori, R.; Corsi, I.; Galletti, A.M.R.; Vitolo, S. Hydrothermal carbonization of sewage sludge: A critical analysis of process severity, hydrochar properties and environmental implications. Waste Manag. 2019, 93, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.B.; Dubey, B.K. Co-hydrothermal carbonization of food waste with yard waste for solid biofuel production: Hydrochar characterization and its pelletization. Waste Manag. 2020, 118, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, S.; Cui, D.; Pan, S.; Xu, F.; Xu, F.; Wang, Z.; Li, G. Co-hydrothermal carbonization of corn stover and food waste: Characterization of hydrochar, synergistic effects, and combustion characteristic analysis. J. Environ. Chem. Eng. 2022, 10, 108716. [Google Scholar] [CrossRef]
- Wang, L.; Chi, Y.; Du, K.; Zhou, Z.; Wang, F.; Huang, Q. Hydrothermal treatment of food waste for bio-fertilizer production: Formation and regulation of humus substances in hydrochar. Sci. Total Environ. 2022, 838, 155900. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.; Ding, S.; Zhao, M.; Ji, Y.; Xie, W.; Feng, Z.; Feng, Y. Hydrothermal carbonization of kitchen waste: An analysis of solid and aqueous products and the application of hydrochar to paddy soil. Sci. Total Environ. 2022, 850, 157953. [Google Scholar] [CrossRef]
- Cavali, M.; Libardi, N., Jr.; de Sena, J.D.; Woiciechowski, A.L.; Soccol, C.R.; Filho, P.B.; Bayard, R.; Benbelkacem, H.; de Castilhos, A.B., Jr. A review on hydrothermal carbonization of potential biomass wastes, characterization and environmental applications of hydrochar, and biorefinery perspectives of the process. Sci. Total Environ. 2023, 857, 159627. [Google Scholar] [CrossRef]
- Hwang, I.-H.; Aoyama, H.; Matsuto, T.; Nakagishi, T.; Matsuo, T. Recovery of solid fuel from municipal solid waste by hydrothermal treatment using subcritical water. Waste Manag. 2012, 32, 410–416. [Google Scholar] [CrossRef]
