Comparison of the Preparation Process of Rare Earth Oxides from the Water Leaching Solution of Waste Nd-Fe-B Magnets’ Sulfate Roasting Products
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Experimental Materials
2.2. Experimental Method
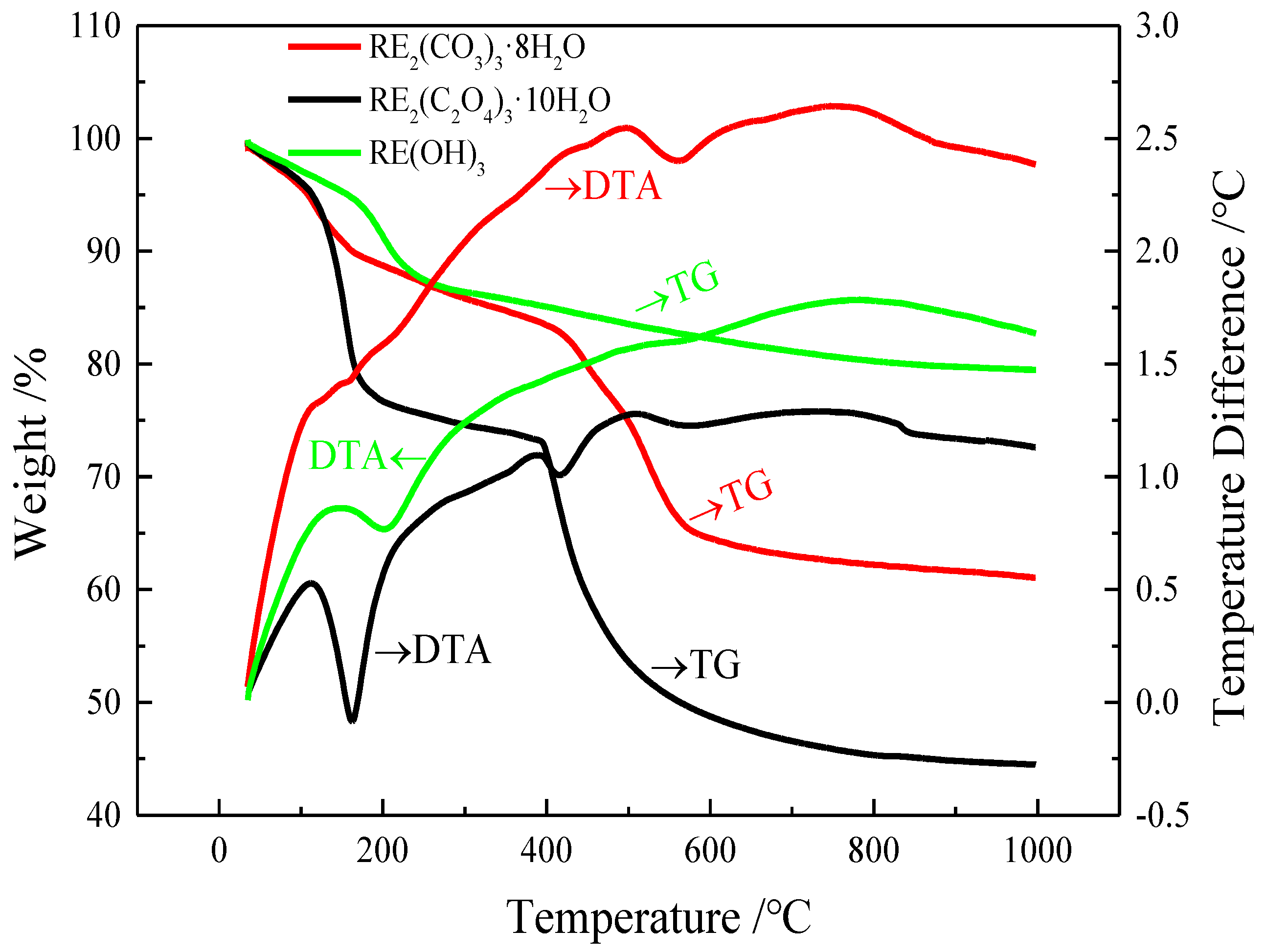
2.3. Experimental Analysis Method
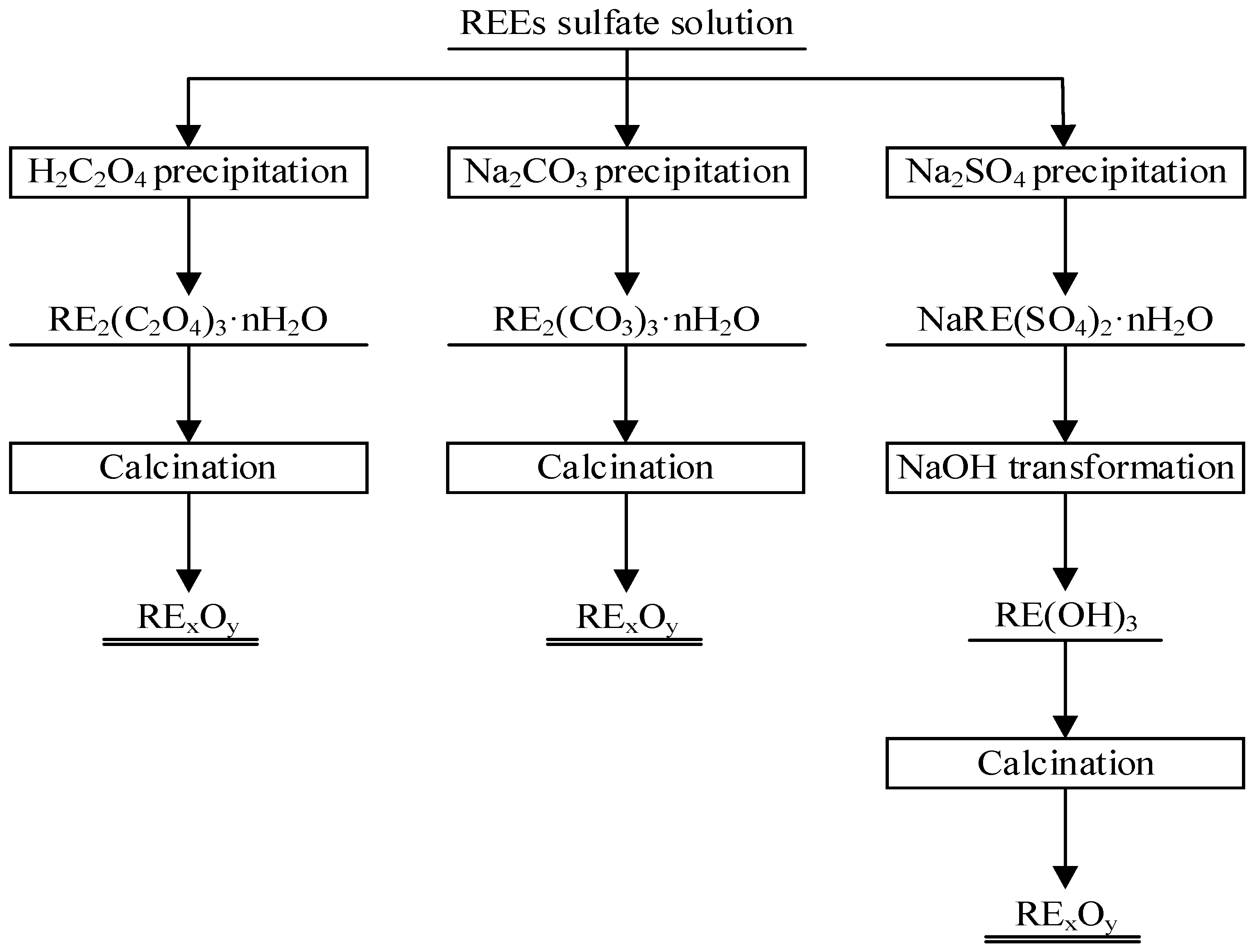
3. Results and Discussion
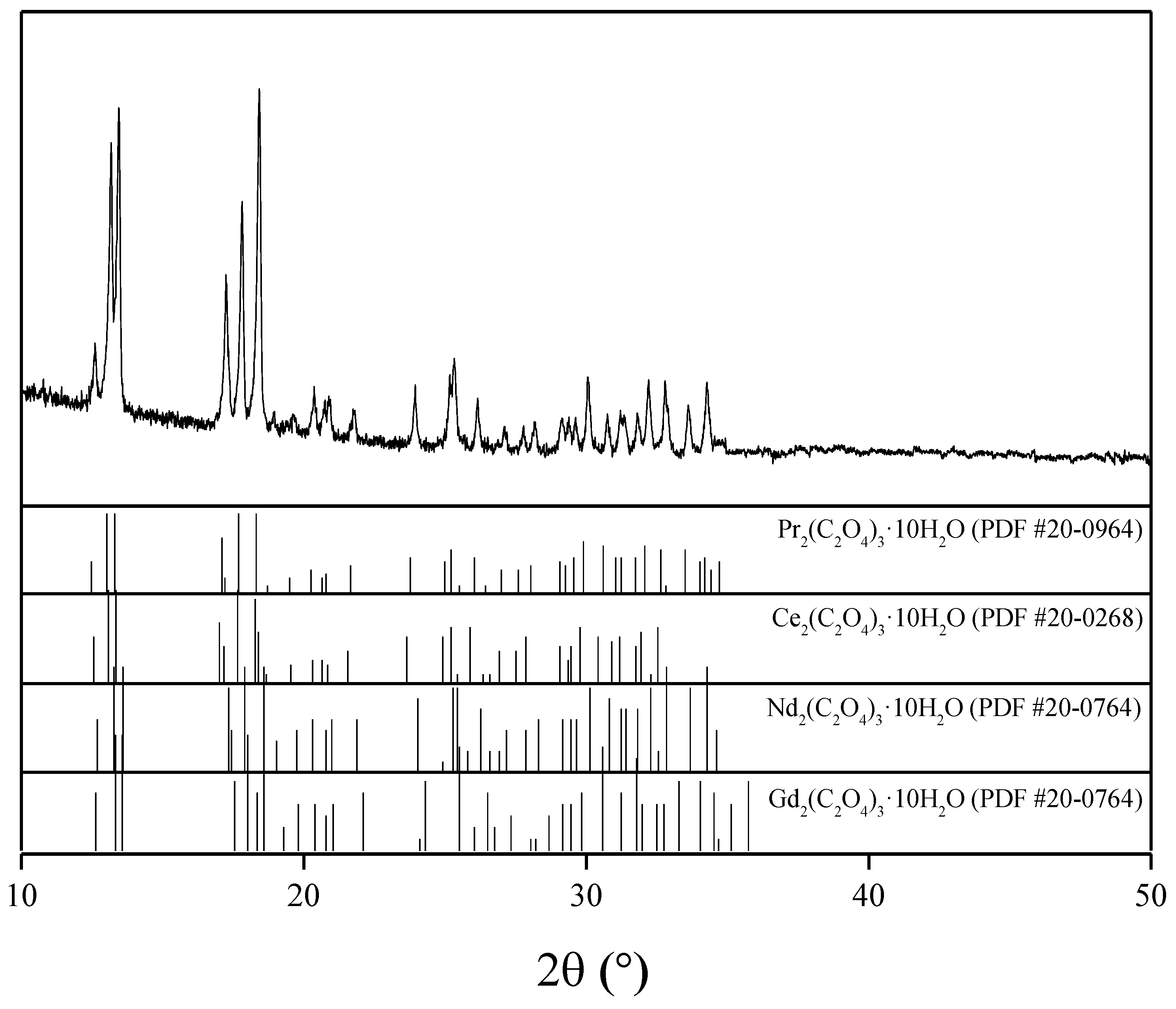
3.1. Oxalic Acid Precipitation Method
3.2. Double Sulfates Precipitation–Alkali Conversion Method
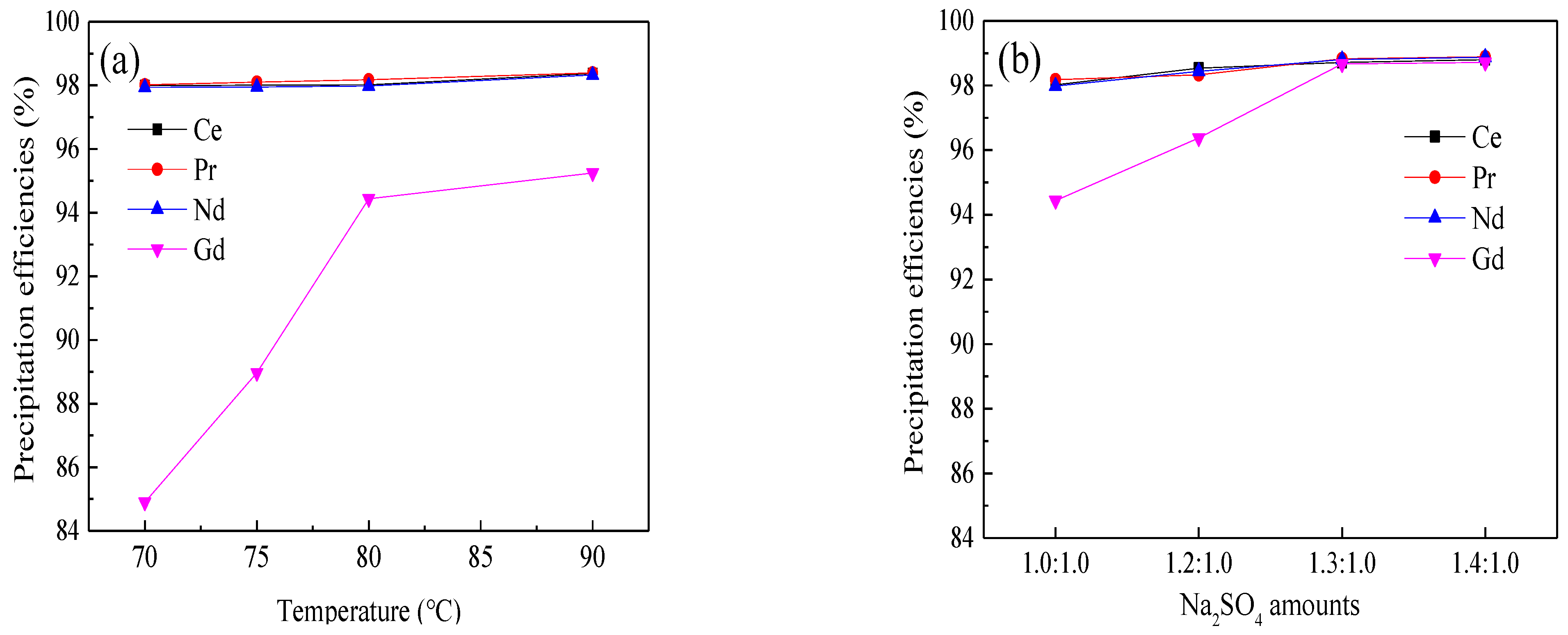
3.2.1. Double Sulfates Precipitation of REEs
3.2.2. Alkali Transformation of Double Sulfates Precipitation
3.3. Sodium Carbonate Precipitation Method
3.4. Rare Earth Oxides Preparation
3.5. Cost Comparison of Three Preparation Methods
4. Conclusions
- (1)
- Under optimal conditions, the REEs recovery efficiencies of the oxalic acid precipitation–calcination, sodium carbonate precipitation–calcination, and double sulfates precipitation–alkali conversion–calcination methods were 99.44%, 99.12%, and 97.95%; the purities of obtained the RExOy were 99.83%, 98.33%, and 98.04%, respectively.
- (2)
