Study of Solidifying Surplus Sludge as Building Material Using Ordinary Portland Cement
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Property Analysis of Surplus Sludge
2.3. Solidification Experiment of the Surplus Sludge
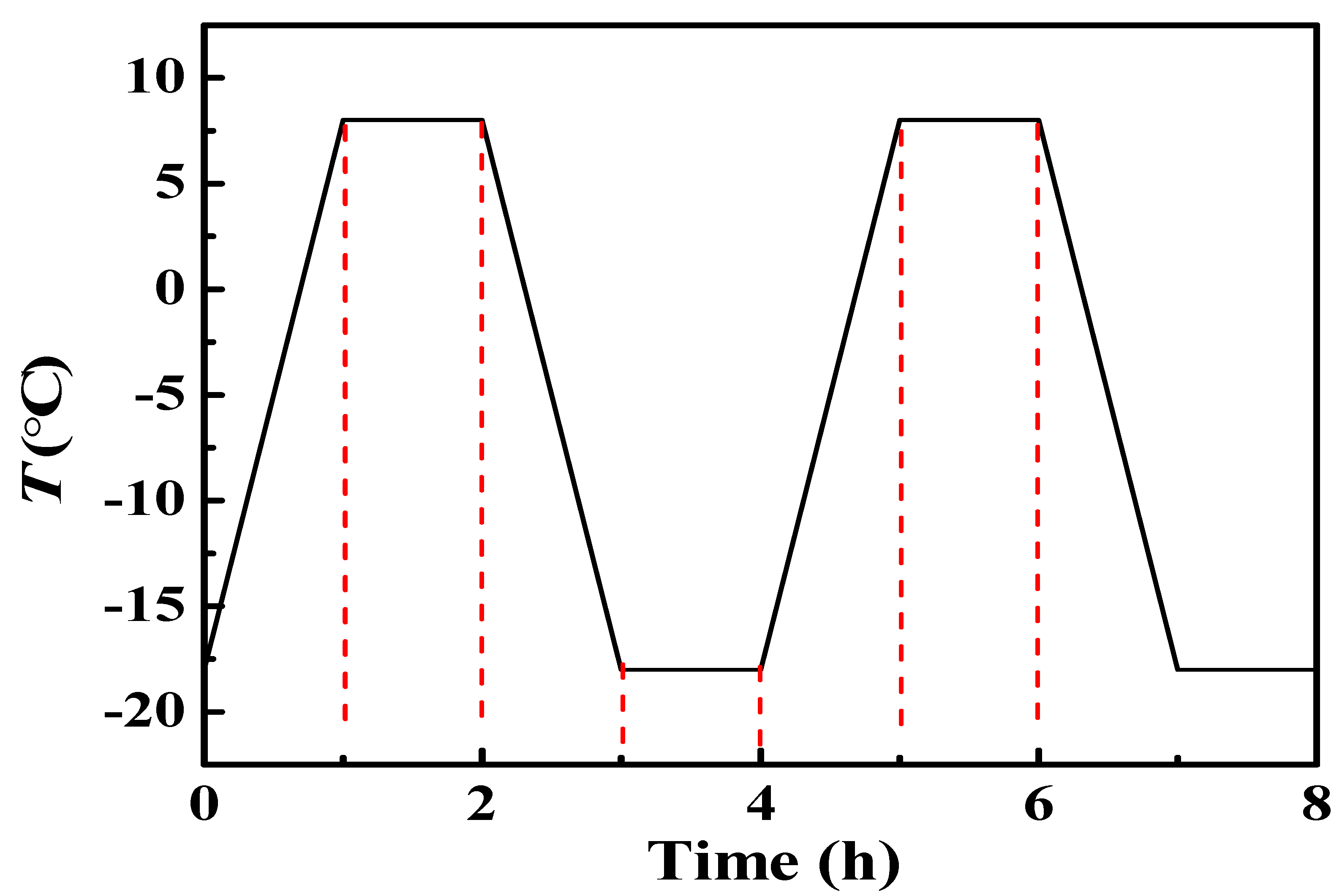
2.4. Freeze-Thaw Recycle Experiment
2.5. Characterization
3. Results and Discussion
3.1. The Characterization of Surplus Sludge and Solidified Blocks
3.1.1. FTIR Spectra of Raw Materials and Solidified Blocks
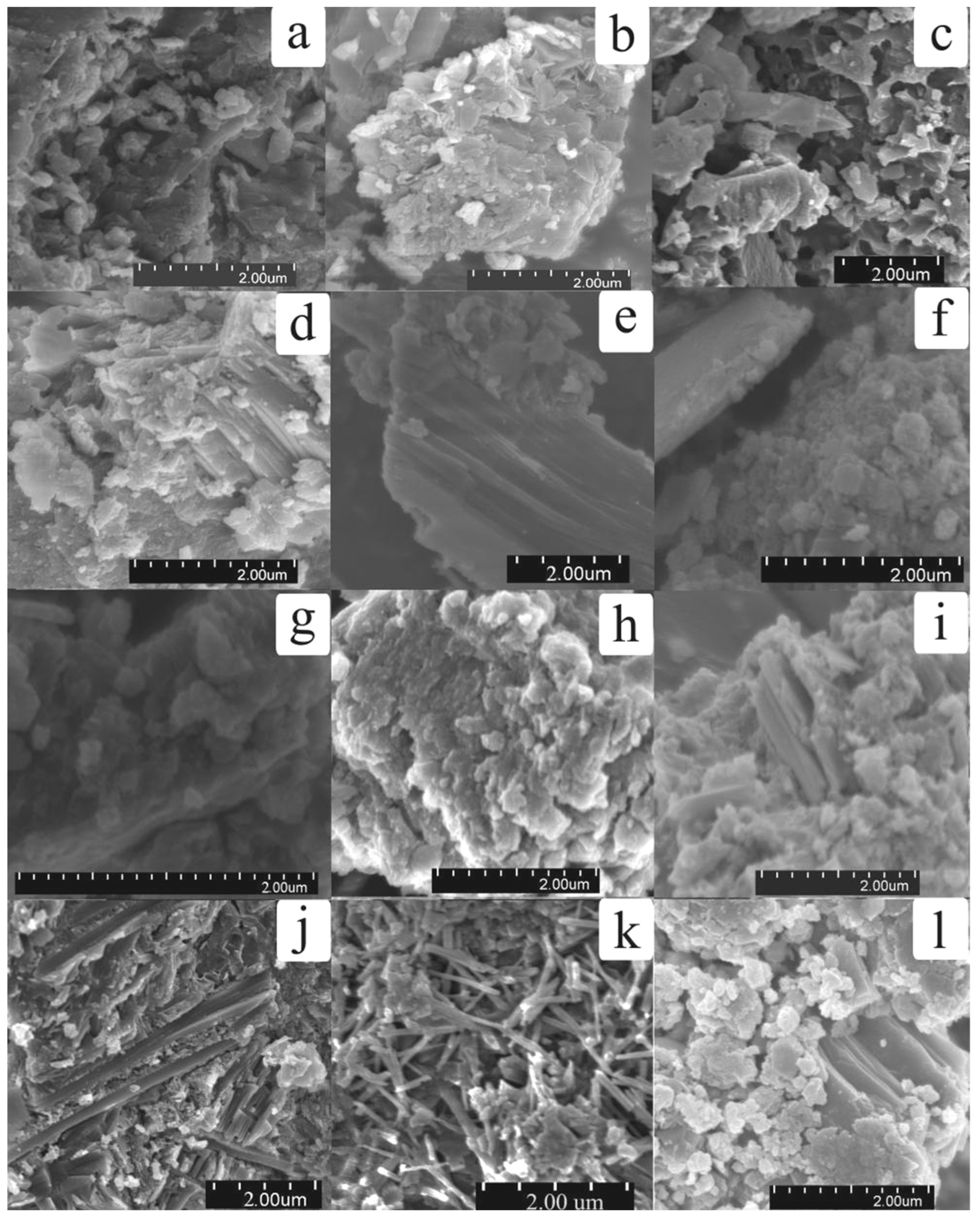
3.1.2. SEM Micrographs of Solidified Blocks
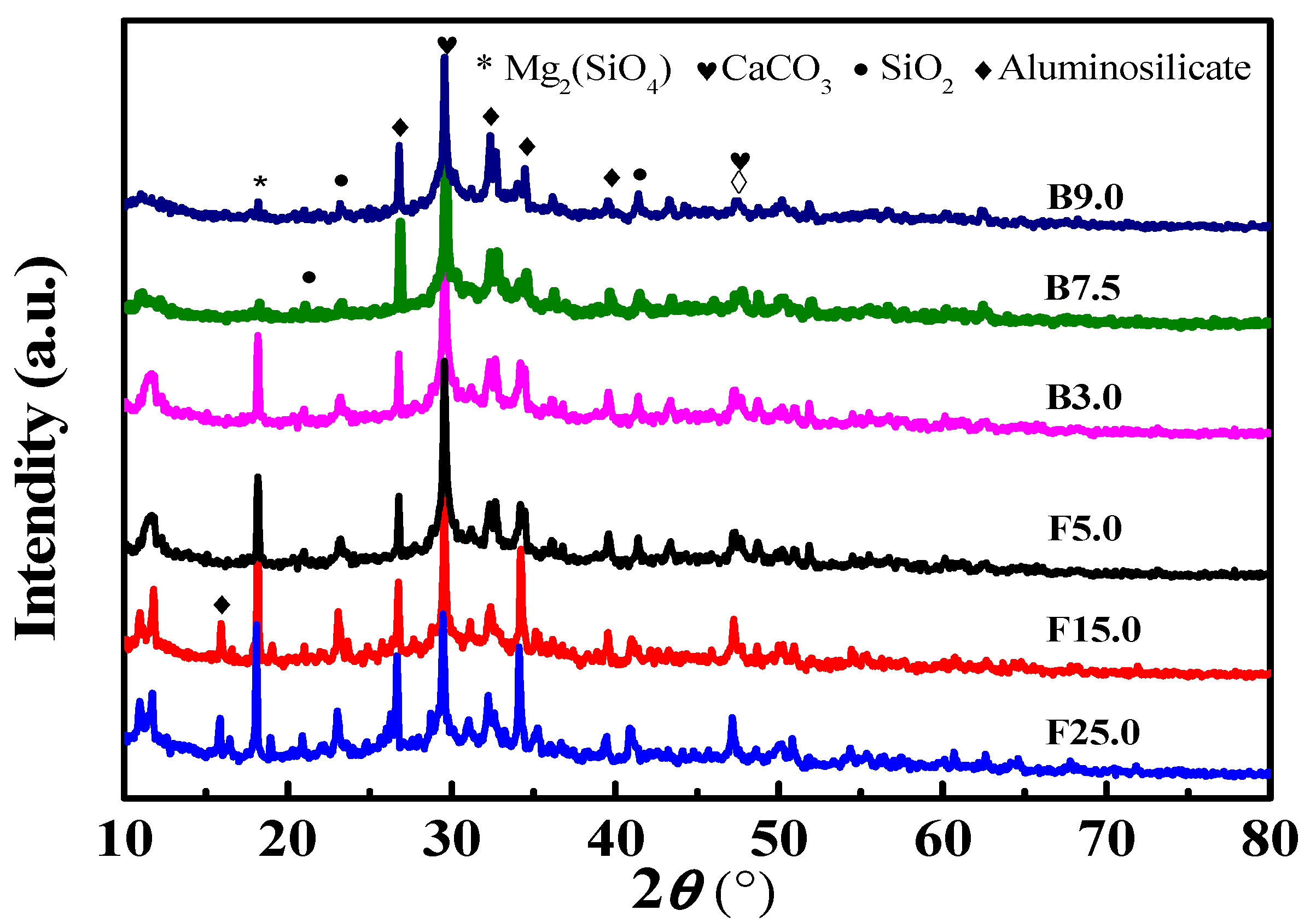
3.1.3. X-ray Diffraction Analysis (XRD) of Solidified Blocks
3.2. Effects of DS and Rl/S on RC of Solidified Blocks
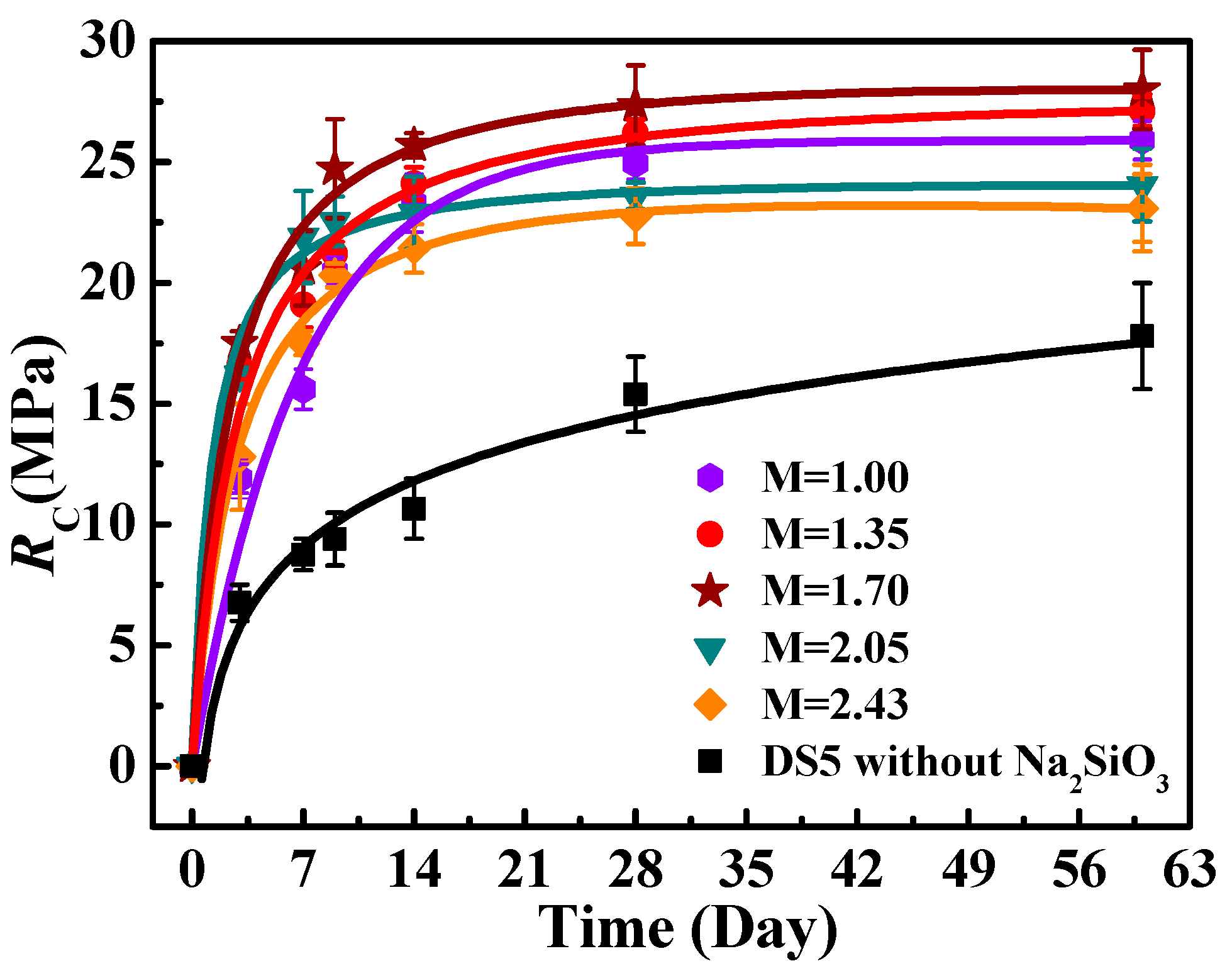
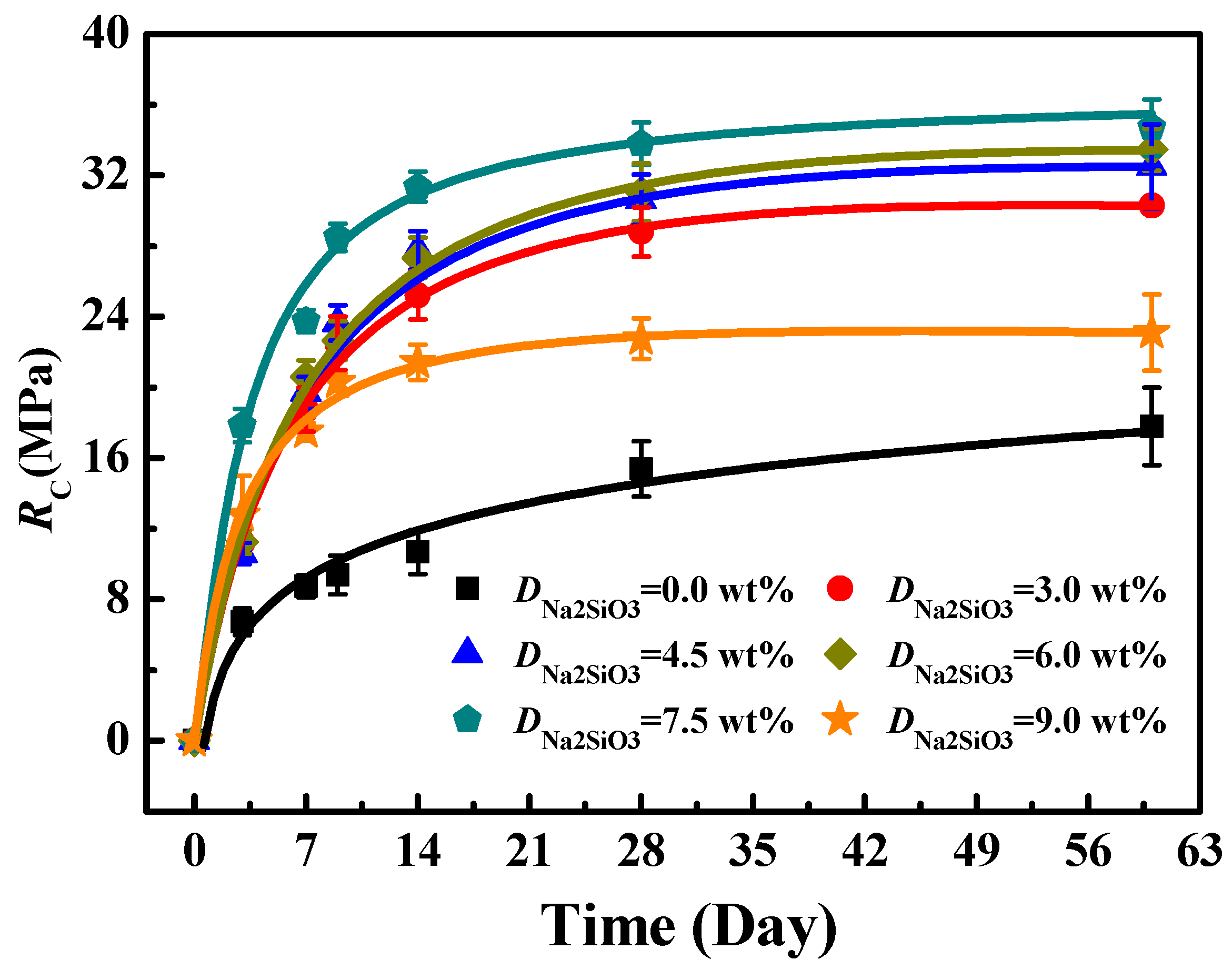
3.3. The Effect of Na2SiO3 on RC of Solidified Blocks
3.4. The Effect of Fly Ash on RC of Solidified Blocks
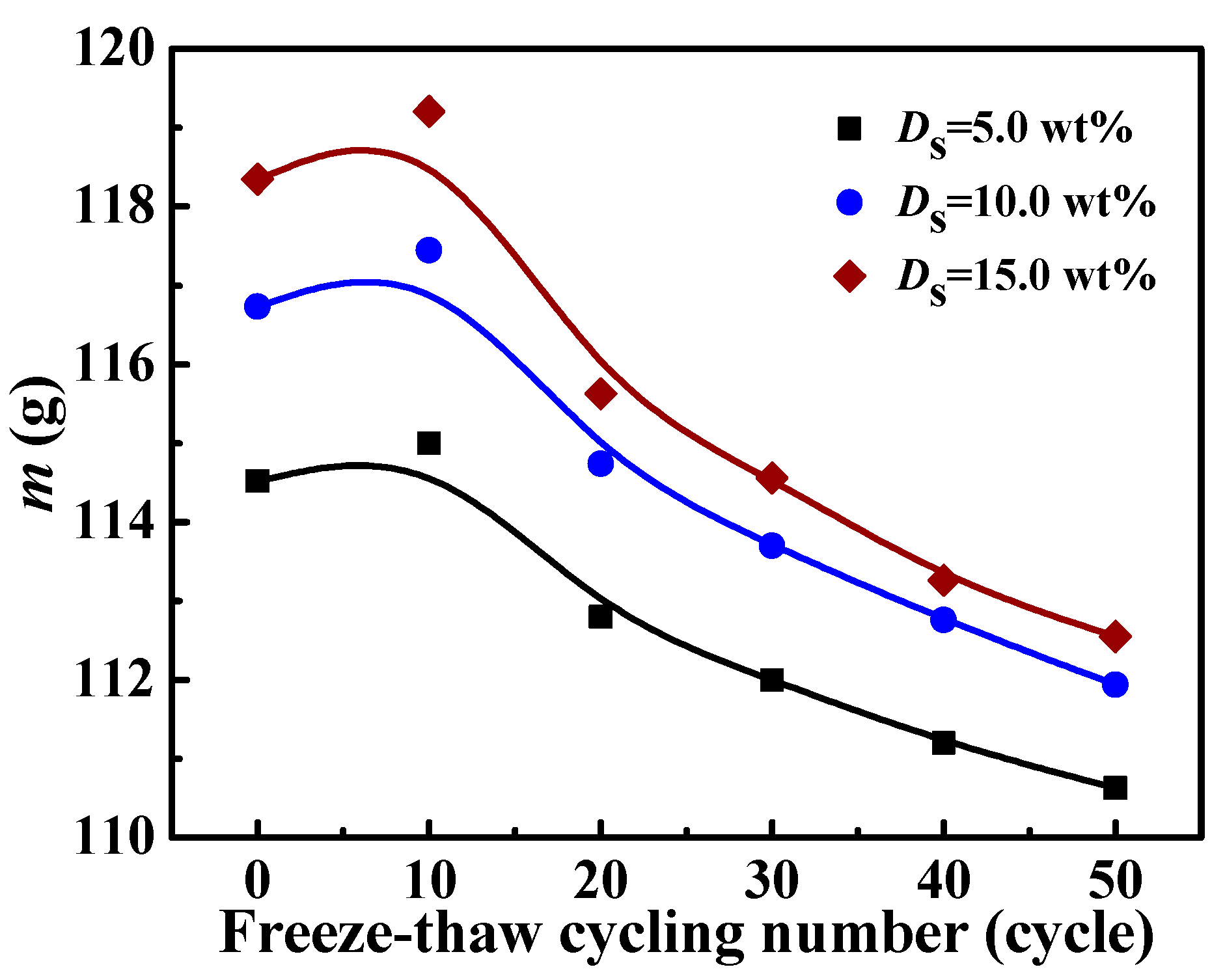
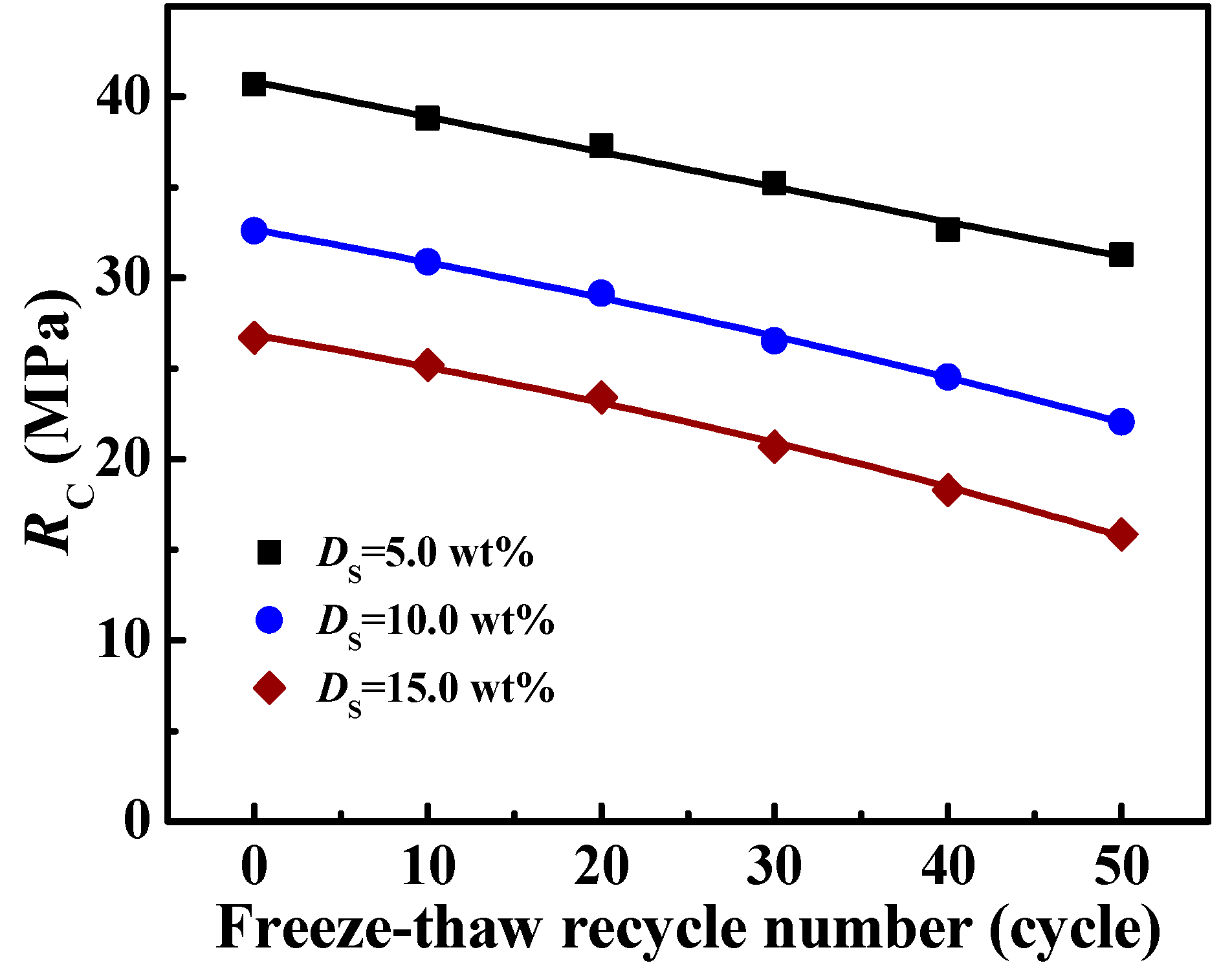
3.5. The Effect of Freeze-Thaw Cycling on the RC of Solidified Blocks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liew, C.S.; Yunus, N.M.; Chidi, B.S.; Lam, M.K.; Goh, P.S.; Mohamad, M.; Sin, J.C.; Lam, S.M.; Lim, J.W.; Lam, S.S. A review on recent disposal of hazardous sewage sludge via anaerobic digestion and novel composting. J. Hazard. Mater. 2022, 423, 126995. [Google Scholar] [CrossRef] [PubMed]