- Berge, N.D.; Ro, K.S.; Mao, J.; Flora, J.R.V.; Chappell, M.A.; Bae, S. Hydrothermal Carbonization of Municipal Waste Streams. Environ. Sci. Technol. 2011, 45, 5696–5703. [Google Scholar] [CrossRef]
- Lu, X.; Berge, N.D. Influence of feedstock chemical composition on product formation and characteristics derived from the hydrothermal carbonization of mixed feedstocks. Bioresour. Technol. 2014, 166, 120–131. [Google Scholar] [CrossRef]
- Pala, M.; Kantarli, I.C.; Buyukisik, H.B.; Yanik, J. Hydrothermal carbonization and torrefaction of grape pomace: A comparative evaluation. Bioresour. Technol. 2014, 161, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, Z.; Zhang, Y.; Savage, P.E. Synergistic and antagonistic interactions during hydrothermal liquefaction of soybean oil, soy protein, cellulose, xylose, and lignin. ACS Sustain. Chem. Eng. 2018, 6, 14501–14509. [Google Scholar] [CrossRef]
- Fox, J.T.; Zook, A.N.; Freiss, J.; Appel, B.; Appel, J.; Ozsuer, C.; Sarac, M. Thermal conversion of blended food production waste and municipal sewage sludge to recoverable products. J. Clean. Prod. 2019, 220, 57–64. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Li, H.; Zhu, Y.; Li, S.; Peng, C.; Wang, B.; Wang, Z.; Xi, Y.; Wang, S.; et al. Co-hydrothermal carbonization of food waste-woody biomass blend towards biofuel pellets production. Bioresour. Technol. 2018, 267, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ma, X.; Yao, Z.; Chen, X. The properties and combustion behaviors of hydrochars derived from co-hydrothermal carbonization of sewage sludge and food waste. Bioresour. Technol. 2019, 285, 121347. [Google Scholar] [CrossRef]
- Mazumder, S.; Saha, P.; Reza, M.T. Co-hydrothermal carbonization of coal waste and food waste: Fuel characteristics. Biomass Convers. Biorefinery 2020, 12, 3–13. [Google Scholar] [CrossRef]
- Su, H.; Liao, W.; Wang, J.; Hantoko, D.; Zhou, Z.; Feng, H.; Jiang, J.; Yan, M. Assessment of supercritical water gasification of food waste under the background of waste sorting: Influences of plastic waste contents. Int. J. Hydrog. Energy 2020, 45, 21138–21147. [Google Scholar] [CrossRef]