- The recovery of REEs from RE2(SO4)3 leaching solution via the sodium carbonate precipitation–calcination method has the greatest potential for industrial application. This process has the lowest cost for preparing RExOy, and the recovery efficiencies of the REEs and the purity of the RExOy are ideal.
- (3)
- By combining the obtained research results with previous studies, a new process for the selective recovery of REEs from NdFeB waste can be formed, which mainly involves ammonium sulfate roasting, REEs water leaching separation, sodium carbonate precipitation, and calcination. The results can also provide theoretical guidance for innovations in secondary resource recovery processes for REEs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.X.; Wang, S.; Li, C. Recycling and life cycle analysis of rare earth elements. Chin. J. Rare Met. 2016, 40, 945–954. [Google Scholar]
- Fu, L.W.; Wang, J.L.; Lei, X.; Wang, H.Q. Research progress on the recycling and utilization of Nd-Fe-B wastes. Nonferr. Met. Sci. Eng. 2020, 11, 92–97. [Google Scholar]
- Li, S.J.; Cui, Z.J.; Li, W.T.; Wang, D.; Wang, Z. Technical actuality and prospect of NdFeB waste recycling. Mater. Rep. 2021, 35, 3001–3009. [Google Scholar]
- Takeda, O.; Okabe, T.H.; Umetsu, Y. Phase equilibrium of the system Ag-Fe-Nd, and Nd extraction from magnet scraps using molten silver. J. Alloys Compd. 2004, 379, 305–313. [Google Scholar] [CrossRef]
- Takeda, O.; Okabe, T.H.; Umetsu, Y. Recovery of neodymium from a mixture of magnet scrap and other scrap. J. Alloys Compd. 2006, 408–412, 387–390. [Google Scholar] [CrossRef]
- Uda, T. Recovery of rare earths from magnet sludge by FeCl2. Mater. Trans. 2002, 43, 55–62. [Google Scholar] [CrossRef]
- Hua, Z.S.; Wang, J.; Wang, L.; Zhao, Z.; Li, X.; Xiao, Y.; Yang, Y. Selective extraction of rare earth elements from NdFeB scrap by molten chlorides. ACS Sustain. Chem. Eng. 2014, 2, 2536–2543. [Google Scholar] [CrossRef]
- Saito, T.; Sato, H.; Ozawa, S.; Yu, J.; Motegi, T. The extraction of Nd from waste NdFeB alloys by the glass slag method. J. Alloys Compd. 2003, 353, 189–193. [Google Scholar] [CrossRef]
- Nakamoto, M.; Kubo, K.; Katayama, Y.; Tanaka, T.; Yamamoto, T. Extraction of rare earth elements as oxides from a neodymium magnetic sludge. Metall. Mater. Trans. B 2011, 43, 468–476. [Google Scholar] [CrossRef]
- Deng, Y.C.; Wu, S.L.; Jiang, Y.J.; Li, H.X. Recycling NdFeB scrap by direct reduction and melting separation. Chin. Rare Earths 2015, 36, 8–13. [Google Scholar]
