Statistical Optimisation of Used-Cooking-Oil Degradation by Burkholderia vietnamiensis AQ5-12 and Burkholderia sp. AQ5-13
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of UCO
2.2. Bacterial Culture Maintenance and Media Preparation
2.3. Assessment of UCO Degradation by B. vietnamiensis AQ5-12 and Burkholderia sp. AQ5-13
2.4. Determination of UCO Degradation by Gravimetric Method
2.5. Bacterial Growth and UCO Degradation Optimisation Using OFAT
2.6. Statistical Optimisation Using Response-Surface Methodology (RSM)
2.6.1. Screening for Significant Factors Using Plackett–Burman Design (PBD)
2.6.2. Optimisation of UCO Degradation by B. vietnamiensis AQ5-12 and Burkholderia sp. AQ5-13 Using CCD
2.7. Statistical Analysis
3. Results
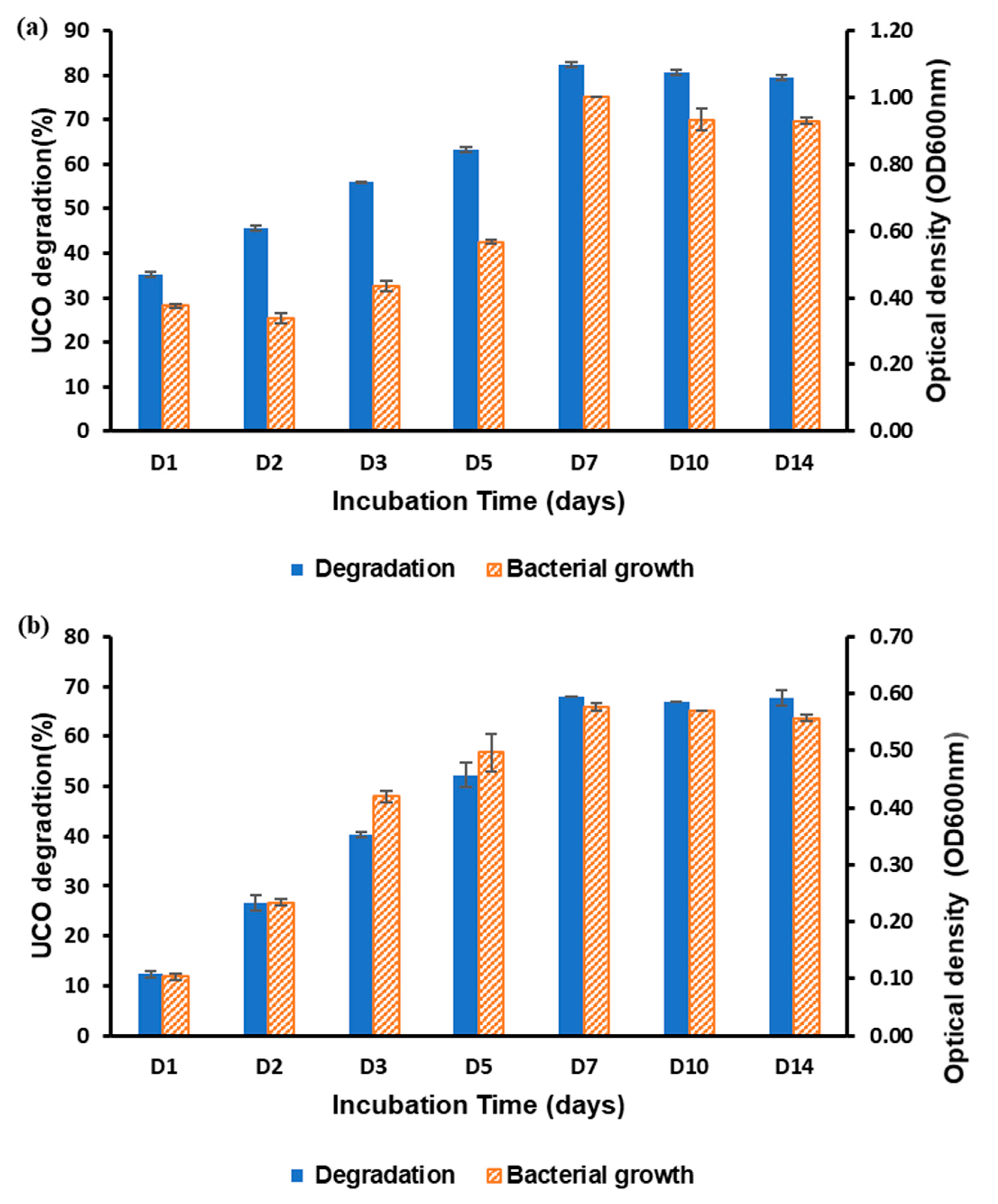
3.1. Assessing UCO Degradation by B. vietnamiensis AQ5-12 and Burkholderia sp. AQ5-13
3.2. Optimisation of Each Parameter for UCO Degradation Using OFAT
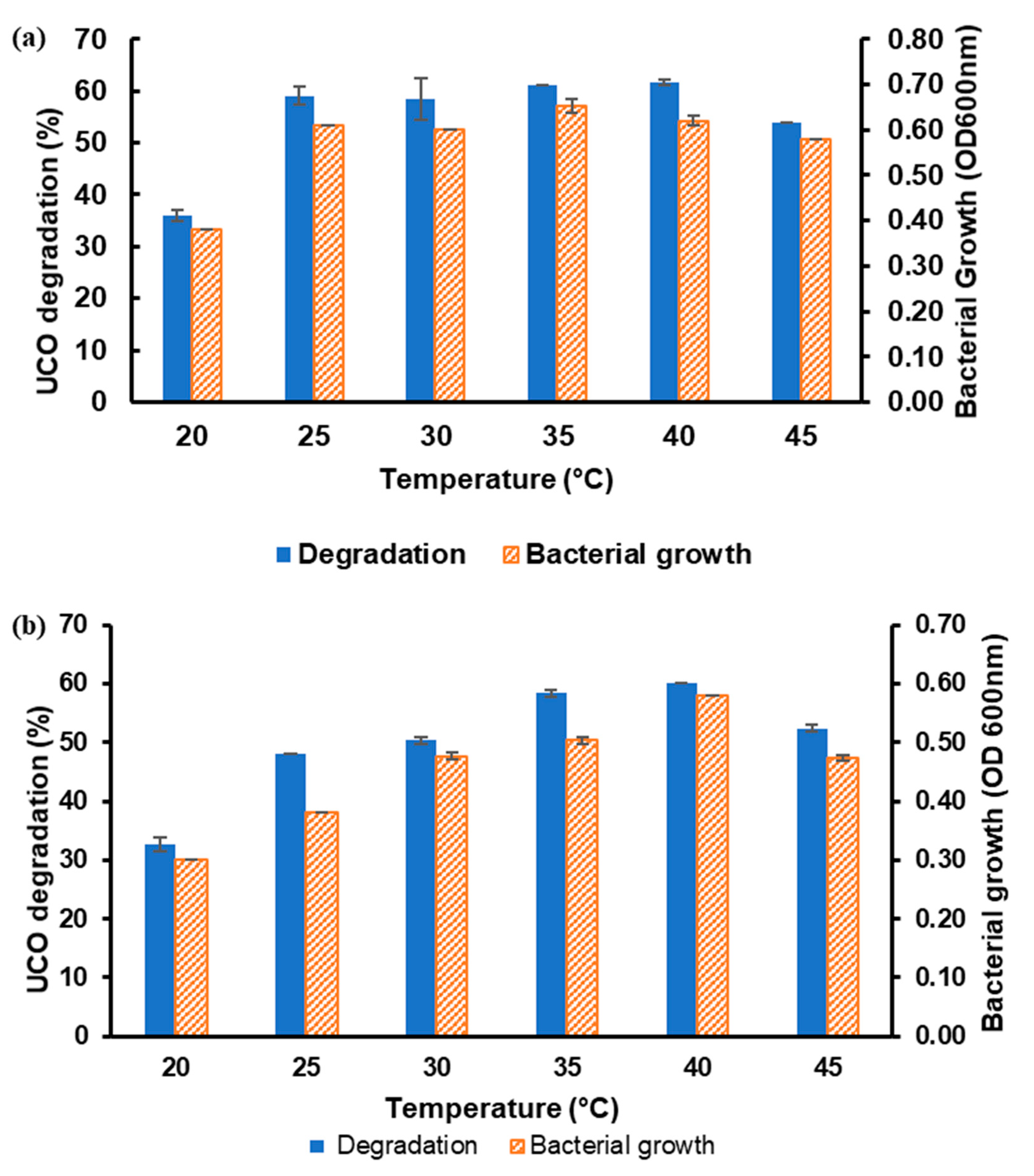
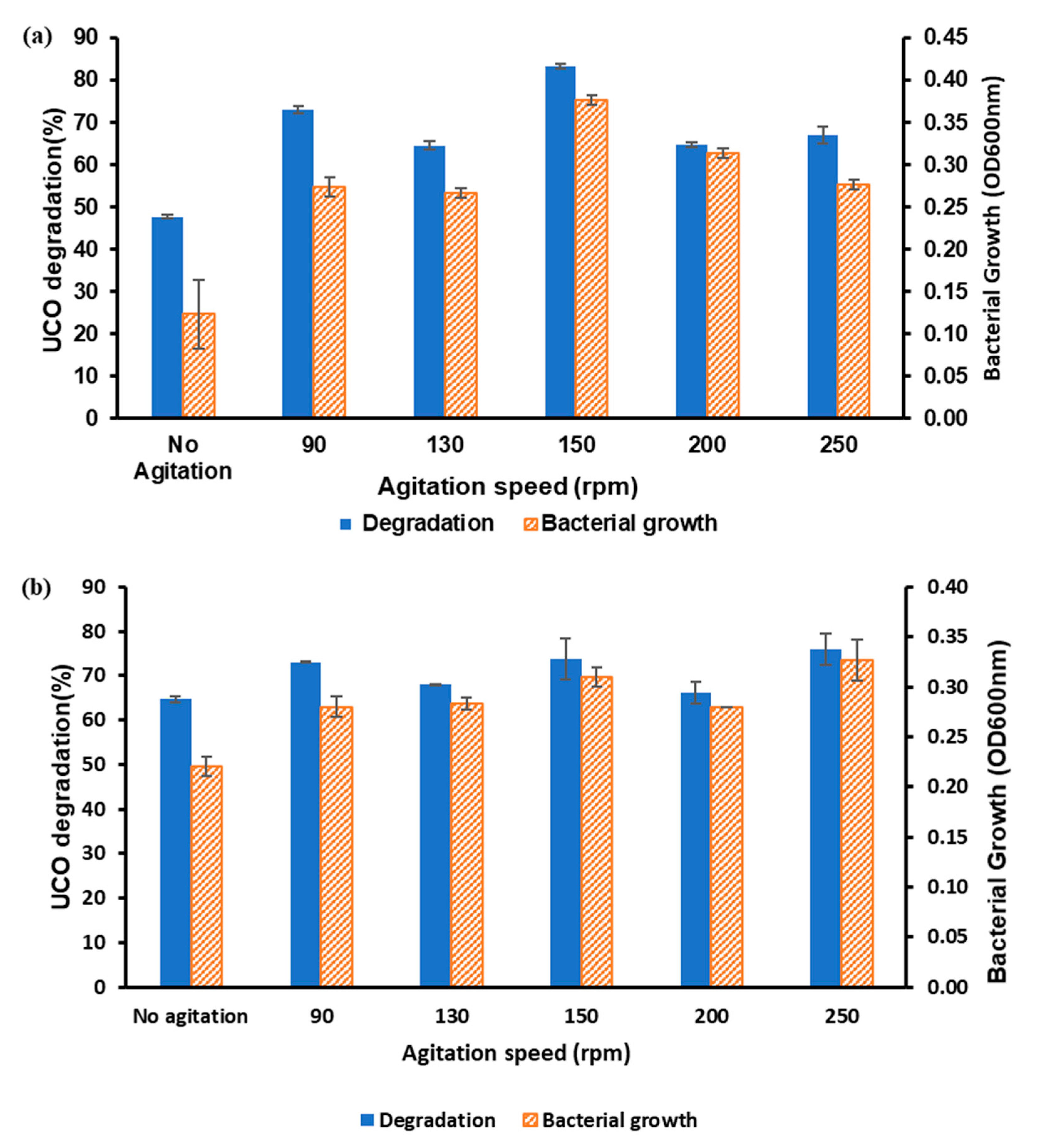
3.2.1. Effects of Temperature and Agitation Speed
3.2.2. Effects of Substrate Concentration and Nitrogen Source
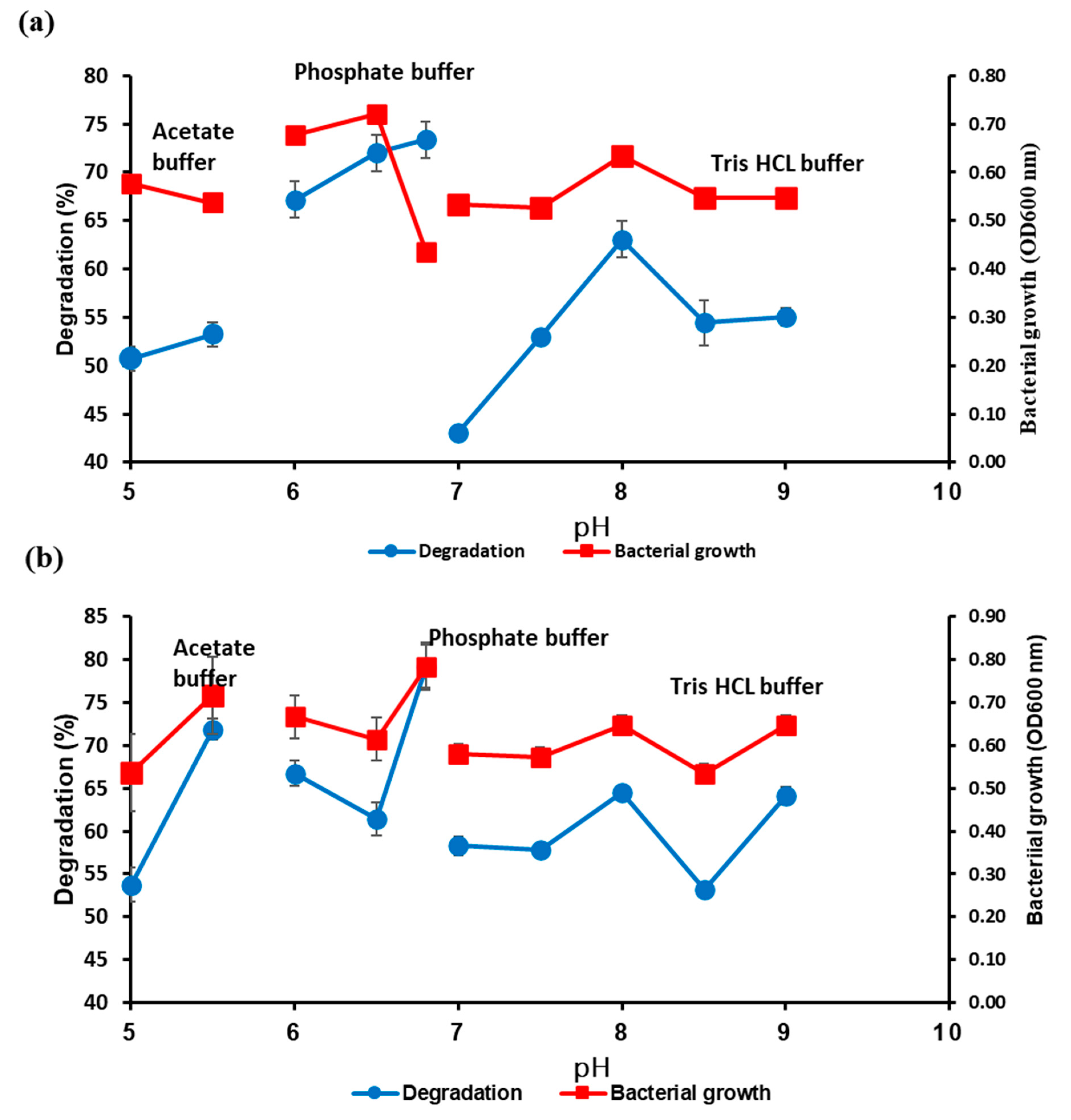
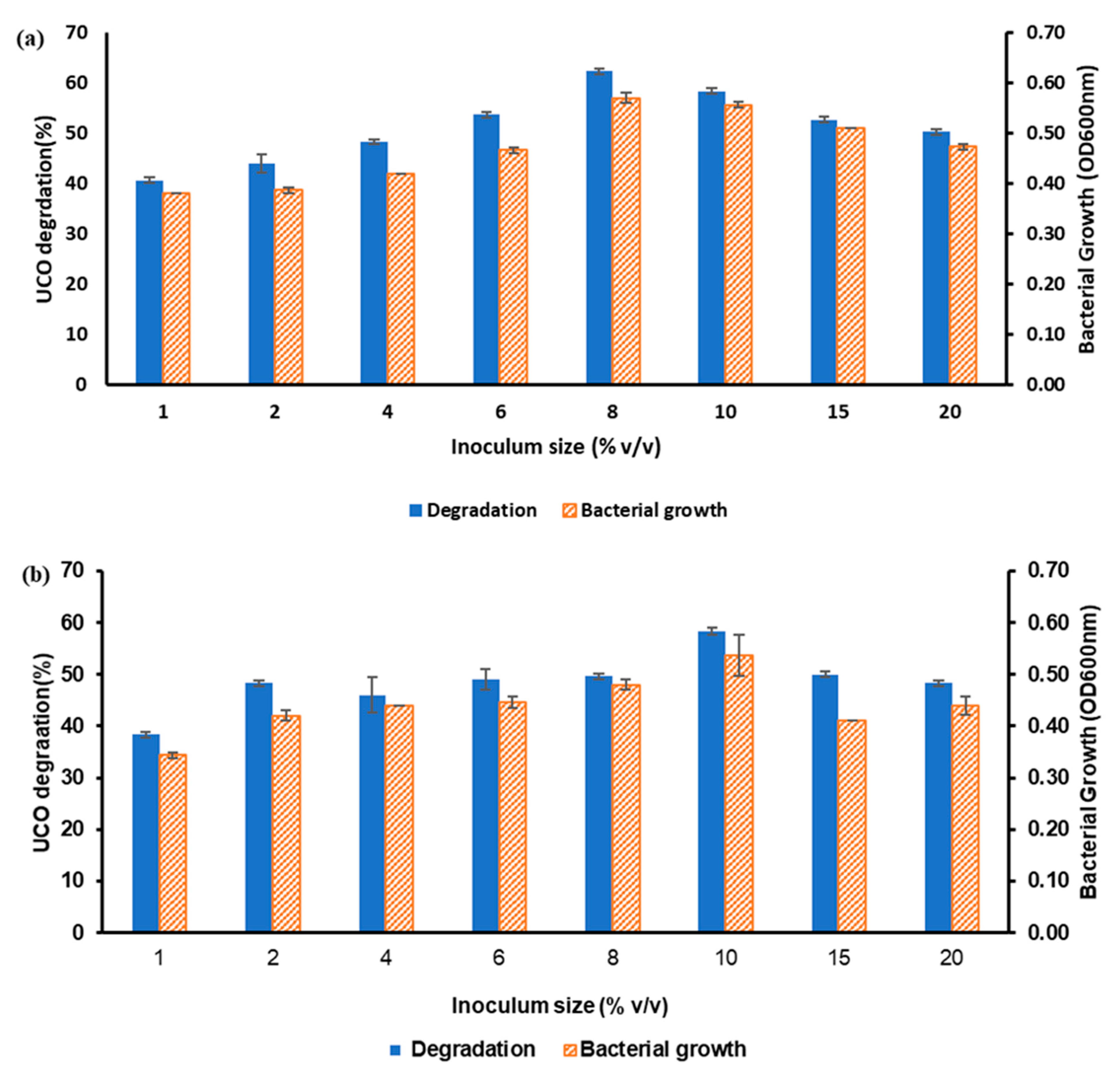
3.2.3. Effects of pH, Yeast Extract Concentration, and Inoculum Size
3.3. Optimisation of Bacterial Growth and Degradation by Response-Surface Methodology (RSM)
3.3.1. Plackett–Burman Design (PBD) Assessing the Significant Factors
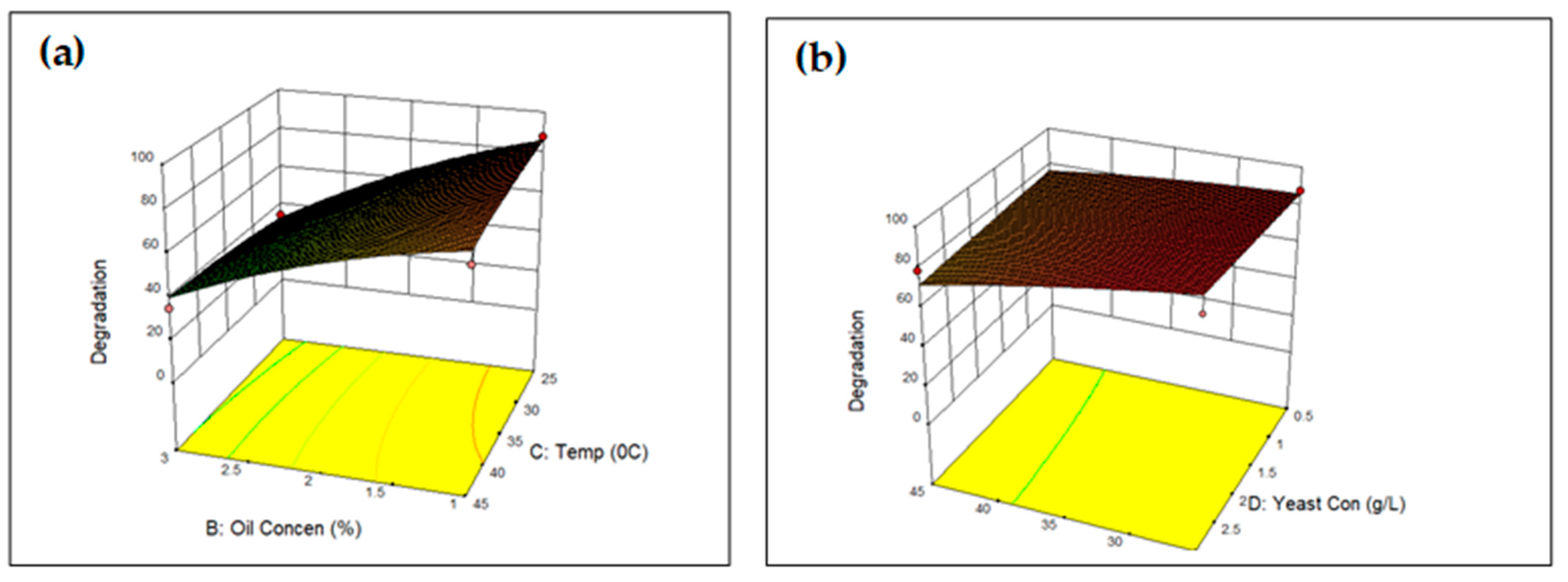
3.3.2. Optimisation of UCO Degradation Using Central Composite Design (CCD)
| Source | Sum of Squares | DF | Mean Square | F Value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 7537.23 | 9 | 837.47 | 28.49 | <0.0001 | Significant |
| A | 207.45 | 1 | 207.45 | 7.06 | 0.0240 | |
