Preparation of Calcined Kaolin by Efficient Decarburization of Coal-Series Kaolinite in a Suspended Bed Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. TG–IR Experiments
2.3. Kinetic Methods
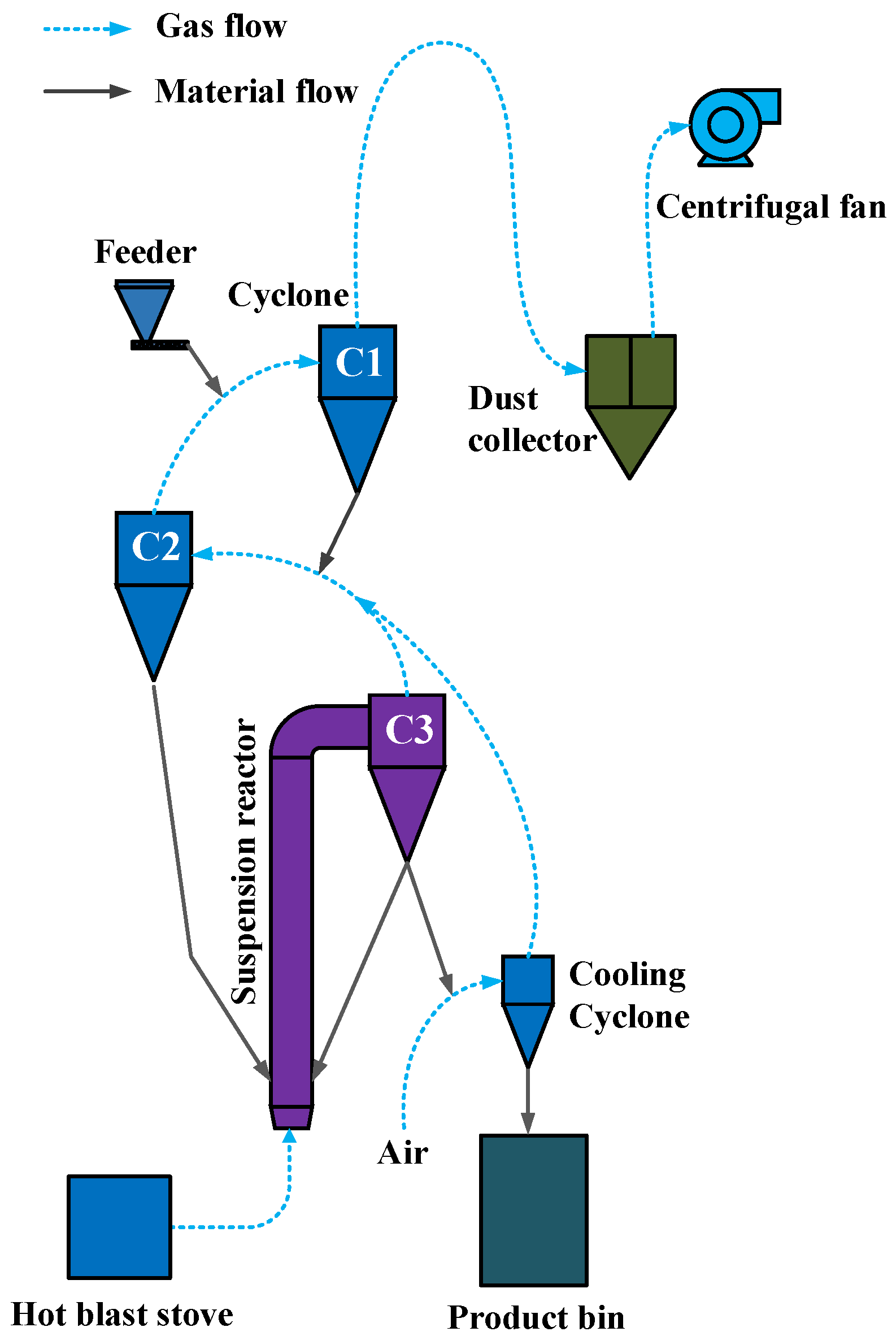
2.4. Pilot System for Suspension Calcination
2.5. Characterization of Calcined Kaolin
3. Results and Discussions
3.1. Thermal Analysis
3.2. IR Spectral Analysis
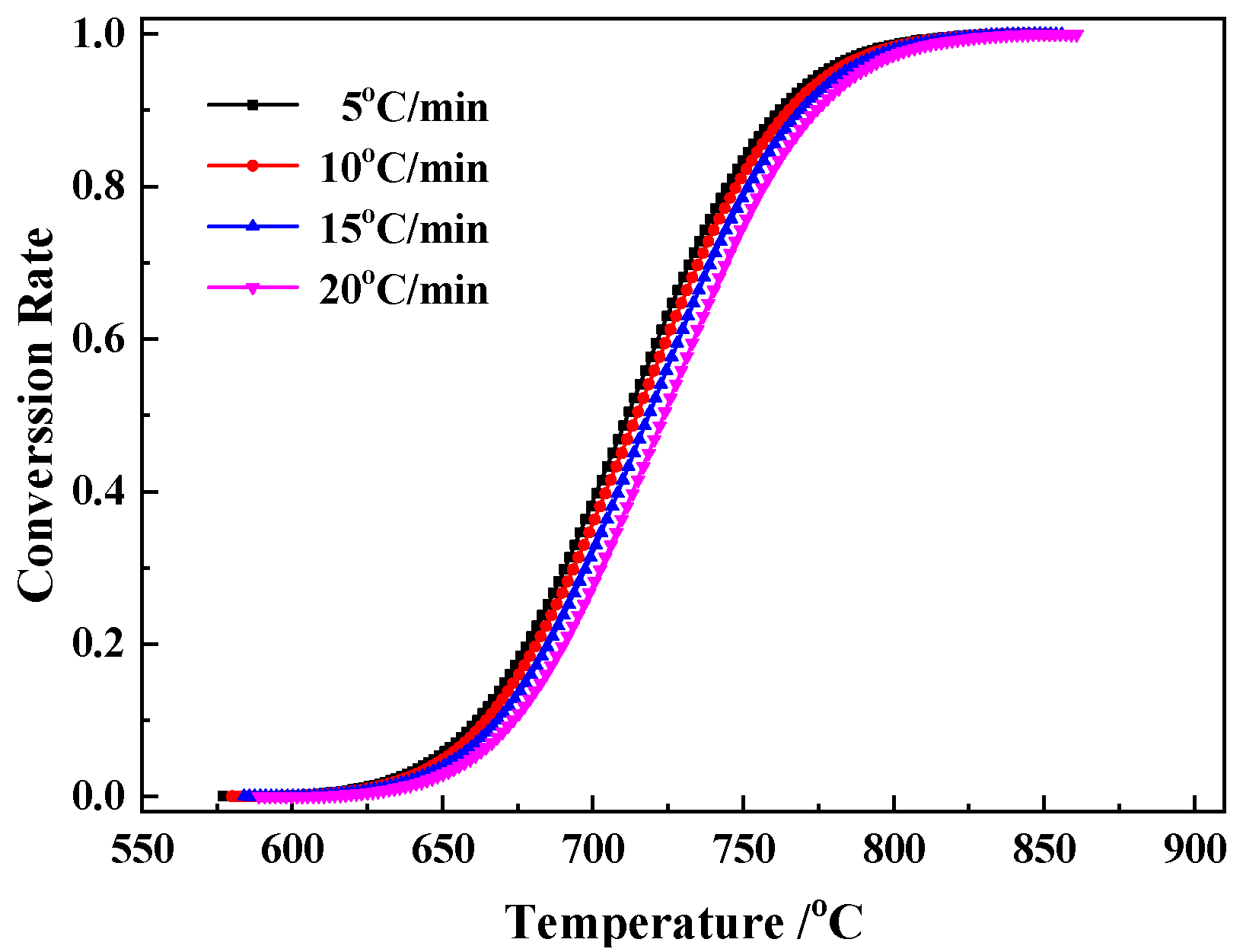
3.3. Kinetic Analysis
3.4. Product Characterization
3.4.1. Carbon Content Analysis
3.4.2. XRD Analysis
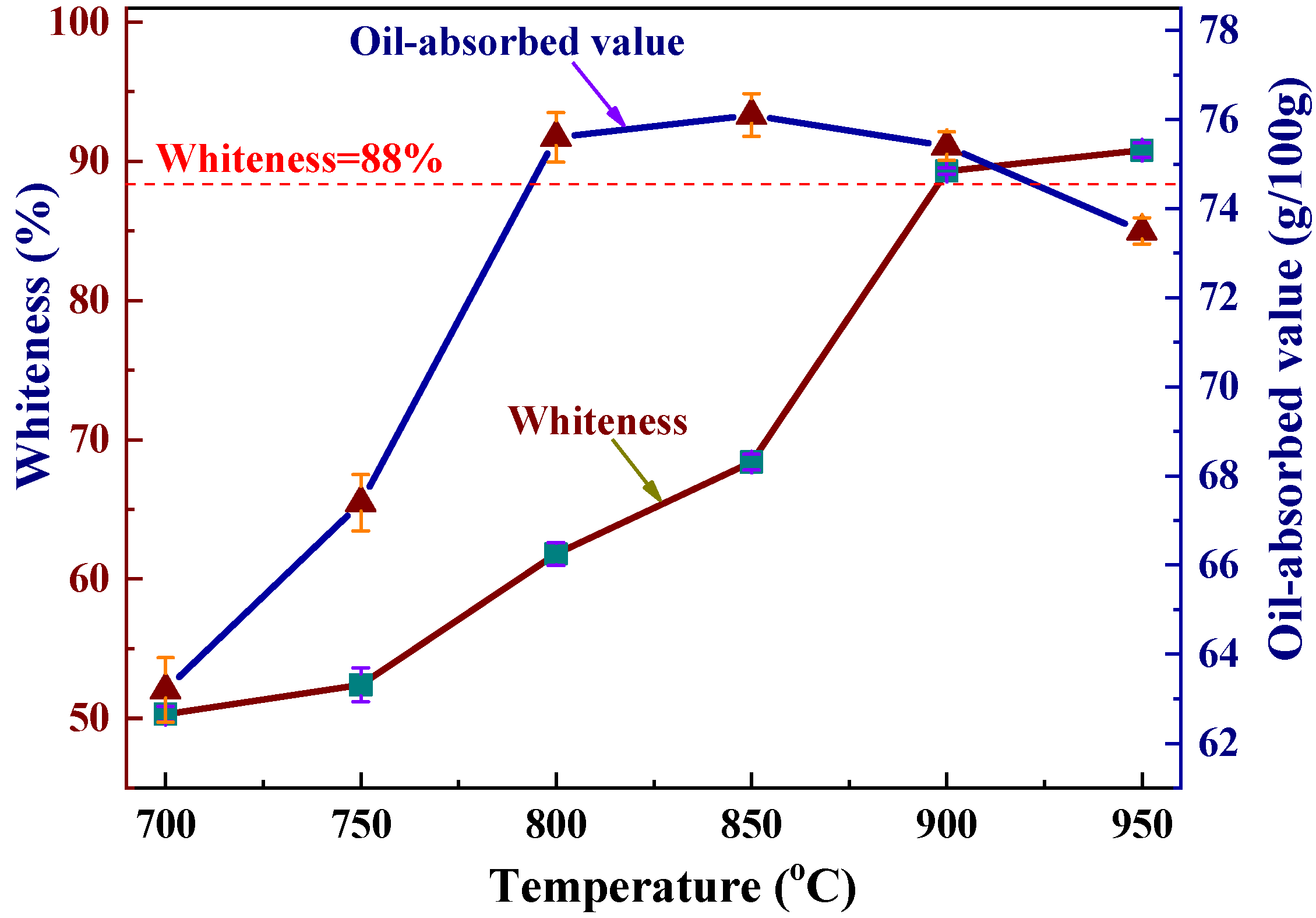
3.4.3. Analysis of Whiteness and Oil-Absorbed Value
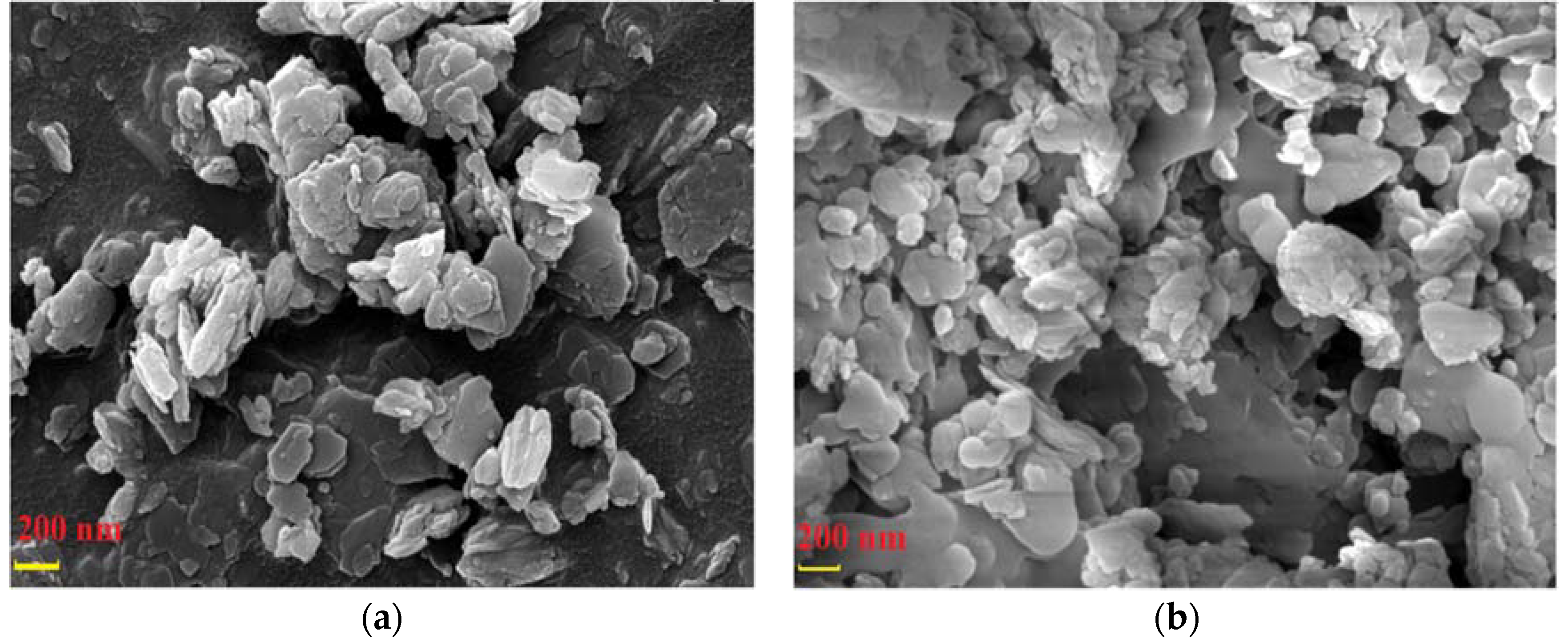
3.4.4. SEM Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosseini, M.; Ahmadi, A. Biological beneficiation of kaolin: A review on iron removal. Appl. Clay Sci. 2015, 107, 238–245. [Google Scholar] [CrossRef]
- Vesely, D.; Kalendova, A.; Manso, M. Properties of calcined kaolins in anticorrosion paints depending on PVC, chemical composition and shape of particles. Prog. Org. Coat. 2012, 74, 82–91. [Google Scholar] [CrossRef]
- Morsy, F.; El-Sherbiny, S.; Hassan, M.S.; Mohammed, H.F. Modification and evaluation of Egyptian kaolinite as pigment for paper coating. Powder Technol. 2014, 264, 430–438. [Google Scholar] [CrossRef]
