Targeted Metabolic Analysis and MFA of Insect Cells Expressing Influenza HA-VLP
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Culture Media
2.2. Baculovirus Amplification and Storage
2.3. Production of HA-VLPs
2.4. Purification of HA-VLPs
2.5. Analytics
2.5.1. Cell Concentration and Viability
2.5.2. Metabolite Analysis
2.5.3. Baculovirus Titration
2.5.4. Hemagglutination Assay
2.5.5. SDS-PAGE and Western Blot
2.5.6. Transmission Electron Microscopy
2.6. Mathematical Equations for Estimation of Reaction Rates
2.7. Metabolic Flux Analysis
2.8. Principal Component Analysis
2.9. Data Availability Statement
3. Results
3.1. Production of HA-VLPs
3.2. The impact of HA-VLPs’ Valency and HA Subtype on the Metabolic Profile of Insect Cells
3.2.1. Infection Kinetics and HA-VLPs’ Expression
3.2.2. Major Metabolite Consumption and Production Rates
3.2.3. Intracellular Metabolic Fluxes Estimated by MFA
3.2.4. Clustering HA-VLPs’ Production Processes Using PCA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roldão, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like Particles in Vaccine Development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, B.; Lateef, Z.; Walker, G.F.; Young, S.L.; Ward, V.K. Virus-like Particle Vaccines: Immunology and Formulation for Clinical Translation. Expert Rev. Vaccines 2018, 17, 833–849. [Google Scholar] [CrossRef] [PubMed]
- Lua, L.H.L.; Connors, N.K.; Sainsbury, F.; Chuan, Y.P.; Wibowo, N.; Middelberg, A.P.J. Bioengineering Virus-Like Particles as Vaccines. Biotechnol. Bioeng. 2014, 111, 425–440. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Voyer, J.; Li, Y.; Chen, X. Flagellin/Virus-like Particle Hybrid Platform with High Immunogenicity, Safety, and Versatility for Vaccine Development. ACS Appl. Mater. Interfaces 2022, 14, 21872–21885. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.B.; Silva, R.J.S.; Sousa, M.F.Q.; Peixoto, C.; Roldão, A.; Carrondo, M.J.T.; Alves, P.M. Bioanalytics for Influenza Virus-Like Particle Characterization and Process Monitoring. Front. Bioeng. Biotechnol. 2022, 10, 805176. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Chu, K.-B.; Yoon, K.-W.; Eom, G.-D.; Mao, J.; Kim, M.-J.; Lee, S.-H.; Moon, E.-K.; Quan, F.-S. Multiple Neuraminidase Containing Influenza Virus-Like Particle Vaccines Protect Mice from Avian and Human Influenza Virus Infection. Viruses 2022, 14, 429. [Google Scholar] [CrossRef]
