1. Introduction
Experimental thermodynamic and transport data at elevated pressures provide important information to chemical engineers for process development, and are a requisite for many studies of the thermodynamics of binary systems [
1]. The increasing industrial and academic interest in high- and low-pressure data for gas–alcohol systems is confirmed by a large number of publications in this area [
2,
3,
4,
5,
6,
7]. Within this literature, sub- or supercritical carbon dioxide–methanol/ethanol systems have been studied extensively [
8,
9,
10,
11,
12], whereas sub- or supercritical carbon dioxide–other alcohol systems have received limited attention [
13,
14,
15].
Low molecular mass alcohols, such as isopropanol, are often used as entrainers to modify the polarity of supercritical carbon dioxide in extraction processes and are also used as a cosolvent in supercritical fluid chromatography.
Most of the literature data are available for alcohol–carbon dioxide systems, more than for binary systems with other gases as sub- and supercritical fluids.
Carbon dioxide has been shown to be the most used supercritical fluid for these processes, due to its excellent thermodynamic and transport properties and low price. Carbon dioxide has a low critical temperature of 304.25 K and critical pressure of 73.8 bar [
16].
Argon is used in some high-temperature industrial processes where ordinarily non-reactive substances are oxidized. Therefore, an inert atmosphere of argon is used to protect them against oxidation [
17]. Argon is relatively easy to produce in the liquefaction process of air, and has a relatively low cost [
18]. On the other hand, the manipulation is more complex, and instead of a high-pressure pump, a gas booster has to be employed for the experiments with argon. The critical data for argon are 48.6 bar and 150.7 K [
16].
Sulphur hexafluoride as a pseudospherical molecule of considerable size and mass has also been used because it is completely non-reactive. The critical data for sulphur hexafluoride are 37.5 bar and 318.7 K [
16]. Systems with sulphur hexafluoride have not been investigated well, due to their environmental impact and the high price of this gas.
The current research work was focused on a systematic study to obtain thermodynamic and transport properties experimentally, including viscosity, density, and interfacial tension of binary systems containing gas and isopropanol. Data for investigated systems are fundamental in several supercritical fluid applications, especially extractions where isopropanol is often used as an entrainer. As already mentioned, carbon dioxide is the most used supercritical fluid, while argon is an alternative supercritical fluid with relatively low critical pressure and temperature and it is a good media, especially for applications in the food industry. On the other hand, sulphur hexafluoride was selected as there is no sufficient data for binary systems with this gas. Known viscosity range is important in different applications including particle formation, while density data are necessary due to its impact on solubility. In addition, interfacial tension is a key parameter in many colloidal systems, including emulsions and foams. Therefore, it can be used to study phenomena as diverse as the formation, shape, and stability of liquid drops.
Experiments were performed at two different temperatures (313.15 K and 333.15 K) and at pressures up to 100 bar for carbon dioxide and up to 500 bar for argon and sulphur hexafluoride. A variable-volume high-pressure optical view cell was used to determine viscosity, where a mixing rule was applied. A vibrating U-tube densimeter was employed to determine the density. Pendant drop tensiometry was used to determine interfacial tension.
In summary, the aim of the current study was to obtain thermodynamics data (P-x,y equilibrium data, density and transport data (viscosity and interfacial tension) for the binary systems of isopropanol with carbon dioxide, argon, and sulphur hexafluoride, which have not yet been studied in detail and have not been reported in the literature. As mentioned above, these data are essential to design a physical process that yields the final products with added value, which means with a high proportion of compound of interest according to the sustainability standards.
2. Materials and Methods
To determine viscosity, a variable-volume high-pressure optical view cell was used, while a vibrating U-tube densimeter was applied to determine the density. Pendant drop tensiometry was used in this study, as it offers a simple and elegant solution to determine interfacial tension, which is a central parameter in many colloidal systems, including emulsions and foams, and can be used to examine phenomena as diverse as the formation, shape, and stability of liquid drops [
19].
2.1. Materials
The isopropanol (CAS Reg. No. 33539-2.5L) was obtained from Honeywell (Charlotte, North Carolina, NC, USA) with 99.8 vol.% purity. The carbon dioxide (Cat. No. 124-38-9) with a purity of 99.99 vol.% and argon (CAS Reg. No. 7440-37-1) with a purity of 99.998 vol.% were obtained from MESSER (MG, Ruše, Slovenia). The sulphur hexafluoride (CAS Reg. No. 2551-62-4) was purchased from Istrabenz plini (Koper, Slovenia) with a purity of 99.93 vol.%. All the chemicals were used as received without further purification.
2.2. Apparatus and Procedure
2.2.1. Viscosity
A variable-volume high-pressure optical view cell described previously by Borjan et al. (2022) was used for the determination of viscosity [
20]. The calculation of nondimensional numbers, both Reynolds and Newton, from the measured voltage, mixing, and electric current, was employed for the determination of the viscosity of two binary gas-saturated isopropanol systems [
21]. The viscosity data for pure sulphur hexafluoride gas is unknown, and, thus, the calculations for the binary sulphur hexafluoride saturated isopropanol system was not possible. For the other two systems, the isothermal series of measurements was comprised partially of more than 20 data points at two temperatures (313.15 K and 333.15 K). The individual points were measured exactly at the nominal temperatures, with the deviations of the experimental temperatures within ± 0.5 K. At least three experiments were performed for each measured point. The obtained data were similar and, therefore, the results were comparable and repeatable (deviation was ± 3.4%).
2.2.2. Density
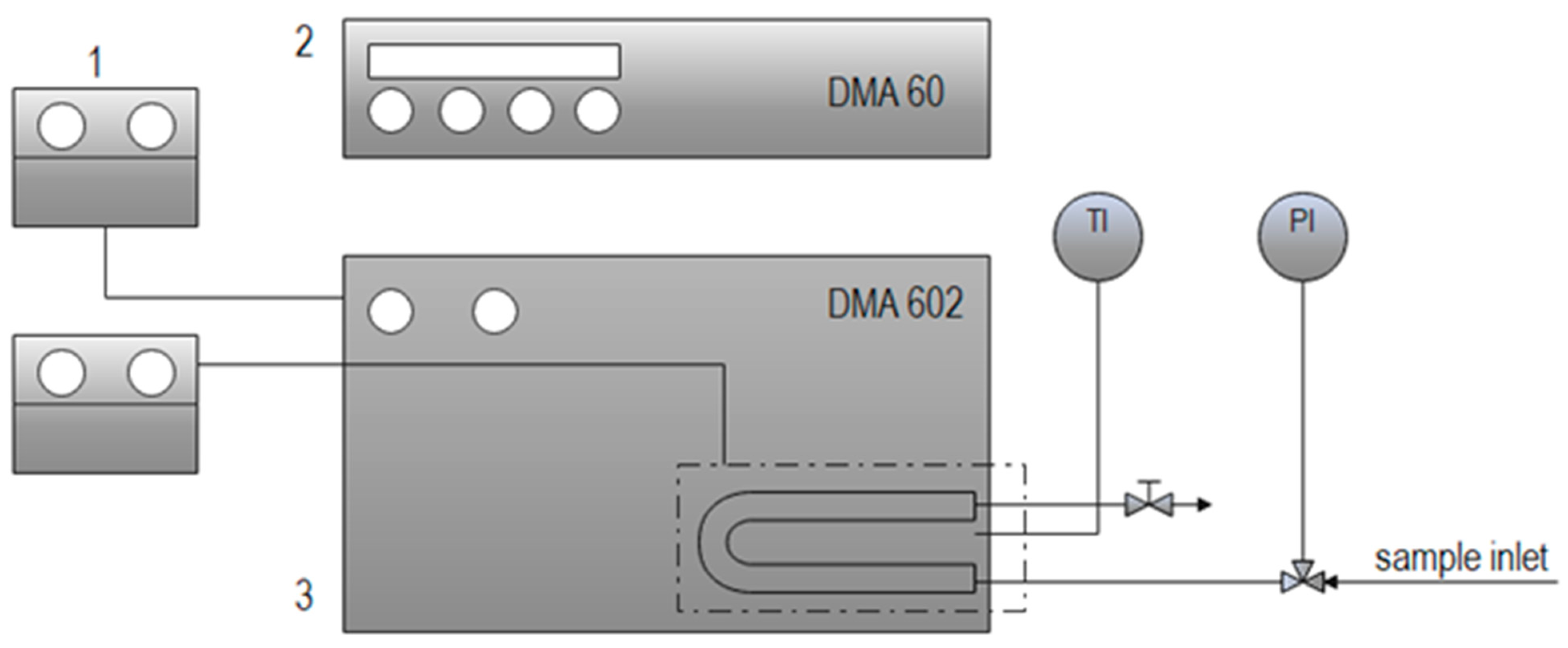
The densities of gas-saturated isopropanol systems were measured using an Anton Paar DMA 602 (Graz, Austria) measuring cell consisting of a vibrating U-tube, with a capacity of 0.7 cm
3, which was set into oscillation by an electronic excitation system (following the scheme shown in
Figure 1).
