Abstract
Previously, we showed that the crude methanol extracts of Leucas indica flowers exhibited antioxidant properties and in the current study, crude methanol flower extracts of L. indica showed anticancerous properties as evidenced cytotoxicity (MTT assay test) against the selected cancerous cell lines HeLa, HCT116, HL-60, and MCF-7. Therefore, further analysis was performed to isolate and purify the bioactive compound using activity-guided repeated fractionation of the methanol extract by silica gel column chromatography. After collection of different fractions, all the fractions were subjected to TLC analysis and the fractions which yielded the same compounds on TLC were further analyzed for physicochemical and spectroscopic analyses, e.g., UV, IR, 1H NMR, 13C NMR, COSY, HSQC, and mass spectroscopy. The bioactive compound isolated was elucidated as 6-hydroxy-3-(4-hydroxyphenyl)-7-(3,4,5-trihydroxy-6-)(hydroxymethyl)tetrahydro-2H-pyran-2yl)-4H-chromen-4-one. Based on the antioxidant and anticancerous properties, L. indica might be a promising source of useful natural products and the newly bioactive compound might offer opportunities to develop new anticancerous drugs.
1. Introduction
The increased incidences of cancer and mortality rate (9.9 million of cancer deaths in 2020) due to cancer are of major concern all over the globe including India. According to the International Agency for Research (https://gco.iarc.fr/, accessed on 5 May 2020) on Cancer, it is estimated that, worldwide, 19.3 million new cancer cases and 10.0 million cancer deaths occurred in 2020 [1]. It has been shown that the magnitude of new cancer cases was in the order of 2.3 million new cases of breast cancer > lung (11.45%) > colorectal (10.0%) > prostate (7.3%) > stomach (5.6%) cancers and the magnitude of cancer deaths were in the order of lung cancer (18% with 1.8 million deaths) > colorectal (9.4%) > liver (8.3%) > stomach (7.7%) > female breast (6.9%) cancers [1]. According to the data obtained in 2018 from 185 countries, India had the highest incidences of mouth and oral cancer [2]. Thus, efforts to control the cancer disease are one of the major objectives of cancer research. Though conventional therapies such as chemotherapy and radiation therapy and their combination are promising to some extent, side effects associated with these therapies often limit their usage [3]. In addition, usage of chemotherapeutic drugs leads to rapid excretion, relatively poor half-life, and the development of resistance by cancerous cells against chemotherapeutic drugs also question the use of these drugs. These aspects particularly pushed many cancer biologists and pharmacologists to search for novel compounds with anticancerous properties from natural sources [4,5,6]. Thus, identification of bioactive leads from crude extracts of plants is one of the key steps in development of plant-based drugs against cancer disease [7]. This can be achieved via rapid detection of biologically active natural products using efficient screening of the crude extracts entailing both biological assays and HPLC analysis coupled with other advanced techniques such as MS and NMR [8]. This in turn allows ample structural information of bioactive compounds efficiently [9].
Plant-derived products in cancer therapy are well acknowledged because of their minimal side effects [10]. Currently, a few plant-based drugs are being used to treat cancer. However, a wide array of plants exist which are potential sources of antioxidant, antimicrobial, antiapoptotic, and anticancerous properties. Thus, there is a need to purify and characterize bioactive compounds from plants and tested for in vitro and in vivo biological properties [11]. If a promising tumor killing is achieved by the bioactive lead, the parameters such as safety analysis, adverse effects, pharmacokinetic properties, and its interactions must be explored. The present study was aimed to isolate, purify, and characterize bioactive principle from the Leucas indica flowers using analytical techniques and its antioxidant, antimicrobial, and anticancerous properties were tested.
Lamiaceae (Labiatae) family is one of the largest and most evolved angiosperms [12]. Among the Lamiaceae family members, the genus Leucas are widely distributed throughout Asia, including India [13]. Many species of Leucas are widely used in traditional medical applications such as cough, cold, diarrhea, and inflammatory skin disorder. Further, phytoconstituents such as lignans, flavonoids, coumarins, steroids, terpenes, fatty acids, and aliphatic long-chain compounds have been isolated from these plants. The phytoconstituents of these plants have been reported to exhibit properties such as anti-inflammatory, antimicrobial, anti-diarrheal, antioxidant activities. Leucas indica (Linn) is a family member of Lamiaceae group and commonly known as Tummi in this part of India. The flowers and leaves of L. indica species is used to treat typhoid fever, while the leaves are used to treat pneumonia and night blindness [14,15,16,17]. With respect to flowers of this family, several studies have shown that the flowers of Leucas species were used to treat cough and colds, migraine, jaundice, emmenagouge, typhoid, and used as poultice to treat headache [13]. Further, antimicrobial activity [18] and red blood corpuscles (RBC) membrane-stabilizing activity [19] of flower extracts of L. aspera was demonstrated. In our preliminary studies, we found that the methanolic extracts obtained from the flowers and leaves of L. indica exhibited antioxidant properties [20]. Since flower extracts had been associated with antioxidant properties, further experiments were designed to determine the anticancerous properties of methanolic flower extracts of L. indica. The other objective of this study was to isolate, purify, and characterize the bioactive principle(s) or metabolite(s) in methanolic flower extracts using bioanalytical techniques such as thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), mass spectroscopy (MS), and nuclear magnetic resonance (NMR).