- Liew, C.S.; Kiatkittipong, W.; Lim, J.W.; Lam, M.K.; Ho, Y.C.; Ho, C.D.; Ntwampe, S.K.O.; Mohamad, M.; Usman, A. Stabilization of heavy metals loaded sewage sludge: Reviewing conventional to state-of-the-art thermal treatments in achieving energy sustainability. Chemosphere 2021, 277, 130310. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, F.; Li, Q.; Xue, C.; Xia, X.; Yu, H.; Zhao, Q.; Jiang, J.; Bai, S. Development, current state and future trends of sludge management in China: Based on exploratory data and CO2-equivaient emissions analysis. Environ. Int. 2020, 144, 106093. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhai, Y.; Li, S.; Wang, B.; Wang, T.; Liu, Y.; Qiu, Z.; Li, C. Hydrothermal carbonization of sewage sludge: Effect of feed-water pH on hydrochar’s physicochemical properties, organic component and thermal behavior. J. Hazard. Mater. 2020, 388, 122084. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, Z.; Wu, G.; Wu, Q.; Zhang, F.; Niu, Z.; Hu, H.Y. Characteristics of water quality of municipal wastewater treatment plants in China: Implications for resources utilization and management. J. Clean. Prod. 2016, 131, 1–9. [Google Scholar] [CrossRef]
- Wang, T.; Shi, F.; Zhang, Q.; Qian, X.; Hashimoto, S. Exploring material stock efficiency of municipal water and sewage infrastructures in China. J. Clean. Prod. 2018, 181, 498–507. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Yang, W.N.; Ngo, H.H.; Guo, W.S.; Jin, P.K.; Dzakpasu, M.; Yang, S.J.; Wang, Q.; Wang, X.C.; Ao, D. Current status of urban wastewater treatment plants in China. Environ. Int. 2016, 92–93, 11–22. [Google Scholar] [CrossRef]