- Buyondo, A.; Kasedde, H.; Kirabira, J. A comprehensive review on kaolin as pigment for paint and coating: Recent trends of chemical-based paints, their environmental impacts and regulation. Case Stud. Chem. Environ. Eng. 2022, 6, 100244. [Google Scholar] [CrossRef]
- Balard, H.; Papirer, E. Characterization and modification of fillers for paints and coatings. Prog. Org. Coat. 1993, 22, 1–17. [Google Scholar] [CrossRef]
- Ahmed, N. Comparative study on the role of kaolin, calcined kaolin and chemically treated kaolin in alkyd-based paints for protection of steel. Pigment Resin Technol. 2013, 42, 3–14. [Google Scholar] [CrossRef]
- He, J.; Yao, Y.; Lu, W.; Long, G.; Bai, Q.; Wang, H. Cleaning and upgrading of coal-series kaolin fines via decarburization using triboelectric separation. J. Clean. Prod. 2019, 228, 956–964. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Y.; Han, Y.; Gao, P. Effects of carbonaceous matter additives on kinetics, phase and structure evolution of coal-series kaolin during calcination. Appl. Clay Sci. 2018, 165, 124–134. [Google Scholar] [CrossRef]
- Kogel, J.; Trivedi, N.; Barker, J.; Krukowski, S. Industrial Minerals & Rocks: Commodities, Markets, and Uses, 7th ed.; Society for Mining, Metallurgy, and Exploration, Inc. (SME): Littleton, CO, USA, 2006; pp. 35–48. [Google Scholar]
- Bish, D. Rietveld refinement of the kaolinite structure at 1.5 K. Clays Clay Miner. 1993, 41, 738–744. [Google Scholar] [CrossRef]
- Pan, F.; Lu, X.; Wang, T.; Wang, Y.; Zhang, Z.; Yan, Y.; Yang, S. Synthesis of large-mesoporous γ-Al2O3 from coal-series kaolin at room temperature. Mater. Lett. 2013, 91, 136–138. [Google Scholar] [CrossRef]
