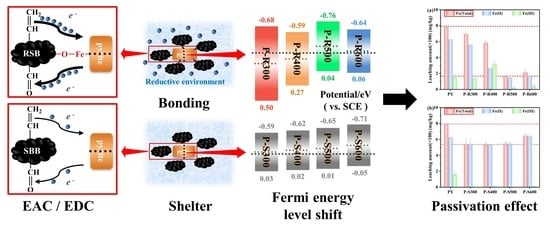
Straw Biochar at Different Pyrolysis Temperatures Passivates Pyrite by Promoting Electron Transfer from Biochar to Pyrite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pyrite Pretreatment
2.2. Preparation of Biochar Materials
2.3. Coating Processing
2.4. Testing and Analysis
2.5. Preparation of Pyrite and Composite Working Electrode
3. Results and Discussion
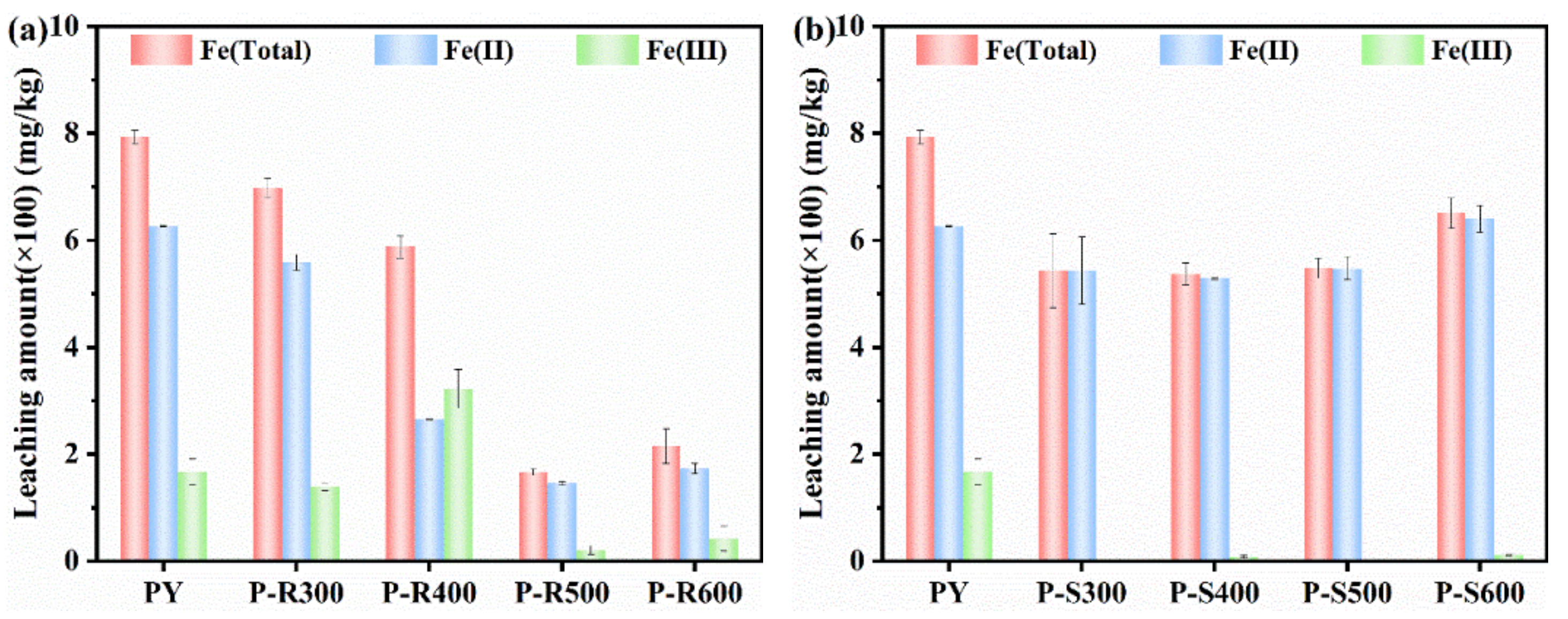
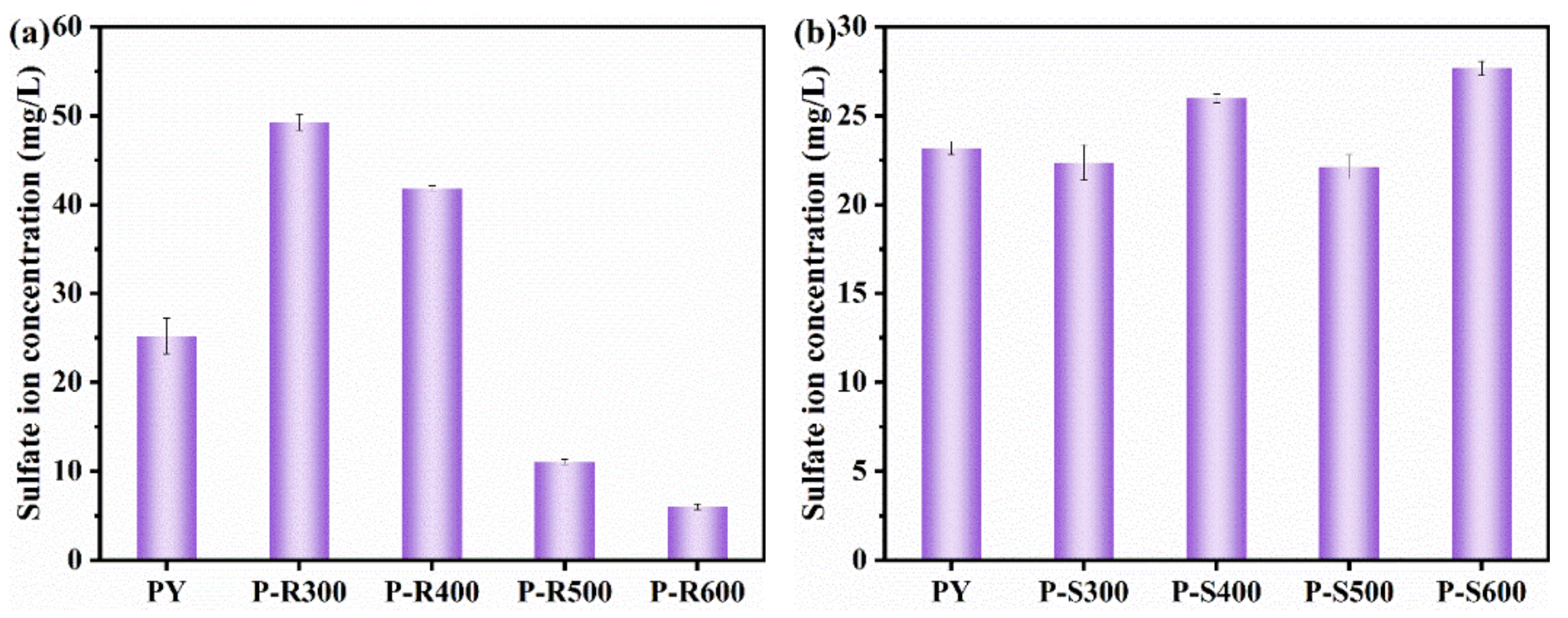
3.1. Ion Leaching of Pyrite
3.2. The Bonding Mechanism between Pyrite and Biochar
3.3. Effects of Electron Transfer of Biochar and the Band Structure of Pyrite on Passivation
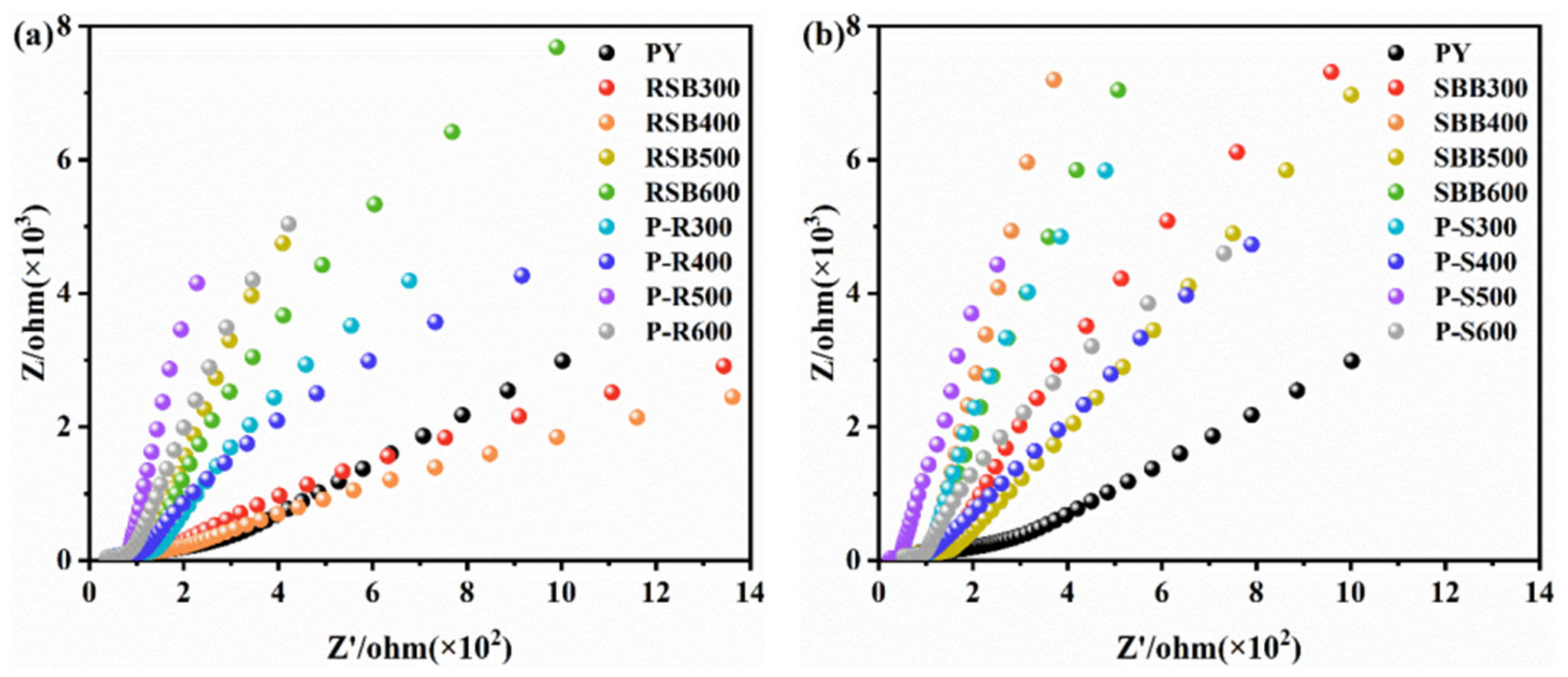
3.3.1. Effect of Electron Transfer of Biochar on Passivation
3.3.2. Effect of the Energy-Band Structure of Pyrite on Passivation
3.3.3. The Passivation Mechanism between Biochar and Pyrite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibert, O.; de Pablo, J.; Luis Cortina, J.; Ayora, C. Chemical characterisation of natural organic substrates for biological mitigation of acid mine drainage. Water Res. 2004, 38, 4186–4196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, H.; He, X.; Guo, S.; Xia, Y.; Zhou, Y.; Liu, K.; Yang, S. Research progress, problems and prospects of mine water treatment technology and resource utilization in China. Crit. Rev. Environ. Sci. Technol. 2020, 50, 331–383. [Google Scholar] [CrossRef]