A square-wave output signal was produced corresponding to the natural vibration frequency of the cell and its contents [
22]. In principle, two samples of known density, nitrogen and Milli-Q water, were used as calibration fluids for characteristic constant determination. Based on the oscillating period of nitrogen
τN2 and Milli-Q water
τmQ, determined experimentally, and the known densities,
φN2 and
φmQ, the characteristic constant, K, of the device was calculated using Equation (1) [
23]:
When the U-tube was filled with a sample (gas and isopropanol) under the same experimental conditions, the oscillating periods of the gas-saturated isopropanol systems,
τIPA/CO2, were measured, and the density of the sample,
ρIPA/CO2, determined by Equation (2) [
23]:
To reach equilibrium, approximately 20 min was needed at each pressure to stabilize the system. The U-tube was thermostated by means of an external temperature-controlled circulating bath, which controlled the temperature within ±5 × 10
−3 K. The temperature and pressure inside the U-tube were measured with an Anton Paar CKT 100 (Graz, Austria) platinum resistance thermometer with an uncertainty of ±0.01 K, and a manometer (Nuova Fima EN837-1, Invorio, Italy) with an accuracy of 0.25% for pressures lower than 600 bar. The detailed operating procedure can be found in the literature [
23]. The reported uncertainty in the density of the reference fluids was generally less than 0.1%, with an estimation of ±0.05 kg m
−3. At least three experiments were performed for each measured pressure at both temperatures, and the data obtained matched, so the results were comparable, as well as repeatable (deviation was ±1.2%).
2.2.3. Interfacial Tension
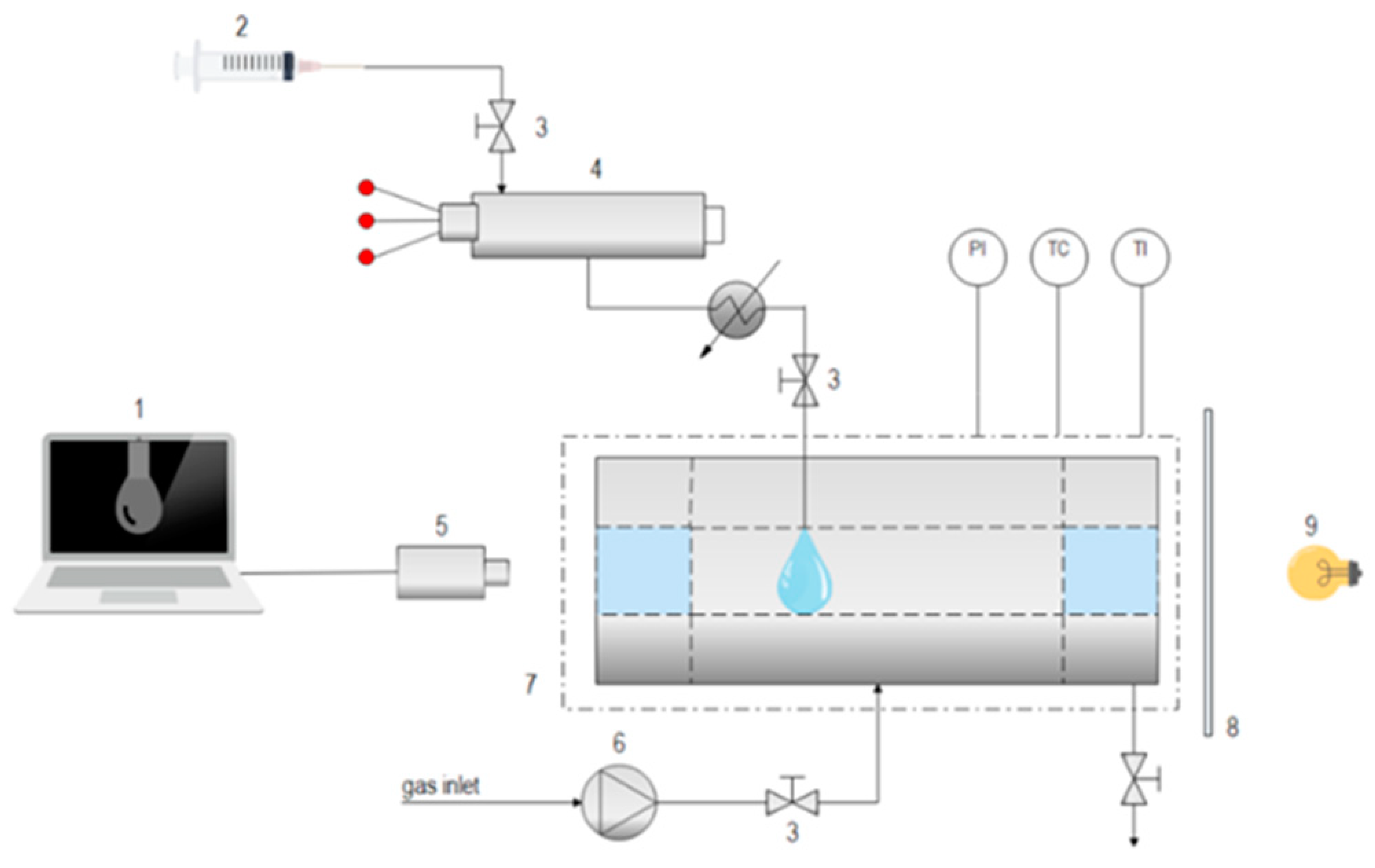
The main equipment for the determination of interfacial tension was a variable-volume high-pressure optical view cell, which was described previously in the section related to
P-x,y equilibrium data, and viscosity measurements. As mixing of the system was not required, the stirrer had been removed before interfacial tension measurements. Also, a high-pressure manual pump (mod. 750.1100, SITEC, Zurich, Switzerland) had been connected to inject isopropanol, as shown in
Figure 2. Pendant drops of adequate size were formed on a stainless-steel tip that was placed vertically in the middle of the view cell. Measurements of the dynamic drop volume were taken with a digital camera (Basler Aca 1300–200 um, Tamron, Japan) equipped with a CCTV lens. Because of possible destruction and the fake reflection of other sources, a glass diffuser was placed between the light source and the hanging drop.
