Mechanisms of Separation and Crystal Growth of Mullite Grains during Preparation of Mullite-Based Ceramics from High Alumina Coal Fly Ash
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
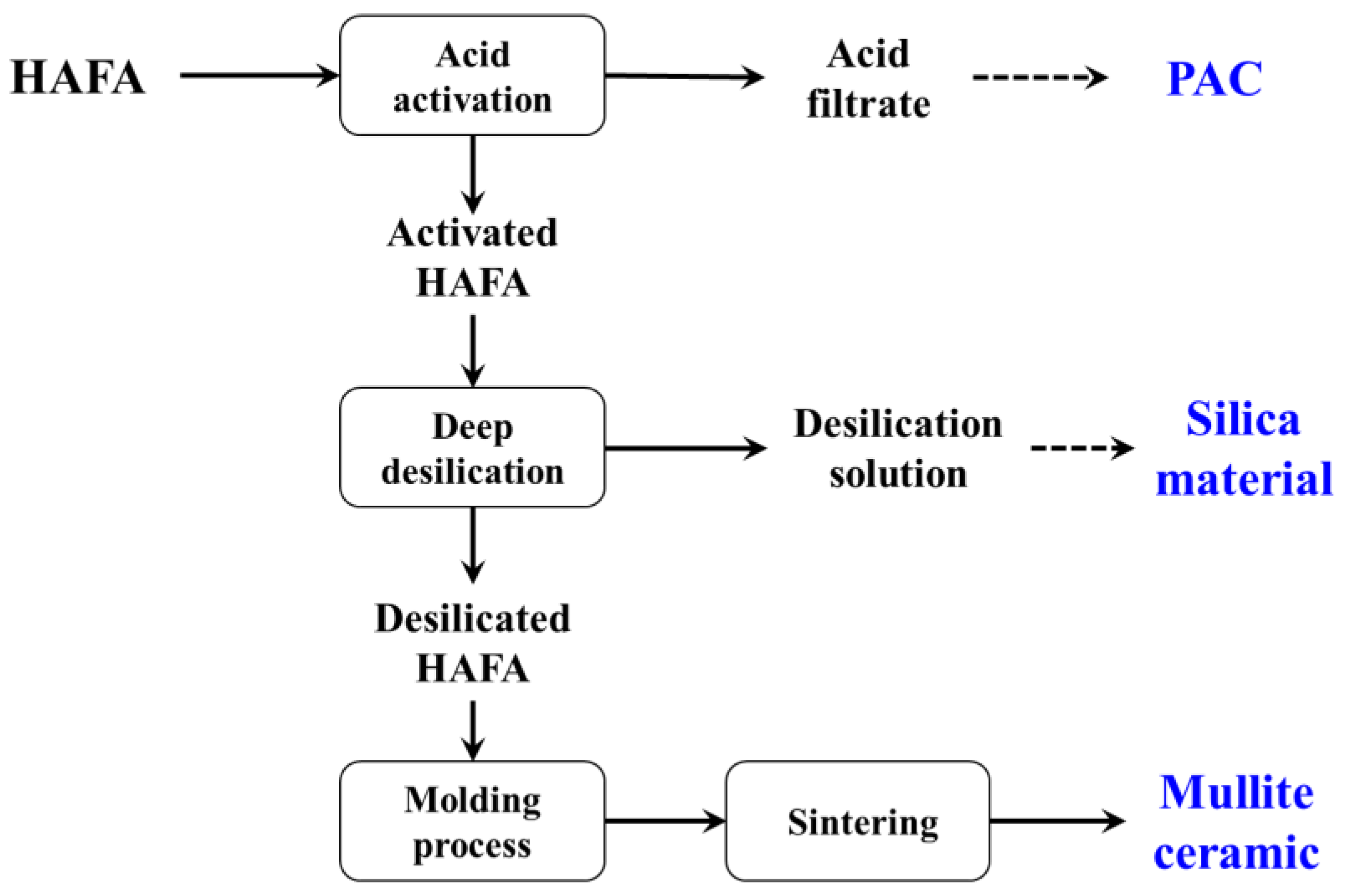