- Liu, J.; Yin, M.; Zhang, W.; Tsang, D.C.W.; Wei, X.; Zhou, Y.; Xiao, T.; Wang, J.; Dong, X.; Sun, Y.; et al. Response of microbial communities and interactions to thallium in contaminated sediments near a pyrite mining area. Environ. Pollut. 2019, 248, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-J.; Ding, K.-B.; Zhang, P.; Qiu, H.; Cloquet, C.; Wen, H.-J.; Morel, J.-L.; Qiu, R.-L.; Tang, Y.-T. Cadmium stable isotope variation in a mountain area impacted by acid mine drainage. Sci. Total Environ. 2019, 646, 696–703. [Google Scholar] [CrossRef]
- Diao, Z.; Shi, T.; Wang, S.; Huang, X.; Zhang, T.; Tang, Y.; Zhang, X.; Qiu, R. Silane-based coatings on the pyrite for remediation of acid mine drainage. Water Res. 2013, 47, 4391–4402. [Google Scholar] [CrossRef]
- Fan, R.; Qian, G.; Short, M.D.; Schumann, R.C.; Brienne, S.; Smart, R.S.C.; Gerson, A.R. Passivation of pyrite for reduced rates of acid and metalliferous drainage using readily available mineralogic and organic carbon resources: A laboratory mine waste study. Chemosphere 2021, 285, 131330. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, W.; Peng, Q.; Shu, X. Stabilization and Utilization of Pyrite under Light Irradiation: Discussion of Photocorrosion Resistance. ACS Omega 2020, 5, 28693–28701. [Google Scholar] [CrossRef]
- Shu, X.; Dang, Z.; Zhang, Q.; Yi, X.; Lu, G.; Guo, C.; Yang, C. Passivation of metal-sulfide tailings by covalent coating. Miner. Eng. 2013, 42, 36–42. [Google Scholar] [CrossRef]
- Ouyang, Y.; Liu, Y.; Zhu, R.; Ge, F.; Xu, T.; Luo, Z.; Liang, L. Pyrite oxidation inhibition by organosilane coatings for acid mine drainage control. Miner. Eng. 2015, 72, 57–64. [Google Scholar] [CrossRef]
