Optimization of Total Saponin Extraction from Polyscias fruticosa Roots Using the Ultrasonic-Assisted Method and Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Samples
2.2. Extraction Process
2.3. Total Saponin Content Determination
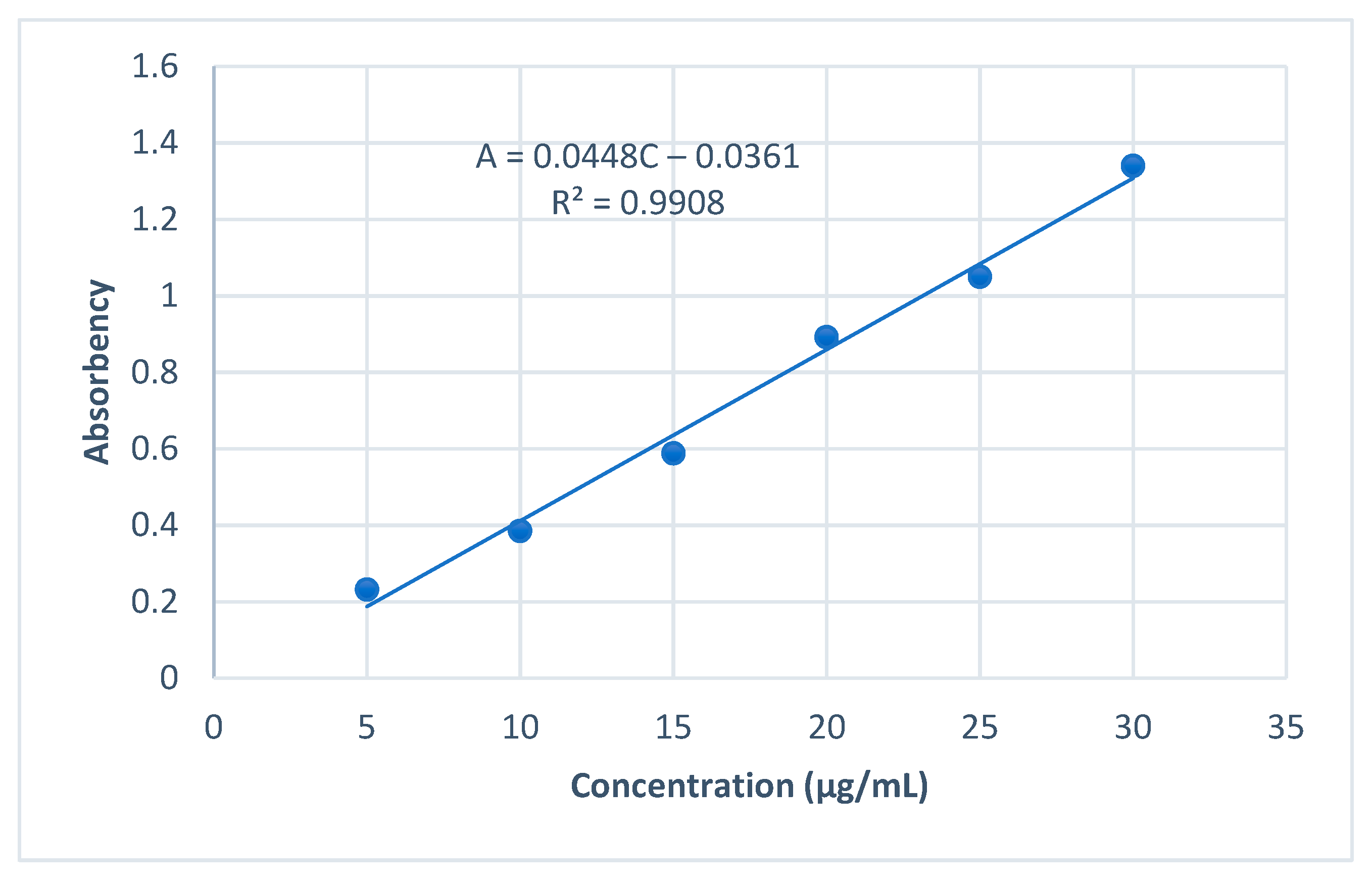
2.3.1. The Standard Curve
2.3.2. Determination of TSC
2.4. Extraction Yield
2.5. Experiment Design and Optimization
2.6. Cytotoxicity Assays
2.7. Statistical Analysis
3. Results and Discussion
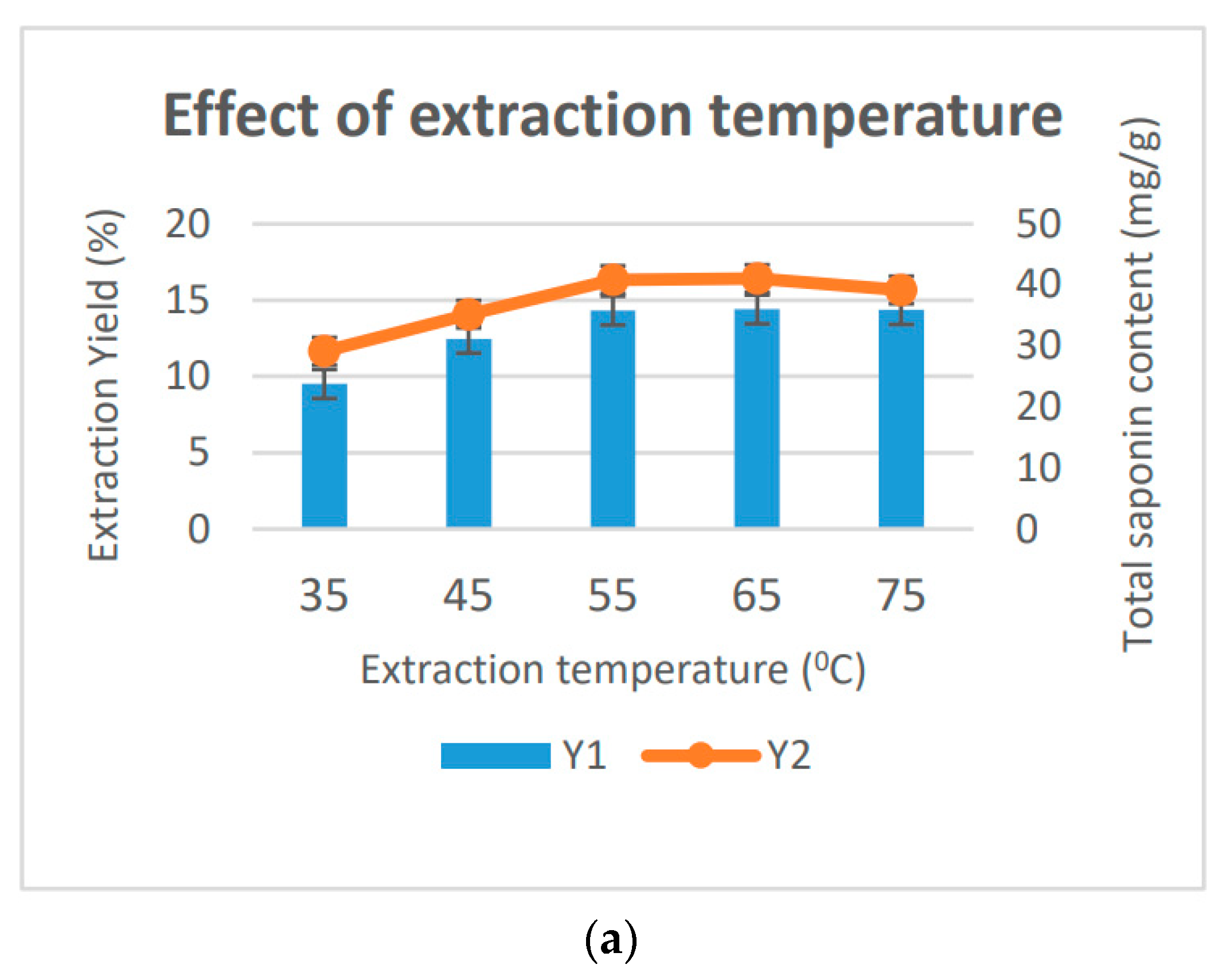
3.1. Optimization of the Saponin Extraction Process
3.2. Statistical Prediction and Analysis Model
3.3. Response Surfaces Analysis
3.4. Cytotoxic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vo, V.C. Dictionary of Vietnamese Medicinal Plants; Publishing House Medicine: Ho Chi Minh City, Vietnam, 1997. [Google Scholar]
- Nguyen, T.-T.-D.; Nguyen, Q.-D.; Nguyen, T.-V.-L. Kinetic Study on Chlorophyll and Antioxidant Activity from Polyscias Fruticosa (L.) Harms Leaves via Microwave-Assisted Extraction. Molecules 2021, 26, 3761. [Google Scholar] [CrossRef]
- Huan, V.D.; Yamamura, S.; Ohtani, K.; Kasai, R.; Yamasaki, K.; Nham, N.T.; Chau, H.M. Oleanane Saponins from Polyscias fruticosa. Phytochemistry 1998, 47, 451–457. [Google Scholar] [CrossRef]