2.2. Experiments
2.3. Characterization
3. Results and Discussion
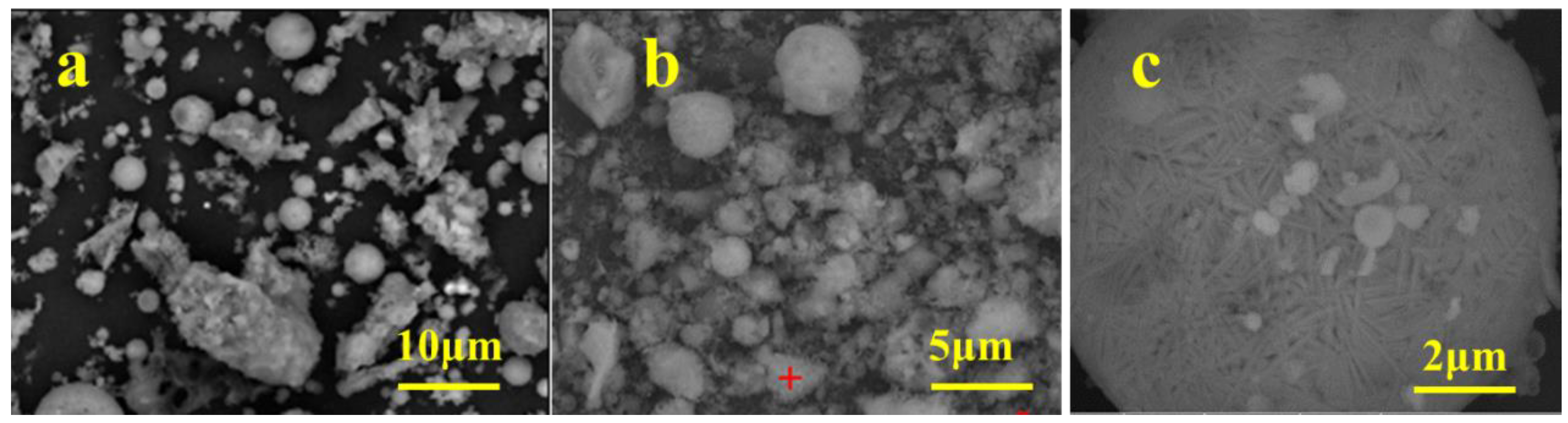
3.1. Properties of HAFA
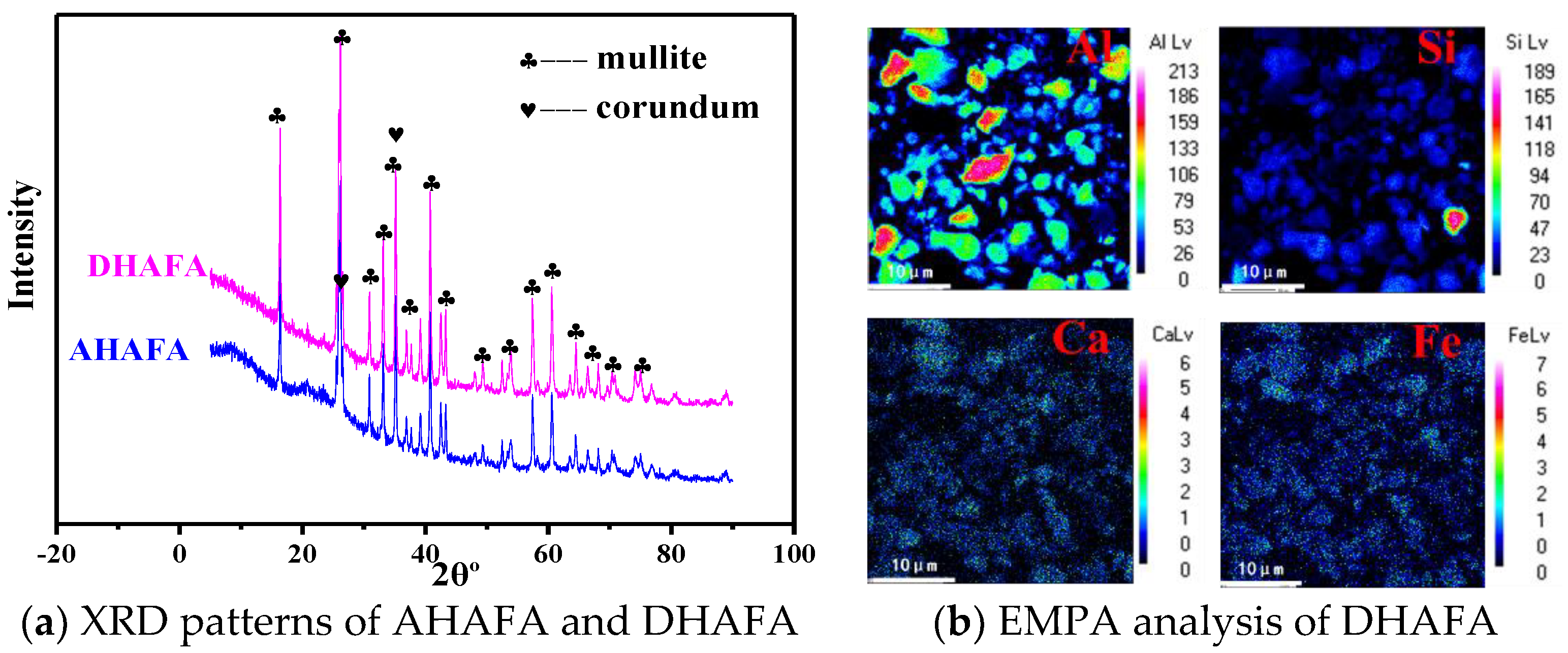
3.2. Investigation of Activation–Deep Desilication Process