- Gong, B.; Li, D.; Niu, Z.; Liu, Y.; Dang, Z. Inhibition of pyrite oxidation using PropS-SH/sepiolite composite coatings for the source control of acid mine drainage. Environ. Sci. Pollut. Res. Int. 2021, 28, 11090–11105. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Kim, K.; Equeenuddin, S.M.; Powell, M.A. Current approaches for mitigating acid mine drainage. Rev. Environ. Contam. Toxicol. 2013, 226, 1–32. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Xu, Y. PropS-SH/SiO2 nanocomposite coatings for pyrite oxidation inhibition to control acid mine drainage at the source. J. Hazard. Mater. 2017, 338, 313–322. [Google Scholar] [CrossRef]
- Li, D.; Gong, B.; Liu, Y.; Dang, Z. Self-healing coatings based on PropS-SH and pH-responsive HNT-BTA nanoparticles for inhibition of pyrite oxidation to control acid mine drainage. Chem. Eng. J. 2021, 415, 128993. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Alhar, M.A.M.; Thompson, D.F.; Oliver, I.W. Mine spoil remediation via biochar addition to immobilise potentially toxic elements and promote plant growth for phytostabilisation. J. Environ. Manag. 2021, 277, 111500. [Google Scholar] [CrossRef]
- Qin, J.; Niu, A.; Liu, Y.; Lin, C. Arsenic in leafy vegetable plants grown on mine water-contaminated soils: Uptake, human health risk and remedial effects of biochar. J. Hazard. Mater. 2021, 402, 123488. [Google Scholar] [CrossRef]
- Penido, E.S.; Martins, G.C.; Mendes, T.B.M.; Melo, L.C.A.; do Rosário Guimarães, I.; Guilherme, L.R.G. Combining biochar and sewage sludge for immobilization of heavy metals in mining soils. Ecotoxicol. Environ. Saf. 2019, 172, 326–333. [Google Scholar] [CrossRef]