| B | 366.17 | 1 | 368.17 | 12.52 | 0.0054 | |
| C | 215.68 | 1 | 215.68 | 7.34 | 0.0220 | |
| A2 | 872.60 | 1 | 872.60 | 29.68 | 0.0003 | |
| B2 | 3568.70 | 1 | 3568.70 | 121.40 | <0.0001 | |
| C2 | 685.62 | 1 | 686.62 | 23.32 | 0.0007 | |
| AB | 200 | 1 | 200.00 | 6.80 | 0.0261 | |
| AC | 512 | 1 | 512.00 | 17.42 | 0.0019 | |
| BC | 1568.00 | 1 | 1568.00 | 53.34 | <0.0001 | |
| Residual | 293.97 | 10 | 29.40 | |||
| Lack of Fit | 215.14 | 5 | 43.03 | 2.73 | 0.1473 | Not significant |
| Pure Error | 78.83 | 5 | 15.77 | |||
| Cor Total | 7831.20 | 19 | ||||
| R2 | 0.9626 | Pred R2 | 0.7631 | |||
| Adj R2 | 0.9287 | Adeq Precision | 16.218 |
| Source | Sum of Squares | DF | Mean Square | F Value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 23,033.97 | 18 | 1279.66 | 21.62 | <0.0001 | Significant |
| A—pH | 6.81 | 1 | 6.81 | 0.12 | 0.7367 | |
| B—Used-oil concentration | 15,431.21 | 1 | 15,431.21 | 260.76 | <0.0001 | |
| C—Temperature | 66.63 | 1 | 66.63 | 1.13 | 0.2968 | |
| D—Yeast concentration | 4.81 | 1 | 4.81 | 0.081 | 0.7775 | |
| E—Ammonium sulphate | 5.01 | 1 | 5.01 | 0.085 | 0.7731 | |
| A2 | 4.25 | 1 | 4.25 | 0.072 | 0.7906 | |
| B2 | 1262.53 | 1 | 1262.53 | 21.33 | <0.0001 | |
| C2 | 86.65 | 1 | 86.65 | 1.46 | 0.2354 | |
| AB | 16.84 | 1 | 16.84 | 0.28 | 0.5976 | |
| AC | 97.31 | 1 | 97.31 | 1.64 | 0.2092 | |
| AD | 35.88 | 1 | 35.88 | 0.61 | 0.4421 | |
| AE | 10.62 | 1 | 10.62 | 0.18 | 0.6748 | |
| BC | 513.65 | 1 | 513.65 | 8.68 | 0.0061 | |
| BD | 0.28 | 1 | 0.28 | 4.702×103 | 0.9458 | |
| BE | 1.70 | 1 | 1.70 | 0.029 | 0.8667 | |
| CD | 296.60 | 1 | 296.60 | 5.01 | 0.0325 | |
| CE | 36.68 | 1 | 36.68 | 0.62 | 0.4371 | |
| DE | 10.21 | 1 | 10.21 | 0.17 | 0.6808 | |
| Residual | 1834.48 | 31 | 59.18 | |||
| Lack of Fit | 1679.37 | 24 | 69.97 | 3.16 | 0.0609 | Not significant |