3.3. Optimization and Characterization of Mullite Properties
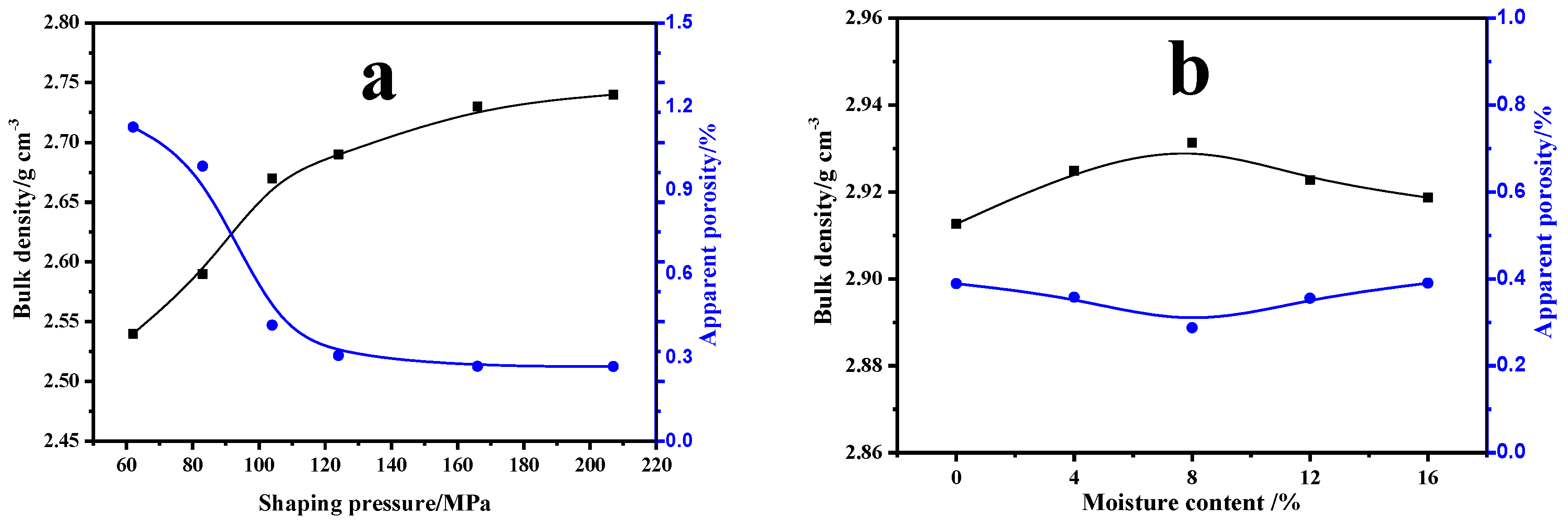
3.3.1. Effects of Forming Process on Mullite Properties
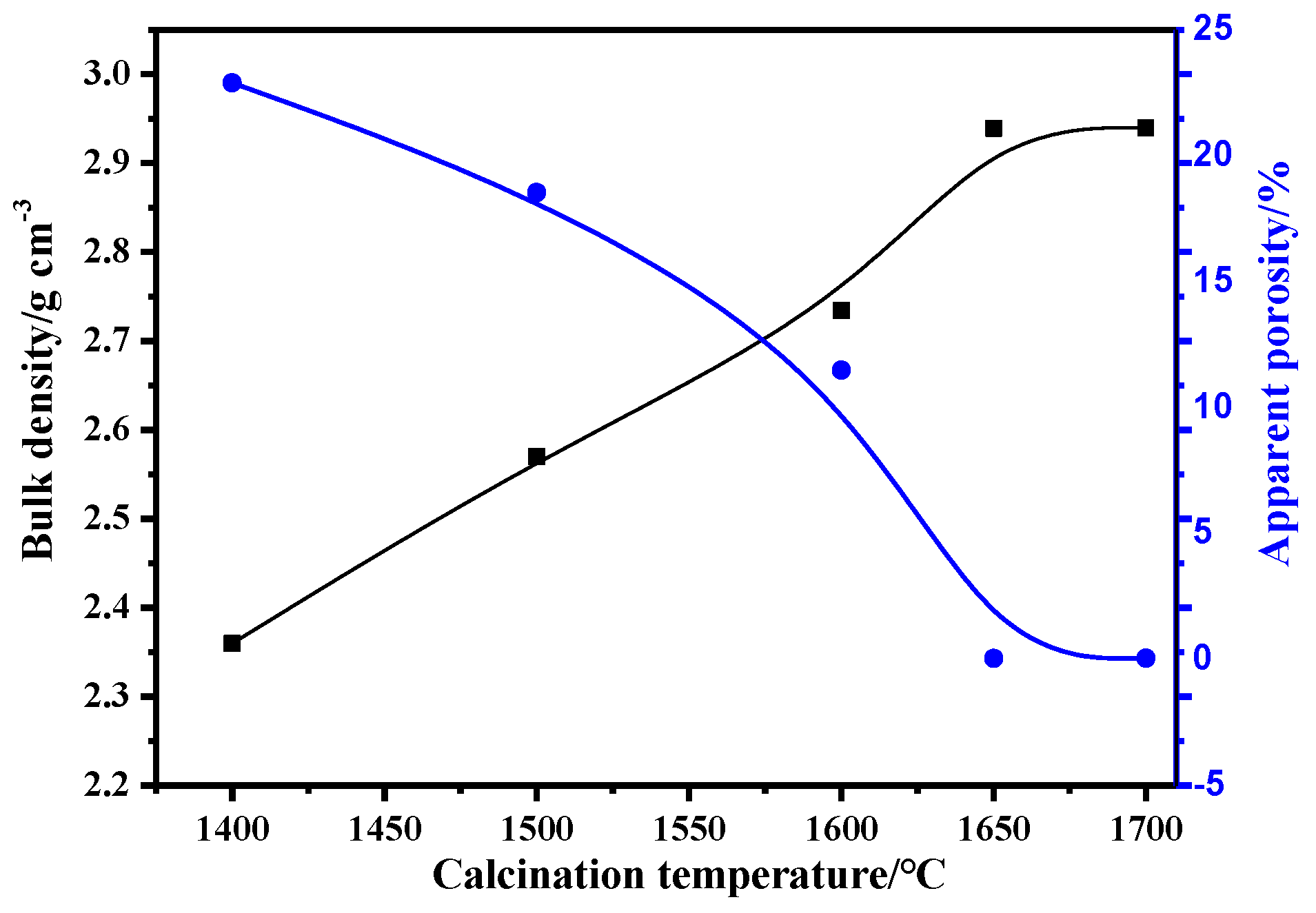