- Puga, A.P.; Melo, L.C.A.; de Abreu, C.A.; Coscione, A.R.; Paz-Ferreiro, J. Leaching and fractionation of heavy metals in mining soils amended with biochar. Soil Tillage Res. 2016, 164, 25–33. [Google Scholar] [CrossRef]
- Gasco, G.; Alvarez, M.L.; Paz-Ferreiro, J.; Mendez, A. Combining phytoextraction by Brassica napus and biochar amendment for the remediation of a mining soil in Riotinto (Spain). Chemosphere 2019, 231, 562–570. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, K.; Zhan, W.; Huang, L.; Liu, Y.; Li, T.; Yang, Z.; Liao, Q.; Chen, R.; Zhang, C.; et al. Highly effective stabilization of Cd and Cu in two different soils and improvement of soil properties by multiple-modified biochar. Ecotoxicol. Environ. Saf. 2021, 207, 111294. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, M.; Shi, J.; Zhang, C.; Zhang, J.; Rinklebe, J.; Yin, W.; Wang, S.; Wang, X. The significant role of electron donating capacity and carbon structure of biochar to electron transfer of zero-valent iron. Chemosphere 2022, 287, 132381. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, T.; Subdiaga, E.; Obst, M.; Haderlein, S.B.; Maisch, M.; Kretzschmar, R.; Angenent, L.T.; Kappler, A. Aggregation-dependent electron transfer via redox-active biochar particles stimulate microbial ferrihydrite reduction. Sci. Total Environ. 2020, 703, 135515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, J.; Pu, J.; Cai, C.; Tyson, G.W.; Yuan, Z.; Hu, S. Biochar-Mediated Anaerobic Oxidation of Methane. Environ. Sci. Technol. 2019, 53, 6660–6668. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Kim, S.; Mao, L.; Fujitsuka, M.; Zhang, J.; Wang, X.; Majima, T. Metal-Free Photocatalyst for H2 Evolution in Visible to Near-Infrared Region: Black Phosphorus/Graphitic Carbon Nitride. J. Am. Chem. Soc. 2017, 139, 13234–13242. [Google Scholar] [CrossRef]