2. Materials and Methods
2.1. Chemicals
The cell culture reagents including media, and antibiotics and the chemicals including HPLC reagents and silica gel, were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Merck, India, respectively. Dimethyl sulfoxide, fetal bovine serum, HEPES buffer, phenol red, glucose, sodium bicarbonate, and streptomycin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Solvents such as ethanol, methanol, chloroform, silica gel, and TLC were purchased from Merck (Mumbai, India).
2.2. Collection of Plant Material
In India, almost 40 species of Leucas family members are available including L. indica. The flowers of L. indica were collected from Marlapudi village, Nellore district, Andhra Pradesh. India. The plant and its floral parts were authenticated by Dr. Padal, Department of Botany, Andhra University, Visakapatnam and based on the herbarium records, the voucher specimen number of L. indica was 22237. The flowers were thoroughly washed under tap water to clean from the extraneous materials, dried, and pulverized to powder in a sterilized blender mechanically. The resultant coarse powder obtained after sieving was stored in air tight container until further use. Based on the phytoscreening studies, we showed that both the aerial parts, leaves and flowers (methanolic extracts) of L. indica exhibited antioxidant properties in vitro [20]. In the present study, methanolic extracts of flowers (powdered form) was further analyzed for anticancerous properties. The schematic illustration of the work design is shown in Figure 1.
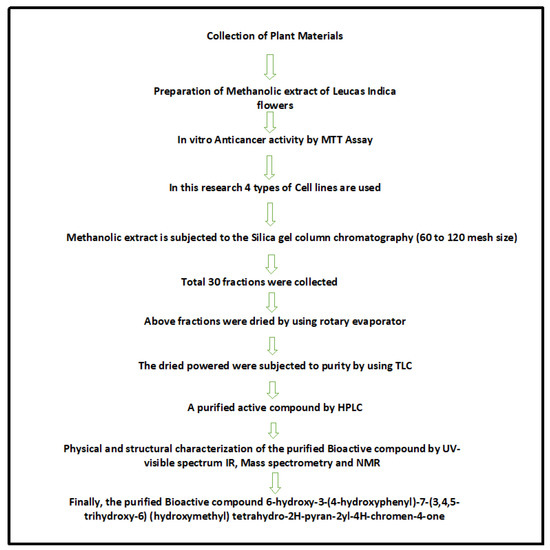
Figure 1.
Schematic illustration of current research work.
2.3. Cell Viability Assay
2.3.1. Preparation of Cell Suspension
MTT assay is one of the widely used techniques to screen the ability of drugs with anticancerous properties and the basic principle of MTT assay underlies the ability of cellular system to convert yellow colored MTT to purple colored formazan by oxidoreductases and thus the amount of formazan formed is directly proportional to the number of viable cells [21]. For in vitro anti-cancerous activity of bioactive compound, four different cell lines, HeLa (ATCC CCL-2), HCT116 (ATCC CCL 247), HL-60 (ATCC CCL-240), and MCF-7 (ATCC HTB-22) cell lines, which represent cervical, colon, leukemia, and breast cancer, respectively, were selected. Cell culture studies were performed at Jayagen Biologics Analytical Laboratory, Chennai. Briefly, a subculture of HeLa, HCT-1160, HL-60, and MCF-7 in Dulbecco’s Modified Eagle’s Medium (DMEM) was subjected to trypsinization. The disaggregated cells after proper homogenization was seeded in a 24-well plate (approximately 104 cells/mL) containing DMEM supplemented with 10% FCS (heat inactivated), 1% antibiotic (equal concentrations of (100 U/mL) of penicillin and streptomycin) and incubated at 37 °C in a humidified CO2-incubator with 5% CO2. Cells were treated with different concentrations (0 to 300 µg/mL) of methanolic extracts of L. indica flowers in dimethylsulfoxide (DMSO) over a period of 24 h. This step was followed by the addition of 15 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (5 mg/mL) reagent to the culture media and further incubated at 37 °C in a CO2 incubator. After 4 h of incubation, the MTT containing medium was aspirated followed by the addition of 200 μL of DMSO and 25 μL of Sorenson glycine buffer (0.1 M glycine and 0.1 M NaCl, pH 10.5) to lyse the cells and dissolve the water-insoluble formazan crystals. Absorbance values of the lysates were determined at 570 nm on a microplate (Lark LIPR-9608). The absorbance values of vehicle and plant extract treated cells were incorporated in the given formula to calculate the percentage inhibition: [(Mean OD of vehicle treated cells − Mean OD of plant extract treated cells)/Mean OD of vehicle treated cells] × 100.
IC50 values were calculated using AAT Bioquest IC50 Calculator and were expressed in molar concentration. IC50 represents the concentration at which a substance exerts half of its maximal inhibitory effect.
2.3.2. Silica Gel Column Chromatography
Briefly, 500 g of dried powdered flowers of L. indica were extracted using methanol via maceration and the resultant extract was concentrated using vacuum rotary evaporator. This step yielded 175 g of crude extract. A portion of crude extract (20 g) was subjected to silica gel column chromatography (60 cm × 3 cm) through a column of silica gel (60 to 120 mesh size) eluted with different ratios of solvents, chloroform to methanol as the mobile phase. The percentage of addition of chloroform to methanol (0% to 100%, with 20% increment) and the fractions for each ratio are mentioned in Table 1. A total of 30 fractions (50 mL per fraction) of eluent were collected as solvent drips from the bottom of the column. All the fractions were subjected to dryness using a rotary evaporator (IKA Rotary evaporator RV 10 digital FLEX) at 40 °C and tested for their purity using thin-layer chromatography.