- Wang, Y.J.; Liu, Y.H.; Guo, J.X.; Wang, S.L.; Weng, G.Q. Recovery of rare earth metals form NdFeB waste materials using hydrochloric acid. Hydrometall. China 2006, 25, 195–197. [Google Scholar]
- Wu, J.P.; Deng, G.F.; Deng, L.L.; Lin, C.X.; Pan, X.B. Rare earth recovery from NdFeB magnet scrap. Nonferr. Met. Sci. Eng. 2016, 7, 119–124. [Google Scholar]
- Huang, J.S.; Qi, M.F. Review on recovery technology of Nd-Fe-B waste materials. China Resour. Compr. Util. 2008, 26, 4–5. [Google Scholar]
- Lai, W.H.; Liu, M.; Li, C.Y.; Suo, L.H.; Yue, M. Recovery of a composite powder from NdFeB slurry by co-precipitation. Hydrometallurgy 2014, 150, 27–33. [Google Scholar] [CrossRef]
- Lin, H.C. Study on process of recovering neodymium oxide from the Nd-Fe-B permanent magnet scraps. World Nonferr. Met. 2007, 4, 59–61. [Google Scholar]
- Liu, Z.Q.; Chen, H.J. The industrial practice of recycling rare earth from NdFeB scraps. Mater. Res. Appl. 2009, 3, 134–137. [Google Scholar]
- Abrahami, S.T.; Xiao, Y.; Yang, Y. Rare-earth elements recovery from post-consumer hard-disc drives. Miner. Process. Extr. Metall. 2015, 124, 106–115. [Google Scholar] [CrossRef]
- Liu, F.P.; Chen, F.X.; Wang, L.J.; Ma, S.B.; Wan, X.B.; Wang, J.L. Selective separation of rare earths from spent Nd-Fe-B magnets using two-stage ammonium sulfate roasting followed by water leaching. Hydrometallurgy 2021, 203, 105626. [Google Scholar] [CrossRef]