- Islam, T.; Li, Y.; Cheng, H. Biochars and Engineered Biochars for Water and Soil Remediation: A Review. Sustainability 2021, 13, 9932. [Google Scholar] [CrossRef]
- Jeong, C.Y.; Dodla, S.K.; Wang, J.J. Fundamental and molecular composition characteristics of biochars produced from sugarcane and rice crop residues and by-products. Chemosphere 2016, 142, 4–13. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, J.; Zhang, L.; Zhong, S.; Ru, X.; Shu, X. Sulfur defect and Fe(III) (hydr)oxides on pyrite surface mediate tylosin adsorption in lake water: Effect of solution chemistry and dissolved organic matter. Environ. Sci. Pollut. Res. Int. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Bai, X.; Ma, W.; Zhang, Q.; Zhang, L.; Zhong, S.; Shu, X. Photon-induced redox chemistry on pyrite promotes photoaging of polystyrene microplastics. Sci. Total Environ. 2022, 829, 154441. [Google Scholar] [CrossRef]
- Yuan, T.; Yuan, Y.; Zhou, S.; Li, F.; Liu, Z.; Zhuang, L. A rapid and simple electrochemical method for evaluating the electron transfer capacities of dissolved organic matter. J. Soils Sediments 2011, 11, 467–473. [Google Scholar] [CrossRef]
- Bi, R.; Lu, Q.; Yu, W.; Yuan, Y.; Zhou, S. Electron transfer capacity of soil dissolved organic matter and its potential impact on soil respiration. J. Soils Sediments 2013, 13, 1553–1560. [Google Scholar] [CrossRef]