- Dong, B.; Liu, X.; Dai, L.; Dai, X. Changes of heavy metal speciation during high-solid anaerobic digestion of sewage sludge. Bioresour. Technol. 2013, 131, 152–158. [Google Scholar] [CrossRef]
- Breulmann, M.; van Afferden, M.; Müller, R.A.; Schulz, E.; Fühner, C. Process conditions of pyrolysis and hydrothermal carbonization affect the potential of sewage sludge for soil carbon sequestration and amelioration. J. Anal. Appl. Pyrolysis 2017, 124, 256–265. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Efaq, A.N.; Bala, J.D.; Norli, I.; Abdel-Monem, M.O.; Kadir, M.O.A. Removal of pathogenic bacteria from sewage-treated effluent and biosolids for agricultural purposes. Appl. Water Sci. 2018, 8, 74. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Anders, A.; Weigand, H.; Cakir, H.; Kornhaas, U.; Platen, H. Phosphorus recycling from activated sludge of full-scale wastewater treatment plants by fast inversion of the biological phosphorus elimination mechanism. J. Environ. Chem. Eng. 2021, 9, 106403. [Google Scholar] [CrossRef]
- Shehu, M.S.; Abdul Manan, Z.; Alwi, S.R. Optimization of thermo-alkaline disintegration of sewage sludge for enhanced biogas yield. Bioresour. Technol. 2012, 114, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, J.; Liu, H.; Zhang, J. Sludge ozonation: Disintegration, supernatant changes and mechanisms. Bioresour. Technol. 2009, 100, 1505–1509. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge-the current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef]