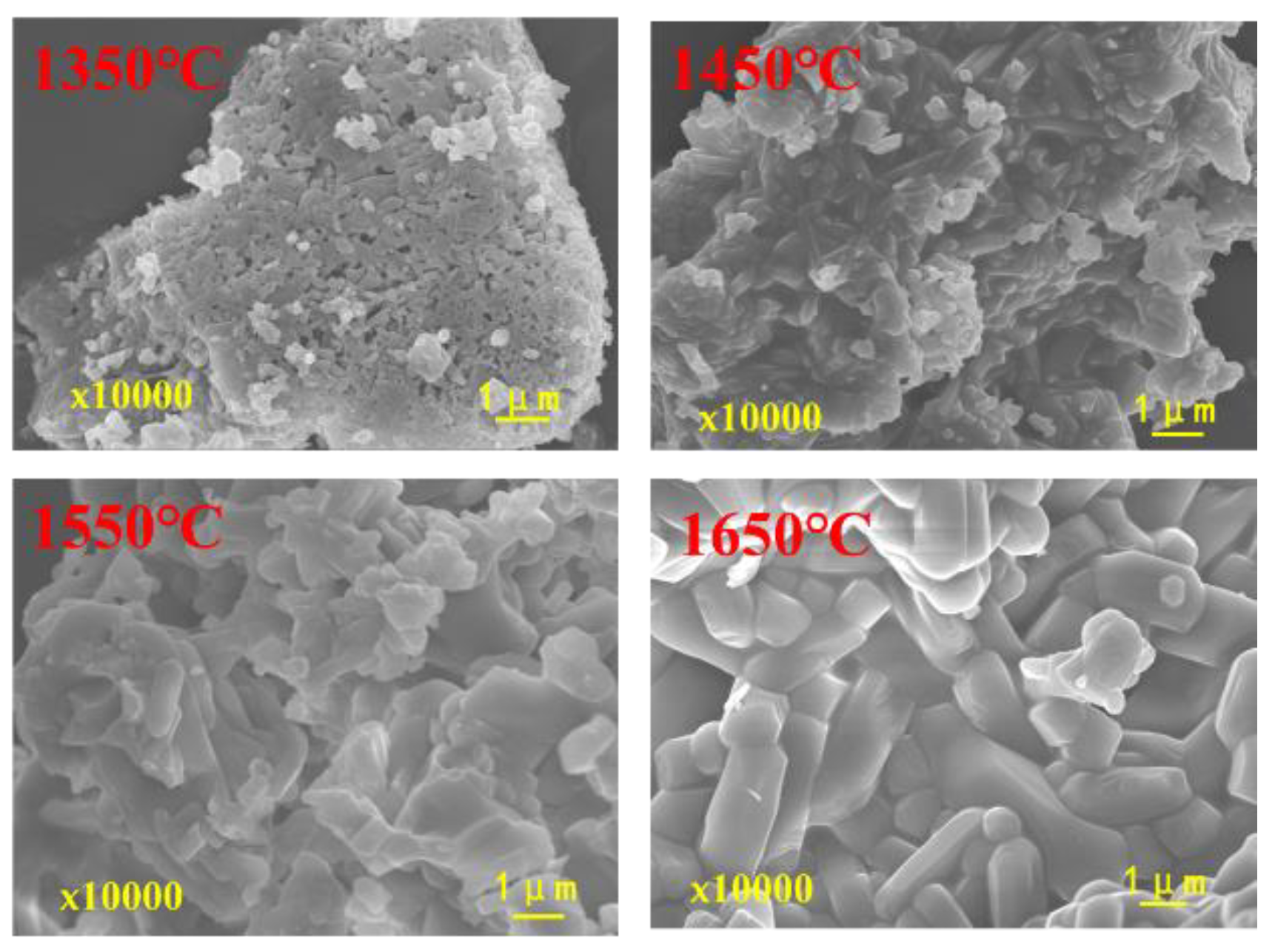
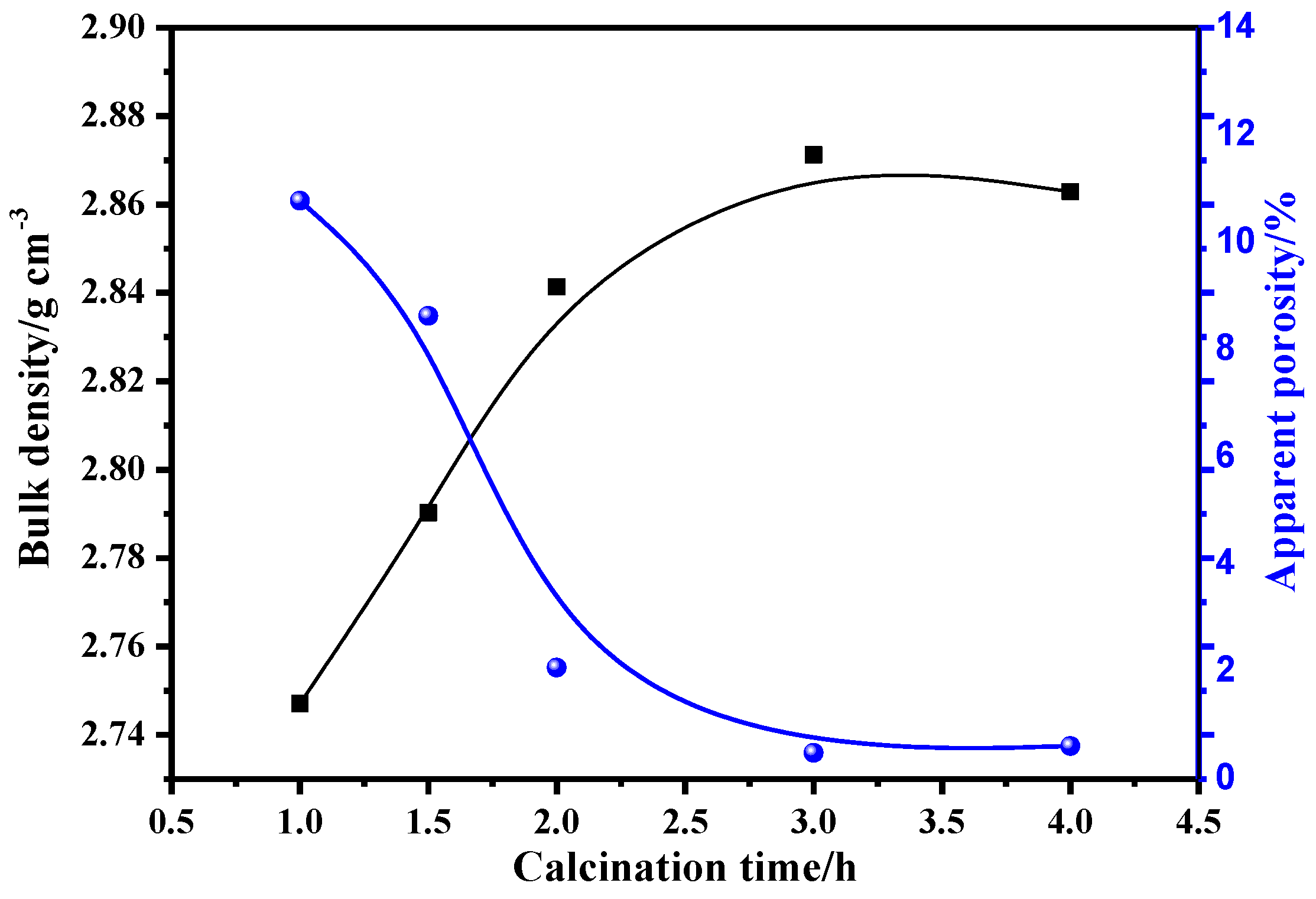
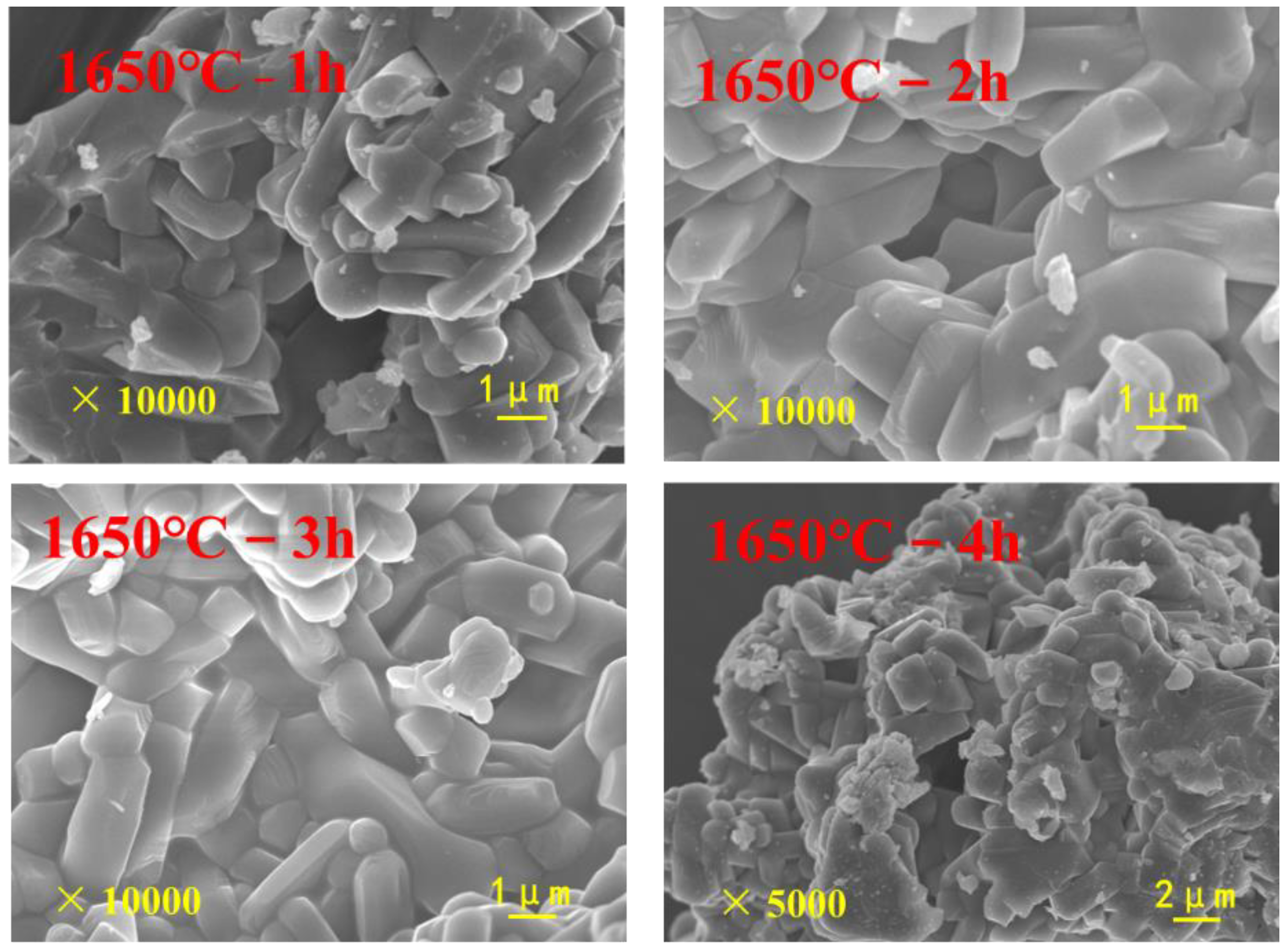
3.3.2. Effects of Calcination Process on the Properties of Mullite-Based Ceramics