The images thus obtained were processed using the OpenDrop software (Melbourne, Victoria) which requires data on the needle diameter (1.56 mm), inner density (the density of pure isopropanol at a particular pressure and temperature) [
16], outer density (the density of pure gas at a particular pressure and temperature) [
16] and gravitational acceleration (9.81 m s
−2). Data for both densities were found in the NIST Chemistry WebBook [
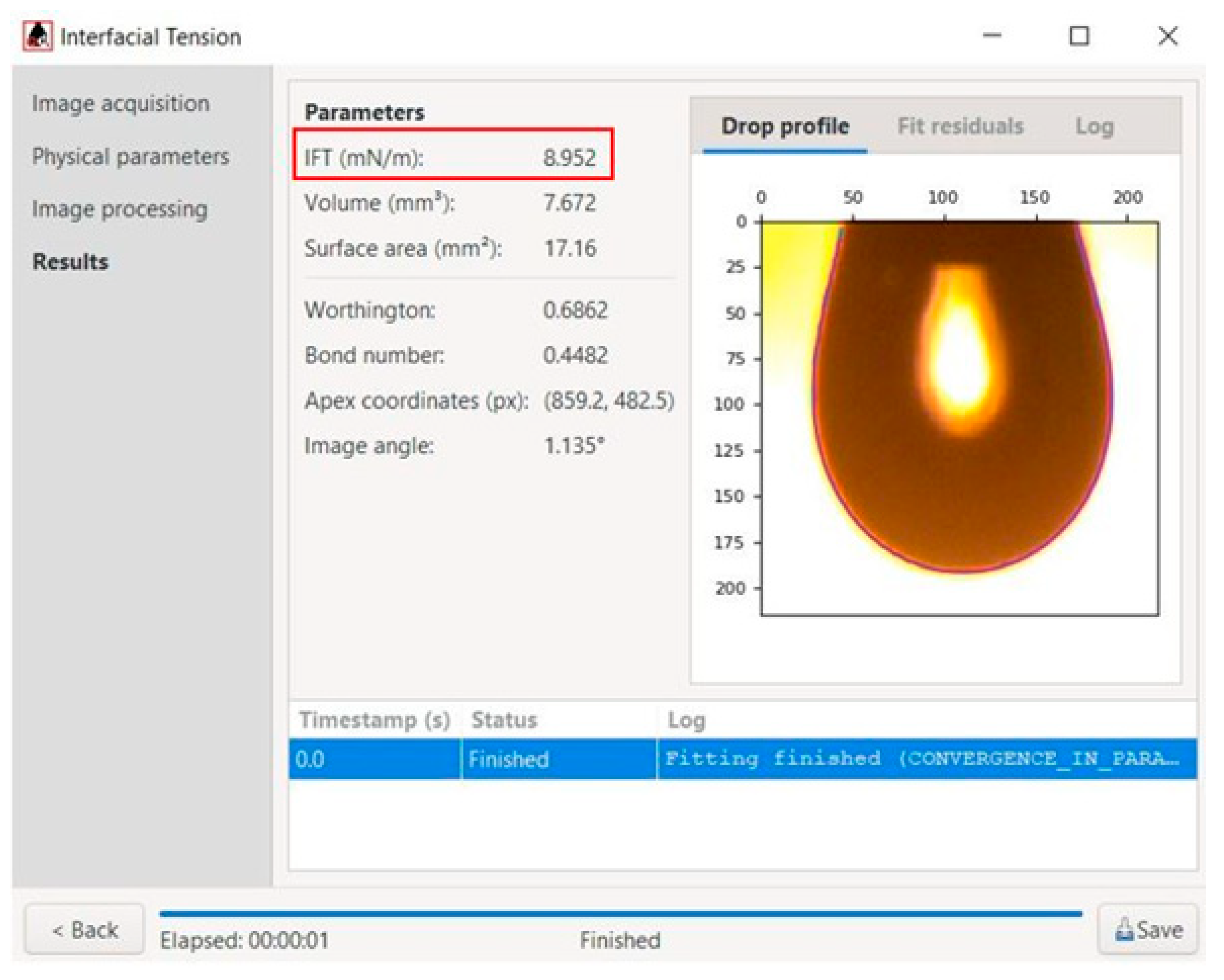
16]. After recognizing the drop region, the software calculated the value of its interfacial tension (IFT), as shown in
Figure 3. Each experiment was performed at least three times. The obtained data were matching, and therefore the results were similar and repeatable (deviation was ±3.7%).
3. Results and Discussion
3.1. Viscosity
3.1.1. Validation of the Method
Figure 4 shows the viscosity of pure gas as a function of pressure compared to the literature data. Numerical values used for diagrams are provided in
Table S1 in Supplementary material section. It is clear that, for pure carbon dioxide as well as for argon, the obtained results are similar at both temperatures (313.15 K and 333.15 K) to those published in the literature. Due to that, this method has been used for further viscosity measurements of binary isopropanol–gas systems.
3.1.2. Viscosity of the Binary Systems
Figure 5 shows the dependence of viscosity on pressure for the carbon dioxide-saturated isopropanol binary system at temperatures of 313.15 K and 333.15 K and pressures up to 95 bar. Numerical values used for diagram are provided in
Table S1 in Supplementary material section. The results show that this dependence is linear, and, as expected, decreases with increasing pressure at a constant temperature. Additionally, as can be seen from
Figure 5, the viscosities at 333.15 K are lower than at 313.15 K for similar pressures, and, therefore, the viscosity also decreases with temperature. As such, the experimental viscosity values can be adjusted to isothermal values by means of a linear correction. Because the concentration of molecules in the gas phase increases with increasing pressure, the concentration of dissolved gas molecules in the solution at equilibrium is also higher at higher pressures. Therefore, increasing pressure increases the concentration of gas in the sample, and increases and leads to a higher viscosity; this trend can be seen predominantly at pressures above 60 bar. The relation between the pressure and the concentration of gas shows a tendency to saturate the sample. Accordingly, a higher concentration of gas in the sample results in a slightly higher viscosity. At low pressures, up to 60 bar, the viscosity is almost constant. A possible explanation is that the mobility of the alcohol molecule chains enables poor saturation with gas at low pressure, as, due to the lower equilibrium composition the viscosity is higher [
24].
A similar dependence is noted for the argon-saturated isopropanol binary system at temperatures of 313.15 K and 333.15 K and pressures up to 500 bar. As can be seen in
Figure 6, the dependence is linear, and viscosity decreases with both pressure, and temperature. Numerical values used for diagrams are provided in
Table S2 in Supplementary material section. The viscosity of the carbon dioxide-saturated isopropanol system is in the range from 6.72 × 10
−6 Pa·s to 8.62 × 10
−6 Pa·s at 313.15 K, and in the range from 5.92 × 10
−6 Pa·s to 6.63 × 10
−6 Pa·s. at 333.15 K. For the argon-saturated isopropanol system, it is in the range from 1.29 × 10
−5 Pa·s to 1.59 × 10
−5 Pa·s at 313.15 K, and in the range from 5.24 × 10
−6 Pa·s to 6.83 × 10
−6 Pa·s. at 333.15 K. Unfortunately, there are no data available in the literature for the viscosity of these systems for comparison.