Table 1.
Solvents used for purification from Leucas indica using column (silica: 20 mm) chromatography.
2.3.3. Thin Layer Chromatography (TLC)
TLC was performed on a pre-coated silica gel TLC plates grade F254 (E-Merck, Darmstadt, Germany) to determine the number of compounds present in the given sample. A total of 5 μL of sample was spotted at 1 cm from the bottom of silica gel plates using capillary tubes. Different solvents at various combinations and concentrations (Table 1) were used for metabolite profiling. Development of the chromatogram was carried out in closed tanks by using methanol as the mobile phase, in which the atmosphere was saturated with eluent vapor by wetting a filter paper lining. The chromatogram was visualized under UV light (365 nm and 254 nm) and iodine vapor. The Rf values of the compounds were calculated using the following formula.
2.3.4. Purification of the Active Compound by High Performance Liquid Chromatography (HPLC)
The identical fractions were pooled and concentrated under vacuum and further subjected to purity using HPLC (Shimadzu, Japan). The HPLC spectrum of the purified compound was obtained using Silica C18 P column (Shodex, 4.6 mm × 150 mm). About 20 µL of the sample was injected using automated syringe and separated using the solvent system of Hexane–Ethyl acetate (85:15) at a flow rate of 1.0 mL/min. A highly sensitive UV detector at 254 nm was used to detect the peak(s) and collected manually.
2.4. Physical and Structural Characterization of the Purified Bioactive Compound
2.4.1. UV-Visible Spectrum
UV-Visible spectrum of the isolated compound was recorded on a UV-Visible spectrometer (Shimadzu UV-2450) at room temperature. About 1 mg of compound was dissolved in 20 mL of methanol/chloroform and used to record the spectrum in the wavelength range of (λ) 200 to 800 nm.
2.4.2. Infrared (IR) Spectroscopy
Infrared spectrum of the compound was measured using Nicolet iS10 Fourier transform infrared (FTIR) spectrometer (Thermo Scientific, Co., Waltham, MA, USA) according to the manufacturer’s protocol. IR spectra for the purified compounds were recorded on a Bruker series FTIR spectrometer using KBr pellets.
2.4.3. Mass Spectrometry
Determinations of small molecular weight compounds in L. indica flower extracts were analyzed using A JEOL GCmate II Benchtop double-focusing magnetic sector mass spectrometer operating in electron ionization (EI) mode and for analysis, TSS-20001 software was used. Mass spectra of low and high resolutions were acquired at a resolving power of 1000 and 5000 (20% height definition each), respectively and scanning range from m/z 80 to m/z 900 at 0.3 s per scan delay for low-resolution mass spectra and scanning range from m/z 65 to m/z 750 at 1 s per scan for high-resolution spectra. To assess the quality, samples obtained at high resolution were introduced into the electron ionization (flow rate: 1 mL/min). The conditions used for the analysis of samples using the mass spectrometer were as follows: (a) operation mode: positive ion mode; (b) spray voltage: 2.4 kV; (c) capillary temperature and voltage: 250 and 29.0 V, respectively and (d) ion isolation window: 1.5 u and (e) inject time: 100 ms.
2.4.4. Nuclear Magnetic Resonance Spectroscopy (NMR)
The one-dimensional 1H NMR and 13C NMR spectra were recorded using Advance III Bruker 400 MHz NMR spectrometer (Bruker, Billerica, MA, USA). The samples were dissolved in deuterated solvents (CDCl3) and tetramethylsilane (TMS) as internal standard. The observed chemical shift values (δ) were given in ppm and the coupling constant (J) in Hz. After data acquisition, the prediction of structure of NMR spectra was performed by Chemdraw Software v20.0 (https://perkinelmerinformatics.com, accessed on 5 May 2020).
3. Results
To measure the anticancer activity of L. indica methanolic flower extracts, different cell lines MCF-7 (breast cancer cell line), HeLa (cervical cell line), HCT-116 (Human colon carcinoma cell line), and HL-60 (Human promyelocytic leukemia cell line) were treated with increasing concentrations of flower extracts (0 to 300 μg/mL) for 48 h. According to MTT assay, the half maximal inhibitory concentration IC50 value of methanolic extracts of flowers was also determined (Figure 2, Table 2).
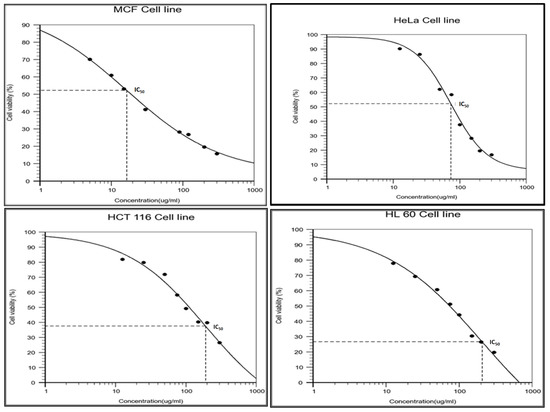
Figure 2.
Determination of IC50 value of crude methanolic extract of Leucas indica flower against selected cancerous cell lines.

Table 2.
In vitro cytotoxicity assay of purified compound from Leucas indica against selected cancer cell lines.