- Cheung, P.P.H.; Watson, S.J.; Choy, K.-T.; Fun Sia, S.; Wong, D.D.Y.; Poon, L.L.M.; Kellam, P.; Guan, Y.; Malik Peiris, J.S.; Yen, H.-L. Generation and Characterization of Influenza A Viruses with Altered Polymerase Fidelity. Nat. Commun. 2014, 5, 4794. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Lapedes, A.S.; de Jong, J.C.; Bestebroer, T.M.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Mapping the Antigenic and Genetic Evolution of Influenza Virus. Science 2004, 305, 371–376. [Google Scholar] [CrossRef]
- Bouvier, N.M.; Palese, P. The Biology of Influenza Viruses. Vaccine 2008, 26 (Suppl. 4), D49–D53. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Krammer, F. Universal Influenza Virus Vaccines and Therapeutic Antibodies. Clin. Microbiol. Infect. 2017, 23, 222–228. [Google Scholar] [CrossRef]
- Nachbagauer, R.; Feser, J.; Naficy, A.; Bernstein, D.I.; Guptill, J.; Walter, E.B.; Berlanda-Scorza, F.; Stadlbauer, D.; Wilson, P.C.; Aydillo, T.; et al. A Chimeric Hemagglutinin-Based Universal Influenza Virus Vaccine Approach Induces Broad and Long-Lasting Immunity in a Randomized, Placebo-Controlled Phase I Trial. Nat. Med. 2021, 27, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Sautto, G.A.; Kirchenbaum, G.A.; Abreu, R.B.; Ecker, J.W.; Pierce, S.R.; Kleanthous, H.; Ross, T.M. A Computationally Optimized Broadly Reactive Antigen Subtype–Specific Influenza Vaccine Strategy Elicits Unique Potent Broadly Neutralizing Antibodies against Hemagglutinin. J. Immunol. 2020, 204, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, D.P.; Correia, R.; Carrondo, M.J.T.; Roldão, A.; Teixeira, A.P.; Alves, P.M. Combining Stable Insect Cell Lines with Baculovirus-Mediated Expression for Multi-HA Influenza VLP Production. Vaccine 2018, 36, 3112–3123. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Zuo, H.; Zhu, F.; Hu, Z.; Chen, L.; Yang, Y.; Lv, P.; Yao, Q.; Chen, K. The Interaction between Baculoviruses and Their Insect Hosts, Developmental & Comparative Immunology. Dev. Comp. Immunol. 2018, 83, 114–123. [Google Scholar] [CrossRef]