- Chen, W.T.; Zhang, Y.H.; Zhang, J.X.; Yu, G.; Schideman, L.C.; Zhang, P.; Minarick, M. Hydrothermal liquefaction of mixed-culture algal biomass from wastewater treatment system into bio-crude oil. Bioresour. Technol. 2014, 152, 130–139. [Google Scholar] [CrossRef]
- Santos Santana, M.; Pereira Alves, R.; da Silva Borges, W.M.; Francisquini, E.; Guerreiro, M.C. Hydrochar production from defective coffee beans by hydrothermal carbonization. Bioresour. Technol. 2020, 300, 122653. [Google Scholar] [CrossRef]
- McGaughy, K.; Toufiq Reza, M. Hydrothermal carbonization of food waste: Simplified process simulation model based on experimental results. Biomass Convers. Biorefin. 2017, 8, 283–292. [Google Scholar] [CrossRef]
- Venna, S.; Sharma, H.B.; Reddy, P.H.P.; Chowdhury, S.; Dubey, B.K. Landfill leachate as an alternative moisture source for hydrothermal carbonization of municipal solid wastes to solid biofuels. Bioresour. Technol. 2021, 320 Pt B, 124410. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Cho, W.; Lee, J.-Y. Characterization of bio-coal made via hydrothermal carbonization of mixed organic waste. J. Korea Soc. Waste Manag. 2020, 37, 93–101. [Google Scholar] [CrossRef]
- Liu, H.; Ding, J.; Chen, L.; Ding, L. A novel fluorescence assay based on self-doping biomass carbon dots for rapid detection of dimethoate. J. Photochem. Photobiol. A 2020, 400, 112724. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Li, Y.; He, Z.; Xu, Q.; Chen, Y.; Street, J.; Guo, H.; Nelles, M. Multicolor carbon nanodots from food waste and their heavy metal ion detection application. RSC Adv. 2018, 8, 23657–23662. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Feng, M.; Zhan, H. Generation of nitrogen-doped photoluminescent carbonaceous nanodots via the hydrothermal treatment of fish scales for the detection of hypochlorite. RSC Adv. 2015, 5, 44636–44641. [Google Scholar] [CrossRef]
