Microbial Communities and Metabolites of Whole Crop Corn Silage Inoculated with Lentilactobacillus plantarum and Lentilactobacillus buchneri
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Experimental Design
2.2. Silage Quality and Aerobic Stability Analysis
2.2.1. Fermentation Quality Analysis
2.2.2. Microbial Population Analysis
2.2.3. Aerobic Stability Analysis
2.3. Bacterial and Microbial Community Analysis
2.4. Metabolite Analysis
2.5. Statistical Analysis
3. Results
3.1. Effects of LAB on the Fermentation Quality and Aerobic Stability of Whole Crop Corn Silage
3.2. α-Diversity Analysis of Bacterial Community of Whole Crop Corn Silage
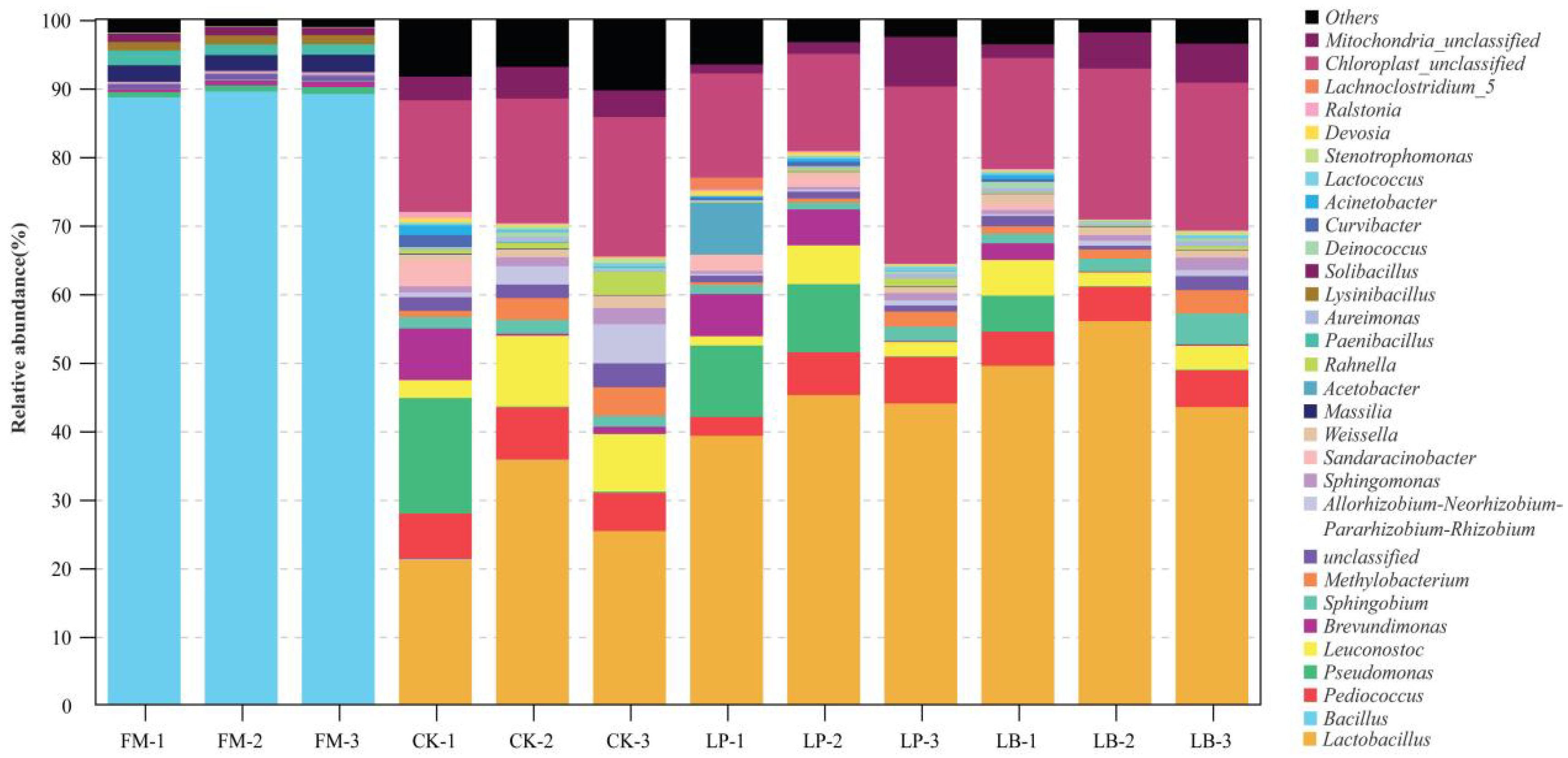
3.3. Effects of LAB on the Bacterial Community of Whole Crop Corn Silage
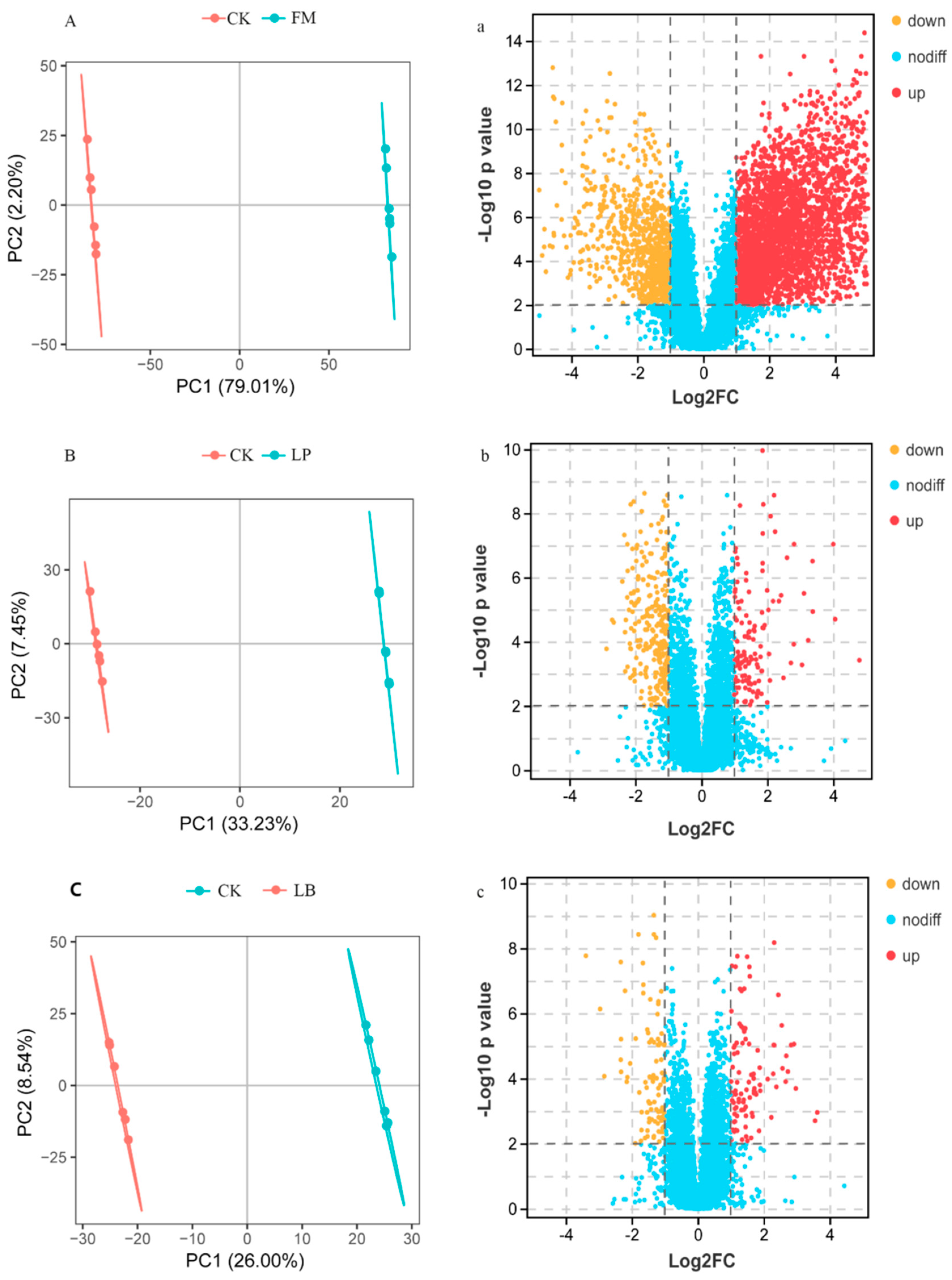
3.4. Metabolite Analysis of Whole Crop Corn Silage
4. Discussion
4.1. Effects of LAB on the Fermentation Quality and Aerobic Stability of Whole Crop Corn Silage
4.2. Effects of LAB on the Bacterial Community of Whole Crop Corn Silage
4.3. Effects of LAB on the Metabolites of Whole Crop Corn Silage
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Han, H.; Sun, L.; Na, N.; Xu, H.; Chang, S.; Jiang, Y.; Xue, Y. Bacterial succession pattern during the fermentation process in whole-plant corn silage processed in different geographical areas of Northern China. Processes 2021, 9, 900. [Google Scholar] [CrossRef]
- Ferraretto, L.; Shaver, R.; Luck, B.D. Silage review: Recent advances and future technologies for whole-plant and fractionated corn silage harvesting. J. Dairy Sci. 2018, 101, 3937–3951. [Google Scholar] [CrossRef] [PubMed]
- WAN, X.-R.; WU, J.-P.; LEI, Z.-M.; HE, Y.-Q.; Wu, R. Effect of lactic acid bacteria on corn silage quality and stability after aerobic exposure. Acta Prataculturae Sin. 2016, 25, 204. [Google Scholar]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.C.; Adesogan, A.T.; Lara, E.C.; Rabelo, C.H.S.; Berchielli, T.T.; Teixeira, I.A.M.A.; Siqueira, G.R.; Reis, R.A. Effects of feeding corn silage inoculated with microbial additives on the ruminal fermentation, microbial protein yield, and growth performance of lambs1. J. Anim. Sci. 2014, 92, 5640–5650. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.; Nadeau, E.; McAllister, T.; Contreras-Govea, F.; Santos, M.; Kung, L., Jr. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Nsereko, V.L.; Smiley, B.K.; Rutherford, W.M.; Spielbauer, A.; Forrester, K.J.; Hettinger, G.H.; Harman, E.K.; Harman, B.R. Influence of inoculating forage with lactic acid bacterial strains that produce ferulate esterase on ensilage and ruminal degradation of fiber. Anim. Feed Sci. Technol. 2008, 145, 122–135. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Shatz, O.; Chen, Y.; Yosef, E.; Nikbahat, M.; Ben-Ghedalia, D.; Miron, J. Effect of Lactic Acid Bacteria Inoculants on In Vitro Digestibility of Wheat and Corn Silages. J. Dairy Sci. 2007, 90, 4754–4762. [Google Scholar] [CrossRef]
- He, L.; Wang, C.; Xing, Y.; Zhou, W.; Pian, R.; Chen, X.; Zhang, Q. Ensiling characteristics, proteolysis and bacterial community of high-moisture corn stalk and stylo silage prepared with Bauhinia variegate flower. Bioresour. Technol. 2020, 296, 122336. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, W.; Guo, X.; Zheng, M.; Chen, X.; Zhang, Q. Citric acid influences the dynamics of the fermentation quality, protease activity and microbial community of Mulberry Leaf Silage. Fermentation 2021, 7, 185. [Google Scholar] [CrossRef]
- Liu, B.; Huan, H.; Gu, H.; Xu, N.; Shen, Q.; Ding, C. Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 2019, 273, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Wang, C.; Huang, Z.; Zhang, Y.; Sun, L.; Xue, Y.; Guo, X. Effects of Lactic Acid Bacteria-Inoculated Corn Silage on Bacterial Communities and Metabolites of Digestive Tract of Sheep. Fermentation 2022, 8, 320. [Google Scholar] [CrossRef]
- Lin, C.; Bolsen, K.; Brent, B.; Fung, D. Epiphytic lactic acid bacteria succession during the pre-ensiling and ensiling periods of alfalfa and maize. J. Appl. Bacteriol. 1992, 73, 375–387. [Google Scholar] [CrossRef]