- Granados, R.R.; Guoxun, L.; Derksen, A.C.G.; McKenna, K.A. A New Insect Cell Line from Trichoplusia Ni (BTI-Tn-5B1-4) Susceptible to Trichoplusia Ni Single Enveloped Nuclear Polyhedrosis Virus. J. Invertebr. Pathol. 1994, 64, 260–266. [Google Scholar] [CrossRef]
- Stolt-Bergner, P.; Benda, C.; Bergbrede, T.; Besir, H.; Celie, P.H.N.; Chang, C.; Drechsel, D.; Fischer, A.; Geerlof, A.; Giabbai, B.; et al. Baculovirus-Driven Protein Expression in Insect Cells: A Benchmarking Study. J. Struct. Biol. 2018, 203, 71–80. [Google Scholar] [CrossRef]
- Drews, M.; Doverskog, M.; Ohman, L.; Chapman, B.E.; Jacobsson, U.; Kuchel, P.W.; Häggström, L. Pathways of Glutamine Metabolism in Spodoptera Frugiperda (Sf9) Insect Cells: Evidence for the Presence of the Nitrogen Assimilation System, and a Metabolic Switch by 1H/15N NMR. J. Biotechnol. 2000, 78, 23–37. [Google Scholar] [CrossRef]
- Wang, Y.; Oberley, L.W.; Murhammer, D.W. Antioxidant Defense Systems of Two Lipidopteran Insect Cell Lines. Free. Radic. Biol. Med. 2001, 30, 1254–1262. [Google Scholar] [CrossRef]
- Martensen, P.; Justesen, J. Specific Inhibitors Prevent Proteolytic Degradation of Recombinant Proteins Expressed in HighFiveTM Cells. BioTechniques 2001, 30, 782–792. [Google Scholar] [CrossRef]
- Keith, M.B.; Farrell, P.J.; Iatrou, K.; Behie, L.A. Screening of Transformed Insect Cell Lines for Recombinant Protein Production. Biotechnol. Prog. 1999, 15, 1046–1052. [Google Scholar] [CrossRef]
- Krammer, F.; Schinko, T.; Palmberger, D.; Tauer, C.; Messner, P.; Grabherr, R. Trichoplusia Ni Cells (High Five) Are Highly Efficient for the Production of Influenza A Virus-Like Particles: A Comparison of Two Insect Cell Lines as Production Platforms for Influenza Vaccines. Mol. Biotechnol. 2010, 45, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, H.; Konishi, E. Production of Japanese Encephalitis Virus-Like Particles in Insect Cells. Bioengineered 2013, 4, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.; Bernal, V.; Saelens, X.; Lozano, A.B.; Bernal, C.; Sevilla, A.; Carrondo, M.J.T.; Alves, P.M. Metabolic Profiling of Insect Cell Lines: Unveiling Cell Line Determinants behind System’s Productivity. Biotechnol. Bioeng. 2014, 111, 816–828. [Google Scholar] [CrossRef]