- Yin, X.W.; Liu, M.; Lai, W.H.; Dong, C.B.; Yue, M.; Suo, H.L. Recycle rare earth elements from NdFeB waste with oxalate precipitation method. Chin. J. Rare Met. 2014, 6, 1093–1098. [Google Scholar]
- Tobias, E.; Daniel, G.; Florian, S. Hydrometallurgical recycling of sintered NdFeB magnets. Erzmetall 2013, 66, 209–219. [Google Scholar]
- Tom, V.H.; Bart, B.; Tom, V.G.; Koen, B. From NdFeB magnets towards the rare-earth oxides: A recycling process consuming only oxalic acid. RSC Adv. 2014, 4, 64099–64111. [Google Scholar]
- Tang, J.; Wei, C.F.; Zhao, D.W.; Lin, H.; Tian, G.H. Nd2O3 recovery from sintered NdFeB scrap. Rare Met. Cem. Carbides 2009, 37, 9–11+18. [Google Scholar]
- Luo, L.; Tang, H.W.; Yang, B.J. Technological study on rare earth recovery form rare earth sulfate solution. Rare Met. Cem. Carbides 2015, 43, 12–15. [Google Scholar]
- Zhang, L.L.; Deng, X.Y.; Deng, Y.G.; Yang, H.; Xia, Y.; Wei, Y.; Yang, G.H. Study on ammonium bicarbonate preparation crystalline mixed rare earth carbonate. J. Huang Shi Inst. Technol. 2011, 27, 20–24. [Google Scholar]