| Pure Error | 155.11 | 7 | 22.16 | |||
| Cor Total | 24,868.45 | 49 | ||||
| R2 | 0.9262 | Pred R2 | 0.7824 | |||
| Adj R2 | 0.8834 | Adeq Precision | 22.453 |
3.4. Validation of the Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panadare, D.C.; Rathod, V.K. Applications of Waste Cooking Oil Other Than Biodiesel: A Review. IJCCE 2015, 12, 55–76. Available online: http://www.ijche.com/article_11253_54b41ee620eb7a8972ee3e37776dad5f.pdf (accessed on 19 April 2019).
- Williams, J.B.; Clarkson, C.; Mant, C.; Drinkwater, A.; May, E. Fat, oil and grease deposits in sewers : Characterisation of deposits and formation mechanisms. Water Res. 2012, 46, 6319–6328. [Google Scholar] [CrossRef] [PubMed]
- Phongjarus, N.; Suvaphat, C.; Srichai, N.; Ritchie, R.J. Photoheterotrophy of photosynthetic bacteria (Rhodopseudomonas palustris) growing on oil palm and soybean cooking oils. Environ. Technol. Innov. 2018, 10, 290–304. [Google Scholar] [CrossRef]
- Ren, J.B.; Fan, H.; Niu, D.; Gu, Y.; Li, C. Biodegradation of Waste Cooking Oils by Klebsiella quasivariicola IUMR-B53 and Characteristics of Its Oil-Degrading Enzyme. Waste Biomass Valori. 2021, 12, 1243–1252. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, Z.H.; Yin, H.; Dang, Z.; Wu, P.X.; Zhu, N.W.; Lin, Z.; Liu, Y. Global review of phthalates in edible oil: An emerging and nonnegligible exposure source to human. Sci. Total Environ. 2020, 704, 135369. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Belwal, T.; Li, L.; Ramola, S.; Aadil, R.M.; Abdullah; Xu, Y.; Zisheng, L. Utilization of wastewater from edible oil industry, turning waste into valuable products: A review. Trends Food. Sci. Technol. 2020, 99, 21–33. [Google Scholar] [CrossRef]