- Monteiro, F.; Bernal, V.; Alves, P.M. The Role of Host Cell Physiology in the Productivity of the Baculovirus-Insect Cell System: Fluxome Analysis of Trichoplusia Ni and Spodoptera Frugiperda Cell Lines. Biotechnol. Bioeng. 2017, 114, 674–684. [Google Scholar] [CrossRef]
- Vieira, H.L.A.; Estêvão, C.; Roldão, A.; Peixoto, C.C.; Sousa, M.F.Q.; Cruz, P.E.; Carrondo, M.J.T.; Alves, P.M. Triple Layered Rotavirus VLP Production: Kinetics of Vector Replication, MRNA Stability and Recombinant Protein Production. J. Biotechnol. 2005, 120, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.; Meneses, L.; Richheimer, C.; Alves, P.M.; Carrondo, M.J.T.; Duarte, A.R.C.; Paiva, A.; Roldão, A. Improved Storage of Influenza HA-VLPs Using a Trehalose-Glycerol Natural Deep Eutectic Solvent System. Vaccine 2021, 39, 3279–3286. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.B.; Moreira, A.S.; Gomes, J.; Carrondo, M.J.T.; Thornton, D.J.; Alves, P.M.; Costa, J.; Peixoto, C. A Detection and Quantification Label-Free Tool to Speed up Downstream Processing of Model Mucins. PLoS ONE 2018, 13, e0190974. [Google Scholar] [CrossRef]
- Mena, J.; Ramirez, O.; Palomares, L. Titration of Non-Occluded Baculovirus Using a Cell Viability Assay. BioTechniques 2003, 34, 260–264. [Google Scholar] [CrossRef]
- Roldão, A.; Oliveira, R.; Carrondo, M.J.T.; Alves, P.M. Error Assessment in Recombinant Baculovirus Titration: Evaluation of Different Methods. J. Virol. Methods 2009, 159, 69–80. [Google Scholar] [CrossRef]
- Klamt, S.; Saez-Rodriguez, J.; Gilles, E. Structural and Functional Analysis of Cellular Networks with CellNetAnalyzer. BMC Syst. Biol. 2007, 1, 2. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, S.; Huang, Y.; Chen, F.; Chen, L.; Ding, D.; Zheng, Y.; Li, H.; Xiao, J.; Feng, J.; et al. Virus-like Particle Vaccines for Poliovirus Types 1, 2, and 3 with Enhanced Thermostability Expressed in Insect Cells. Vaccine 2019, 37, 2340–2347. [Google Scholar] [CrossRef] [PubMed]
- Imasaki, T.; Wenzel, S.; Yamada, K.; Bryant, M.L.; Takagi, Y. Titer Estimation for Quality Control (TEQC) Method: A Practical Approach for Optimal Production of Protein Complexes Using the Baculovirus Expression Vector System. PLoS ONE 2018, 13, e0195356. [Google Scholar] [CrossRef] [PubMed]