- Yao, G.; Guo, Y.; Le, Y.; Jin, B.; He, R.; Zhong, H.; Jin, F. Energy valorization of food waste: Rapid conversion of typical polysaccharide components to formate. Ind. Eng. Chem. Res. 2020, 59, 17069–17075. [Google Scholar] [CrossRef]
- Chengli, Y.; Jinmiao, Z. Mineralization of hydroxyapatite induced by eggshell as calcium source with hydrothermal synthesis method. Crystallogr. Rep. 2020, 65, 1242–1247. [Google Scholar] [CrossRef]
- Alif, M.F.; Aprillia, W.; Arief, S. A hydrothermal synthesis of natural hydroxyapatite obtained from Corbicula moltkiana freshwater clams shell biowaste. Mater. Lett. 2018, 230, 40–43. [Google Scholar] [CrossRef]
- Panahi-Kalamuei, M.; Amiri, O.; Salavati-Niasari, M. Green hydrothermal synthesis of high quality single and few layers graphene sheets by bread waste as precursor. J. Mater. Res. Technol. 2020, 9, 2679–2690. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Q. Functional materials development from kitchen waste. Procedia Environ. Sci. 2012, 16, 70–74. [Google Scholar] [CrossRef]
- Chung, W.; Oh, M.; Cho, W.; Park, S.-K.; Lee, J.-Y. A study on the adsorption of heavy metal with food waste bio-char using hydrothermal carbonization (HTC). J. Korea Soc. Waste Manag. 2016, 33, 137–144. [Google Scholar] [CrossRef]
- Feng, Y.; Sun, H.; Han, L.; Xue, L.; Chen, Y.; Yang, L.; Xing, B. Fabrication of hydrochar based on food waste (FWHTC) and its application in aqueous solution rare earth ions adsorptive removal: Process, mechanisms and disposal methodology. J. Clean. Prod. 2019, 212, 1423–1433. [Google Scholar] [CrossRef]
- Jin, F.; Zhang, G.; Jin, Y.; Watanabe, Y.; Kishita, A.; Enomoto, H. A new process for producing calcium acetate from vegetable wastes for use as an environmentally friendly deicer. Bioresour. Technol. 2010, 101, 7299–7306. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zhang, W.; Cao, S.; Wang, G.; Zhou, Z. Carbon-based Fe3O4 nanocomposites derived from waste pomelo peels for magnetic solid-phase extraction of 11 triazole fungicides in fruit samples. Nanomaterials 2018, 8, 302. [Google Scholar] [CrossRef]