- Chen, P.; Wu, J.; Liu, R.; Chen, A.; Chen, Y.; Shi, Y.; Li, C.; Zhang, K.; Gui, D.; Wang, Y. A low-cost and simple approach to prepare mullite ceramics via DCC-HVCI using ammonia as dispersant. Ceram. Int. 2018, 44, 21779–21785. [Google Scholar] [CrossRef]
- Huang, T.; Lei, S.; Liu, M.; Ji, Y.; Liu, X.; Peng, Y. Dry separation of iron minerals from low-grade coal-series kaolin. J. Wuhan Univ. Technol. Mater. Sci. Edit. 2015, 30, 935–940. [Google Scholar] [CrossRef]
- Gámiz, E.; Melgosa, M.; Sánchez-Maraňón, M.; Martín-García, J.; Delgado, R. Relationships between chemico-mineralogical composition and color properties in selected natural and calcined Spanish kaolins. Appl. Clay Sci. 2005, 28, 269–282. [Google Scholar] [CrossRef]
- Saikia, N.; Bharali, D.; Sengupta, P.; Bordoloi, D.; Goswamee, R.; Saikia, P.; Borthakur, P. Characterization, beneficiation and utilization of a kaolinite clay from Assam, India. Appl. Clay Sci. 2003, 24, 93–103. [Google Scholar] [CrossRef]
- Xu, X.; Lao, X.; Wu, J.; Zhang, Y.; Xu, X.; Li, K. Microstructural evolution, phase transformation, and variations in physical properties of coal series kaolin powder compact during firing. Appl. Clay Sci. 2015, 115, 76–86. [Google Scholar] [CrossRef]