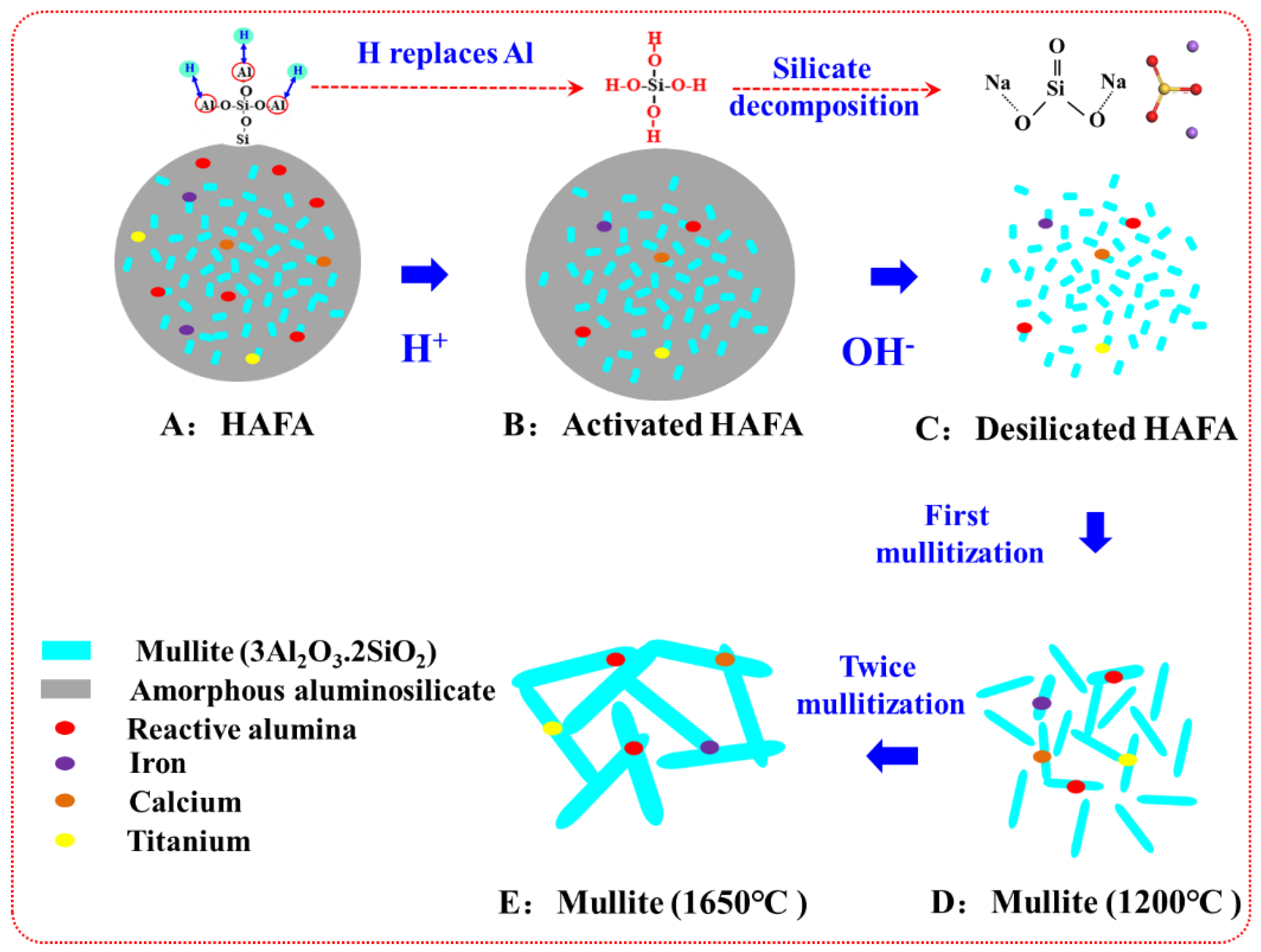
3.4. Mechanism of Activation–Deep Desilication–Sintering Process
4. Conclusions
- (1)
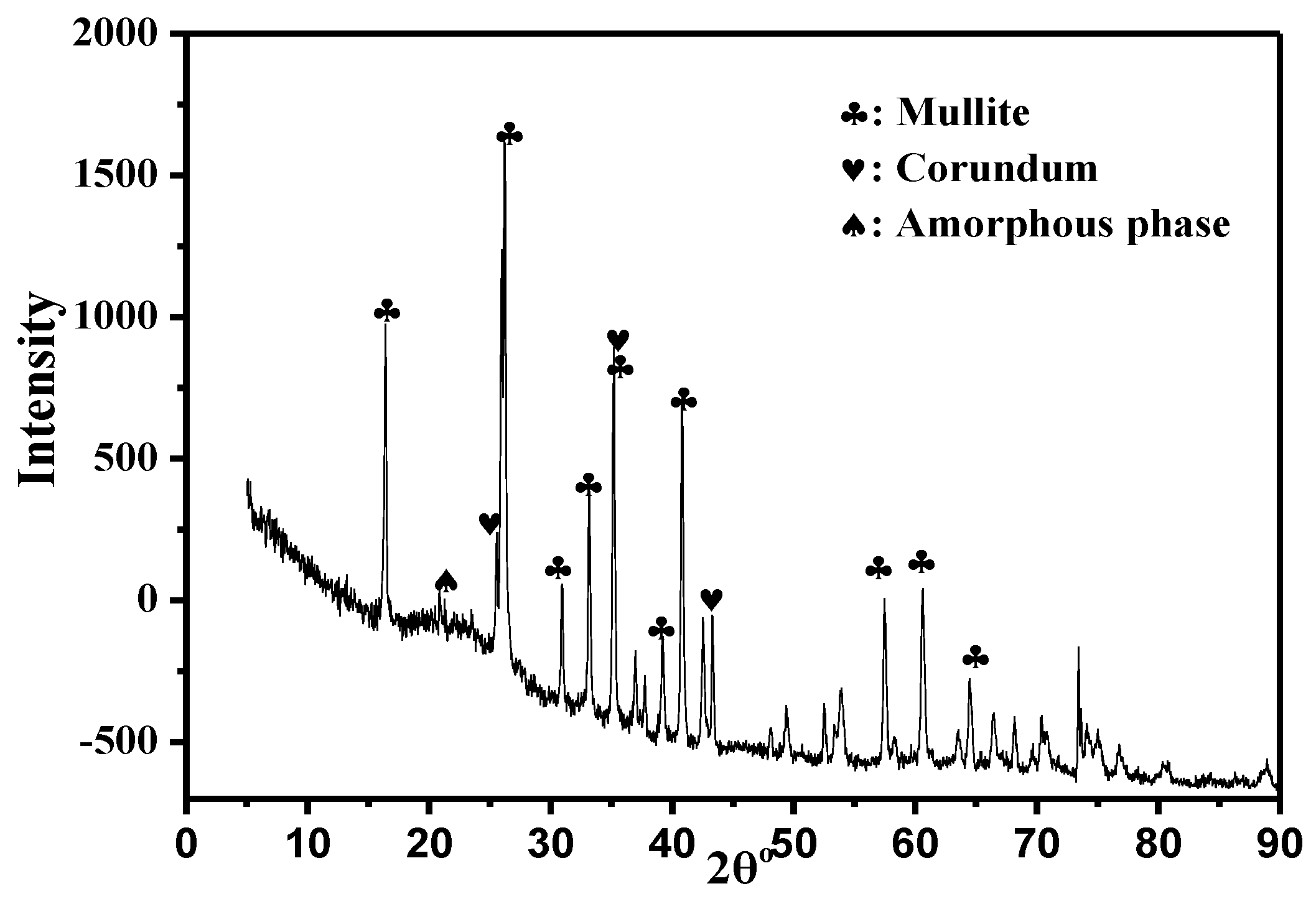
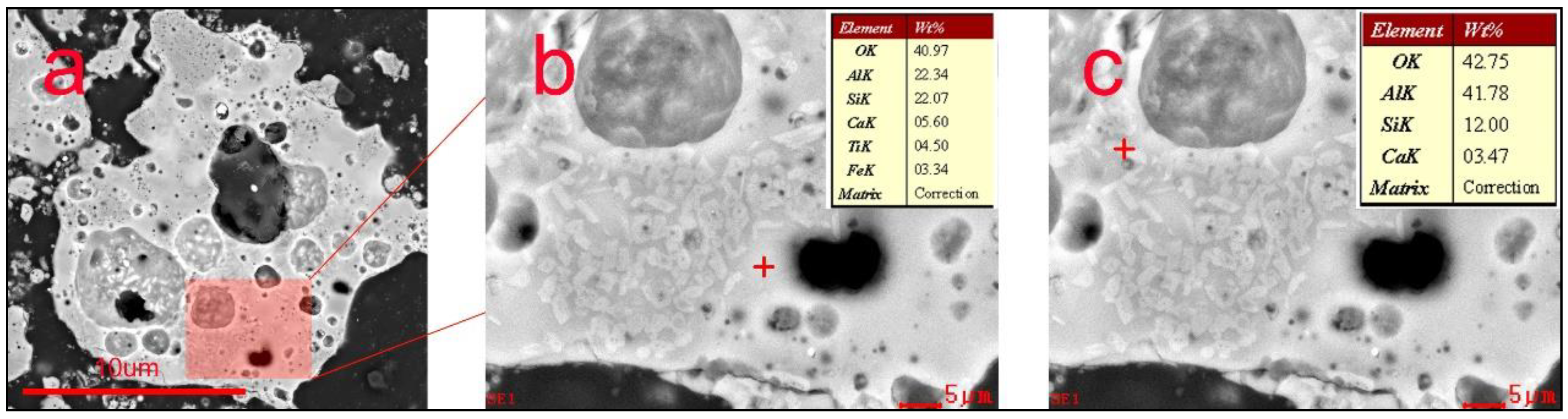