- Peng, C.; Zhai, Y.; Zhu, Y.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Production of char from sewage sludge employing hydrothermal carbonization: Char properties, combustion behavior and thermal characteristics. Fuel 2016, 176, 110–118. [Google Scholar] [CrossRef]
- Suarez-Iglesias, O.; Urrea, J.L.; Oulego, P.; Collado, S.; Diaz, M. Valuable compounds from sewage sludge by thermal hydrolysis and wet oxidation-A review. Sci. Total Environ. 2017, 584–585, 921–934. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Wei, L.; Li, J.; Xue, M.; Wang, S.; Li, Q.; Qin, K.; Jiang, J.; Ding, J.; Zhao, Q. Adsorption behaviors of Cu2+, Zn2+ and Cd2+ onto proteins, humic acid, and polysaccharides extracted from sludge EPS: Sorption properties and mechanisms. Bioresour. Technol. 2019, 291, 121868. [Google Scholar] [CrossRef]
- Hii, K.; Baroutian, S.; Parthasarathy, R.; Gapes, D.J.; Eshtiaghi, N. A review of wet air oxidation and thermal hydrolysis technologies in sludge treatment. Bioresour. Technol. 2014, 155, 289–299. [Google Scholar] [CrossRef]
- Manara, P.; Zabaniotou, A. Towards sewage sludge based biofuels via thermochemical conversion—A review. Renew. Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Werle, S.; Wilk, R.K. A review of methods for the thermal utilization of sewage sludge: The polish perspective. Renew. Energy 2010, 35, 1914–1919. [Google Scholar] [CrossRef]