The results indicated that the IC50 value of MCF-7 cell line treated with methanolic flower extract was found to be 16 μg/mL as compared with IC50 values of cell lines treated with plant extract (73.549 µg/mL against HeLa cell lines, 190.587 µg/mL against HCT-116 cell lines, and 206.465 µg/mL against HL 60 cell lines). The cytotoxic ability of methanolic extracts of flowers against the selected cell lines were in the order of MCF-7 > HeLa > HCT-116 > HL-60). In contrast, samples treated with DMSO did not exhibit cytotoxic effect. These findings suggest that growth inhibitory principles were present in methanolic extracts of flowers and effective against MCF-7 cell lines based on IC50 value.
In order to identify the active metabolite(s) and its structure, the flower extracts were subjected to analytical techniques such as silica gel column chromatography, TLC, HPLC, MS, and NMR. Among the fractions collected via silica gel column chromatography, the fractions 11 to 15 eluted (Table 1) with respect to chloroform to methanol ratio (60 mL: 40 mL) showed identical compounds when subjected to iodine vapors on TLC plate (Figure 3).
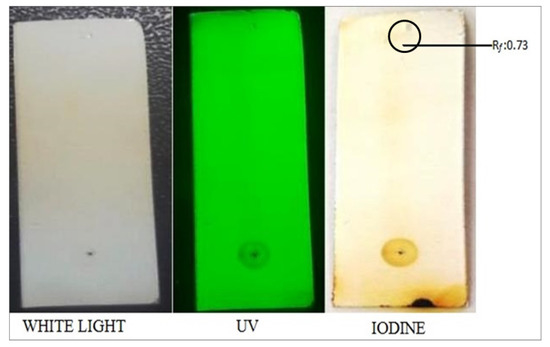
Figure 3.
Thin-layer chromatography of the purified compound from the extracts of Leucas indica flowers.
The identical compounds were carefully separated from the silica plate, pooled, and subjected to preparative HPLC for further purification. HPLC analysis revealed a single peak with a retention time 3.670 min. (Figure 4).
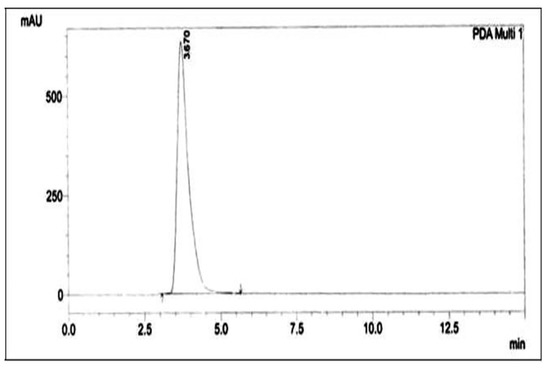
Figure 4.
HPLC chromatogram of the purified compound from the extracts of Leucas indica flowers.
UV spectroscopy showed characteristic absorption maxima at 270 nm corresponding to π–π* transition, indicating the presence of C=C double bonds in the HPLC-purified compound (Figure 5).
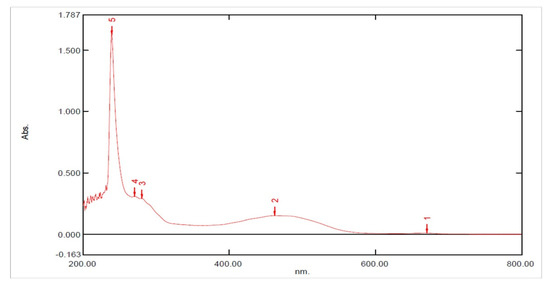
Figure 5.
Absorption (UV) spectrum of the purified compound from the extracts of Leucas indica flowers. The peaks observed in UV spectra from 1 to 5 represent different electronic excitations of organic molecules.
The IR spectrum of the isolated compound showed different peaks at 3445 cm−1 (broad band) and 1737 cm−1 (stretching band), indicating the presence of an OH group and carbonyl (C=O) group, respectively. The spectrum also showed a peak at 1464 cm−1 indicating the presence of C=C stretching, C–O stretching, and OH bending. The aliphatic CH and aromatic CH stretching was observed at 2917 cm−1 and 2855 cm−1, respectively (Figure 6).
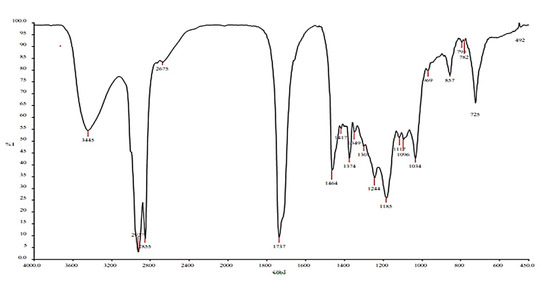
Figure 6.
Fourier transform infrared (FTIR) spectrum of the purified compound from the extracts of Leucas indica flowers.
The mass spectrum of bioactive compound exhibited a molecular ion [M]+ peak with a molecular weight of m/z 416.1462 (Figure 7) and no other prominent peaks were observed.
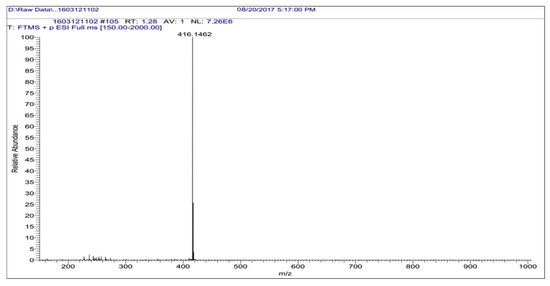
Figure 7.