- Food and Agriculture Organisation of the United Nations. “Chapter 6 : Selected Uses of Fats and Oils in Food,” Fats and Oils in Human Nutrition. 1994. Available online: https://www.fao.org/3/v4700e/V4700E0b.htm#Chapter%206%20:%20Selected%20uses%20of%20fats%20and%20oils%20in%20food (accessed on 15 March 2019).
- Choe, E.; Min, D.B. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, 77–80. [Google Scholar] [CrossRef]
- Mannu, A.; Garroni, S.; Ibanez Porras, J.; Mele, A. Available technologies and materials for waste cooking oil recycling. Processes 2020, 8, 366. [Google Scholar] [CrossRef]
- Hazrat, M.A.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Rufford, T.E. Emission characteristics of waste tallow and waste cooking oil based termary biodiesel fuels. Energy Procedia 2019, 160, 842–847. [Google Scholar] [CrossRef]
- Ananey-Obiri, D.; Matthews, L.; Azahrani, M.H.; Ibrahim, S.A.; Galanakis, C.M.; Tahergorabi, R. Application of protein-based edible coatings for fat uptake reduction in deep-fat fried foods with an emphasis on muscle food proteins. Trends Food Sci. Technol. 2018, 80, 167–174. [Google Scholar] [CrossRef]
- Joshi, R.; Sharma, R.; Kuila, A. Lipase production from Fusarium incarnatum KU377454 and its immobilization using Fe3O4 NPs for application in waste cooking oil degradation. Bioresour. Technol. Rep. 2019, 5, 134–140. [Google Scholar] [CrossRef]
- Zahri, K.N.M.; Zulkharnain, A.; Claudio, G.; Sabri, S.; Khalil, K.A.; Convey, P.; Ahmad, S.A. The use of response surface methodology as a statistical tool for the optimisation of waste and pure canola oil biodegradation by antarctic soil bacteria. Life 2021, 11, 456. [Google Scholar] [CrossRef]
- Čipinytė, V.; Grigiškis, S.; Baškys, E. Selection of fat-degrading microorganisms for the treatment of lipid-contaminated environment. Biologija 2009, 55, 84–92. [Google Scholar] [CrossRef]