- Chen, C.; Lan, G.; Tuan, W. Microstructural evolution of mullite during the sintering of kaolin powder compacts. Ceram. Int. 2000, 26, 715–720. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, S.; Lin, M.; Li, Y.; Ye, Z.; Fan, Y. Assessment of pozzolanic activity of calcined coal-series kaolin. Appl. Clay Sci. 2017, 143, 159–167. [Google Scholar] [CrossRef]
- Souri, A.; Golestani-Fard, F.; Naghizadeh, R.; Veiseh, S. An investigation on pozzolanic activity of Iranian kaolins obtained by thermal treatment. Appl. Clay Sci. 2015, 103, 34–39. [Google Scholar] [CrossRef]
- Sun, T.; Ge, K.; Wang, G.; Geng, H.; Shui, Z.; Cheng, S.; Chen, M. Comparing pozzolanic activity from thermal-activated water-washed and coal-series kaolin in Portland cement mortar Construct. Build. Mater. 2019, 227, 117092. [Google Scholar] [CrossRef]
- Cheng, S.; Jiu, S.; Li, H. Kinetics of Dehydroxylation and Decarburization of Coal Series Kaolinite during Calcination: A Novel Kinetic Method Based on Gaseous Products. Materials 2021, 14, 1493. [Google Scholar] [CrossRef]
- Grigore, M.; Sakurovs, R.; French, D.; Sahajwalla, V. Mineral matter in coals and their reactions during coking. Int. J. Coal Geol. 2008, 76, 301–308. [Google Scholar] [CrossRef]
- Budrugeac, P. On the use of the model-free way method for kinetic analysis of thermoanalytical data–advantages and limitations. Thermochim. Acta 2021, 706, 179063. [Google Scholar] [CrossRef]
- John, P.; Patrick, K. Kinetic analyses using simultaneous TG/DSC measurements: Part I: Decomposition of calcium carbonate in argon. Thermochim. Acta 2002, 388, 115–128. [Google Scholar]