- He, L.; Wang, C.; Xing, Y.; Zhou, W.; Pian, R.; Yang, F.; Chen, X.; Zhang, Q. Dynamics of proteolysis, protease activity and bacterial community of Neolamarckia cadamba leaves silage and the effects of formic acid and Lactobacillus farciminis. Bioresour. Technol. 2019, 294, 122127. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.N.; Li, Y.F.; Jeong, E.C.; Kim, H.J.; Kim, J.G. Effects of formic acid and lactic acid bacteria inoculant on main summer crop silages in Korea. J. Anim. Sci. Technol. 2021, 63, 91. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, L.; Ma, G.; Jiang, X.; Yang, J.; Lv, J.; Zhang, Y. Cellulase Interacts with Lactic Acid Bacteria to Affect Fermentation Quality, Microbial Community, and Ruminal Degradability in Mixed Silage of Soybean Residue and Corn Stover. Animals 2021, 11, 334. [Google Scholar] [CrossRef]
- Schmidt, R.; Emara, M.; Kung, L., Jr. The use of a quantitative real-time polymerase chain reaction assay for identification and enumeration of Lactobacillus buchneri in silage. J. Appl. Microbiol. 2008, 105, 920–929. [Google Scholar] [CrossRef]
- Mari, L.; Schmidt, R.; Nussio, L.; Hallada, C.; Kung, L., Jr. An evaluation of the effectiveness of Lactobacillus buchneri 40788 to alter fermentation and improve the aerobic stability of corn silage in farm silos. J. Dairy Sci. 2009, 92, 1174–1176. [Google Scholar] [CrossRef]
- Soundharrajan, I.; Park, H.S.; Rengasamy, S.; Sivanesan, R.; Choi, K.C. Application and Future Prospective of Lactic Acid Bacteria as Natural Additives for Silage Production—A Review. Appl. Sci. 2021, 11, 8127. [Google Scholar] [CrossRef]
- Muck, R.E. Microbiologia da silagem e seu controle com aditivos. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef]
- Danner, H.; Holzer, M.; Mayrhuber, E.; Braun, R. Acetic Acid Increases Stability of Silage under Aerobic Conditions. Appl. Environ. Microbiol. 2003, 69, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, N.K.; Kung, L., Jr. The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 2000, 83, 526–535. [Google Scholar] [CrossRef]