- Rahman, K.S.M.; Rahman, J.T.; Lakshmanaperualsamy, P.; Banat, I. Towards efficient crude oil degradation by a mixed bacterial consortium. Bioresour. Technol. 2002, 85, 257–261. [Google Scholar] [CrossRef]
- Zahri, K.N.M.; Zulkharnain, A.; Sabri, S.; Gomez-fuentes, C.; Ahmad, S.A. Research trends of biodegradation of cooking oil in Antarctica from 2001 to 2021: A bibliometric analysis based on the scopus database. Int. J. Environ. Res. Public Health 2021, 18, 2050. [Google Scholar] [CrossRef] [PubMed]
- Brooksbank, A.; Latchford, J.; Mudge, S. Degradation and modification of fats, oils and grease by commercial microbial supplements degradation and modification of fats, oils and grease by commercial microbial supplements. World. J. Microbiol. Biotechnol. 2007, 23, 977–985. [Google Scholar] [CrossRef]
- Huang, L.; Ma, T.; Li, D.; Liang, F.; Liu, R.L.; Li, G. Optimization of nutrient component for diesel oil degradation by Rhodococcus erythropolis. Mar. Pollut. Bull. 2008, 56, 1714–1718. [Google Scholar] [CrossRef] [PubMed]
- Okino-Delgado, C.H.; Prado, D.Z.; do Facanali, R.; Fernandes, C.; Zambuzzi, W.F.; Fleuri, L.F. Bioremediation of cooking oil waste using lipases from wastes. PLoS ONE 2017, 12, e0186246. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, B.; Mondal, N.K.; Chattoraj, S. Optimisation using central composite design (CCD) and the desirability function for sorption of methylene blue from aqueous solution onto Lemna major. Karbala Int. J. Mod. 2016, 2, 145–155. [Google Scholar] [CrossRef]
- Sihag, S.; Pathak, H. Biodegradation of 2T engine oil using soil microbe and gravimetric analysis. IJSER 2006, 7, 1286–1295. Available online: www.pelagiaresearchlibrary.com (accessed on 20 April 2019).