3.2. Density
The liquid densities of gas-saturated isopropanol binary systems are determined at two temperatures (313.15 K and 333.15 K) and pressures up to 65 bar for carbon dioxide, 270 bar for argon, and 350 bar for sulphur hexafluoride. Density is found to be a linear function of pressure and temperature, as shown in
Figure 7, and the densities of these binary systems increase with pressure and decrease with temperature. Numerical values used for diagram are provided in
Tables S3 and S4 in Supplementary material section. Kariznovi et al. (2013) investigated a similar binary system (carbon dioxide–1-propanol) at temperatures of 303.2 K and 323.2 K and pressures up to 100 bar, and obtained the same density dependence of pressure and temperature [
25]. The density of the carbon dioxide-saturated isopropanol system is in the range from 770 kg m
−3 to 780 kg m
−3 at 313.15 K and between 740 kg m
−3 and 750 kg m
−3 at 333.15 K. The density of the argon-saturated isopropanol system is in the range from 770 kg m
−3 to 790 kg m
−3 at 313.15 K, and between 740 kg m
−3 and 770 kg m
−3 at 333.15 K. The density of the sulphur hexafluoride saturated isopropanol system is in the range from 760 kg m
−3 to 790 kg m
−3 at 313.15 K, and between 740 kg m
−3 and 760 kg m
−3 at 333.15 K. According to previously described results, the densities of these binary systems under the same high-pressure conditions at 313.15 K are in the following order: Carbon dioxide–isopropanol > argon–isopropanol > sulphur hexafluoride–isopropanol; and in the following order at 333.15 K: Argon–isopropanol > carbon dioxide–isopropanol > sulphur hexafluoride–isopropanol. For the density of the researched systems, there are no data in the literature for comparison.
3.3. Interfacial Tension
Equilibrium composition has a direct impact on droplet size, as increased equilibrium composition caused a smaller droplet size. Because of this, experiments are conducted up to pressures at which it is possible to form stable droplets.
As determined in our previous research article, the critical pressure of the mixture of carbon dioxide–isopropanol, where the transition to one phase occurs, is 68.8 bar at 313.13 K and 93.6 bar at 333.15 K. Above these pressures, only one phase is present. Interfacial tension had, therefore, been determined up to 69.4 bar at 313.15 K, and up to 90.3 bar at 333.15 K.
For the mixture sulphur hexafluoride–isopropanol phase, inversion is observed at about 37 bar according to our preliminary experiments. Consequently, interfacial tension had been determined up to 36.9 bar at 333.15 K and up to 28.3 bar at 313.15 K.
After reconsideration of the results, it can be concluded that the critical point of the mixture is not the key parameter that affects the formation of a drop. This is evident in the case of the argon–isopropanol system. The critical pressure of the mixture argon–isopropanol has not been determined, since the transition to one phase has not been observed in the range of the performed measurements. The drop has been formed up to the pressures above 200 bar.
Figure 8 shows the interfacial tension of a carbon dioxide–isopropanol system at a temperature of 313.15 K and pressure up to 70 bar, and at a temperature of 333.15 K and pressure up to 90 bar. It can be seen that pressure has a significant effect on interfacial tension, and that it is reduced by elevated pressure. For instance, at a constant temperature of 313.15 K, interfacial tension decreases from 24.90 × 10
−3 N m
−1 at 8.6 bar to 1.88 × 10
−3 N m
−1 at 69.4 bar. Additionally, at a higher temperature, this decrease is sharper. Dittmar et al. (2003) researched the interfacial tension of a carbon dioxide-ethanol binary system. They reported the same behavior as in this article, the decreasing of interfacial tension with increasing of both pressure and temperature [
27].
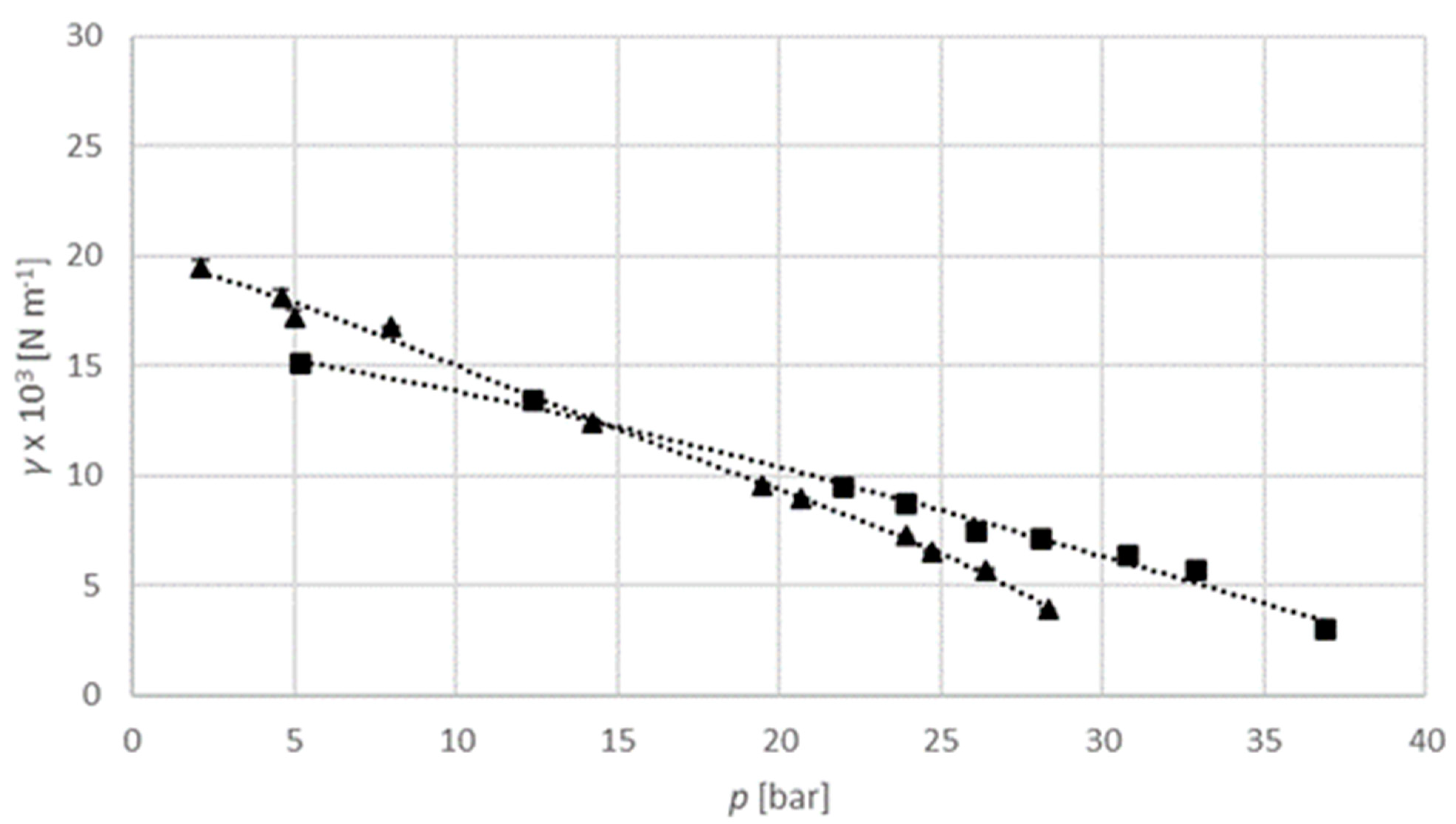
The interfacial tension of an argon–isopropanol system is examined at a temperature of 313.15 K and a pressure of up to 200 bar, and a temperature of 333.15 K and a pressure of up to 150 bar (
Figure 9). The interfacial tension decreases with elevated pressure. The temperature has a less significant influence than for the carbon dioxide–isopropanol system described above.