- Nishino, N.; Wada, H.; Yoshida, M.; Shiota, H. Microbial Counts, Fermentation Products, and Aerobic Stability of Whole Crop Corn and a Total Mixed Ration Ensiled With and Without Inoculation of Lactobacillus casei or Lactobacillus buchneri. J. Dairy Sci. 2004, 87, 2563–2570. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Lu, Q.; Sun, L.; Du, S.; Liu, T.; Hou, M.; Ge, G.; Wang, Z.; Jia, Y.J. Effects of Lactic Acid Bacteria Additives on the Quality, Volatile Chemicals and Microbial Community of Leymus chinensis Silage During Aerobic Exposure. Front. Microbiol. 2022, 13, 1–17. [Google Scholar] [CrossRef]
- Bai, J.; Xu, D.; Xie, D.; Wang, M.; Li, Z.; Guo, X. Effects of antibacterial peptide-producing Bacillus subtilis and Lactobacillus buchneri on fermentation, aerobic stability, and microbial community of alfalfa silage. Bioresour. Technol. 2020, 315, 123881. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.-T.; Huang, Y.; Wu, C.-R.; Peng, C.; Zheng, Y.-L.; Chen, C.; Hao, J.J. Addition of lactic acid bacteria can promote the quality and feeding value of Broussonetia papyrifera (Paper Mulberry) Silage. Fermentation 2022, 8, 25. [Google Scholar] [CrossRef]
- Ricciardi, A.; Storti, L.V.; Giavalisco, M.; Parente, E.; Zotta, T. The Effect of Respiration, pH, and Citrate Co-Metabolism on the Growth, Metabolite Production and Enzymatic Activities of Leuconostoc mesenteroides subsp. cremoris E30. Foods 2022, 11, 535. [Google Scholar] [CrossRef]
- Fujimatsu, T.; Endo, K.; Yazaki, K.; Sugiyama, A.J. Secretion dynamics of soyasaponins in soybean roots and effects to modify the bacterial composition. Plant Direct 2020, 4, e00259. [Google Scholar] [CrossRef]
- Zou, S.; Zhao, K.; Tang, H.; Zhang, Z.; Zhang, B.; Liu, Z.; Zheng, Y. Improved production of D-pantothenic acid in Escherichia coli by integrated strain engineering and fermentation strategies. J. Biotechnol. 2021, 339, 65–72. [Google Scholar] [CrossRef]
- Xu, J.; Patassini, S.; Begley, P.; Church, S.; Waldvogel, H.J.; Faull, R.L.; Unwin, R.D.; Cooper, G.J. Cerebral deficiency of vitamin B5 (d-pantothenic acid; pantothenate) as a potentially-reversible cause of neurodegeneration and dementia in sporadic Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2020, 527, 676–681. [Google Scholar] [CrossRef]