- Zhao, Y.; Zhang, J.; Sun, T.; Shan, Y.; Zhao, H. Reduced equations to estimate kinetic parameters from non-isothermal TG-DTG or DSC curves. Thermochim. Acta 1993, 223, 101–108. [Google Scholar]
- Jiu, S.; Zhao, B.; Chen, Y. High-Efficiency Desulfurization of High-Sulfur Bauxite Calcined in a Conveyor Bed: Kinetics, Process, and Application. Processes 2022, 10, 1586. [Google Scholar] [CrossRef]
- Henderson, J.; Tant, M.; Moore, G.; Wiebelt, J. Determination of kinetic parameters for the thermal decomposition of phenolic ablative materials by a multiple heating rate method. Thermochim. Acta 1981, 44, 253–264. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C. Isothermal and nonisothermal kinetics of thermally stimulated reactions of solids. Int. Rev. Phys. Chem. 1998, 3, 407–433. [Google Scholar] [CrossRef]
- Blaine, R.; Kissinger, H. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Juan, J.; Francisco, P.; Guillermo, R. On the integration of kinetic models using a high-order Taylor series method. J. Chemom. 1992, 6, 231–246. [Google Scholar]
- Hu, R.; Shi, Q. Thermal Analysis Kinetics, 1st ed.; Science Press: Beijing, China, 2001; pp. 127–154. [Google Scholar]
- Cejnar, M.; Kobler, H.; Hunyor, S. Quantitative photoplethysmography: Lambert-Beer law or inverse function incorporating light scatter. J. Biomed. Phys. Eng. 1993, 15, 151–154. [Google Scholar] [CrossRef]