The interfacial tension of a sulphur hexafluoride–isopropanol system is determined at a pressure of up to 28 bar at a temperature of 313.15 K, and a pressure of up to 37 bar at a temperature of 333.15 K, as can be seen in
Figure 10. As described above, the interfacial tension decreases with elevated pressure. The temperature has a less significant influence than for the other two systems.
Numerical values used for diagrams are provided in
Table S5 in Supplementary material section. The interfacial tension of the carbon dioxide–isopropanol system is in the range from 1.88 × 10
−3 N m
−1 to 24.90 × 10
−3 N m
−1 at 313.15 K, and in the range from 1.40 × 10
−3 N m
−1 to 19.55 × 10
−3 N m
−1 at 333.15 K. For the argon–isopropanol system it is in the range from 10.30 × 10
−3 N m
−1 to 25.09 × 10
−3 N m
−1 at 313.15 K, and in the range from 11.95 × 10
−3 N m
−1 to 18.74 × 10
−3 N m
−1 at 333.15 K. For the sulphur hexafluoride–isopropanol system it is in the range from 3.91 × 10
−3 N m
−1 to 19.45 × 10
−3 N m
−1 at 313.15 K, and in the range from 3.02 × 10
−3 N m
−1 to 15.08 × 10
−3 N m
−1 at 333.15 K. Unfortunately, there are no data available in the literature for the viscosity of these three systems, nor for the interfacial tension.
Pressure has a significant effect on droplet size. With increasing pressure, the droplet size decreases; finally, at a certain pressure at a critical value at which it is impossible to push the droplet through the needle. This corresponds to the occurrence of the phase transition; for the mixture of carbon dioxide–isopropanol at 68.8 bar at 313.13 K, and at 93.6 bar at 333.15 K. Interfacial tension had been determined up to 69.4 bar at 313.15 K and up to 90.3 bar at 333.15 K, and for the mixture of sulphur hexafluoride–isopropanol at about 37 bar. Interfacial tension had been determined up to 36.9 bar at 333.15 K and up to 28.3 bar at 313.15 K (
Figure 10).
4. Conclusions
Binary systems of gas and isopropanol were investigated, and viscosity, density and interfacial tension were determined at temperatures of 313.15 K and 333.15 K and pressures up to 100 bar for carbon dioxide and 500 bar for argon and sulphur hexafluoride, using a vibrating tube densimeter for density and a variable-volume high-pressure optical view cell with some modifications for the other measurements. The results are novel, and attained by a simple, economic method that is also adjustable for various multi-compound mixtures at elevated pressures.
Accurate prediction of thermodynamic and mass transfer data is fundamental in various engineering and industrial operations to design processes involving mass transfer (e.g., conventional and supercritical extractions, multiphase chemical reactions, distillation, carbon sequestration, membrane separation processes, absorption, and adsorption). These data, measured at ambient conditions, can be found in the literature for numerous binary and ternary systems, since the literature that reports data on the systems with supercritical fluids is still relatively scarce. According to the obtained pressure-viscosity dependence, which was almost linear, the pressure did not have a great impact on viscosity. The capability to measure viscosity at high pressure, however, presents a number of engineering trials and several innovative viscometers have consequently been devised and operated. The main challenge is still the determination of the data at elevated pressures and temperatures in multicompound systems.
The densities of these binary systems under the same high-pressure conditions at 313.15 K were in the following order: Carbon dioxide–isopropanol > argon–isopropanol > sulphur hexafluoride–isopropanol; and in the following order at 333.15 K: Argon–isopropanol > carbon dioxide–isopropanol > sulphur hexafluoride–isopropanol.
The interfacial tension decreased with elevated pressure. Pressure had a significant effect on droplet size—with increasing pressure the droplet size decreased to a pressure value at which it was impossible to make the droplet, while temperature had less impact on the values of interfacial tension. After a reconsideration of the results, we may conclude that the critical point of the mixture is not the key parameter that affects the formation of a drop. Furthermore, it was concluded that composition droplet size decreased with increased equilibrium.
The data obtained are fundamental for the design of processes that lead to end products with added value, i.e., with a high proportion of targeted compounds in accordance with sustainability standards.
A recent study of solubility for the same systems (carbon dioxide-isopropanol, ar-gon-isopropanol, and sulphur hexafluoride-isopropanol) is currently under investigation.
 , measured data at 313.15 K;
, measured data at 313.15 K;  , measured data at 333.15 K; ×, pure gas at 313.15 K [16];
, measured data at 333.15 K; ×, pure gas at 313.15 K [16];  , pure gas at 333.15 K [16].
, pure gas at 333.15 K [16].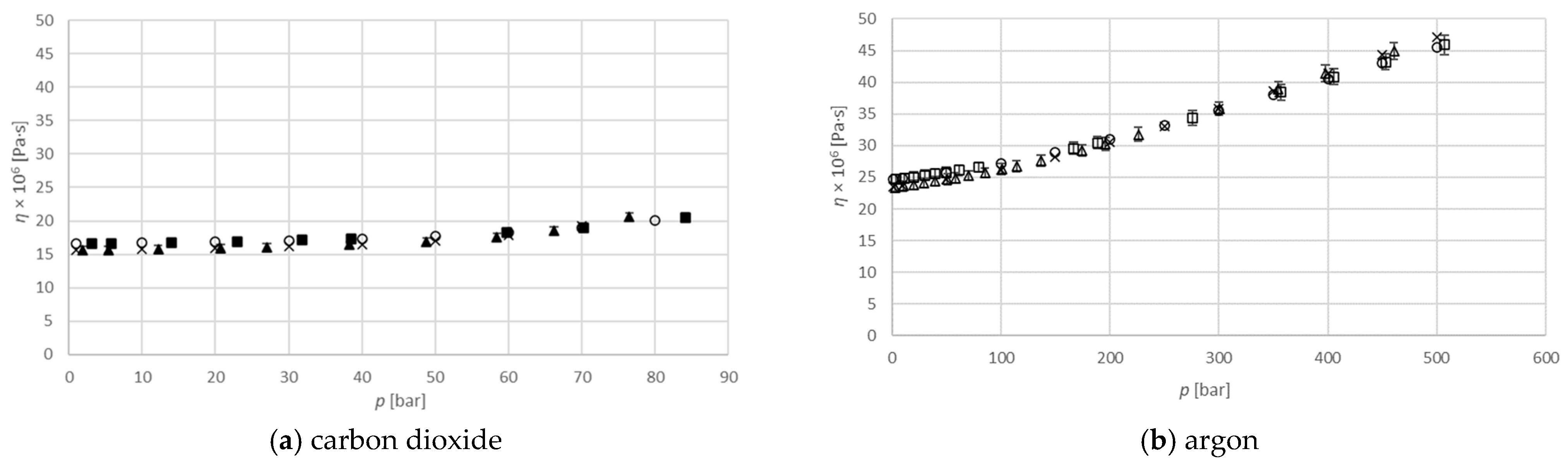
 , measured data at 313.15 K;
, measured data at 313.15 K;  , measured data at 333.15 K.
, measured data at 333.15 K.
 , measured data at 313.15 K;
, measured data at 313.15 K;  , measured data at 333.15 K.