- Fan, J.; De Bruyn, M.; Zhu, Z.; Budarin, V.; Gronnow, M.; Gomez, L.D.; Macquarrie, D.; Clark, J. Microwave-enhanced formation of glucose from cellulosic waste. Chem. Eng. Process. 2013, 71, 37–42. [Google Scholar] [CrossRef]
- Kannan, S.; Gariepy, Y.; Raghavan, V. Optimization of enzyme hydrolysis of seafood waste for microwave hydrothermal carbonization. Energy Fuels 2015, 29, 8006–8016. [Google Scholar] [CrossRef]
- Kaur, G.J.; Kumar, D.; Orsat, V.; Singh, A. Assessment of carrot rejects and wastes for food product development and as a biofuel. Biomass Convers. Biorefinery 2020, 12, 757–768. [Google Scholar] [CrossRef]
- Yu, X.; Yin, J.; Wang, K.; Shen, D.; Long, Y.; Chen, T. Enhancing food waste hydrolysis and the production rate of volatile fatty acids by prefermentation and hydrothermal pretreatments. Energy Fuels 2016, 30, 4002–4008. [Google Scholar] [CrossRef]
- Chen, W.-T.; Zhang, Y.; Lee, T.H.; Wu, Z.; Si, B.; Lee, C.-F.F.; Lin, A.; Sharma, B.K. Renewable diesel blendstocks produced by hydrothermal liquefaction of wet biowaste. Nat. Sustain. 2018, 1, 702–710. [Google Scholar] [CrossRef]
- Kaushik, R.; Parshetti, G.K.; Liu, Z.; Balasubramanian, R. Enzyme-assisted hydrothermal treatment of food waste for co-production of hydrochar and bio-oil. Bioresour. Technol. 2014, 168, 267–274. [Google Scholar] [CrossRef]
- Shao, Y.; Long, Y.; Zhou, Y.; Jin, Z.; Zhou, D.; Shen, D. 5-Hydroxymethylfurfural production from watermelon peel by microwave hydrothermal liquefaction. Energy 2019, 174, 198–205. [Google Scholar] [CrossRef]
- Mahssin, Z.Y.; Hassan, N.A.; Yaacob, H.; Puteh, M.H.; Amin, N.A.S.; Zainol, M.M.; Hainin, M.R. Characterization of asphalt binder containing hydrothermal liquefied composition extracted from food waste. IOP Conf. Ser. Earth Environ. Sci. 2019, 220, 012013. [Google Scholar] [CrossRef]