- Kong, F.; Fan, X.; Kong, A.; Zhou, Z.; Zhang, X.; Shan, Y. Covalent Phenanthroline Framework Derived FeS@Fe3C Composite Nanoparticles Embedding in N-S-Codoped Carbons as Highly Efficient Trifunctional Electrocatalysts. Adv. Funct. Mater. 2018, 28, 1803973. [Google Scholar] [CrossRef]
- Brigante, M.; Pecini, E.; Avena, M. Magnetic mesoporous silica for water remediation: Synthesis, characterization and application as adsorbent of molecules and ions of environmental concern. Microporous Mesoporous Mater. 2016, 230, 1–10. [Google Scholar] [CrossRef]
- Tang, J.; Huang, Y.; Gong, Y.; Lyu, H.; Wang, Q.; Ma, J. Preparation of a novel graphene oxide/Fe-Mn composite and its application for aqueous Hg(II) removal. J. Hazard. Mater. 2016, 316, 151–158. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. [Google Scholar] [CrossRef]
- Guo, X.; Yang, C.; Wu, Y.; Dang, Z. The influences of pH and ionic strength on the sorption of tylosin on goethite. Environ. Sci. Pollut. Res. 2014, 21, 2572–2580. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Burton, E.D.; Wang, H.; Shaheen, S.M.; Rinklebe, J.; Luttge, A. Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: An integrated spectroscopic and microscopic examination. Environ. Pollut. 2018, 232, 31–41. [Google Scholar] [CrossRef]
- Tan, X.; Zhu, S.; Show, P.L.; Qi, H.; Ho, S.H. Sorption of ionized dyes on high-salinity microalgal residue derived biochar: Electron acceptor-donor and metal-organic bridging mechanisms. J. Hazard. Mater. 2020, 393, 122435. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Corrigendum to “The thermal performance of the polysaccharides extracted from hardwood: Cellulose and hemicellulose” [Carbohydr. Polym. 