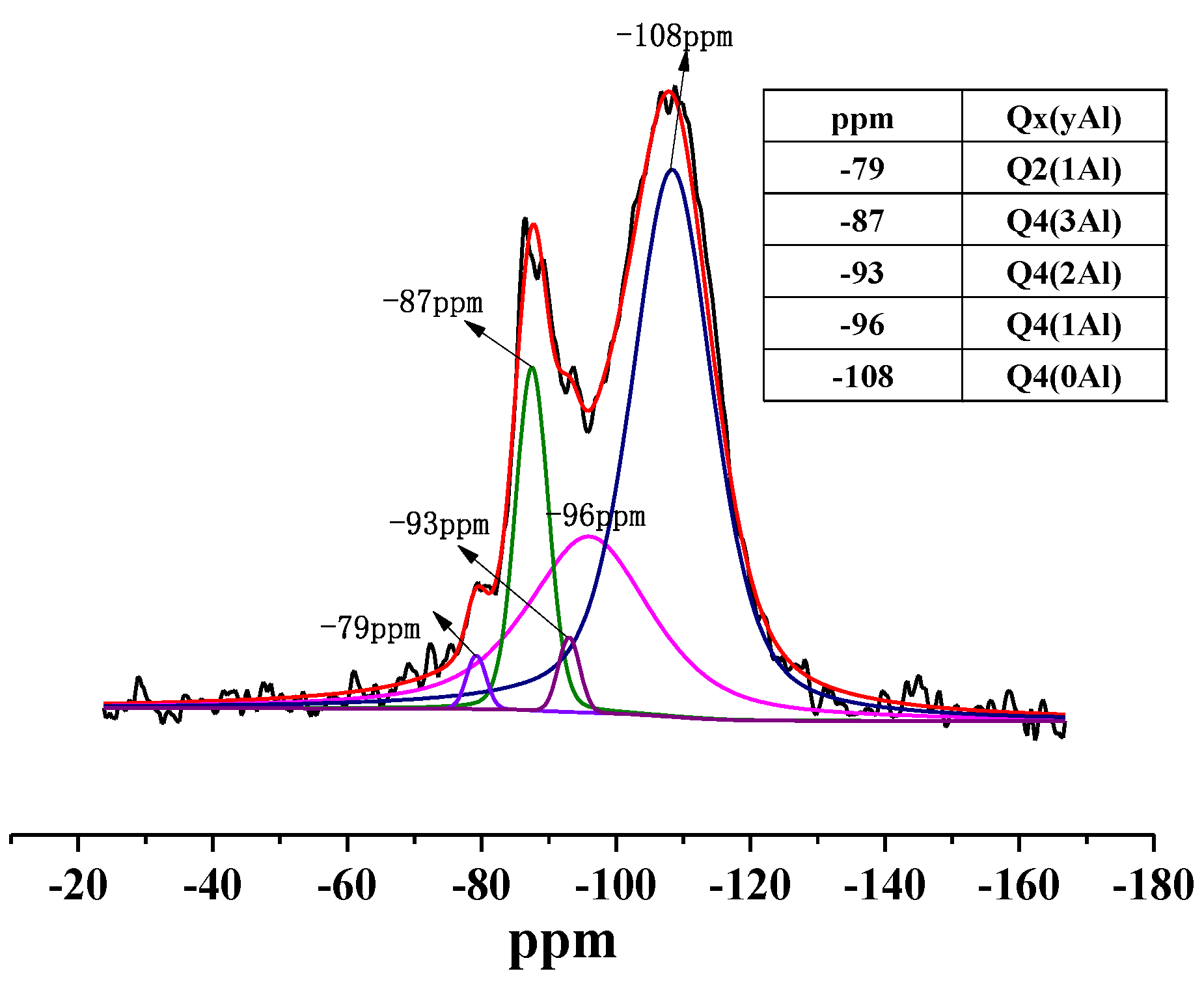
- Mineral phases in HAFA include mullite/corundum/amorphous aluminosilicate. Crystal phases (mullite/corundum) are wrapped by an amorphous phase, which is mainly in the form of Q4(3,2,1,0Al).