- Chua, G.K.; Tan, F.H.Y.; Chew, F.N.; Mohd-Hairul, A.R. Nutrients content of food wastes from different sources and its pre-treatment. AIP Conf. Proc. 2019, 2124, 020031. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, X.; Ye, J.; Sun, Y.; Wang, W.; Zhang, Z. Evaluation of biogas production from different biomass wastes with/without hydrothermal pretreatment. Renew. Energy 2011, 36, 3313–3318. [Google Scholar] [CrossRef]
- Jia, X.; Xi, B.; Li, M.; Xia, T.; Hao, Y.; Liu, D.; Hou, J. Evaluation of biogasification and energy consumption from food waste using short-term hydrothermal pretreatment coupled with different anaerobic digestion processes. J. Clean. Prod. 2017, 152, 364–368. [Google Scholar] [CrossRef]
- Redwood, M.D.; Orozco, R.L.; Majewski, A.J.; Macaskie, L.E. An integrated biohydrogen refinery: Synergy of photofermentation, extractive fermentation and hydrothermal hydrolysis of food wastes. Bioresour. Technol. 2012, 119, 384–392. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Nie, X.; Li, R.; Song, M. In-situ emission characteristics of odorous gases from two food waste processing plants. J. Mater. Cycles Waste Manag. 2013, 15, 510–515. [Google Scholar] [CrossRef]
- Villa Montoya, A.C.; da Silva Mazareli, R.C.; Silva, E.L.; Varesche, M.B.A. Improving the hydrogen production from coffee waste through hydrothermal pretreatment, co-digestion and microbial consortium bioaugmentation. Biomass Bioenergy 2020, 137, 105551. [Google Scholar] [CrossRef]
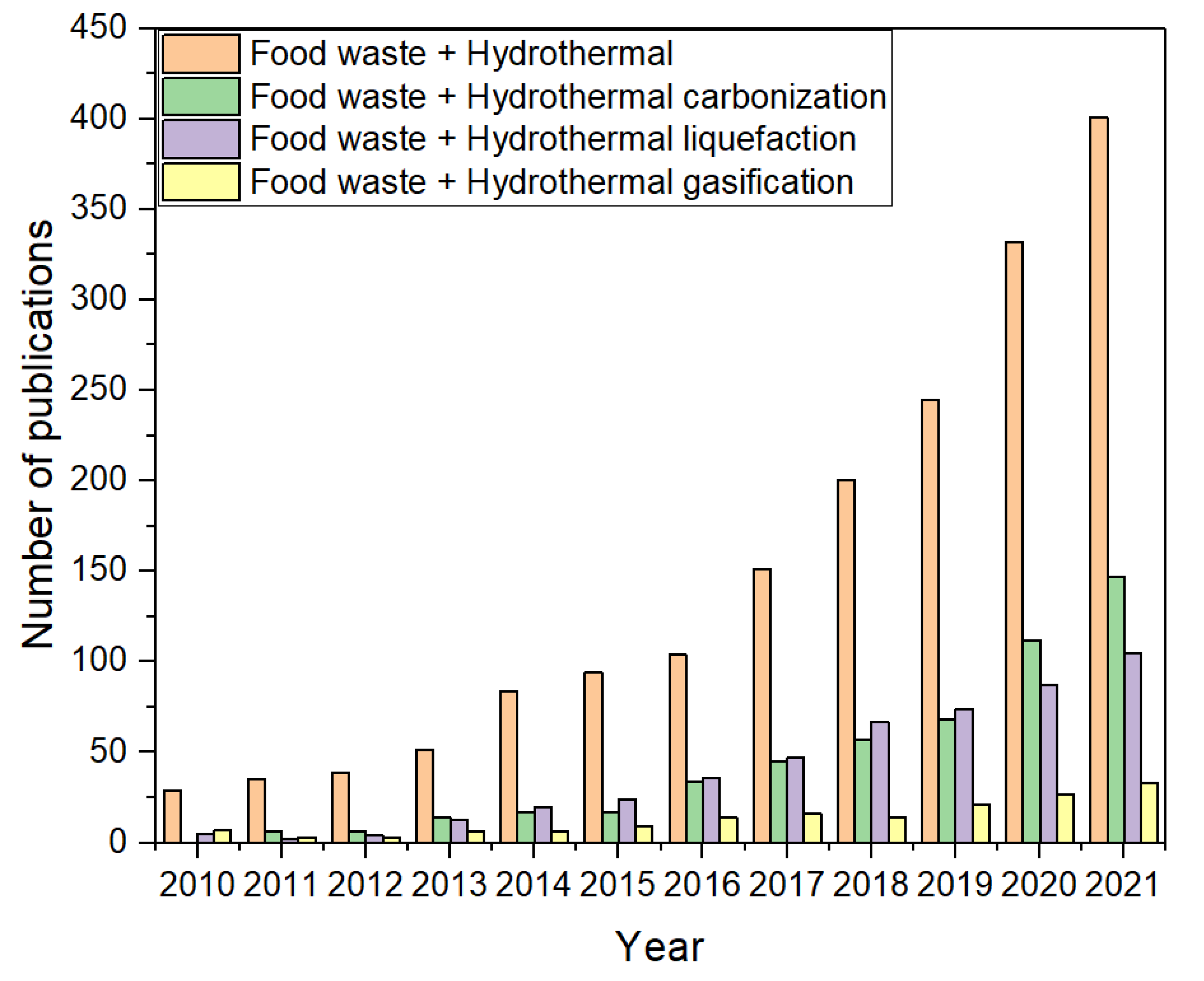


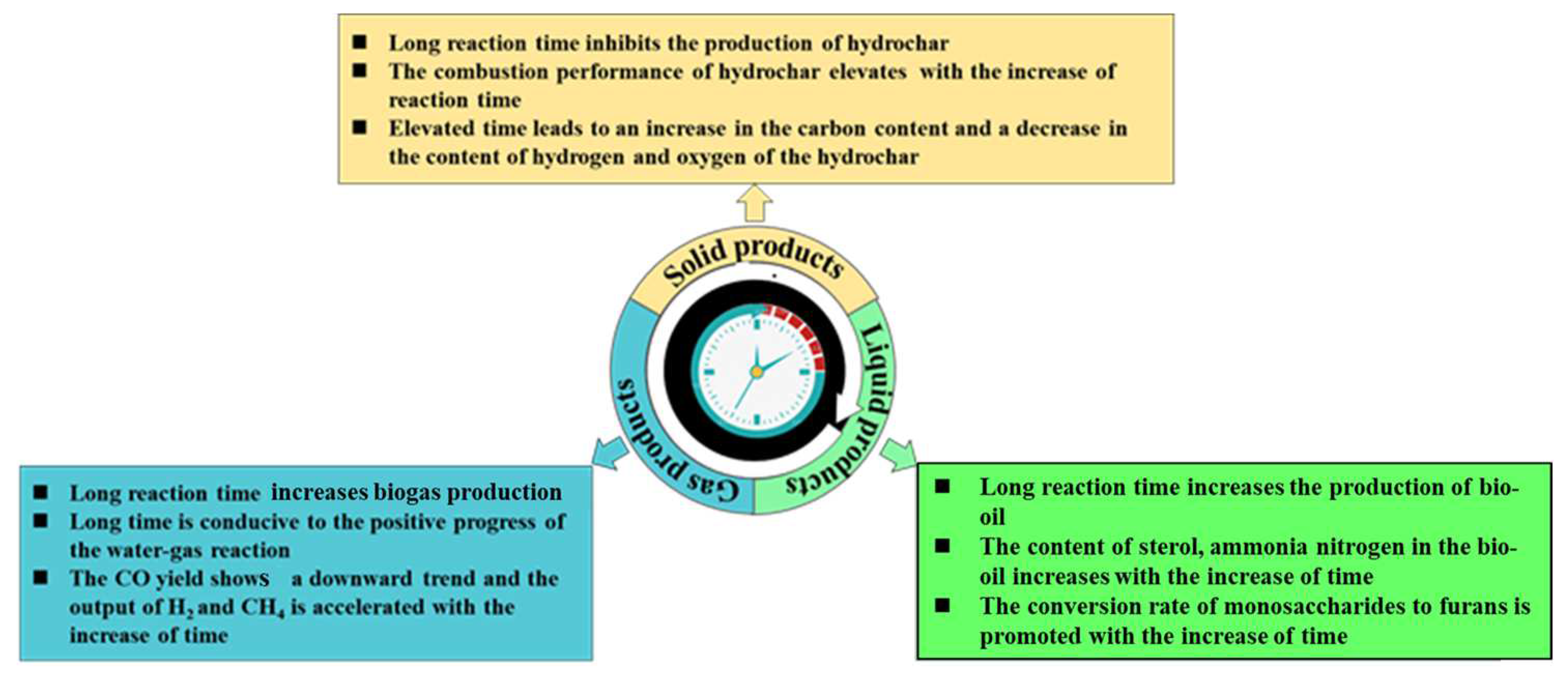
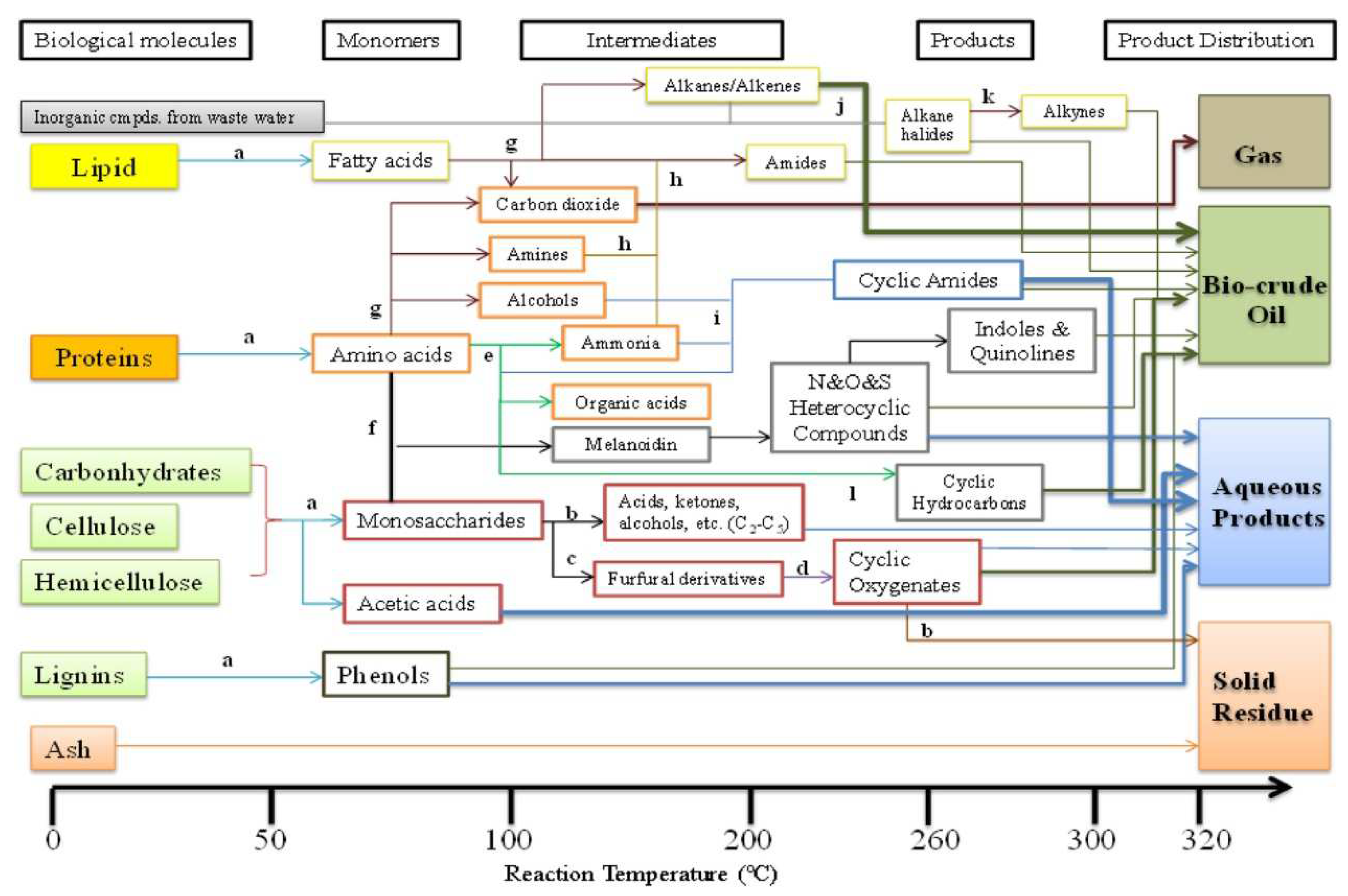


| Treatment Process | Advantages | Disadvantages |
|---|---|---|
| Landfill | Low cost, simple technology, more used in developing countries | Pollution of groundwater, occupying a large amount of land, and no resource recovery |
| Incineration | High degree of reduction, suitable for handling hazardous or toxic garbage | High cost, long capital recovery cycle, and low economic efficiency |