| Elements | SiO2 | Al2O3 | CaO | TiO2 | Fe2O3 | K2O | MgO | SO3 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| Content | 45.26 | 36.74 | 2.21 | 1.21 | 0.65 | 0.12 | 0.07 | 0.06 | 0.10 |
| Chemical Composition | Kaolinite | Boehmite | Quartz | Rutile | Hematite | Coal |
|---|---|---|---|---|---|---|
| Percentage content | 87.32 | 5.70 | 2.37 | 1.35 | 0.36 | 2.78 |
| Serial Number of G(α) | Correlation Coefficient r | Activation Energy E (kJ/mol) | Reaction Mechanism | Order |
|---|---|---|---|---|
| 29 | 0.996479 | 214.56 | Surface reaction rate controlling mechanism | 1 |
| 30 | 0.995928 | 215.75 | 2 | |
| 31 | 0.993684 | 201.76 | 3 | |
| 32 | 0.993635 | 201.77 | 4 | |
| 15 | 0.998703 | 182.78 | Nucleation and growth rate controlling mechanism | 5 |
| Method | Heating Rate (°C/min) | Activation Energy E (kJ/mol) | Preexponential Factor A (s−1) | Correlation Coefficient r |
|---|---|---|---|---|
| General integral method | 5 | 210.62 | 2.79 × 109 | 0.995259 |
| 10 | 211.21 | 1.56 × 109 | 0.995328 | |
| 15 | 204.70 | 4.88 × 108 | 0.994881 | |
| 20 | 217.39 | 1.57 × 109 | 0.996899 | |
| Average value | 214.56 | 1.60 × 109 | 0.996479 | |
| Kissinger method | 216.35 | —— | 0.995554 |
| Temperature (°C) | 700 | 750 | 800 | 850 | 900 | 950 |
|---|---|---|---|---|---|---|
| Time (s) | 205.6 | 56.1 | 17.4 | 5.9 | 2.2 | 0.91 |
| Temperature (°C) | Al2O3 (wt.%) | C (wt.%) | Decarburization Rate (%) | |