- Morya, R.; Salvachúa, D.; Thakur, I.S. Burkholderia: An Untapped but Promising Bacterial Genus for the, Conversion of Aromatic Compounds. Trends Biotechnol. 2020, 38, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Manogaran, M.; Ahmad, S.A.; Yasid, N.A.; Yakasai, H.M.; Shukor, M.Y.S. Characterisation of the simultaneous molybdenum reduction and glyphosate degradation by Burkholderia vietnamiensis AQ5—12 and Burkholderia sp. AQ5—13. 3 Biotech 2018, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, J.; Nawawi, N.M.; Ibrahim, A.L. Rhodococcus UKMP-5M, an endogenous lipase producing actinomycete from Peninsular Malaysia. Biologia 2014, 69, 123–132. [Google Scholar] [CrossRef]
- Patowary, K.; Patowary, R.; Kalita, M.C.; Deka, S. Characterization of biosurfactant produced during degradation of hydrocarbons using crude oil as sole source of carbon. Front. Microbiol. 2017, 8, 279. [Google Scholar] [CrossRef]
- Bashir, M.J.K.; Aziz, H.A.; Aziz, S.Q.; Amr, S.A. An overview of wastewater treatment processes optimization using response surface methodology (RSM). In Proceedings of the 4th International Engineering Conference (IEC4), Gaza, Palestine, 15–16 October 2012; Available online: https://www.researchgate.net/profile/Shuokr-Qarani-Aziz/publication/234840337_An_Overview_of_Wastewater_Treatment_Processes_Optimization_Using_Response_Surface_Methodology_RSM/links/61280bfd2b40ec7d8bc8477c/An-Overview-of-Wastewater-Treatment-Processes-Optimization-Using-Response-Surface-Methodology-RSM.pdf (accessed on 26 September 2019).
- Roslee, A.F.A.; Gomez-Fuentes, C.; Zakaria, N.N.; Shaharuddin, N.A.; Zulkharnain, A.; Khalil, K.A.; Convey, P.; Ahmad, S.A. Growth optimisation and kinetic profiling of diesel biodegradation by a cold-adapted microbial consortium isolated from trinity peninsula, antarctica. Biology 2021, 10, 493. [Google Scholar] [CrossRef]
- Sanusu, S.N.A.; Halmi, M.I.E.; Abdullah, S.R.S.; Hassan, H.A.; Hamzah, F.M.; Idris, M. Comparative process optimization of pilot-scale total petroleum hydrocarbon (TPH) degradation by Paspalum scrobiculatum L. Hack using response surface methodology (RSM) and artificial neural networks (ANNs). Ecol. Eng. 2016, 97, 524–534. [Google Scholar] [CrossRef]
- Tong, S.; Chen, N.; Wang, H.; Liu, H.; Tao, C.; Feng, C.; Zhang, B.; Hao, C.; Pu, J.; Zhao, J. Bioresource Technology Optimization of C/N and current density in a heterotrophic/biofilm-electrode autotrophic denitrification reactor (HAD-BER). Bioresour. Technol. 2014, 171, 389–395. [Google Scholar] [CrossRef]
- Kanmani, P.; Karthik, S.; Aravind, J.; Kumaresan, K. The use of response surface methodology as a statistical tool for media optimization in lipase production from the dairy rffluent isolate Fusarium Solani. Int. Sch. Res. Not. 2013, 2013, 528708. [Google Scholar] [CrossRef]
- Reddy, L.V.A.; Wee, Y.; Yun, J.; Ryu, H. Optimization of alkaline protease production by batch culture of Bacillus sp. RKY3 through Plackett—Burman and response surface methodological approaches. Bioresour. Technol. 2008, 99, 2242–2249. [Google Scholar] [CrossRef]
- Manogaran, M.; Shukor, M.Y.; Yasid, N.A.; Ahmad, S.A. Optimisation of culture composition for glyphosate degradation by Burkholderia vietnamiensis strain AQ5-12. 3 Biotech 2008, 8, 108. [Google Scholar] [CrossRef]
- Karigar, C.; Rao, S. Role of microbial enzymes in the bioremediation of pollutants : A review. Enzym. Res. 2011, 1, 805187. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications : A comprehensive review. Microb. Cell. Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesteri fi cation of high free fatty acid oil (waste cooking oil) to biodiesel : A review. Biotechno. Adv. 2020, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Ławniczak, Ł.; Wo, M.; Heipieper, H.J. Microbial degradation of hydrocarbons—basic principles for bioremediation : A review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef] [PubMed]
- Kataria, J.; Mohapatra, S.K.; Kundu, K. Biodiesel production from waste cooking oil using heterogeneous catalysts and its operational characteristics on variable compression ratio CI engine. J. Energy Inst. 2019, 92, 275–287. [Google Scholar] [CrossRef]
- Saha, S.; Jeon, B.H.; Kurade, M.B.; Chatterjee, P.K.; Chang, S.W.; Markkandan, K.; Salama, E.S.; Govindwar, S.P.; Roh, H.S. Microbial acclimatization to lipidic-waste facilitates the efficacy of acidogenic fermentation. Chem. Eng. J. 2019, 358, 188–196. [Google Scholar] [CrossRef]
- Moxley, E.; Puerta-Fernández, E.; Gómez, E.J.; Gonzalez, J.M. Influence of Abiotic Factors Temperature and Water Content on Bacterial 2-Chlorophenol Biodegradation in Soils. Front. Environ. Sci. 2019, 7, 1–5. [Google Scholar] [CrossRef]
- Bhumibhamon, O.; Koprasertsak, A.; Funthong, S. Biotreatment of High Fat and Oil Wastewater by Lipase Producing Microorganisms. Kasetsart 2002, 36, 261–267. [Google Scholar]