Mass Spectroscopic analysis the purified compound from the extracts of Leucas indica flowers.
1H NMR (Figure 8A) and 13C NMR analysis (Figure 8B) of the purified compound showed the following: 1H NMR spectrum (CDCl3, 400 MHz): δ 8.16 (d, J = 5 Hz, 1H), 7.70 (m, 4H), 7.27 (s, 6H), 7.05 (d, J = 5 Hz, 3H), 6.93 (d, J = 5 Hz, 2H), 6.30 (d, J = 15 Hz, 3H), 3.95–3.90 (m, 6H), 3.65 (s, 4H), 3.41–3.38 (m, 8H), 2.85 (s, 7H), 2.40–2.00 (m, 33), 1.25–0.87 (s, 27H). 13C NMR (CDCl3, 100 MHz): δ 180.92, 148.56, 147.28, 126.52, 123.69, 115.27, 109.87, 55.98, 49.59, 31.92, 30.66, 29.90, 29.69, 29.65, 29.36, 22.68, 17.63, 14.11, 10.79.
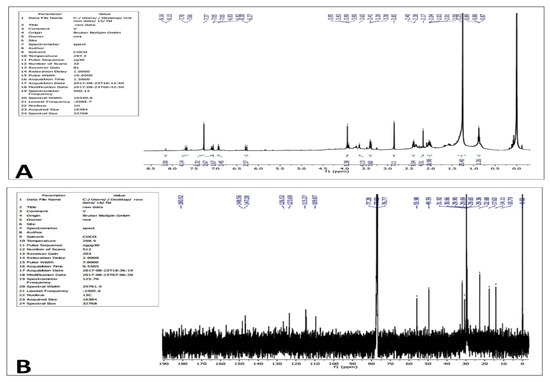
Figure 8.
1HNMR (A) and 13CNMR (B) spectrum of the purified compound from the extracts of Leucas indica flowers.
Distortionless Enhancement by Polarization Transfer (DEPT) experiments were performed to generate separate 13C subspectra for methyl (CH3), methylene (CH2), and methine (CH) signals. DEPT 45 (Figure 9A) yielded spectra with positive CH, CH2, and CH3 signals (all protonated carbons); DEPT 90 (Figure 9B) yielded spectra with only CH signals, while DEPT 135 (Figure 9C) yielded spectra with CH and CH3 signals in the opposite phase to CH2 signals. The pulse sequences of all spectrums are shown as follows:
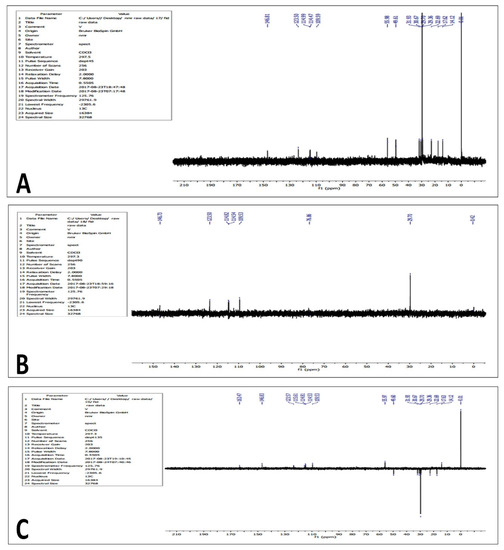
Figure 9.
DEPT 45 (A), 90 (B) and 135 (C) NMR analysis of the purified compound from the extracts of Leucas indica flowers.
- 3C DEPT 45 NMR: δ 146.81, 123.50, 114.99, 114.47, 105.59, 55.98, 49.61, 31.93, 30.63, 30.67, 29.70, 29.36, 22.69, 17.62, 14.12.
- 13C DEPT 90 NMR: δ 146.73, 123.50, 114.82, 114.54, 109.53, 76.86, 29.70.
- 13C DEPT 135 NMR: δ 163.47, 146.83, 123.57, 115.61, 114.81, 114.53, 109.53, 55.97, 49.60, 31.93, 30.67, 29.70, 29.36, 22.69, 17.63, 14.12.
Two-dimensional NMR experiments such as correlation spectroscopy (COSY) (Figure 10) and heteronuclear single quantum coherence (HSQC) (Figure 11) revealed the occurrence of proton-to-proton and proton-to-carbon interactions, respectively.
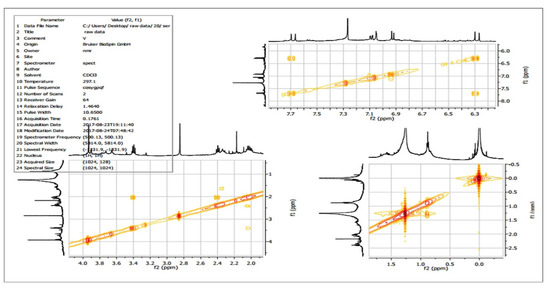
Figure 10.
H-1H COSY NMR analysis of the purified compound from the extracts of Leucas indica flowers.