- (2)
- During deep desilication, the active Al-O- in the amorphous aluminosilicate phase is decomposed and replaced by H+, which helps to form an active OH site, and the amorphous phase becomes flocculent. The Al/Si ratio was increased from the original 1.17 to 2.80 through this method.
- (3)
- During the sintering period, the mullite grains grew into rod-like structures, which helped to form a stable, intricate structure under the following optimal conditions: forming pressure, 168 MPa; moisture content, 8%; calcination temperature, 1650 °C; and calcination time, 2 h. The bulk density of the mullite was enhanced to above 2.85 g/cm3, and the apparent porosity was controlled below 0.5%.
Author Contributions
Funding
Conflicts of Interest
References
- Tchamba, A.B.; Mbessa, M.; Metekong, J.V.S.; Yang, L.; Tankeu, S.N.; Nkeng, G.E.; Njopwouo, D.; Bier, T.A. Mechanical and microstructural properties of cameroonian bauxite ceramics for ballistic applications. Int. J. Appl. Ceram. Technol. 2020, 17, 949–962. [Google Scholar] [CrossRef]
- Lisboa, J.; Rocha, F.; de Oliveira, D.S. Application of multivariate analysis in the assessment of ceramic raw materials. Clays Clay Miner. 2016, 64, 767–787. [Google Scholar] [CrossRef]
- Zhang, J.B.; Yang, Z.B.L.N.; Li, S.P.; Li, H.Q. Investigation on the occurrence morphology and quantitative analysis of amorphous silicon in coal fly ash. Clean Coal Technol. 2019, 25, 116–121. [Google Scholar]
- Mohebbi, M.; Rajabipour, F.; Madadian, E. A framework for identifying the host phases in Coal-derived fly ash. Fuel 2022, 314, 122806. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Menendez, R.; Diaz-Somoano, M.; Martinez-Tarazona, M.R. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 2. Characterization of ceramic cenosphere and salt concentrates. Fuel 2004, 83, 585–603. [Google Scholar] [CrossRef]
- Zhan, X.Y.; Kirkelund, G.M. Electrodialytic remediation of municipal solid waste incineration fly ash as pre-treatment before geopolymerisation with coal fly ash. J. Hazard. Mater. 2021, 412, 125220. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Z.; Wu, D.; Kong, H. Synthesis and properties of zeolite/hydrated iron oxide composite from coal fly ash as efficient adsorbent to simultaneously retain cationic and anionic pollutants from water. Fuel 2014, 116, 71–76. [Google Scholar] [CrossRef]
- Yuan, Q.X.; Zhang, Y.S.; Wang, T.; Wang, J.W.; Romero, C.E. Mechanochemical stabilization of heavy metals in fly ash from coal-fired power plants via dry milling and wet milling. Waste Manag. 2021, 135, 428–436. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Dong, X.; Hampshire, S.; Zhu, L.; Zhu, Z.; Li, L. Feasible recycling of industrial waste coal fly ash for preparation of anorthite-cordierite based porous ceramic membrane supports with addition of dolomite. J. Eur. Ceram. Soc. 2016, 36, 1059–1071. [Google Scholar] [CrossRef]
- Cheng, B.C.; Liu, R.T.; Li, X.H.; Castillo, E.R.; Chen, M.J.; Li, S.C. Asturian fly ash as a heavy metals removal material. Constr. Build. Mater. 2022, 319, 125831. [Google Scholar] [CrossRef]
- Fan, C.C.; Wang, B.M.; Ai, H.M.; Qi, Y.; Liu, Z. A comparative study on solidification/stabilization characteristics of coal fly ash-based geopolymer and Portland cement on heavy metals in MSWI fly ash. J. Clean. Prod. 2021, 319, 128790. [Google Scholar] [CrossRef]
- Bukhari, S.S.; Behin, J.; Kazemian, H.; Rohani, S. Conversion of coal fly ash to zeolite utilizing microwave and ultrasound energies: A review. Fuel 2015, 140, 250–266. [Google Scholar] [CrossRef]
- Cao, P.X.; Li, G.H.; Jiang, H.; Zhang, X.; Luo, J.; Rao, M.J.; Jiang, T. Extraction and value-added utilization of alumina from coal fly ash via one-step hydrothermal process followed by carbonation. J. Clean. Prod. 2021, 323, 129174. [Google Scholar] [CrossRef]
- Ma, Y.Q.; Stopic, S.; Xakalashe, B.; Ndlovu, S.; Forsberg, K.; Friedrich, B. A cleaner approach for recovering Al and Ti from coal fly ash via microwave-assisted baking, leaching, and precipitation. Hydrometallurgy 2021, 206, 105754. [Google Scholar] [CrossRef]
- Liu, X.T.; Wang, B.D.; Xiao, Y.F.; Wang, X.H.; Zhao, L.J.; Yu, G.Z.; Sun, Q. Alumina Extraction from Alumina Rich Fly Ash Generated from Inner-Mongolia Chinese Coal. Adv. Mater. Res. 2014, 1065–1069, 1725–1731. [Google Scholar] [CrossRef]
- Li, C.; Zhou, Y.; Tian, Y.; Zhao, Y.; Wang, K.; Li, G.; Chai, Y. Preparation and characterization of mullite whisker reinforced ceramics made from coal fly ash. Ceram. Int. 2019, 45, 5613–5616. [Google Scholar] [CrossRef]
- Han, G.H.; Yang, S.Z.; Peng, W.J.; Huang, Y.F.; Wu, H.Y.; Chai, W.C.; Liu, J.T. Enhanced recycling and utilization of mullite from coal fly ash with a flotation and metallurgy process. J. Clean. Prod. 2018, 178, 804–813. [Google Scholar] [CrossRef]