- Den Hartigh, L.J. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef] [PubMed]
| Item | CK | LP | LB | SEM | p Value |
|---|---|---|---|---|---|
| DM (%) | 32.1 b | 37.0 a | 32.9 b | 0.80 | 0.001 |
| pH | 3.89 | 3.93 | 3.86 | 0.01 | 0.089 |
| Lactic acid bacteria (log cfu/g) | 3.60 | 5.28 | 4.11 | 0.35 | 0.128 |
| Coliform bacteria (log cfu/g) | <2.00 | <2.00 | <2.00 | <0.01 | - |
| Yeasts (log10 cfu/g FM) | <2.00 b | 4.24 a | 2.60 ab | 0.73 | 0.023 |
| Lactic acid (%DM) | 5.55 | 3.64 | 5.39 | 0.99 | 0.737 |
| Acetic acid (%DM) | 2.00 | 1.45 | 2.28 | 0.44 | 0.788 |
| Aerobic Stability (h) | 68 | 36 | 128 | 17.9 | 0.081 |
| Item | OTUs | Shannon | Simpson | Chao1 | Good’s Coverage |
|---|---|---|---|---|---|
| CS | 183 | 3.58 | 0.82 | 195 | 1.00 |
| CK | 350 | 5.09 | 0.93 | 359 | 1.00 |
| LP | 257 | 4.42 | 0.89 | 264 | 1.00 |
| LB | 259 | 4.52 | 0.90 | 269 | 1.00 |
| SEM | 24.8 | 0.17 | 0.01 | 29.1 | - |
| p value | 0.182 | 0.000 | 0.000 | 0.275 | - |
| Phylum | CS | CK | LP | LB | SEM | p-Value |
|---|---|---|---|---|---|---|
| Firmicutes | 94.1 | 43.9 | 53.9 | 60.1 | 5.92 | 0.000 |
| Proteobacteria | 5.03 | 32.3 | 25.4 | 17.0 | 3.58 | 0.011 |
| Cyanobacteria | 0.00 | 18.3 | 18.43 | 19.9 | 2.64 | 0.001 |
| Bacteroidetes | 0.10 | 1.37 | 0.39 | 0.53 | 0.15 | 0.001 |
| Actinobacteria | 0.02 | 0.42 | 0.31 | 0.36 | 0.05 | 0.006 |
| Deinococcus-Thermus | 0.00 | 0.36 | 0.17 | 0.54 | 0.09 | 0.127 |
| Acidobacteria | 0.04 | 0.48 | 0.31 | 0.09 | 0.08 | 0.184 |
| Planctomycetes | 0.00 | 0.10 | 0.03 | 0.02 | 0.01 | 0.001 |
| Verrucomicrobia | 0.00 | 0.10 | 0.01 | 0.02 | 0.01 | 0.016 |
| Unclassified | 2.49 | 0.95 | 1.36 | 0.74 | 0.25 | 0.024 |
| Genus | CS | CK | LP | LB | SEM | p-Value |
|---|---|---|---|---|---|---|
| Lactobacillus | 0.04 | 27.4 | 42.8 | 49.6 | 5.88 | 0.000 |
| Bacillus | 89.0 | 0.05 | 0.00 | 0.00 | 11.62 | 0.000 |
| Pediococcus | 0.00 | 6.61 | 5.27 | 5.12 | 0.82 | 0.001 |
| Pseudomonas | 0.86 | 5.74 | 6.81 | 1.82 | 1.62 | 0.552 |
| Leuconostoc | 0.00 | 7.11 | 3.04 | 3.57 | 0.97 | 0.046 |