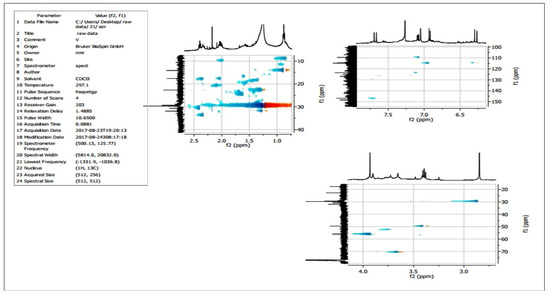
Figure 11.
HSQC NMR analysis of the purified compound from the extracts of Leucas indica flowers.
Based on the results, one-dimensional and two-dimensional NMR data along with mass spectroscopy revealed that the structure of purified compound was found to be 6-hydroxy-3-(4-hydroxyphenyl)-7-(3,4,5-trihydroxy-6-)(hydroxymethyl)tetrahydro-2H-pyran-2yl)-4H-chromen-4-one (Figure 12).
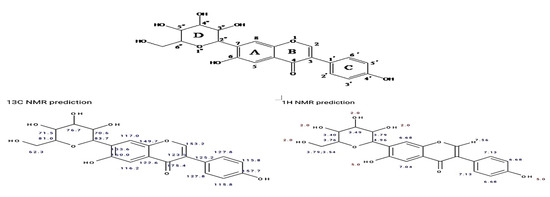
Figure 12.
Structure of purified bioactive compound isolated from Leucas indica flowers. The letters A, B, C, and D were indicated to number the position of carbon atoms.
4. Discussion
In the current study, the findings of the MTT assay indicated that methanolic flower extracts of Leucas indica exhibited growth-inhibiting properties (based on IC50 values) against the cancer cell lines MCF-7, HeLa, HCT-116, and HT-60, suggesting cytotoxic effects of flower extracts and could be ascribed to bioactive compounds. IC50 values are commonly used as a measure of antagonist drug potency in pharmacological research. However, in accordance to the criteria set by the American National Cancer Institute towards the cytotoxicity activity of crude extracts, the crude extract is considered effective if IC50 < 30 μg/mL in the preliminary assay [22]. Even though the methanolic flower extracts of L. indica were able to inhibit the growth of selected cancerous cell lines, significant cytotoxic potential was observed in MCF-7 (breast cancer) cell lines and moderate cytotoxic potential was observed in HeLa (cervical cancer) cell line ((IC50 = 16.429 µg/mL against the MCF-7 cell line, IC50 = 73.549 µg/mL against the HeLa cell line, IC50 = 190.587µg/mL against the HCT-116 cell line, and IC50 = 206.465 µg/mL against the HL 60 cell line). A lower IC50 value of methanolic extract was observed, which may be due to the synergistic action of both cytotoxic and cytoprotective components present in the extract [23]. Further, many studies have shown that phenolic compounds of plant extracts might be associated with cytotoxic effects [24]. A range of photochemicals such as phenols, flavonoids, alkaloids, tannins, saponins, ascorbic acid, carotenoids, and lycopene have been demonstrated in the aerial parts such as flowers and leaves of L. indica [20]. Earlier, it has been shown that the leaf extracts of L. aspera showed in vitro and in vivo anticancer activities [25,26]. Further, it has been shown that the ethanolic flower extracts of L. aspera showed anticancerous properties against Ehrlich ascetic tumor in vitro and in vivo (mouse model) systems [26]. Interestingly, the authors in Ref. [26] demonstrated that the antitumor activities of ethanolic extracts of L. aspera flowers could be attributed to antioxidant properties of phenolic compounds. On the other hand, the studies of Madhu et al. [25] demonstrated that the hot water extract of L. aspera leaves exhibited anticancer properties against HeLa cells in vitro and could be attributed to apoptosis.
Based on the anticancerous properties of methanolic extracts of L. indica flowers, next we analyzed the active compound(s) with growth-inhibiting properties. To accomplish this task, a pipeline wherein the methanolic crude extracts was subjected to analytical techniques such as silica gel column chromatography, TLC, HPLC, mass spectroscopy, and NMR to isolate, purify, and characterize the active compound(s). A total of 35 fractions were collected with respect to different concentrations of chloroform to methanol via silica gel chromatography and among the fractions, i.e., 11 to 15 with respect to chloroform-to-methanol ratio (60% to 40%) showed a single band on the TLC plate (Rf value of 0.73) and the fractions that exhibited a single band on TLC were pooled and when subjected to preparative HPLC, exhibiting a single peak with RT value 3.6 min. and thereby suggesting the occurrence of secondary metabolite in the flower extract of L. indica. The purified compound with respect to peak (HPLC spectrum) was separated and collected using an elution gradient program. The molecular mass of the purified compound was found to be 416.1462 m/z and FTIR analysis revealed the occurrence of several functional groups such as an OH group, carbonyl (C=O) group, and C=C, C–O, and aliphatic and aromatic CH groups. Further, UV-Vis spectroscopy indicated the occurrence of polyphenols such as total phenols, flavones, phenolic acids, and total anthocyanids. Finally, 1D (1H NMR and 13C NMR and DEPT) and 2D (COSY and HSQC) NMR studies indicated that the active compound present in methanolic extracts of L. indica flowers was found to be a structural isomer of coumarin, 6-hydroxy-3-(4-hydroxyphenyl)-7-(3,4,5-trihydroxy-6-)(hydroxymethyl)tetrahydro-2H-pyran-2yl)-4H-chromen-4-one. Earlier, photochemical and spectroscopic (UV, FTIR, NMR, and mass spectroscopy) analysis showed that the bioactive compound isolated from the methanolic extracts of L. aspera leaves was elucidated as 5,7-dihydroxy-2-[14-methoxy-15-propyl phenyl]-4H-chromen-4-one (leucasin) and further, antioxidant properties using a liposome model and DNA protection property using plasmid DNA has been demonstrated for this compound [27]. Previously, a glycoside known as acteoside was isolated from the methanolic extracts of L. indica flowers using preparative HPLC and anticancerous properties against MCF-7 cell lines have been demonstrated [28]. Using NMR studies, acteoside 2-(3,4-Dihydroxyphenyl)-ethyl-O-a-l-rhamnopyranosyl-(1→3)-4-O-caffeoyl-b-Dglucopyranoside has been reported in the aerial parts of L. lavandulaefolia and its antioxidant properties have also been demonstrated [29]. Furthermore, several bioactive compounds have been isolated from the Leucas species [30,31].