- Foo, C.T.; Salleh, M.A.M.; Ying, K.K.; Matori, K.A. Mineralogy and thermal expansion study of mullite-based ceramics synthesized from coal fly ash and aluminum dross industrial wastes. Ceram. Int. 2019, 45, 7488–7494. [Google Scholar] [CrossRef]
- Ji, H.Y.; Mi, X.; Tian, Q.K.; Liu, C.L.; Yao, J.X.; Ma, S.H.; Zeng, G.S. Recycling of mullite from high-alumina coal fly ash by a mechanochemical activation method: Effect of particle size and mechanism research. Sci. Total Environ. 2021, 784, 147100. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, H.; Wu, Q.; Xi, X.; Li, Z. Preparation of Al–Si composite from high-alumina coal fly ash by mechanical–chemical synergistic activation. Ceram. Int. 2017, 43, 6532–6541. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.Z.; Li, S.; Cao, S.; Hu, P.; Zhu, G. Construct and research advance in clean and cyclic utilizations of associated resources in high alumina coal fly ash. Clean Coal Technol. 2018, 24, 1–8. [Google Scholar]
- Lu, J.; Zhang, Z.; Li, Y.; Liu, Z. Effect of alumina source on the densification, phase evolution, and strengthening of sintered mullite-based ceramics from milled coal fly ash. Constr. Build. Mater. 2019, 229, 116851. [Google Scholar] [CrossRef]
- Liu, X.T.; Wang, B.D.; Yu, G.Z.; Xiao, Y.F.; Wang, X.W.; Zhao, L.J.; Sun, Q. Kinetics study of predesilication reaction for alumina recovery from alumina rich fly ash. Mater. Res. Innov. 2014, 18, S2-541–S2-546. [Google Scholar] [CrossRef]
- Zhu, G.; Tan, W.; Sun, J.; Gong, Y.; Zhang, S.; Zhang, Z.; Liu, L. Effects and Mechanism Research of the Desilication Pretreatment for High-Aluminum Fly Ash. Energy Fuels 2013, 27, 6948–6954. [Google Scholar] [CrossRef]
- Liu, X.T.; Wang, B.D.; Xiao, Y.F.; Zhao, L.J.; Sun, Q. Pre-desilication process of alumina-rich fly ash in alkali solution. China Powder Sci. Technol. 2013, 19, 24–27. [Google Scholar]
- Zhu, L.; Dong, Y.C.; Li, L.L.; Liu, J.; You, S.J. Coal fly ash industrial waste recycling for fabrication of mullite-whisker-structured porous ceramic membrane supports. RSC Adv. 2015, 15, 11163–11174. [Google Scholar] [CrossRef]
- Guo, A.; Liu, J.; Xu, R.; Xu, H.; Wang, C. Preparation of mullite from desilication-flyash. Fuel 2010, 89, 3630–3636. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, S.; Zheng, S.; Liu, C.; Han, D.; Wang, X. Mullite-based ceramic tiles produced solely from high-alumina fly ash: Preparation and sintering mechanism. J. Alloys Compd. 2018, 732, 828–837. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Li, S.; Hu, P.; Wu, W.; Wu, Q.; Xi, X. Mechanism of mechanical–chemical synergistic activation for preparation of mullite ceramics from high-alumina coal fly ash. Ceram. Int. 2018, 44, 3884–3892. [Google Scholar] [CrossRef]
| Sample | Al2O3 | CaO | SiO2 | Fe2O3 | Na2O | TiO2 | MgO | Al/Si |
|---|---|---|---|---|---|---|---|---|
| HAFA | 45.87 | 1.85 | 39.37 | 1.60 | 0.33 | 1.62 | 0.2 | 1.17 |
| Sample | SiO2 | Al2O3 | CaO | Fe2O3 | TiO2 | MgO | Na2O | Al/Si |
|---|---|---|---|---|---|---|---|---|
| AHAFA | 44.43 | 45.16 | 0.21 | 0.64 | 1.42 | 0 | 0 | 1.02 |
| DHAFA | 22.94 | 67.09 | 0.12 | 0.73 | 1.44 | 0 | 0 | 2.80 |
| Product Level 1 | Product Level 2 | Product Level 3 | Standard Requirements (M70-2) | |
|---|---|---|---|---|
| Al2O3 (%) | 69.76 | 70.17 | 70.02 | 67–72 |
| TiO2 (%) | 1.42 | 1.47 | 1.48 | ≤3.5 |
| Fe2O3 (%) | 0.74 | 0.67 | 0.71 | ≤1.5 |
| Na2O + K2O (%) | 0.34 | 0.32 | 0.32 | ≤0.4 |
| Bulk density (g/cm3) | 2.87 | 2.92 | 2.94 | ≥2.75 |
| Apparent porosity (%) | 0.37 | 0.42 | 0.41 | ≤5 |
| Refractoriness (CN) | 180 | 180 | 180 | 180 |
| Mullite content (%) | 92.35 | 93.44 | 94.08 | ≥90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, H.; Li, S. Mechanisms of Separation and Crystal Growth of Mullite Grains during Preparation of Mullite-Based Ceramics from High Alumina Coal Fly Ash. Processes 2022, 10, 2416. https://doi.org/10.3390/pr10112416
Zhang J, Li H, Li S. Mechanisms of Separation and Crystal Growth of Mullite Grains during Preparation of Mullite-Based Ceramics from High Alumina Coal Fly Ash. Processes. 2022; 10(11):2416. https://doi.org/10.3390/pr10112416
Chicago/Turabian StyleZhang, Jianbo, Huiquan Li, and Shaopeng Li. 2022. "Mechanisms of Separation and Crystal Growth of Mullite Grains during Preparation of Mullite-Based Ceramics from High Alumina Coal Fly Ash" Processes 10, no. 11: 2416. https://doi.org/10.3390/pr10112416
APA StyleZhang, J., Li, H., & Li, S. (2022). Mechanisms of Separation and Crystal Growth of Mullite Grains during Preparation of Mullite-Based Ceramics from High Alumina Coal Fly Ash. Processes, 10(11), 2416. https://doi.org/10.3390/pr10112416