| Brevundimonas | 0.67 | 2.97 | 3.85 | 0.93 | 0.77 | 0.430 |
| Sphingobium | 0.07 | 1.75 | 1.45 | 2.57 | 0.35 | 0.046 |
| Metabolite | FC (LB/LP) | Important Predictor Variables | p-Value |
|---|---|---|---|
| Gly-Val | 3.32 | 4.20 | 0.00 |
| 9-Oxo-10(E),12(E)-octadecadienoic acid | 3.22 | 3.11 | 0.00 |
| 9Z,11E,13E-Octadecatrienoic acid ethyl ester | 2.92 | 2.97 | 0.00 |
| 5Z,11Z,14Z-Eicosatrienoic acid | 2.86 | 2.79 | 0.00 |
| 8(9)-Epoxy-5Z,11Z,14Z-eicosatrienoic acid | 2.69 | 3.75 | 0.00 |
| 8,11-Tridecadienoic acid | 2.58 | 3.76 | 0.00 |
| Diniconazole | 2.40 | 3.02 | 0.00 |
| Testosterone_Propionate | 2.35 | 2.41 | 0.00 |
| Acylcarnitine 15:0 | 2.30 | 3.11 | 0.00 |
| Bis(2-ethylhexyl) adipate | 2.25 | 2.97 | 0.00 |
| Homoorientin | 2.10 | 2.52 | 0.00 |
| Quercetin-3-O-rhamnoside | 2.04 | 3.15 | 0.00 |
| LysoPC 16:0 | 0.49 | 2.32 | 0.00 |
| Acylcarnitine 25:4 | 0.45 | 3.17 | 0.00 |
| LysoPC 18:2 | 0.44 | 2.61 | 0.00 |
| Pheophorbide a | 0.42 | 2.86 | 0.01 |
| Butanal | 0.36 | 3.11 | 0.00 |
| Metabolite | FC (LP/CK) | Important Predictor Variables | p-Value |
|---|---|---|---|
| Soyasaponin Bb | 6.05 | 4.34 | 0.00 |
| D-Pantothenic acid | 4.71 | 3.86 | 0.00 |
| LysoPC 16:0 | 3.58 | 3.74 | 0.00 |
| LysoPE 16:0 | 2.83 | 2.54 | 0.00 |
| Ser-Phe | 2.67 | 2.73 | 0.00 |
| 4-Hydroxy-7-trifluoromethyl-3- quinolinecarboxylic acid | 2.25 | 1.72 | 0.00 |
| Phytosphingosine | 0.50 | 1.72 | 0.00 |
| 8(9)-Epoxy-5Z,11Z,14Z-eicosatrienoic acid | 0.49 | 2.56 | 0.00 |
| 7-O-Acetylaustroinulin | 0.47 | 1.56 | 0.00 |
| 9-HODE | 0.46 | 1.54 | 0.00 |
| 12,13-Dihydroxy-9Z-octadecenoic acid | 0.46 | 1.66 | 0.00 |
| 2-Hydroxy-3-methoxybenzaldehyde | 0.45 | 1.42 | 0.00 |
| D-erythro-N-stearoylsphingosine | 0.44 | 2.46 | 0.00 |
| Methionine | 0.44 | 1.41 | 0.00 |
| 8Z,14Z-Eicosadienoic acid | 0.43 | 2.30 | 0.00 |
| 5Z,11Z,14Z-Eicosatrienoic acid | 0.43 | 2.12 | 0.00 |
| Testosterone_Propionate | 0.40 | 2.66 | 0.00 |
| Isoquercitrin | 0.39 | 2.39 | 0.01 |
| Bis(2-ethylhexyl) adipate | 0.37 | 2.65 | 0.00 |
| (S)-2,3,4,5-tetrahydropyridine-2-carboxylate | 0.35 | 3.09 | 0.00 |
| Acylcarnitine 25:4 | 0.35 | 3.46 | 0.00 |