- Lutomski, J.; Luan, T.C. Polyacetylenes in the Araliaceae family. Part III. Herba Pol. 1992, 2, 53–61. [Google Scholar]
- Nguyen, T.T.H.; Luong, K.B. Research on anti-depressant and anti-stress effects of Polyscias fruticosa. J. Med. Mater. 2001, 6, 84–86. [Google Scholar]
- Nguyen, T.T.H.; Nguyen, T.A.N. Study on hepatoprotective effects of Dinh Lang based on the mechanism of antioxidant effect. J. Med. Mater. 2004, 9, 85–89. [Google Scholar]
- Nguyen, T.T.H.; Tran, T.M.X. Memory-improving effect of alcohol extract from Dinh lang leaves (Polyscias fruticosa L. Harms, Araliaceae). Ho Chi Minh City J. Med. 2008, 12, 170–175. [Google Scholar]
- Boye, A.; Osei Owusu, A.; Koffuor, G.; Barku, V.; Asiamah, E.; Asante, E. Assessment of Polyscias fruticosa (L.) Harm (Araliaceae) Leaf Extract on Male Fertility in Male Wistar Rats. J. Intercult. Ethnopharmacol. 2018, 1, 45–56. [Google Scholar] [CrossRef]
- Hanh, T.T.H.; Dang, N.H.; Dat, N.T. α-Amylase and α-Glucosidase Inhibitory Saponins from Polyscias Fruticosa Leaves. J. Chem. 2016, 2016, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Ly, H.T.; Nguyen, T.T.H.; Le, V.M.; Lam, B.T.; Mai, T.T.T.; Dang, T.P.T. Therapeutic Potential of Polyscias fruticosa (L.) Harms Leaf Extract for Parkinson’s Disease Treatment by Drosophila Melanogaster Model. Oxidat. Med. Cell. Longev. 2022, 2022, 1–18. [Google Scholar] [CrossRef]