- Li, H.Q.; Li, J.; Zhao, Y.Z.; Zhang, R.X.; Hao, X.K.; Ma, X.D. Study on preparation of yttrium oxide with large particle sizes and spherical shapes by precipitation with ammonium bicarbonate. Chin. Rare Earths 2014, 35, 77–81. [Google Scholar]
- Lu, M.; Qiu, G.H.; Ma, Y.; Zhang, X.B.; Gao, W.Z. Preparation of small size and high total cerium carbonate. Rare Met. Cem. Carbides 2019, 47, 16–18+35. [Google Scholar]
- Gao, X.G.; Sun, M.H.; Sun, M.X.; Feng, C.F.; Sun, J.Z. Study on preparation of barium carbonate by orthogonal test method and sodium bicarbonate. China Resour. Compr. Util. 2019, 37, 17–19. [Google Scholar]
- Chen, X.; Liao, D.H. Magnetization process for strengthening the precipitation of rare earth leach solution by sodium bicarbonate. Min. Metall. Eng. 2020, 40, 99–101. [Google Scholar]
- Wang, S.L.; Liu, Y.L.; Cheng, F.X.; Wu, S.; Liao, C.S. A study on preparation of rare earth Carbonate using sodium carbonate. Min. Metall. 2015, 24, 44–46. [Google Scholar]
- Gao, G.H.; Lai, A.B.; Zhou, X.F.; Liao, C.F.; Liu, J.Y.; Xiao, Y.F. Precipitation crystallization process of yttrium carbonate and its influencing factors. Chin. J. Nonferr. Met. 2020, 30, 2457–2474. [Google Scholar]



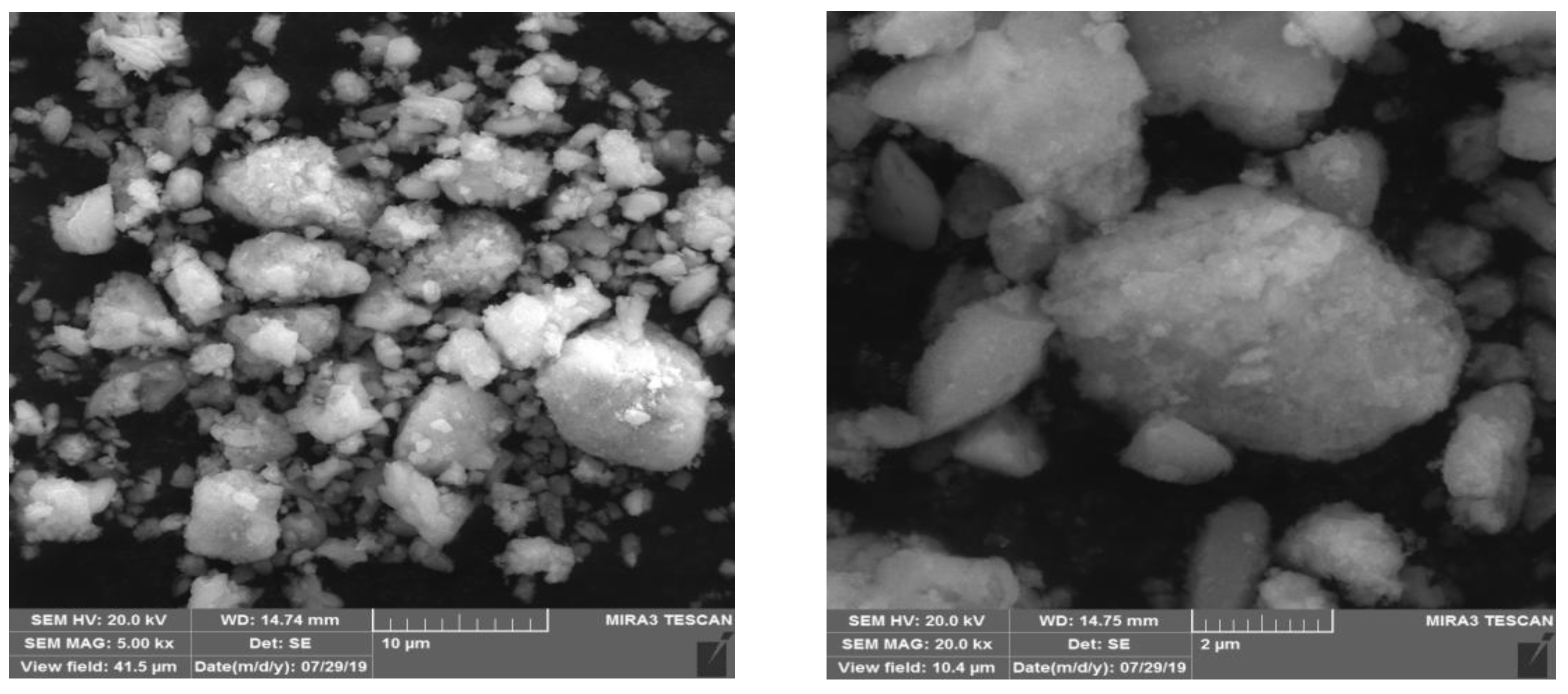
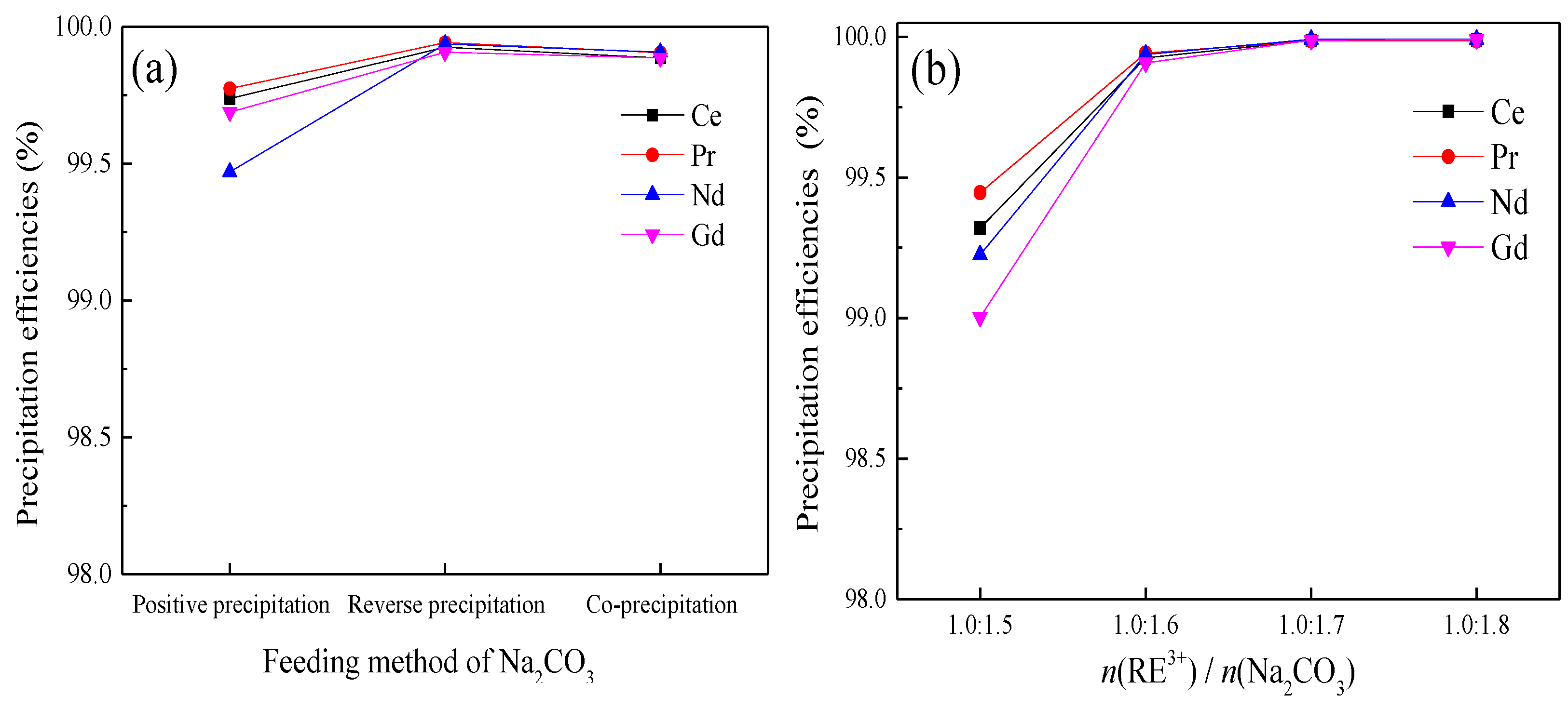