| epsilon-Caprolactam | 0.33 | 2.88 | 0.00 |
| Quercetin 3-galactoside | 0.32 | 2.47 | 0.00 |
| 8,11-Tridecadienoic acid, 13-(3-pentyl-2-oxiranyl)-, (8Z,11Z)- | 0.28 | 3.71 | 0.00 |
| LysoPC 18:2 | 0.26 | 3.84 | 0.00 |
| Acylcarnitine 15:0 | 0.25 | 2.87 | 0.00 |
| Gerberinol | 0.23 | 2.96 | 0.00 |
| Metabolite | FC (LB/CK) | Important Predictor Variables | p-Value |
|---|---|---|---|
| Epsilon-caprolactam | 2.74 | 3.04 | 0.00 |
| Hordenine | 2.65 | 2.86 | 0.00 |
| D-Pantothenic acid | 2.63 | 3.41 | 0.00 |
| Phytosphingosine | 2.48 | 3.69 | 0.00 |
| Diniconazole | 2.31 | 3.39 | 0.00 |
| Karakoline | 2.22 | 2.89 | 0.00 |
| Diosgenin | 2.18 | 2.51 | 0.00 |
| Homoorientin | 2.06 | 2.45 | 0.00 |
| Quercetin-3-O-rhamnoside | 2.06 | 1.75 | 0.00 |
| LysoPE 16:0 | 2.03 | 2.52 | 0.00 |
| Isoquercitrin | 0.47 | 2.98 | 0.00 |
| Quercetin 3-galactoside | 0.35 | 3.55 | 0.00 |
| LysoPC 16:0 | 0.32 | 3.94 | 0.00 |
| Acylcarnitine 25:4 | 0.32 | 4.05 | 0.00 |
| Pheophorbide a | 0.14 | 5.74 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Hao, X.; Li, Y.; Zhang, Q.; Wang, C.; Han, H. Microbial Communities and Metabolites of Whole Crop Corn Silage Inoculated with Lentilactobacillus plantarum and Lentilactobacillus buchneri. Processes 2022, 10, 2369. https://doi.org/10.3390/pr10112369
Guo Q, Hao X, Li Y, Zhang Q, Wang C, Han H. Microbial Communities and Metabolites of Whole Crop Corn Silage Inoculated with Lentilactobacillus plantarum and Lentilactobacillus buchneri. Processes. 2022; 10(11):2369. https://doi.org/10.3390/pr10112369
Chicago/Turabian StyleGuo, Qian, Xia Hao, Yuerui Li, Qing Zhang, Chao Wang, and Hongyan Han. 2022. "Microbial Communities and Metabolites of Whole Crop Corn Silage Inoculated with Lentilactobacillus plantarum and Lentilactobacillus buchneri" Processes 10, no. 11: 2369. https://doi.org/10.3390/pr10112369
APA StyleGuo, Q., Hao, X., Li, Y., Zhang, Q., Wang, C., & Han, H. (2022). Microbial Communities and Metabolites of Whole Crop Corn Silage Inoculated with Lentilactobacillus plantarum and Lentilactobacillus buchneri. Processes, 10(11), 2369. https://doi.org/10.3390/pr10112369