82 (1) (2010) 39–34]. Carbohydr. Polym. 2011, 83, 39–45. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Liu, X.; Duan, X.; Bao, T.; Hao, D.; Chen, Z.; Wei, W.; Wang, D.; Wang, S.; Ni, B.J. High-performance photocatalytic decomposition of PFOA by BiOX/TiO2 heterojunctions: Self-induced inner electric fields and band alignment. J. Hazard. Mater. 2022, 430, 128195. [Google Scholar] [CrossRef]
- Minella, M.; Maurino, V.; Minero, C.; Pelizzetti, E. Thin Film Nanocrystalline TiO2 Electrodes: Dependence of Flat Band Potential on pH and Anion Adsorption. J. Nanosci. Nanotechnol. 2015, 15, 3348–3358. [Google Scholar] [CrossRef]
- Hofmann, O.T.; Rinke, P. Band Bending Engineering at Organic/Inorganic Interfaces Using Organic Self-Assembled Monolayers. Adv. Electron. Mater. 2017, 3, 1600373. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Fu, Z.; Hong, F.; Pang, G.; Dong, T.; Zhang, Y.; Liu, G.; Dong, X.; Wang, J. Non-metal group doped g-C3N4 combining with BiF3:Yb3+, Er3+ upconversion nanoparticles for photocatalysis in UV–Vis–NIR region. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127180. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, X.; Yu, Y.; Yao, C.; Tsang, D.C.W.; Cao, X. Evolution of redox activity of biochar during interaction with soil minerals: Effect on the electron donating and mediating capacities for Cr(VI) reduction. J. Hazard. Mater. 2021, 414, 125483. [Google Scholar] [CrossRef]