|---|---|---|---|---|
| Experimental Data | Forecast Data | |||
| Raw ore | 36.74 | 2.78 | 0.00 | 0.00 |
| 700 | 37.79 | 2.6318 | 7.97 | 4.74 |
| 755 | 38.35 | 2.3105 | 20.37 | 16.59 |
| 800 | 41.36 | 1.5839 | 49.39 | 46.85 |
| 850 | 42.35 | 0.2726 | 91.49 | 91.14 |
| 900 | 42.63 | 0.0023 | 99.93 | 100.0 |
| 950 | 42.70 | 0.0007 | 99.98 | 100.0 |
| Temperature (°C) | 700 | 750 | 800 | 850 | 900 | 950 |
|---|---|---|---|---|---|---|
| Specific surface area (m2/kg) | 17.52 | 18.79 | 20.19 | 20.39 | 20.17 | 19.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Jiu, S.; Li, H. Preparation of Calcined Kaolin by Efficient Decarburization of Coal-Series Kaolinite in a Suspended Bed Reactor. Processes 2022, 10, 2048. https://doi.org/10.3390/pr10102048
Cheng S, Jiu S, Li H. Preparation of Calcined Kaolin by Efficient Decarburization of Coal-Series Kaolinite in a Suspended Bed Reactor. Processes. 2022; 10(10):2048. https://doi.org/10.3390/pr10102048
Chicago/Turabian StyleCheng, Simeng, Shaowu Jiu, and Hui Li. 2022. "Preparation of Calcined Kaolin by Efficient Decarburization of Coal-Series Kaolinite in a Suspended Bed Reactor" Processes 10, no. 10: 2048. https://doi.org/10.3390/pr10102048
APA StyleCheng, S., Jiu, S., & Li, H. (2022). Preparation of Calcined Kaolin by Efficient Decarburization of Coal-Series Kaolinite in a Suspended Bed Reactor. Processes, 10(10), 2048. https://doi.org/10.3390/pr10102048