- Anjum, M.; Al-Makishah, N.H.; Barakat, M.A. Wastewater sludge stabilization using pre-treatment methods. Process Saf. Environ. 2016, 102, 615–632. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Wei, L.; Qin, K.; Ding, J.; Xue, M.; Yang, C.; Jiang, J.; Zhao, Q. Optimization of the co-digestion of sewage sludge, maize straw and cow manure: Microbial responses and effect of fractional organic characteristics. Sci. Rep. 2019, 9, 2374. [Google Scholar] [CrossRef]
- Yang, D.; Hu, C.; Dai, L.; Liu, Z.; Dong, B.; Dai, X. Post-thermal hydrolysis and centrate recirculation for enhancing anaerobic digestion of sewage sludge. Waste Manag. 2019, 92, 39–48. [Google Scholar] [CrossRef]
- Zhao, X.; Kumar, K.; Gross, M.A.; Kunetz, T.E.; Wen, Z. Evaluation of revolving algae biofilm reactors for nutrients and metals removal from sludge thickening supernatant in a municipal wastewater treatment facility. Water Res. 2018, 143, 467–478. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.T.; Feng, D.; Wu, C.L.; Wang, X.; Min, F.L. Effect of chemical conditioners on deep dewatering of urban dewatered sewage sludge in the temporary sludge lagoon. J. Environ. Eng. 2019, 145, 04019063. [Google Scholar] [CrossRef]
- Wei, L.; Xia, X.; Zhu, F.; Li, Q.; Xue, M.; Li, J.; Sun, B.; Jiang, J.; Zhao, Q. Dewatering efficiency of sewage sludge during Fe2+-activated persulfate oxidation: Effect of hydrophobic/hydrophilic properties of sludge EPS. Water Res. 2020, 181, 115903. [Google Scholar] [CrossRef]
- Ge, S.; Foong, S.Y.; Ma, N.L.; Liew, R.K.; Mahari, W.A.W.; Xia, C.; Yek, P.N.Y.; Peng, W.; Nam, W.L.; Lim, X.Y.; et al. Vacuum pyrolysis incorporating microwave heating and base mixture modification: An integrated approach to transform biowaste into eco-friendly bioenergy products. Renew. Sustain. Energy Rev. 2020, 127, 109871. [Google Scholar] [CrossRef]