- Hou, N.; Li, X.; Jiang, X.; Zhang, N.; Wang, R.; Li, D. The role of biochar in the photocatalytic treatment of a mixture of Cr(VI) and phenol pollutants: Biochar as a carrier for transferring and storing electrons. Sci. Total Environ. 2022, 844, 157145. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Band bending in semiconductors: Chemical and physical consequences at surfaces and interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef]

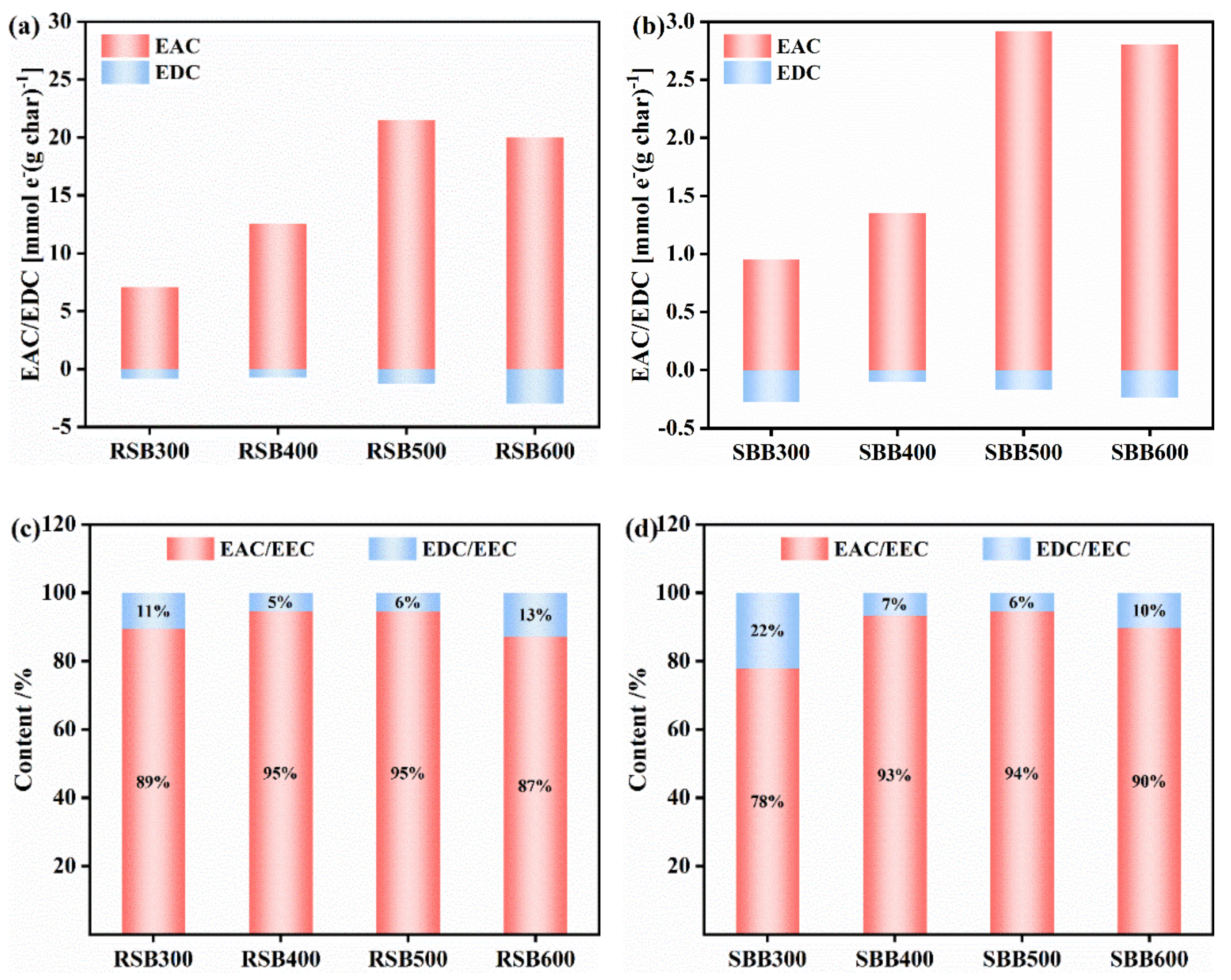

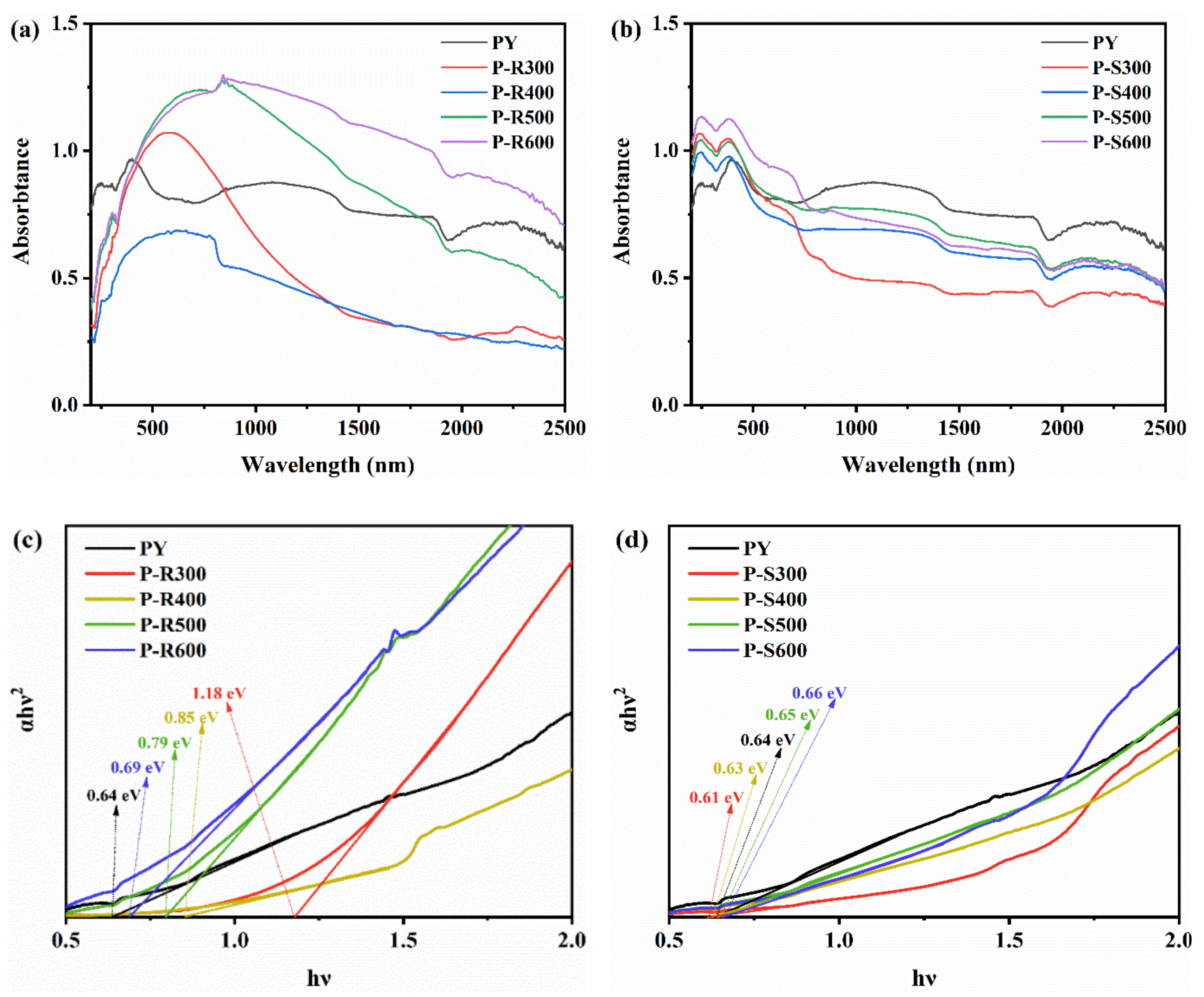
| Samples | P-R300 | P-R400 | P-R500 | P-R600 | P-S300 | P-S400 | P-S500 | P-S600 |
|---|---|---|---|---|---|---|---|---|
| Inhibition rate | 12.1% | 25.9% | 79.1% | 72.9% | 31.5% | 32.3% | 30.9% | 18.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, X.; Tian, W.; Xiong, S.; Zhang, W.; Zhang, Q. Straw Biochar at Different Pyrolysis Temperatures Passivates Pyrite by Promoting Electron Transfer from Biochar to Pyrite. Processes 2022, 10, 2148. https://doi.org/10.3390/pr10102148
Shu X, Tian W, Xiong S, Zhang W, Zhang Q. Straw Biochar at Different Pyrolysis Temperatures Passivates Pyrite by Promoting Electron Transfer from Biochar to Pyrite. Processes. 2022; 10(10):2148. https://doi.org/10.3390/pr10102148
Chicago/Turabian StyleShu, Xiaohua, Wei Tian, Shiqing Xiong, Wenlong Zhang, and Qian Zhang. 2022. "Straw Biochar at Different Pyrolysis Temperatures Passivates Pyrite by Promoting Electron Transfer from Biochar to Pyrite" Processes 10, no. 10: 2148. https://doi.org/10.3390/pr10102148
APA StyleShu, X., Tian, W., Xiong, S., Zhang, W., & Zhang, Q. (2022). Straw Biochar at Different Pyrolysis Temperatures Passivates Pyrite by Promoting Electron Transfer from Biochar to Pyrite. Processes, 10(10), 2148. https://doi.org/10.3390/pr10102148