| Anaerobic digestion | High degree of automation, diversified products, high economic value | Need to screen suitable microorganisms, complex technology, discontinuous cycle, and difficult-to-treat biogas residue |
| Compost | High technology maturity, low cost | Low product value, environmental pollution, long cycle |
| Hydrothermal treatment | The product has high energy utilization value, simple process, low cost, and short cycle | Hydrothermal treatment products need to be further optimized |
| Feedstock | Temperature (°C) | Hydrochar Yield (%) | Heating Value (MJ/kg) | Reference |
|---|---|---|---|---|
| Dog food | 234–295 | 55.0 | 26.0 | [88] |
| Rabbit food | 250 | 43.8 | 29.1 | [89] |
| Restaurant food waste | 225–275 | 45.0 | 33.6 | [27] |
| Sweet corn | 250 | 50.0 | 11.0 | [90] |
| Grape pomace | 175–275 | 46.5 | 24.3–28.3 | [91] |
| Food waste from a student’s hostel mess | 200 | 48.5 | 30.0 | [54] |
| Food waste and yard waste | 220 | 59.8 | 27.6 | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Wang, Z.; Wang, X.; Li, N.; Tao, J.; Zheng, W.; Yan, B.; Cui, X.; Cheng, Z.; Chen, G. A Review on the Hydrothermal Treatment of Food Waste: Processing and Applications. Processes 2022, 10, 2439. https://doi.org/10.3390/pr10112439
Wang C, Wang Z, Wang X, Li N, Tao J, Zheng W, Yan B, Cui X, Cheng Z, Chen G. A Review on the Hydrothermal Treatment of Food Waste: Processing and Applications. Processes. 2022; 10(11):2439. https://doi.org/10.3390/pr10112439
Chicago/Turabian StyleWang, Chuanbin, Zhi Wang, Xutong Wang, Ning Li, Junyu Tao, Wandong Zheng, Beibei Yan, Xiaoqiang Cui, Zhanjun Cheng, and Guanyi Chen. 2022. "A Review on the Hydrothermal Treatment of Food Waste: Processing and Applications" Processes 10, no. 11: 2439. https://doi.org/10.3390/pr10112439
APA StyleWang, C., Wang, Z., Wang, X., Li, N., Tao, J., Zheng, W., Yan, B., Cui, X., Cheng, Z., & Chen, G. (2022). A Review on the Hydrothermal Treatment of Food Waste: Processing and Applications. Processes, 10(11), 2439. https://doi.org/10.3390/pr10112439