- Xu, Z.; Ye, D.; Dai, T.; Dai, Y. Research on preparation of coal waste-based geopolymer and its stabilization/solidification of heavy metals. Integr. Ferroelectr. 2021, 217, 214–224. [Google Scholar] [CrossRef]
- Shen, Z.; Jin, F.; O’Connor, D.; Hou, D. Solidification/stabilization for soil remediation: An old technology with new vitality. Environ. Sci. Technol. 2019, 53, 11615–11617. [Google Scholar] [CrossRef]
- Tuncan, A.; Tuncan, M.; Koyuncu, H. Use of petroleum-contaminated drilling wastes as sub-base material for road construction. Waste Manag. Res. 2000, 18, 489–505. [Google Scholar] [CrossRef]
- Ministry of Construction of the People’s Republic of China. Determination Method for Municipal Sludge in Wastewater Treatment Plant; Ministry of Construction of the People’s Republic of China: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Lv, Q.; Yu, J.; Ji, F.; Gu, L.; Chen, Y.; Shan, X. Mechanical property and microstructure of fly ash-based geopolymer activated by sodium silicate. KSCE J. Civ. Eng. 2021, 25, 1765–1777. [Google Scholar] [CrossRef]
- Kowalski, M.; Kowalska, K.; Wiszniowski, J.; Turek-Szytow, J. Qualitative analysis of activated sludge using FT-IR technique. Chem. Pap. 2018, 72, 2699–2706. [Google Scholar] [CrossRef]