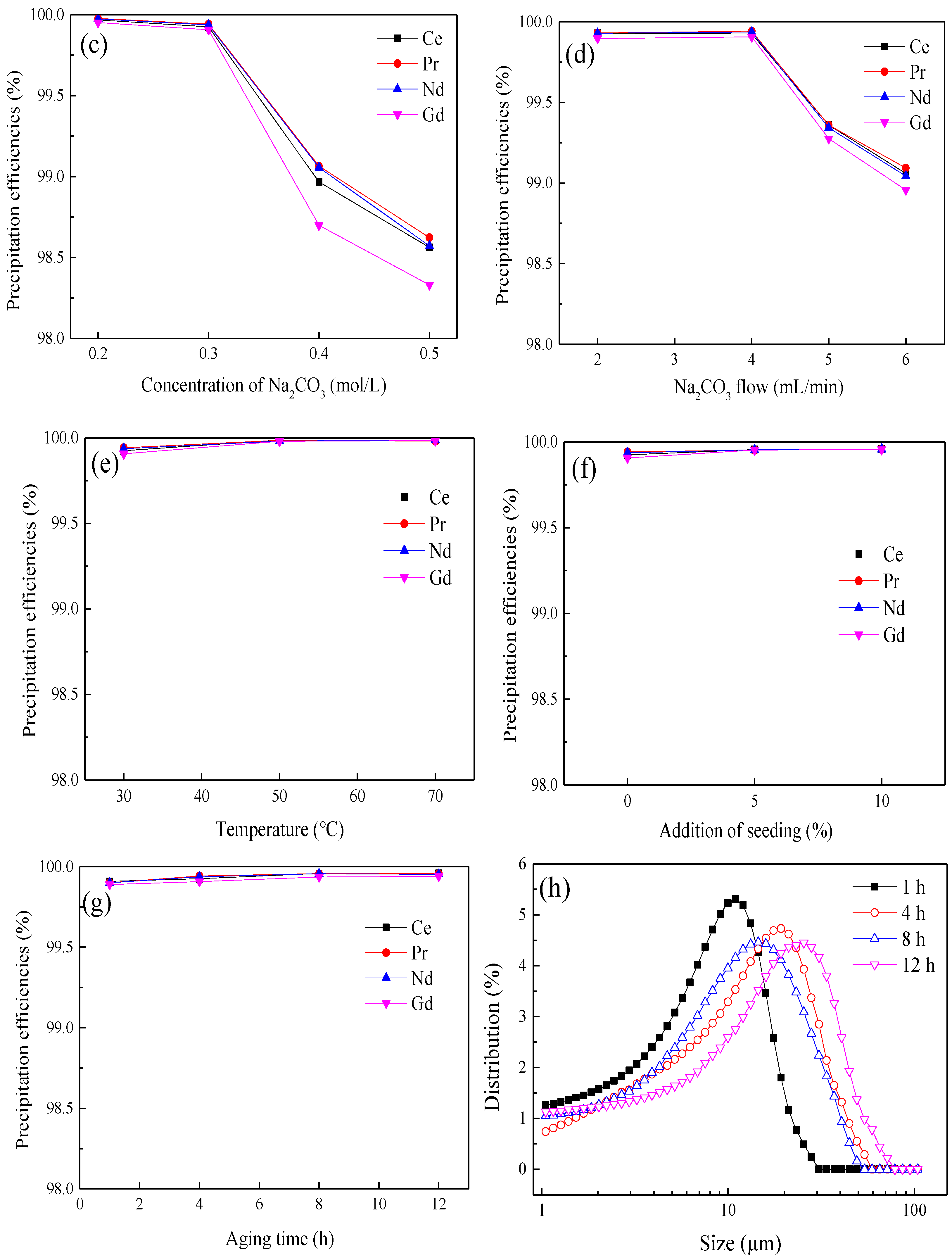

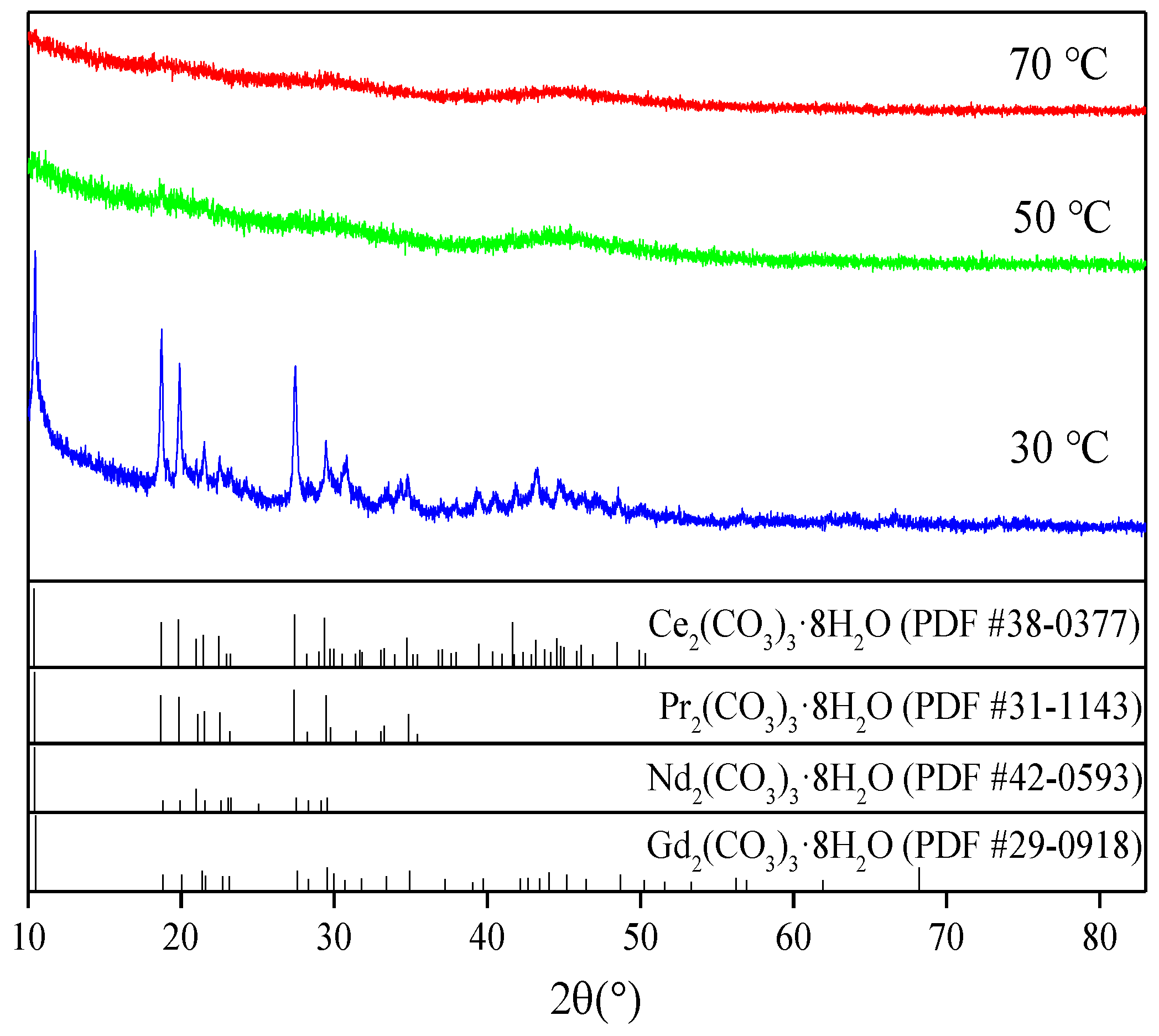
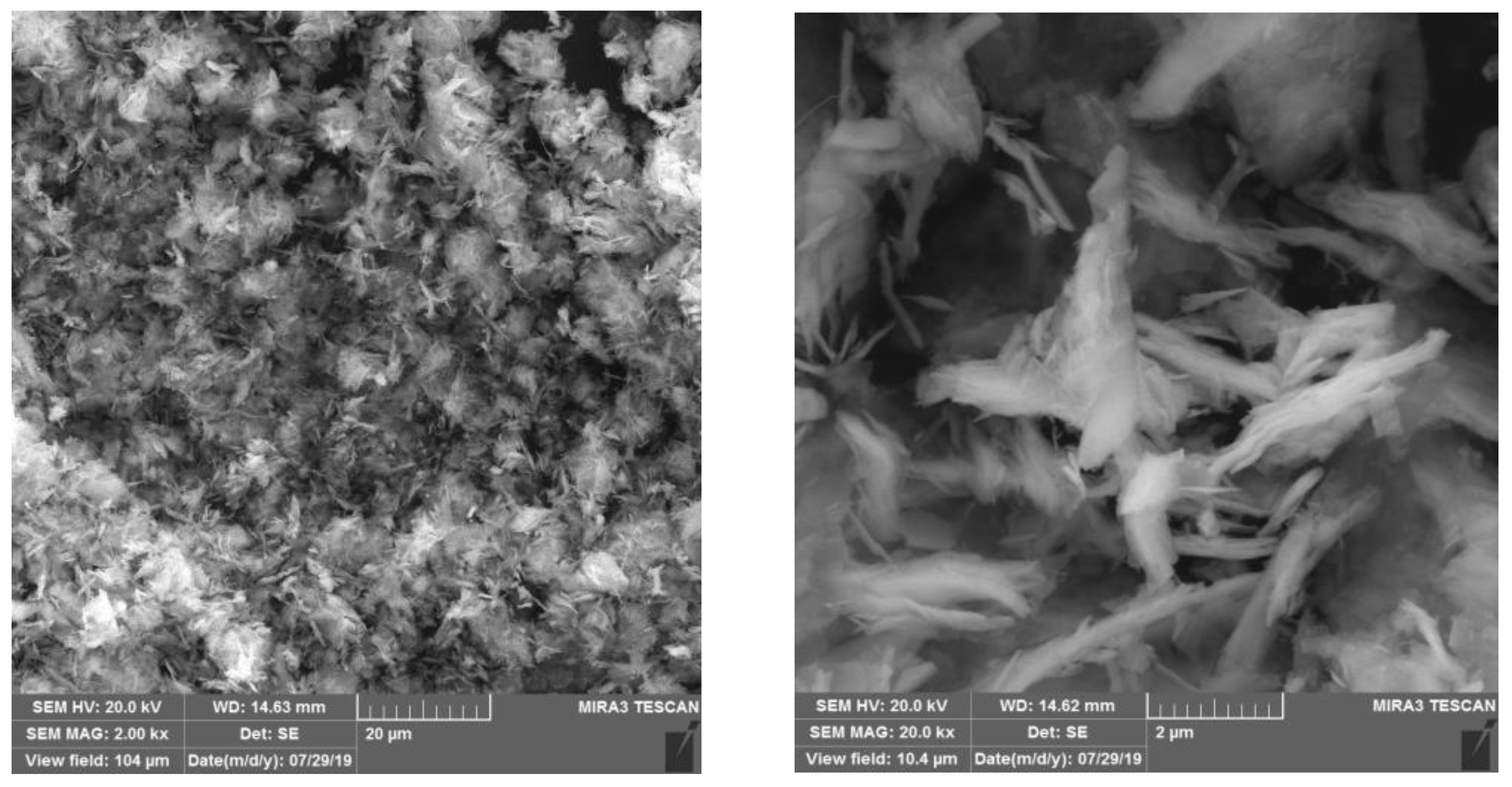

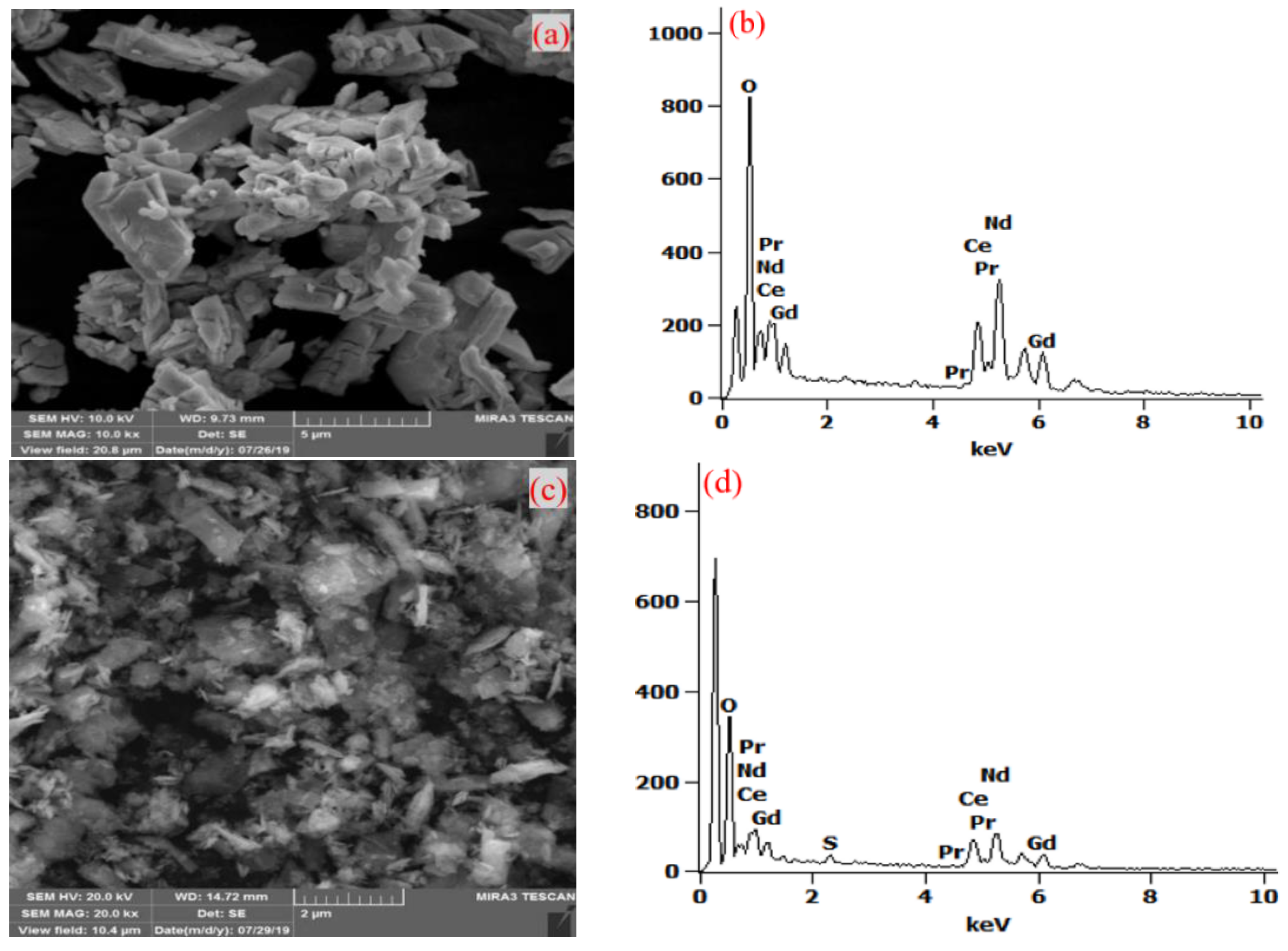
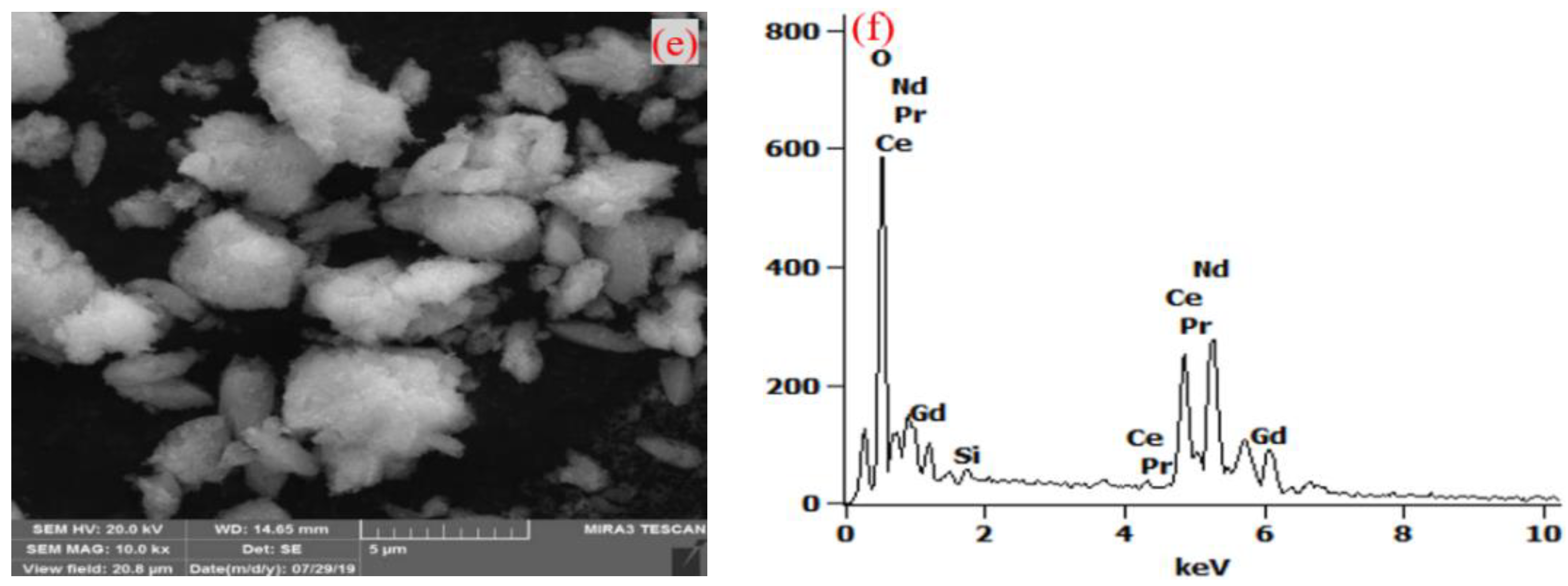
| Composition | Ce | Pr | Nd | Gd | SO42− | Al | Fe | Co | Cu | Si |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (mg/L) | 1720 | 1700 | 5654 | 1510 | 17,290 | 3.12 | 1.10 | 2.45 | 2.08 | 2.05 |
| REO | Ce | Pr | Nd | Gd | Al | Ca | Co | Cu | Fe | Si | SO42− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | 30.51 | 10.03 | 31.54 | 11.67 | 0.0070 | 0.020 | 0.0012 | 0.0047 | 0.002 | 0.0008 | 0.081 |
| b | 30.38 | 9.86 | 30.89 | 11.34 | 0.0054 | 0.024 | 0.0020 | 0.0021 | 0.006 | 0.011 | 0.12 |
| c | 30.16 | 9.68 | 31.61 | 10.78 | 0.01 | 0.034 | 0.00019 | 0.0021 | 0.006 | 0.052 | 0.090 |
| Precipitation Method | REEs Recovery Rate/% | Product Purity/% | H2C2O4 Dosage/t | H2C2O4 Price (CNY/t) | Sum (CNY) | ||
|---|---|---|---|---|---|---|---|
| a | 99.44 | 99.83 | 0.96 | 5500 | 5280 | ||
| Precipitation method | REEs recovery rate/% | Product purity/% | Na2CO3 dosage/t | Na2CO3 price (CNY/t) | Sum (CNY) | ||
| b | 99.12 | 98.33 | 1 | 2600 | 2600 | ||