- Pastor, A.R.; González-Domínguez, G.; Díaz-Salinas, M.A.; Ramírez, O.T.; Palomares, L.A. Defining the Multiplicity and Time of Infection for the Production of Zaire Ebola Virus-like Particles in the Insect Cell-Baculovirus Expression System. Vaccine 2019, 37, 6962–6969. [Google Scholar] [CrossRef]
- Üstün-Aytekin, Ö.; Gürhan, İ.D.; Ohura, K.; Imai, T.; Öngen, G. Monitoring of the Effects of Transfection with Baculovirus on Sf9 Cell Line and Expression of Human Dipeptidyl Peptidase IV. Cytotechnology 2014, 66, 159–168. [Google Scholar] [CrossRef][Green Version]
- Nerome, K.; Matsuda, S.; Maegawa, K.; Sugita, S.; Kuroda, K.; Kawasaki, K.; Nerome, R. Quantitative Analysis of the Yield of Avian H7 Influenza Virus Haemagglutinin Protein Produced in Silkworm Pupae with the Use of the Codon-Optimized DNA: A Possible Oral Vaccine. Vaccine 2017, 35, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Chlanda, P.; Mekhedov, E.; Waters, H.; Sodt, A.; Schwartz, C.; Nair, V.; Blank, P.S.; Zimmerberg, J. Palmitoylation Contributes to Membrane Curvature in Influenza A Virus Assembly and Hemagglutinin-Mediated Membrane Fusion. J. Virol. 2017, 91, e00947-17. [Google Scholar] [CrossRef] [PubMed]
- Bedard, C.; Tom, R.; Kamen, A. Growth, Nutrient Consumption, and End-Product Accumulation in Sf-9 and BTI-EAA Insect Cell Cultures: Insights into Growth Limitation and Metabolism. Biotechnol. Prog. 1993, 9, 615–624. [Google Scholar] [CrossRef]
- Radford, K.; Reid, S.; Greenfield, P. Substrate Limitation in the Baculovirus Expression Vector System. Biotechnol. Bioeng. 1997, 56, 32–44. [Google Scholar] [CrossRef]
- Vallazza, M.; Petri, T. Optimization of the Production of Triabin, a Novel Thrombin Inhibitor, in High FiveTM Insect Cells Infected with a Recombinant Baculovirus. Cytotechnology 1999, 29, 85–92. [Google Scholar] [CrossRef]
- Doverskog, M.; Ljunggren, J.; Öhman, L.; Häggström, L. Physiology of Cultured Animal Cells. J. Biotechnol. 1997, 59, 103–115. [Google Scholar] [CrossRef]
- Koczka, K.; Peters, P.; Ernst, W.; Himmelbauer, H.; Nika, L.; Grabherr, R. Comparative Transcriptome Analysis of a Trichoplusia Ni Cell Line Reveals Distinct Host Responses to Intracellular and Secreted Protein Products Expressed by Recombinant Baculoviruses. J. Biotechnol. 2018, 270, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Geck, R.C.; Toker, A. Nonessential Amino Acid Metabolism in Breast Cancer. Adv. Biol. Regul. 