- Ma, J.; Li, P.; Wang, W.; Wang, S.; Pan, X.; Zhang, F.; Li, S.; Liu, S.; Wang, H.; Gao, G.; et al. Biodegradable poly (amino acid)-gold-magnetic complex with efficient endocytosis for multimodal imaging-guided chemo-photothermal therapy. ACS Nano 2018, 12, 9022–9032. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Yakubu, Y.; Zhou, J.; Ping, D.; Shu, Z.; Chen, Y. Effects of pH dynamics on solidification/stabilization of municipal solid waste incineration fly ash. J. Environ. Manag. 2018, 207, 243–248. [Google Scholar] [CrossRef]
- Li, J.T.; Zeng, M.; Ji, W.X. Characteristics of the cement-solidified municipal solid waste incineration fly ash. Environ. Sci. Pollut. Res. 2018, 25, 36736–36744. [Google Scholar] [CrossRef]
- Hou, Y.F.; Wang, D.M.; Li, Q.; Lu, H.B. Effect of water glass performance on fly ash-based geopolymers. J. Chin. Ceram. Soc. 2008, 36, 61–64. (In Chinese) [Google Scholar] [CrossRef]
- Li, J.L.; Zhu, L.Y.; Zhou, K.P.; Liu, H.W.; Cao, S.P. Damage characteristics of sandstone pore structure under freeze-thaw cycles. Rock Soil Mech. 2019, 40, 3524–3532. (In Chinese) [Google Scholar]







| Chemical Composition | Weight (wt%) | Chemical Composition | Weight (wt%) |
|---|---|---|---|
| SiO2 | 53.97 | MgO | 1.01 |
| Al2O3 | 31.15 | Na2O | 0.89 |
| Fe2O3 | 4.16 | SO3 | 0.73 |
| CaO | 4.01 | P2O5 | 0.67 |
| K2O | 2.04 | Cl | 0.13 |
| TiO2 | 1.13 | NiO | 0.11 |
| Solidified Block | DS/wt% | DP/wt% | Rl/s | DNa2SiO3/wt% | M | WF/wt% |
|---|---|---|---|---|---|---|
| DS0 | 0.0 | 100.0 | 0.35 0.45 0.55 | 0.0 | - | 0.0 |
| DS5 | 5.0 | 95.0 | 0.35 0.45 0.55 | 0.0 | - | 0.0 |
| DS10 | 10.0 | 90.0 | 0.35 0.45 0.55 | 0.0 | - | 0.0 |
| DS15 | 15.0 | 85.0 | 0.35 0.45 0.55 | 0.0 | - | 0.0 |
| B3S0 | 0.0 | 100.0 | 0.45 | 3.0 | 2.43 | 0.0 |