Chromones form the basic nucleus of important compounds including flavonoids and naturally occurring oxygen containing heterocyclic compounds. The important biological roles of chromones such as antimicrobial, antitumor, and antiviral properties are well acknowledged [32,33,34,35]. It has been shown that an appropriate heterocyclic ring containing compounds such as chromones when combined with sulfonamides could act as potent antitumor compounds [36]. In the current study, a new flavonoid, 6-hydroxy-3-(4-hydroxyphenyl)-7-(3,4,5-trihydroxy-6-)(hydroxymethyl)tetrahydro-2H-pyran-2yl)-4H-chromen-4-one, was isolated, purified, and structurally characterized from the methanolic extracts of L. indica flowers using HPLC, MS, and NMR studies.
5. Conclusions
The present study investigated the anticancer and antioxidant properties of methanolic extracts of L. indica flowers. The results indicated that subjecting the flower extracts to silica gel column separation led to a compound that exhibited anticancer properties and antioxidant abilities. The extracted and purified compound was subjected to TLC, HPLC, MS, and NMR for characterization and the results suggested that the purified compound was identified as a flavonoid [6-hydroxy-3-(4-hydroxyphenyl)-7-(3,4,5-trihydroxy-6-)(hydroxymethyl)tetrahydro-2H-pyran-2yl)-4H-chromen-4-one] (subjected to patent processing). Further, in vivo studies using purified compounds provide valuable insights into the mechanism(s) against cancer. Studies are in progress in our laboratory towards this end. From the findings, we identified a compound that exhibited anticancer properties in vitro, thus opening a new realm to explore the anticancerous properties of L. indica which might offer opportunities to develop novel anticancerous drugs.
Author Contributions
M.S.: Methodology, investigation, compilation of data, formal analysis and draft preparation. K.V.: formal analysis and investigation. B.V.S., S.B.S., V.R.D., M.H.R. and J.A.P.: Conceptualization, methodology, software, validation, formal analysis, data curation, writing—original draft preparation, writing—review and editing, project administration, funding acquisition. B.V.S.: visualization, supervision, S.B.S., and J.A.P.: Final approval. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available by contacting the corresponding author.
Acknowledgments
Muthyam Sowjanya thank the Head, Department of Biotechnology, Andhra University, Visakhapatnam, AP, and India for providing laboratory facilities. We also thank the Head, Department of Marine Biology and the Head, Department of Biotechnology, V.S. University, Nellore, AP, India for providing laboratory facilities for further confirmation tests. Muthyam Sowjanya also thank the Jayagen Biologics Analytical Laboratory, Chennai for providing facilities to carry out experiments related to cell lines. MS also thank the support towards the completion of part of the current research work. We thank T. Murali, Department of Chemistry, V.S. University, Nellore for his help towards the prediction of NMR spectra of the compound.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Bray, F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020, 102, 104551. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M. Status and Challenges of Plant-Anticancer Compounds in Cancer Treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar]
- Iqbal, J.; Abbasi, B.H.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer Activity of Natural Compounds from Plant and Marine Environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef]
- Valentino, G.; Graziani, V.; D’Abrosca, B.; Pacifico, S.; Fiorentino, A.; Scognamiglio, M. NMR-Based Plant Metabolomics in Nutraceutical Research: An Overview. Molecules 2020, 25, 1444. [Google Scholar] [CrossRef]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal Plants and Cancer Chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, B.; Kanwar, S.S.; Kumar, A. Lead Phytochemicals for Anticancer Drug Development. Front. Plant Sci. 2016, 7, 1667. [Google Scholar] [CrossRef]
- Hedge, I.C. Labiatae of South-West Asia: Diversity, distribution and endemism. Proc. R. Soc. Edinburgh. Sect. B Boil. Sci. 1986, 89, 23–35. [Google Scholar] [CrossRef]
- Das, S.N.; Patro, V.J.; Dinda, S.C. A review on Ethanobotanical survey of genus Leucas. Pharmacogn. Rev. 2012, 6, 100–106. [Google Scholar] [CrossRef]
- Ramachandran, V.S. Further notes on the ethno botany of Cannanore district, Kerala. J. Econ. Tax Bot. 1987, 11, 47–50. [Google Scholar]