2016, 62, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Correia, R.; Fernandes, B.; Alves, P.M.; Carrondo, M.J.T.; Roldão, A. Improving Influenza HA-Vlps Production in Insect High Five Cells via Adaptive Laboratory Evolution. Vaccines 2020, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.; Vidigal, J.; Correia, R.; Carrondo, M.J.T.; Alves, P.M.; Teixeira, A.P.; Roldão, A. Adaptive Laboratory Evolution of Stable Insect Cell Lines for Improved HIV-Gag VLPs Production. J. Biotechnol. 2020, 307, 139–147. [Google Scholar] [CrossRef]
- Ramon, C.; Gollub, M.G.; Stelling, J. Integrating–Omics Data into Genome-Scale Metabolic Network Models: Principles and Challenges. Essays Biochem. 2018, 62, 563–574. [Google Scholar] [CrossRef]
- Tsugawa, H. Advances in Computational Metabolomics and Databases Deepen the Understanding of Metabolisms. Curr. Opin. Biotechnol. 2018, 54, 10–17. [Google Scholar] [CrossRef]
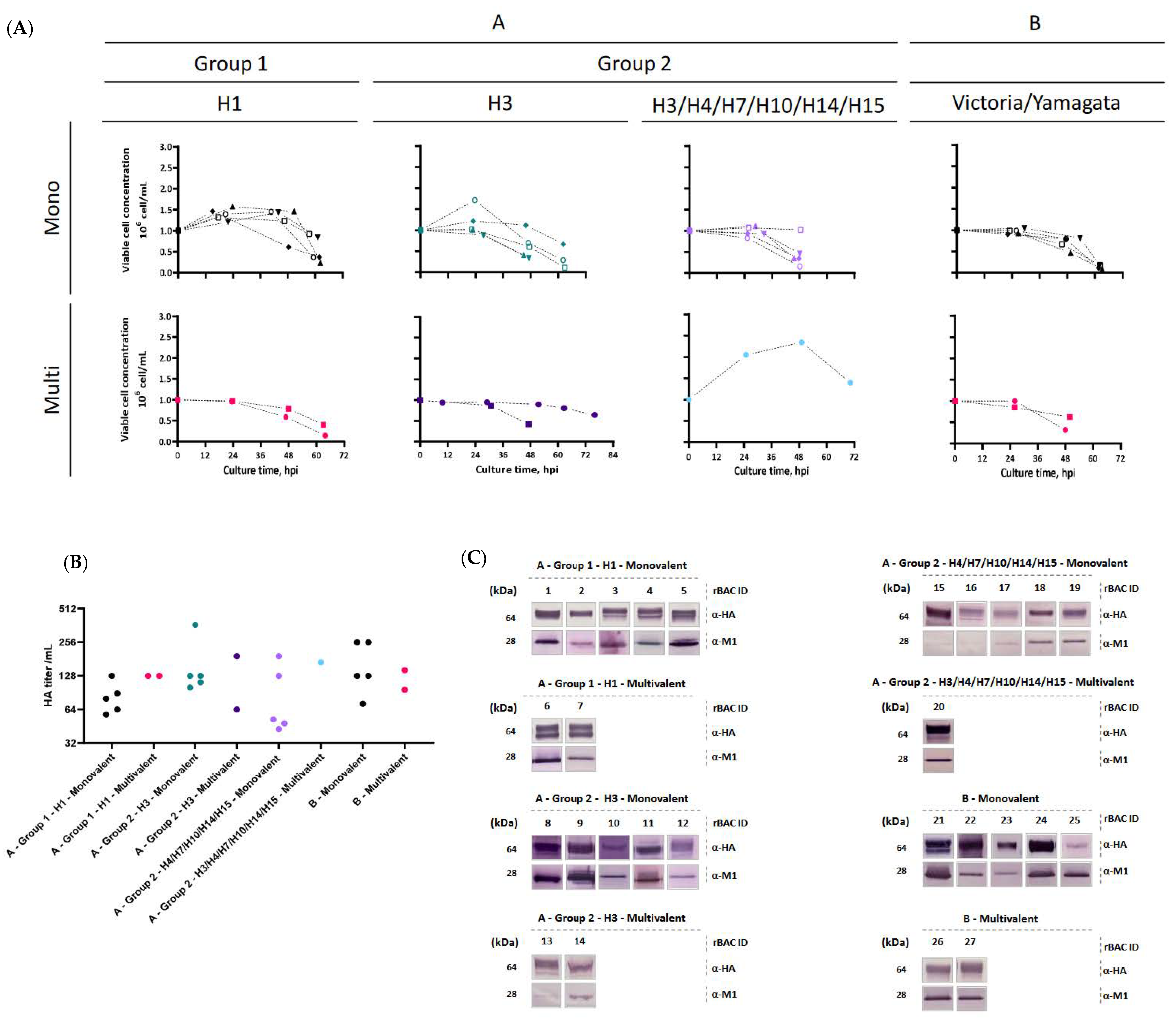
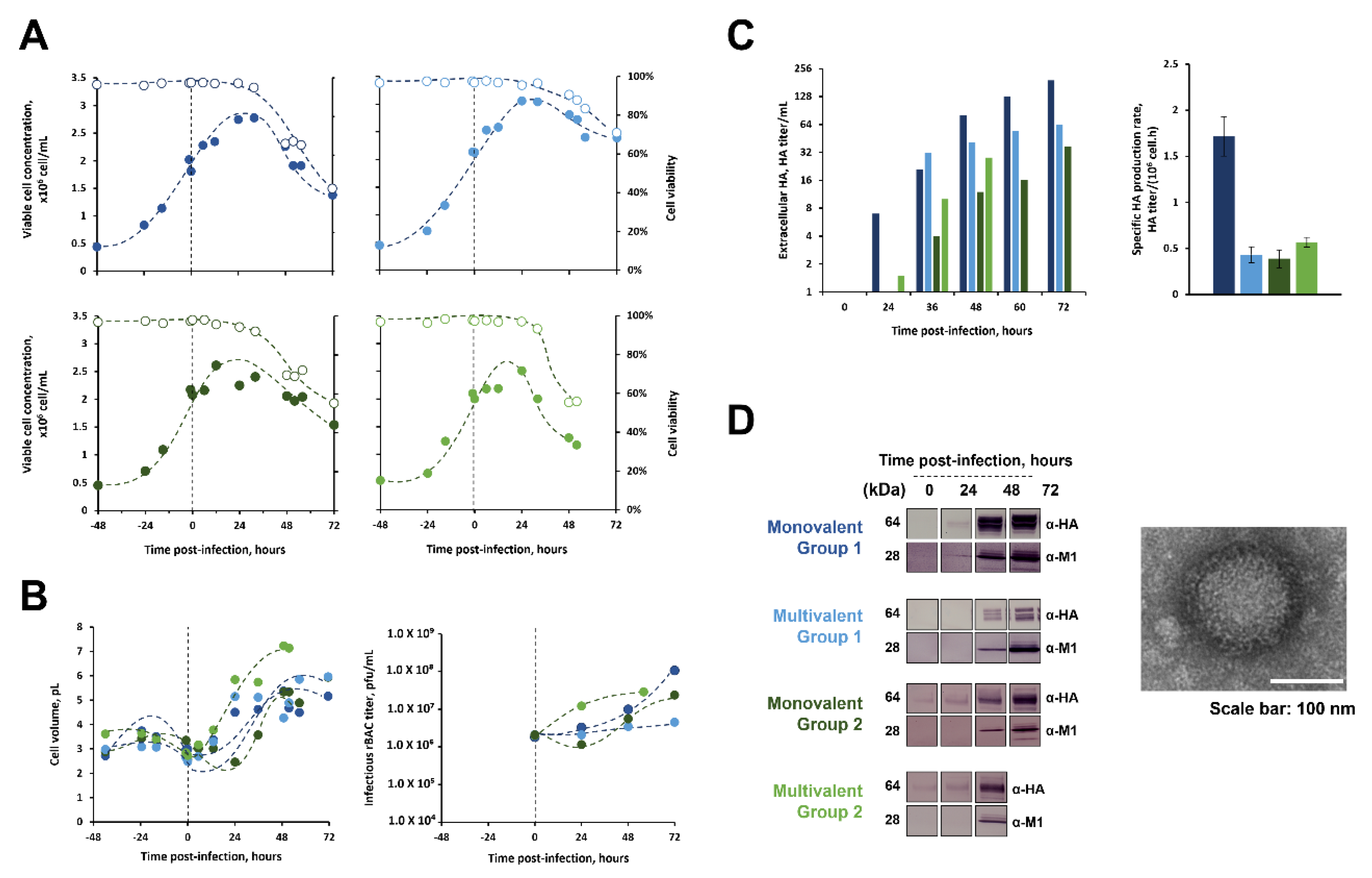
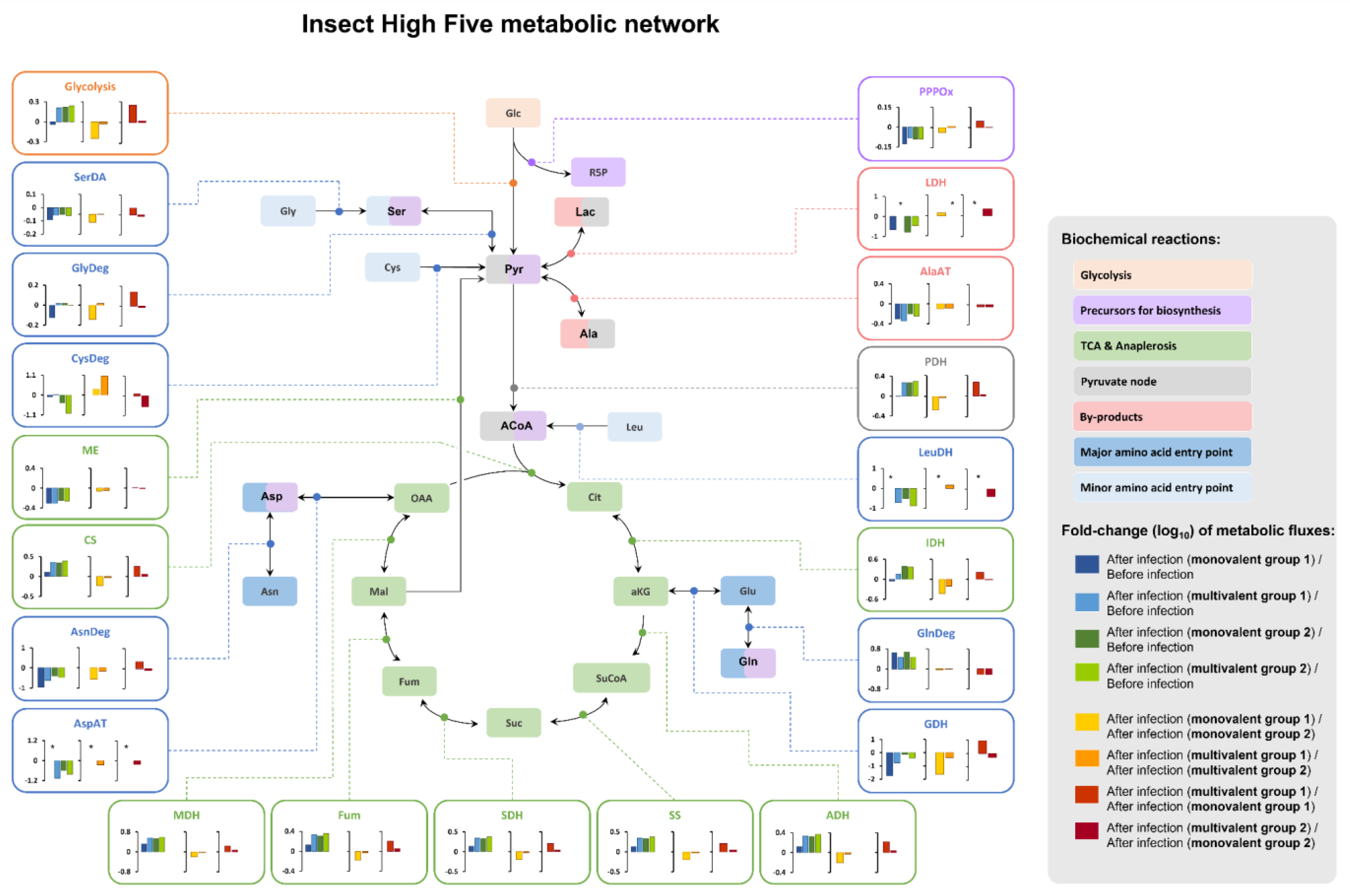
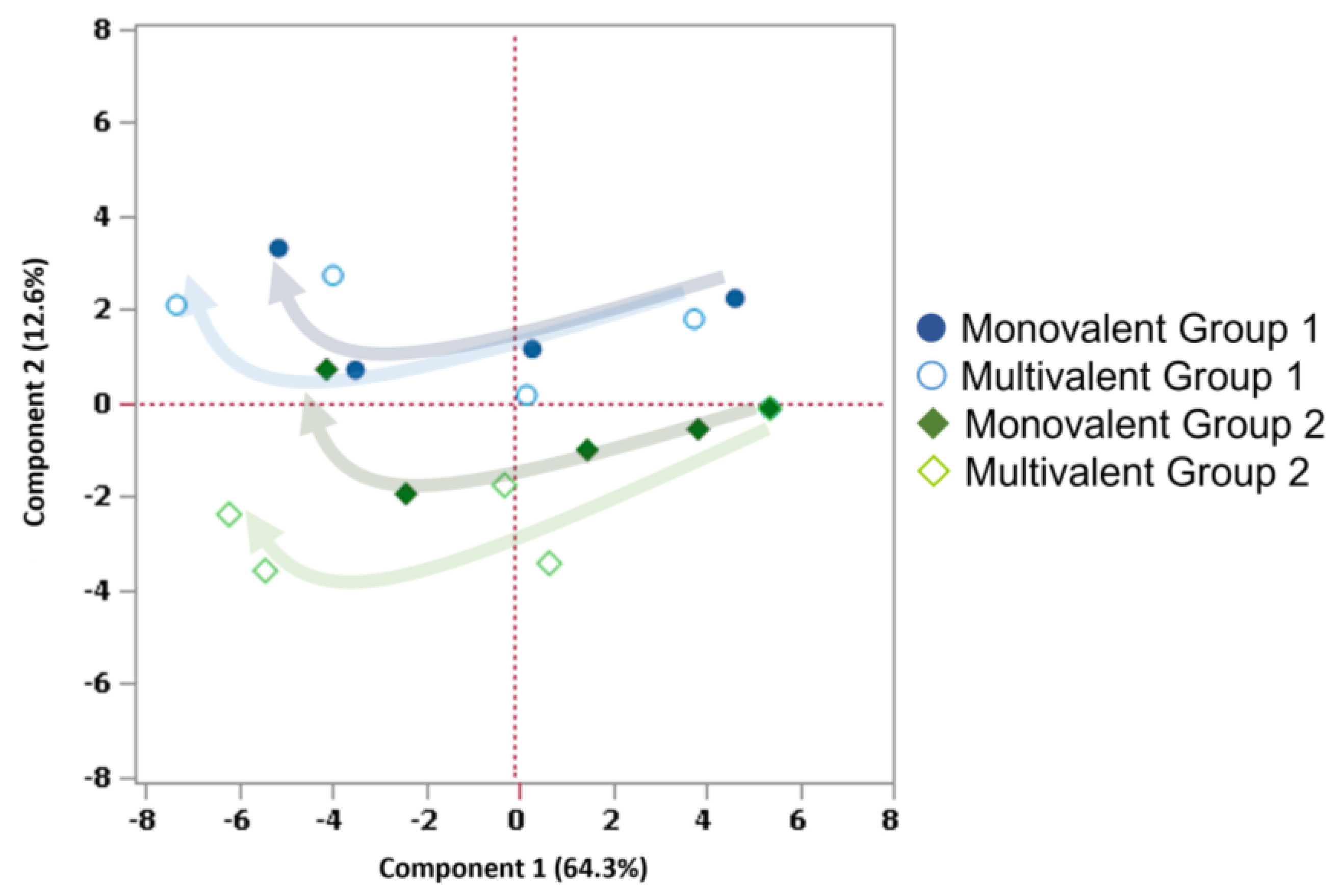
| Influenza Type/Group/Subtype | Valency | Hemagglutinin (HA) from Strain: | Abbr. | rBac ID | ||
|---|---|---|---|---|---|---|
| A | Group 1 | H1 | Monovalent | A/Puerto_Rico/8/1934 | PR34 | 1 |
| A/USSR/90/1978 | USSR78 | 2 | ||||
| A/Texas/36/1991 | Te91 | 3 | ||||
| A/New_Caledonia/20/1999 | NC99 | 4 | ||||
| A/Brisbane/59/2007 | Br07 | 5 | ||||
| Multivalent | PR8 + Tx36 + Bb59 | - | 6 | |||
| PR8 + USSR90 + Tx36 + NC20 + Bb59 | - | 7 | ||||
| Group 2 | H3 | Monovalent | A/Hong_Kong/1/1968 | HK68 | 8 | |
| A/England/321/1977 | En77 | 9 | ||||
| A/Sichuan/2/1987 | Si87 | 10 | ||||
| A/Johannesburg/33/1994 | Jo94 | 11 | ||||
| A/Fujian/411/2002 | Fu02 | 12 | ||||
| Multivalent | HK68 + Si87 + Fu02 | - | 13 | |||
| HK68 + En77 + Si87 + Jo94 + Fu02 | - | 14 | ||||
| H4 | Monovalent | A/mallard/Alberta/47/1998 | Al98 | 15 | ||
| H7 | A/cinnamon teal/Bolivia/4537/2001 | Bo01 | 16 | |||
| H10 | A/quail/NJ/25254-22/1995 | NJ95 | 17 | |||
| H14 | A/herring gull/Astrakhan/267/1982 | As82 | 18 | |||
| H15 | A/Australian shelduck/Western Australia/1762/1979 | WA79 | 19 | |||
| H3/H4/H7/H10/H14/H15 | Multivalent | Jo94 + Al98 + Bo01 + NJ95 + As82 + WA79 | - | 20 | ||