, measured data at 333.15 K.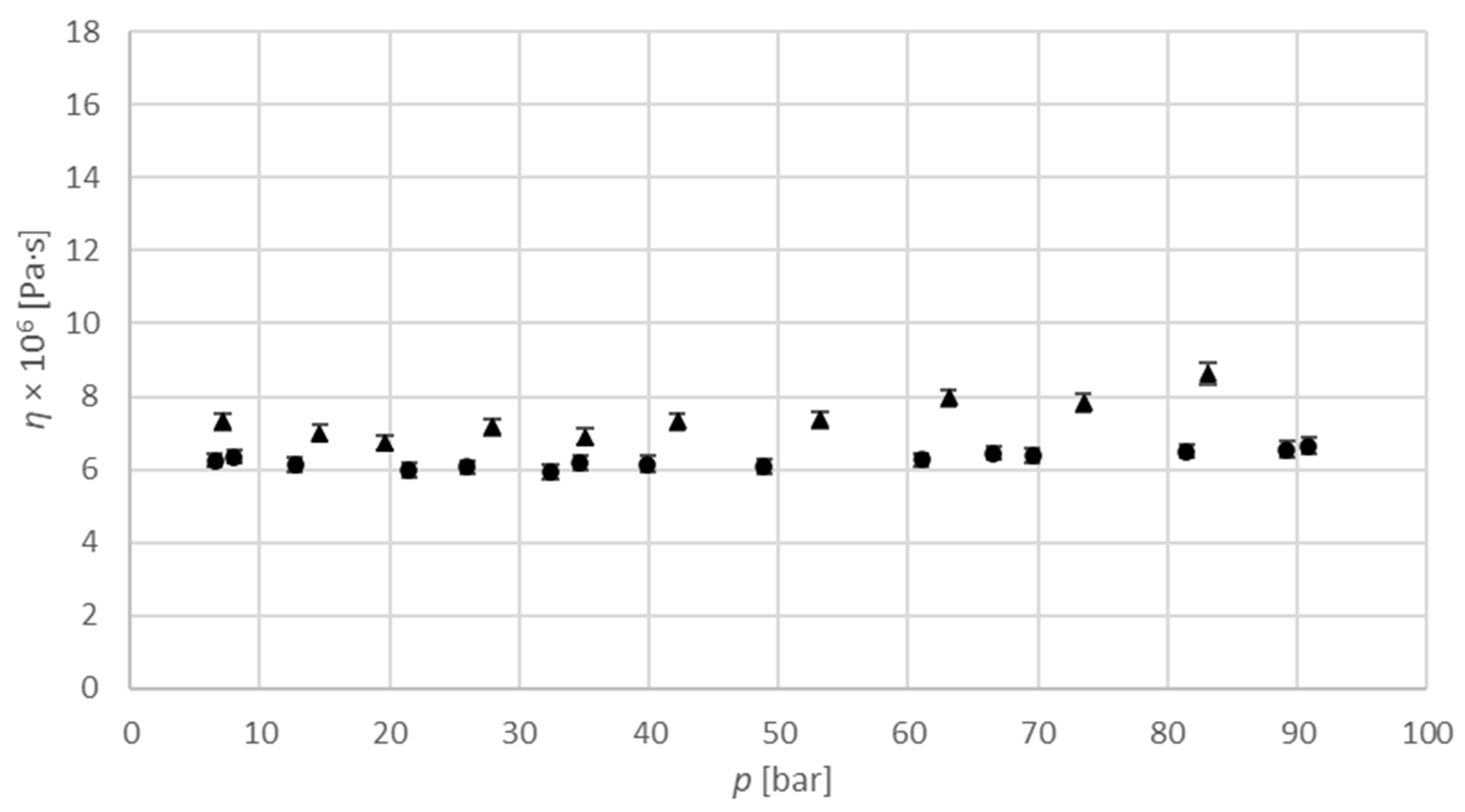
 , measured data at 313.15 K;
, measured data at 313.15 K;  , measured data at 333.15 K.
, measured data at 333.15 K.
 , measured data at 313.15 K;
, measured data at 313.15 K;  , measured data at 333.15 K.
, measured data at 333.15 K.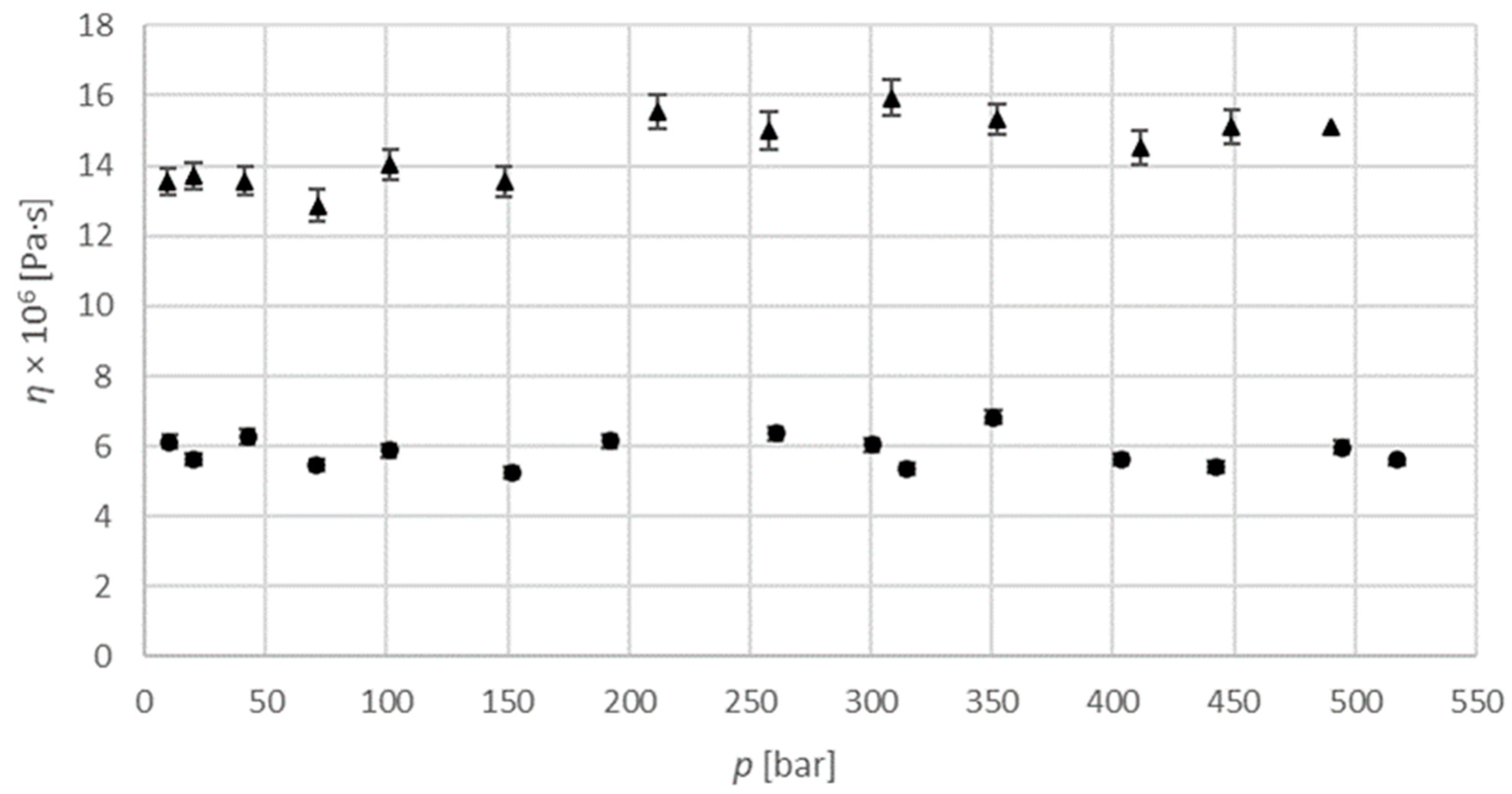
 , measured data CO2 saturated isopropanol at 313.15 K;
, measured data CO2 saturated isopropanol at 313.15 K;  , measured data CO2 saturated isopropanol at 333.15 K;
, measured data CO2 saturated isopropanol at 333.15 K;  , argon saturated isopropanol at 313.15 K;
, argon saturated isopropanol at 313.15 K;  , argon saturated isopropanol at 333.15 K;
, argon saturated isopropanol at 333.15 K;  , SF6 saturated isopropanol at 313.15 K;
, SF6 saturated isopropanol at 313.15 K;  , SF6 saturated isopropanol at 333.15 K; +, Zúñiga-Moreno, et al. (2002) isopropanol at 313.15 K [26]; –, Zúñiga-Moreno et al. (2002) isopropanol at 332.99 K [26].