- Mannu, A.; Ferro, M.; Di Pietro, M.E.; Mele, A. Innovative applications of waste cooking oil as raw material. Sci. Prog. 2019, 102, 153–160. [Google Scholar] [CrossRef]
- Rikmawati, N.A.; Mangunwardoyo, W.; Hanies, A. The use of single and mixed cultures of bacillus cereus InaCC B284 and Pseudomonas aeruginosa InaCC B290 to degrade used-cooking oil measurement of bacterial growth. In Proceedings of the 8th Annual Basic Science International Conference, Malang, Indonesia, 6–7 March 2018; pp. 1–5. [Google Scholar]
- Bharti, V.; Gupta, B.; Kaur, J. Novel bacterial strains Pseudomonas sp. And Bacillus sp. isolated from petroleum oil contaminated soils for degradation of flourene and phenanthrene. Pollution 2019, 5, 657–669. [Google Scholar] [CrossRef]
- Tanaka, D.; Takashima, M.; Mizuta, A.; Tanaka, S.; Sakatoku, A.; Nishikawa, A.; Osawa, T.; Noguchi, M.; Aizawa, S.-I.; Nakamura, S. Acinetobacter sp. Ud-4 Efficiently Degrades Both Edible and Mineral Oils : Isolation and Characterization. Curr. Microbiol. 2010, 60, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Matsumiya, Y.; Wakita, D.; Kimura, A.; Sanpa, S.; Kubo, M. Isolation and characterization of a lipid-degrading bacterium and its application to lipid-containing wastewater treatment. J. Biosci. Bioeng. 2007, 103, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Makky, E.; Azmi, N.; Ismail, J. Optimisation parameter for Mycobacteria confluentis biodegradation of PAHs. Matec Web Conf. 2018, 150, 06035. [Google Scholar] [CrossRef][Green Version]
- Galaction, A.; Ca, D.; Rotaru, R. Kinetic studies on biodegradation of lipids from olive oil mill wastewaters with free and immobilised Bacillus sp. Sci. Study Res. Chem. Chem. Eng. 2012, 13, 49–62. [Google Scholar]
- Serikovna, S.Z.; Serikovich, K.S.; Sakenovna, A.S.; Murzakhmetovich, S.; Khamitovich, A.K. Screening of lipid degrading microorganism for wastewater treatment. Malays. J. Microbiol. 2013, 9, 219–226. [Google Scholar] [CrossRef]
- Mobarak-Qamsari, E.; Kasra-Kermanshahi, R.; Moosavi-nejad, Z. Isolation and identification of a novel, lipase-producing bacterium, Pseudomnas aeruginosa KM110. Iran. J. Microbiol. 2011, 3, 92–98. [Google Scholar]
- Adetunji, A.I. Production strategies and biotechnological relevance of microbial lipases : A review. Braz. J. Microbiol. 2021, 52, 1257–1269. [Google Scholar] [CrossRef]
- Leahy, J.G.; Colwell, R.R. Microbial degradation of hydrocarbons in the environment. Microbiol. Rev. 1990, 54, 305–315. [Google Scholar] [CrossRef]
- Kamble, P.; Raut, A.; Gandhi, M. Isolation and characterization of vegetable oil degrading bacteria. J. Pure. Appl. Microbiol. 2011, 5, 887–990. [Google Scholar]
- Shon, H.K.; Tian, D.; Kwon, D.-Y.; Jin, C.; Lee, T.; Chung, W.J. Degradation of Fat, Oil, and Grease (FOGs) by Lipase-Producing Bacterium Pseudomonas sp. Strain D2D3. J. Microbiol. Biotechnol. 2002, 12, 583–591. [Google Scholar]
- Ganapathy, B.; Yahya, A.; Ibrahim, N. Bioremediation of palm oil mill effluent (POME) using indigenous Meyerozyma guilliermondii. Environ. Sci. Pollut. Res. 2012, 26, 11113–11125. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Zahri, K.N.M.; Convey, P.; Khalil, K.A.; Gomez-Fuentes, C.; Zulkarnain, A.; Alias, S.A.; González-Rocha, G.; Ahmad, S.A. Optimisation of biodegradation conditions for waste canola oil by cold-adapted Rhodococcus sp. AQ5-07 from Antarctica. Electron. J. Biotechnol. 2020, 48, 1–12. [Google Scholar] [CrossRef]
- Ajoku, G.A.; Oduola, M.K. Kinetic model of pH effect on bioremediation of crude petroleum kinetic model of pH effect on bioremediation of crude petroleum contaminated soil. 1. model development. Am. J. Chem. Eng. 2013, 1, 6–10. [Google Scholar] [CrossRef]
- Danikuu, F.M.; Sowley, E.N.K. Biodegradation of shea nut cake by indigenous soil bacteria. J. Medical. Biomedical. Sci. 2014, 3, 9–15. [Google Scholar] [CrossRef]
- Maliji, D.; Olama, Z.; Holail, H. Environmental studies on the microbial degradation of oil hydrocarbons and its application in Lebanese oil polluted coastal and marine ecosystem. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 1–18. [Google Scholar]
- Farag, S.; Soliman, N.A.; Abdel-fattah, Y.R. Statistical optimization of crude oil bio-degradation by a local marine bacterium isolate Pseudomonas sp. sp48. J. Genet. Biotechnol. 2018, 16, 409–420. [Google Scholar] [CrossRef]
- Ramadan, M.A.; El-tayeb, O.M.; Alexander, M. Inoculum size as a factor limiting success of inoculation for biodegradation. Appl. Environ. Microbiol. 1990, 56, 1392–1396. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Sofos, J.N. Effect of inoculum size on the combined temperature, pH and aw limits for growth of Listeria monocytogenes. Int. J. Food Microbiol. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Patel, G.B.; Shah, K.R.; Shindhal, T.; Rakholiya, P.; Varjani, S. Process parameter studies by central composite design of response surface methodology for lipase activity of newly obtained Actinomycete. Environ. Technol. Innov. 2021, 23, 101724. [Google Scholar] [CrossRef]