| B3.0 B4.5 B6.0 B7.5 B9.0 | 5.0 | 95.0 | 0.45 | 3.0 4.5 6.0 7.5 9.0 | 2.43 | 0.0 |
| F5.0 F10.0 F15.0 F20.0 F25.0 | 5.0 | 90.0 85.0 80.0 75.0 70.0 | 0.45 | - | - | 5.0 10.0 15.0 20.0 25.0 |
| F15S0 | 0.0 | 85.0 | 0.45 | - | - | 15.0 |
| Sample | DNa2SiO3/wt% | DS/wt% | DP/wt% | M | WF/wt% | Rl/s |
|---|---|---|---|---|---|---|
| A | 7.5 | 5.0 | 75.0 | 1.00 | 20.0 | 0.35 |
| B | 7.5 | 10.0 | 70.0 | 1.00 | 20.0 | 0.35 |
| C | 7.5 | 15.0 | 65.0 | 1.00 | 20.0 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; He, H.; Wei, J.; Han, T.; Wang, W.; Wang, L.; Han, J.; Zhang, L.; Zhang, Y.; Ma, H. Study of Solidifying Surplus Sludge as Building Material Using Ordinary Portland Cement. Processes 2022, 10, 2234. https://doi.org/10.3390/pr10112234
Liang J, He H, Wei J, Han T, Wang W, Wang L, Han J, Zhang L, Zhang Y, Ma H. Study of Solidifying Surplus Sludge as Building Material Using Ordinary Portland Cement. Processes. 2022; 10(11):2234. https://doi.org/10.3390/pr10112234
Chicago/Turabian StyleLiang, Jiling, Han He, Jianwei Wei, Tingting Han, Wenwu Wang, Lu Wang, Jie Han, Lunqiu Zhang, Yan Zhang, and Haiqiang Ma. 2022. "Study of Solidifying Surplus Sludge as Building Material Using Ordinary Portland Cement" Processes 10, no. 11: 2234. https://doi.org/10.3390/pr10112234
APA StyleLiang, J., He, H., Wei, J., Han, T., Wang, W., Wang, L., Han, J., Zhang, L., Zhang, Y., & Ma, H. (2022). Study of Solidifying Surplus Sludge as Building Material Using Ordinary Portland Cement. Processes, 10(11), 2234. https://doi.org/10.3390/pr10112234