, SF6 saturated isopropanol at 333.15 K; +, Zúñiga-Moreno, et al. (2002) isopropanol at 313.15 K [26]; –, Zúñiga-Moreno et al. (2002) isopropanol at 332.99 K [26].
 , measured data CO2 saturated isopropanol at 313.15 K;
, measured data CO2 saturated isopropanol at 313.15 K;  , measured data CO2 saturated isopropanol at 333.15 K;
, measured data CO2 saturated isopropanol at 333.15 K;  , argon saturated isopropanol at 313.15 K;
, argon saturated isopropanol at 313.15 K;  , argon saturated isopropanol at 333.15 K;
, argon saturated isopropanol at 333.15 K;  , SF6 saturated isopropanol at 313.15 K;
, SF6 saturated isopropanol at 313.15 K;  , SF6 saturated isopropanol at 333.15 K; +, Zúñiga-Moreno, et al. (2002) isopropanol at 313.15 K [26]; –, Zúñiga-Moreno et al. (2002) isopropanol at 332.99 K [26].
, SF6 saturated isopropanol at 333.15 K; +, Zúñiga-Moreno, et al. (2002) isopropanol at 313.15 K [26]; –, Zúñiga-Moreno et al. (2002) isopropanol at 332.99 K [26].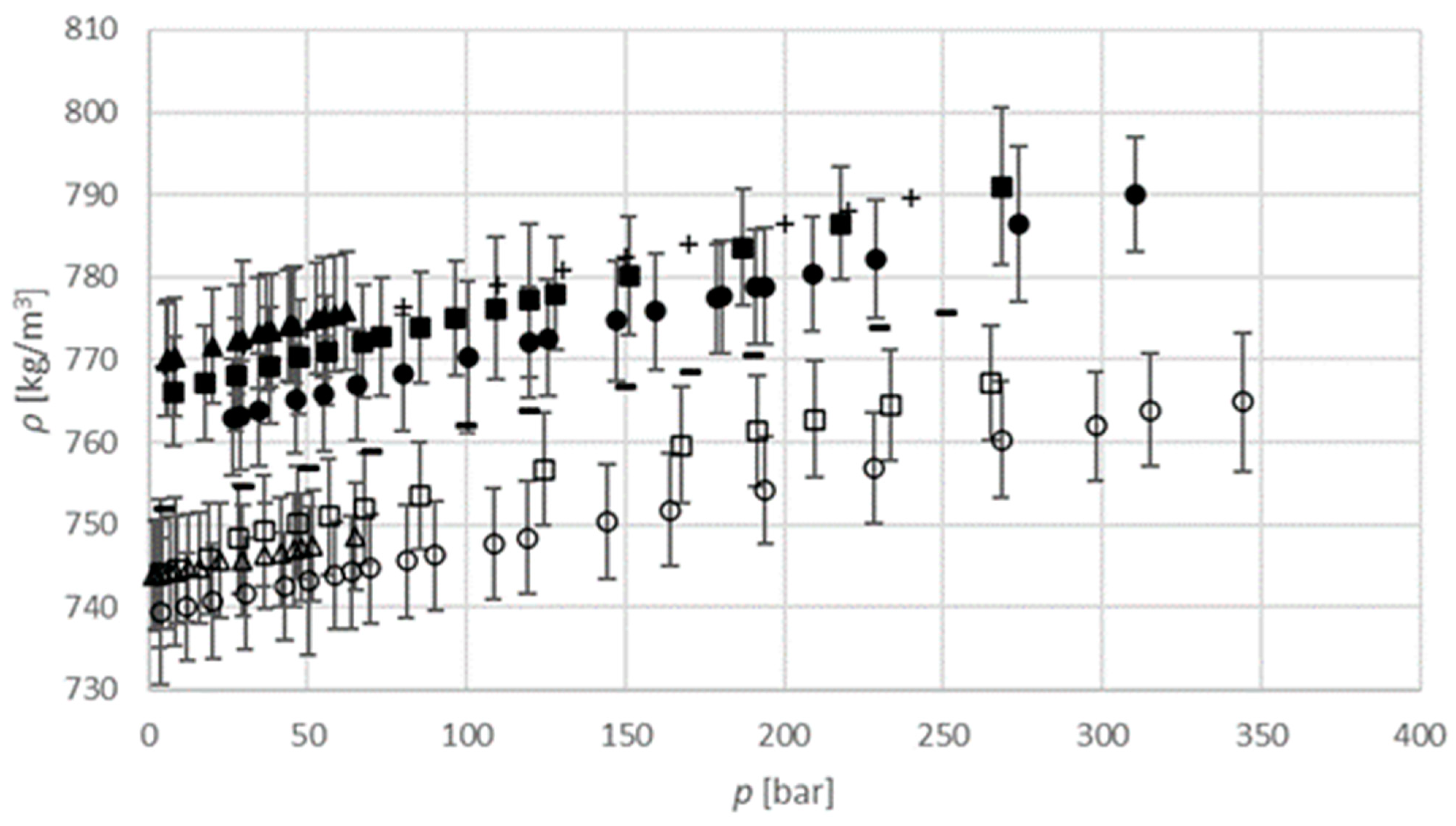
 , measured data at 313.15 K;
, measured data at 313.15 K;  , at 333.15 K.
, at 333.15 K.
 , measured data at 313.15 K;
, measured data at 313.15 K;  , at 333.15 K.