- Abdulrasheed, M.; Zulkharnain, A.; Zakaria, N.N.; Ahmad Roslee, A.F.; Khalil, K.A.; Napis, S.; Convey, P.; Gomez-Fuentes, C.; Ahmad, S.A. Response surface methodology optimization and kinetics of diesel degradation by a cold-adapted antarctic bacterium, Arthrobacter sp. strain AQ5-05. Sustainability 2020, 12, 6966. [Google Scholar] [CrossRef]
- Roslee, A.F.A.; Zakaria, N.N.; Convey, P.; Zulkharnain, A.; Lee, G.L.Y.; Gomez-Fuentes, C.; Ahmad, S.A. Statistical optimisation of growth conditions and diesel degradation by the Antarctic bacterium, Rhodococcus sp. strain AQ5-07. Extremophiles 2020, 24, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.N.; Gomez-fuentes, C.; Khalil, K.A.; Convey, P.; Fareez, A.; Roslee, A.; Zulkharnain, A.; Sabri, S.; Shaharuddin, N.A.; Leyla, C.; et al. Statistical Optimisation of Diesel Biodegradation at Low Temperatures by an Antarctic Marine Bacterial Consortium Isolated from Non-Contaminated Seawater. Microorganisms 2021, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Manogaran, M.; Yunus, M.; Nur, S.; Yasid, A. Isolation and characterisation of glyphosate-degrading bacteria isolated from local soils in Malaysia. Rend. Lincei 2017, 28, 471–479. [Google Scholar] [CrossRef]





| Parameter | Unit | Experimental Values | ||
|---|---|---|---|---|
| Low (−1) | Centre (0) | High (+1) | ||
| pH | - | 6.00 | 7.00 | 8.00 |
| Oil | %(v/v) | 1.00 | 2.00 | 3.00 |
| Temperature | C | 25.0 | 30.0 | 35.0 |
| Yeast extract | g/L | 0.50 | 1.75 | 3.00 |
| Ammonium sulphate | g/L | 0.10 | 0.55 | 1.00 |
| Source | Sum of Squares | DF | Mean Square | F Value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 2483.16 | 10 | 248.32 | 578.79 | 0.0323 | Significant |
| A | 22.20 | 1 | 22.20 | 51.74 | 0.0879 | |
| B | 6.63 | 1 | 6.63 | 15.46 | 0.1585 | |
| C | 124.88 | 1 | 124.88 | 291.08 | 0.0373 | |
| D | 489.75 | 1 | 489.75 | 1141.55 | 0.0188 | |
| E | 159.62 | 1 | 159.62 | 372.05 | 0.0330 | |
| AC | 17.27 | 1 | 17.27 | 40.26 | 0.0995 | |
| AE | 80.27 | 1 | 80.54 | 187.74 | 0.0464 | |
| BE | 4.77 | 1 | 4.77 | 11.11 | 0.1855 | |
| CD | 286.15 | 1 | 286.15 | 666.97 | 0.0246 | |
| CE | 4.71 | 1 | 4.71 | 10.97 | 0.1867 | |
| Residual | 0.43 | 1 | 0.43 | |||
| Cor Total | 2483.59 | 11 | ||||
| R2 | 0.9998 | Pred R2 | N/A | |||
| Adj R2 | 0.9981 | Adeq Precision | 62.712 |
| Source | Sum of Squares | DF | Mean Square | F Value | Prob > F | |
|---|---|---|---|---|---|---|
| Model | 1438.52 | 10 | 143.85 | 533.74 | 0.0055 | Significant |
| A - | 9.63 | 1 | 9.68 | 1339.67 | 0.0174 | |
| B - | 92.95 | 1 | 92.95 | 12,865.20 | 0.0056 | |
| C - | 12.92 | 1 | 12.92 | 1788.09 | 0.0151 | |
| D – | 23.41 | 1 | 23.41 | 3240.30 | 0.0112 | |
| E – | 92.75 | 1 | 92.75 | 12,837.62 | 0.0056 | |
| AB | 1.11 | 1 | 1.11 | 153.95 | 0.0512 | |
| BC | 77.13 | 1 | 77.13 | 10,676.00 | 0.0062 | |
| BD | 22.82 | 1 | 22.82 | 3159.16 | 0.0113 | |
| BE | 6.27 | 1 | 6.27 | 867.27 | 0.0216 | |
| CE | 107.59 | 1 | 107.59 | 14,891.32 | 0.0052 | |
| Residual | 7.225 × 10−0.33 | 1 | 0.33 | |||
| Cor Total | 1438.53 | 11 | ||||
| R2 | 1.0000 | Pred R2 | N/A | |||
| Adj R2 | 0.9999 | Adeq Precision | 440.273 |
| Name of Isolates | Degradation (%) | p-Value | Efficiency (%) | |
|---|---|---|---|---|
| Expected Value | Actual Value | |||
| B. vietnamiensis AQ5-12 | 83.5 | 85.2 | 0.068 | 98.0 |
| Burkholderia sp. AQ5-13 | 89.1 | 90.1 | 0.065 | 98.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.S.; Ahmad, S.A.; Shukor, M.Y.; Yusof, M.T. Statistical Optimisation of Used-Cooking-Oil Degradation by Burkholderia vietnamiensis AQ5-12 and Burkholderia sp. AQ5-13. Processes 2022, 10, 2178. https://doi.org/10.3390/pr10112178
Ahmed MS, Ahmad SA, Shukor MY, Yusof MT. Statistical Optimisation of Used-Cooking-Oil Degradation by Burkholderia vietnamiensis AQ5-12 and Burkholderia sp. AQ5-13. Processes. 2022; 10(11):2178. https://doi.org/10.3390/pr10112178
Chicago/Turabian StyleAhmed, Mariyam Shabeena, Siti Aqlima Ahmad, Mohd Yunus Shukor, and Mohd Termizi Yusof. 2022. "Statistical Optimisation of Used-Cooking-Oil Degradation by Burkholderia vietnamiensis AQ5-12 and Burkholderia sp. AQ5-13" Processes 10, no. 11: 2178. https://doi.org/10.3390/pr10112178
APA StyleAhmed, M. S., Ahmad, S. A., Shukor, M. Y., & Yusof, M. T. (2022). Statistical Optimisation of Used-Cooking-Oil Degradation by Burkholderia vietnamiensis AQ5-12 and Burkholderia sp. AQ5-13. Processes, 10(11), 2178. https://doi.org/10.3390/pr10112178