| Precipitation method | REEs recovery rate/% | Product purity/% | Na2SO4 dosage/t | Na2SO4 price (CNY/t) | NaOH dosage (t) | NaOH price (CNY/t) | Sum (CNY) |
| c | 97.95 | 98.04 | 0.55 | 520 | 1 | 3300 | 3586 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Liu, F.; Wang, L.; Wang, J. Comparison of the Preparation Process of Rare Earth Oxides from the Water Leaching Solution of Waste Nd-Fe-B Magnets’ Sulfate Roasting Products. Processes 2022, 10, 2310. https://doi.org/10.3390/pr10112310
Chen F, Liu F, Wang L, Wang J. Comparison of the Preparation Process of Rare Earth Oxides from the Water Leaching Solution of Waste Nd-Fe-B Magnets’ Sulfate Roasting Products. Processes. 2022; 10(11):2310. https://doi.org/10.3390/pr10112310
Chicago/Turabian StyleChen, Feixiong, Fupeng Liu, Longjun Wang, and Jinliang Wang. 2022. "Comparison of the Preparation Process of Rare Earth Oxides from the Water Leaching Solution of Waste Nd-Fe-B Magnets’ Sulfate Roasting Products" Processes 10, no. 11: 2310. https://doi.org/10.3390/pr10112310
APA StyleChen, F., Liu, F., Wang, L., & Wang, J. (2022). Comparison of the Preparation Process of Rare Earth Oxides from the Water Leaching Solution of Waste Nd-Fe-B Magnets’ Sulfate Roasting Products. Processes, 10(11), 2310. https://doi.org/10.3390/pr10112310