- Maïkere-Faniyo, R.; Van Puyvelde, L.; Mutwewingabo, A.; Habiyaremye, F. Study of Rwandese medicinal plants used in the treatment of diarrhoea I. J. Ethnopharmacol. 1989, 26, 101–109. [Google Scholar] [CrossRef]
- Selvanayahgam, Z.E.; Gnanevendhan, S.G.; Balakrishna, K.; Rao, R.B. Antisnake venom botanicals from ethno medicine. J. Herbs Spices Med. Plants 1994, 2, 45–100. [Google Scholar] [CrossRef]
- Saha, K.; Mukherjee, P.K.; Das, J.; Pal, M.; Saha, B. Wound healing activity of Leucas lavandulaefolia Rees. J. Ethnopharmacol. 1997, 56, 139–144. [Google Scholar] [CrossRef]
- Mangathayaru, K.; Lakshmikant, J.; Sundar, N.S.; Swapna, R.; Grace, X.F.; Vasantha, J. Antimicrobial activity of Leucas aspera flowers. Fitoterapia 2005, 76, 752–754. [Google Scholar] [CrossRef]
- Manivannana, R.; Sukumar, D. The RBC membrane stabilization in an in vitro method by the drug isolated from Leucas aspera. Int. J. Appl Sci Eng. 2007, 5, 133–138. [Google Scholar]
- Sowjanya, M.; Kiran Kumar, M.; Sandeep, B.V. Assessment of Phytochemicals and Antioxidant activities of Leucas indica aerial parts—A comparative study. Int. J. Life Sci. 2016, 4, 29–43. [Google Scholar]
- Mattana, C.M.; Satorres, S.E.; Escobar, F. Antibacterial and cytotoxic activities of acacia aroma extracts. Emir. J. Food Agric. 2012, 24, 308–313. [Google Scholar]
- Suffness, M.; Pezzuto, J.M. Assays related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity; Hostettmann, K., Ed.; Academic Press: London, UK, 1990; pp. 71–133. [Google Scholar]
- Babykutty, S.; Padikkala, J.; Sathiadevan, P.; Vijayakurup, V.; Azis, T.; Srinivas, P.; Gopala, S. Apoptosis induction of Centella asiatica on human breast cancer cells. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 9–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer Plants: A Review of the Active Phytochemicals, Applications in Animal Models, and Regulatory Aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef]
- Madhu, G.C.; Kannaiyan, J.; Paulraj, B.; Veeramani, V. Anti-diabetic, Anti-cancer Activity and Associated Toxicity of Leucas aspera Extract in Wistar Albino Rats. Int. J. Pharm. Sci. Drug Res. 2019, 11, 387–392. [Google Scholar] [CrossRef]
- Jayanthi, M.K. Evaluation of anticancer activity of ethanol extract of Leucas aspera flower. Int. J. Green Pharm. 2020, 14, 257. [Google Scholar]
- Meghashri, S.; Kumar, H.V.; Gopal, S. Antioxidant properties of a novel flavonoid from leaves of Leucas aspera. Food Chem. 2010, 122, 105–110. [Google Scholar] [CrossRef]
- Vinayagam, A.V.A.; Sudha, P.N.S.P.N. In Vitro Cytotoxicity Activity of Acteoside from Leucas Indica Flowers. Indian J. Appl. Res. 2011, 4, 1–3. [Google Scholar] [CrossRef]
- Mostafa, M.; Nilufar, N.; Mosihuzzaman, M.; Talat, M.; Iqbal, M.C.; Attaur, R. Free radical scavenging phenylethanoid glycosides from Leucas indica Linn. Nat. Prod Res. 2007, 21, 354–361. [Google Scholar] [CrossRef]
- Shah, M.; Prajapati, M.; Patel, J.; Modi, K. Leucas aspera: A review. Pharmacogn. Rev. 2010, 4, 85–87. [Google Scholar] [CrossRef]
- Molla, M.I.; Ela, S.P. Leucas Zeylanica Is a Bangladeshi Plant with Significant Medicinal Prospect: A Review. GSC Biol. Pharm. Sci. 2021, 16, 011–018. [Google Scholar] [CrossRef]
- Huang, W.; Ding, Y.; Miao, Y.; Liu, M.-Z.; Li, Y.; Yang, G.-F. Synthesis and antitumor activity of novel dithiocarbamate substituted chromones. Eur. J. Med. Chem. 2009, 44, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- Desideri, N.; Mastromarino, P.; Conti, C. Synthesis and Evaluation of Antirhinovirus Activity of 3-Hydroxy and 3-Methoxy 2-Styrylchromones. Antivir. Chem. Chemother. 2003, 14, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.H.; Lee, K.Y.; Moon, C.S.; Lee, Y.S. Synthesis and anticancer activity of chromone-based analogs of lavendustin A. Eur. J. Med. Chem. 2010, 45, 4288–4292. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Shaikh, A.U.; Naseer, M.M.; Supuran, C.T. In-vitro antibacterial, antifungal and cytotoxic properties of metal-based furanyl derived sulfonamides. J. Enzyme Inhib. Med. Chem. 2006, 21, 771–781. [Google Scholar] [CrossRef]
- Awadallah, F.M.; El-Waei, T.A.; Hanna, M.M.; Abbas, S.E.; Ceruso, M.; Oz, B.E.; Guler, O.O.; Supuran, C.T. Synthesis, carbonic anhydrase inhibition and cytotoxic activity of novel chromone-based sulfonamide derivatives. Eur. J. Med. Chem. 2015, 96, 425–435. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).