| B | - | Victoria/Yamagata | Monovalent | B/Hong Kong/8/1973 | HK73 | 21 |
| Victoria | B/Victoria/2/1987 | Vi87 | 22 | |||
| Yamagata | B/Yamagata/16/1988 | Ya88 | 23 | |||
| Yamagata | B/Jiangsu/10/2003 | Ji03 | 24 | |||
| Victoria | B/Malaysia/2506/2004 | Ma04 | 25 | |||
| - | Multivalent | HK73 + Ji03 + Ma04 | - | 26 | ||
| HK73 + Vi87 + Ya88 + Ji03 + Ma04 | - | 27 | ||||
| Specific Metabolite (j) Production or Consumption Rates (rj), μM/106 Cells.h | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell Growth Phase | Infection Phase | ||||||||||||||
| Group 1 | Group 2 | ||||||||||||||
| Monovalent | Multivalent | Monovalent | Multivalent | ||||||||||||
| Metabolite | Rate | ± | SD | Rate | ± | SE | Rate | ± | SE | Rate | ± | SE | Rate | ± | SE |
| Alanine | 65.4 | ± | 9.4 | 29.6 | ± | 2.2 | 24.9 | ± | 6.2 | 38.6 | ± | 1.0 | 33.5 | ± | 3.2 |
| Ammonium | 31.3 | ± | 6.8 | 9.5 | ± | 1.4 | 6.7 | ± | 1.8 | 11.9 | ± | 2.2 | 7.2 | ± | 5.4 |
| Arginine | −5.1 | ± | 1.8 | −6.4 | ± | 0.9 | −6.5 | ± | 0.4 | −5.6 | ± | 1.4 | −6.1 | ± | 1.1 |
| Asparagine | −67.8 | ± | 2.6 | −10.2 | ± | 4.1 | −19.0 | ± | 5.6 | −28.5 | ± | 3.3 | −25.2 | ± | 2.5 |
| Aspartate | 3.5 | ± | 5.6 | −2.1 | ± | 4.6 | −3.7 | ± | 1.2 | 2.2 | ± | 1.4 | 0.6 | ± | 1.7 |
| Cysteine | −4.1 | ± | 1.2 | −2.8 | ± | 0.0 | −3.7 | ± | 0.0 | −1.8 | ± | 0.2 | −0.64 | ± | 0.05 |
| Glucose | −120.7 | ± | 33.2 | −109.1 | ± | 15.2 | −189.2 | ± | 22.0 | −191.9 | ± | 3.0 | −202.5 | ± | 5.0 |
| Glutamine | −28.3 | ± | 2.5 | −35.2 | ± | 1.4 | −31.0 | ± | 2.5 | −37.8 | ± | 7.2 | −30.9 | ± | 1.5 |
| Glutamate | −2.1 | ± | 11.1 | −0.6 | ± | 3.1 | −2.2 | ± | 1.4 | 2.1 | ± | 2.4 | −2.2 | ± | 4.9 |
| Glycine | −5.6 | ± | 6.4 | −2.0 | ± | 1.3 | −1.6 | ± | 0.4 | −1.7 | ± | 0.6 | −2.1 | ± | 0.9 |
| Histidine | −1.4 | ± | 0.8 | −1.5 | ± | 0.6 | −1.2 | ± | 0.2 | −1.3 | ± | 0.2 | −1.4 | ± | 0.2 |
| Isoleucine | −2.6 | ± | 3.5 | −5.1 | ± | 1.4 | −4.6 | ± | 0.6 | −1.9 | ± | 0.6 | −5.1 | ± | 2.2 |
| Lactate | 18.7 | ± | 6.2 | 4.2 | ± | 0.5 | −8.3 | ± | 2.8 | 3.1 | ± | 0.2 | 6.5 | ± | 4.6 |
| Leucine | −5.0 | ± | 1.9 | −8.0 | ± | 0.8 | −6.1 | ± | 0.4 | −5.6 | ± | 0.4 | −6.1 | ± | 0.6 |
| Lysine | −4.1 | ± | 2.2 | −5.9 | ± | 0.8 | −5.2 | ± | 0.5 | −4.7 | ± | 0.5 | −5.4 | ± | 1.3 |