, at 333.15 K.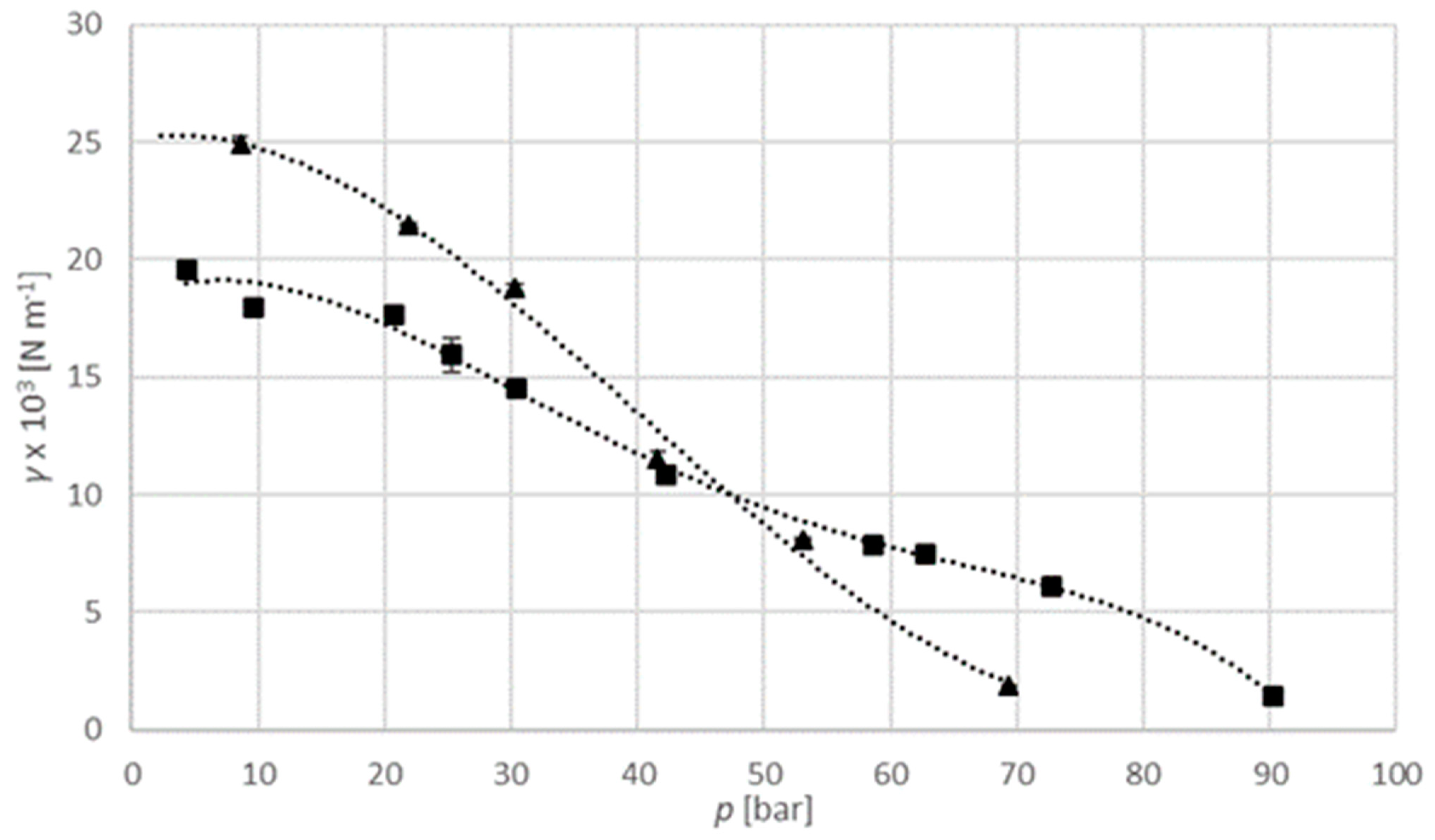
 , measured data at 313.15 K;
, measured data at 313.15 K;  , at 333.15 K.
, at 333.15 K.
 , measured data at 313.15 K;
, measured data at 313.15 K;  , at 333.15 K.
, at 333.15 K.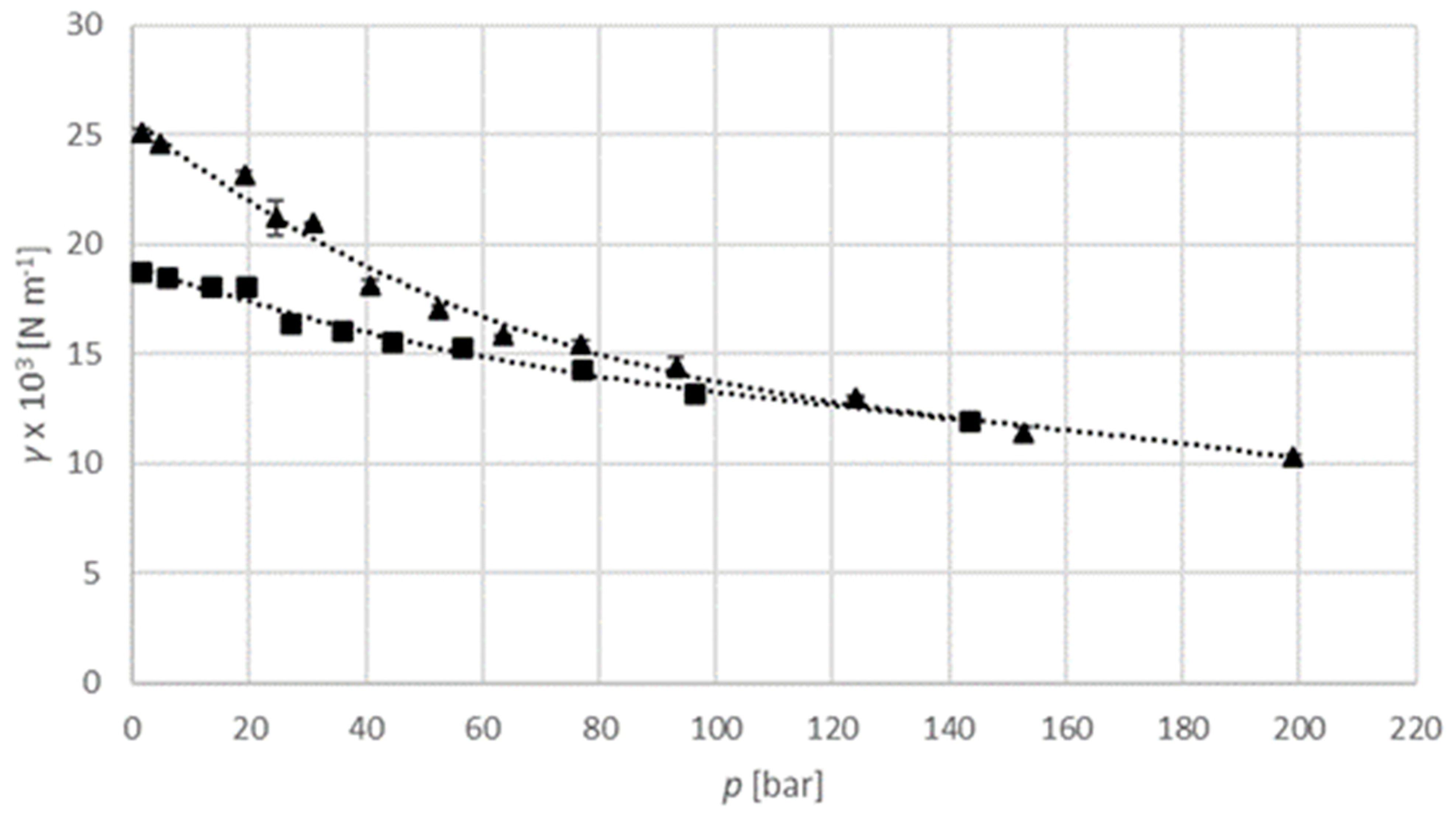
 , measured data at 313.15 K;
, measured data at 313.15 K;  , at 333.15 K.
, at 333.15 K.
 , measured data at 313.15 K;
, measured data at 313.15 K;  , at 333.15 K.
, at 333.15 K.