| Methionine | −2.7 | ± | 4.2 | −3.5 | ± | 1.4 | −3.7 | ± | 0.6 | −3.4 | ± | 1.6 | −3.7 | ± | 2.2 |
| Phenylalanine | −1.9 | ± | 3.3 | −3.3 | ± | 1.4 | −3.6 | ± | 0.6 | −2.9 | ± | 1.3 | −3.6 | ± | 2.3 |
| Proline | −5.0 | ± | 2.3 | −6.5 | ± | 1.2 | −5.6 | ± | 0.8 | −5.4 | ± | 1.6 | −5.2 | ± | 0.2 |
| Serine | −2.3 | ± | 5.8 | −2.0 | ± | 1.1 | −3.2 | ± | 0.6 | −2.3 | ± | 0.2 | −2.8 | ± | 0.1 |
| Threonine | −3.4 | ± | 1.1 | −4.5 | ± | 0.7 | −4.5 | ± | 0.3 | −4.0 | ± | 0.2 | −3.9 | ± | 0.5 |
| Tyrosine | −2.3 | ± | 1.4 | −3.2 | ± | 0.5 | −2.4 | ± | 0.4 | 2.54 | ± | 0.05 | −2.5 | ± | 0.4 |
| Valine | −3.3 | ± | 2.7 | −5.3 | ± | 1.2 | −4.6 | ± | 0.5 | −3.4 | ± | 0.7 | −4.7 | ± | 1.6 |
| Infection Phase | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | |||||||||||
| Monovalent | Multivalent | Monovalent | Multivalent | |||||||||
| Rate | ± | SE | Rate | ± | SE | Rate | ± | SE | Rate | ± | SE | |
| Specific O2 consumption rate (mmol/106 cell.h) | 71 | ± | 2 | 65 | ± | 1 | 92 | ± | 5 | 164 | ± | 8 |
| Specific HA production rate (HA titer/106 cell.h) | 1.7 | ± | 0.2 | 0.4 | ± | 0.1 | 0.4 | ± | 0.1 | 0.6 | ± | 0.1 |
| Specific rBAC production rate (h−1) | 0.013 | ± | 0.005 | 0.0025 | ± | 0.0004 | 0.0131 | ± | 0.0003 | 0.012 | ± | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murad, A.B.; Sousa, M.Q.; Correia, R.; Isidro, I.A.; Carrondo, M.J.T.; Roldão, A. Targeted Metabolic Analysis and MFA of Insect Cells Expressing Influenza HA-VLP. Processes 2022, 10, 2283. https://doi.org/10.3390/pr10112283
Murad AB, Sousa MQ, Correia R, Isidro IA, Carrondo MJT, Roldão A. Targeted Metabolic Analysis and MFA of Insect Cells Expressing Influenza HA-VLP. Processes. 2022; 10(11):2283. https://doi.org/10.3390/pr10112283
Chicago/Turabian StyleMurad, Alexandre B., Marcos Q. Sousa, Ricardo Correia, Inês A. Isidro, Manuel J. T. Carrondo, and António Roldão. 2022. "Targeted Metabolic Analysis and MFA of Insect Cells Expressing Influenza HA-VLP" Processes 10, no. 11: 2283. https://doi.org/10.3390/pr10112283
APA StyleMurad, A. B., Sousa, M. Q., Correia, R., Isidro, I. A., Carrondo, M. J. T., & Roldão, A. (2022). Targeted Metabolic Analysis and MFA of Insect Cells Expressing Influenza HA-VLP. Processes, 10(11), 2283. https://doi.org/10.3390/pr10112283