- Bensita, M.B.; Nilani, P.; Sandhya, S.M. Studies on the Adaptogenic and Antibacterial Properties of Polyscias fructicosa (L.) Harms. Anc. Sci. Life 1999, 18, 231–246. [Google Scholar]
- Do, V.M.; Tran, C.L.; Nguyen, T.P. Polysciosides J and K, Two New Oleanane-Type Triterpenoid Saponins from the Leaves of Polyscias fruticosa (L.) Harms. Cultivating in An Giang Province, Viet Nam. Nat. Prod. Res. 2020, 34, 1250–1255. [Google Scholar] [CrossRef]
- Bernard, B.M.; Pakianathan, N.; Venkataswamy, R.; Divakar, M.C. A Pharmacognostic Report on the Leaf and Root of Polyscias fruticosa (L.) Harms. Anc. Sci. Life 1998, 18, 165–172. [Google Scholar] [PubMed]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical Study and Medical Application of Saponins as Anti-Cancer Agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef]
- Peng, L.; Hu, C.; Zhang, C.; Lu, Y.; Man, S.; Ma, L. Anti-Cancer Activity of Conyza Blinii Saponin against Cervical Carcinoma through MAPK/TGF-β/Nrf2 Signaling Pathways. J. Ethnopharmacol. 2020, 251, 112503. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.C.-S.; Wong, W.-Y.; Lee, M.M.-L.; Chan, B.D.; Lu, C.; Hsiao, W.-L.W. Mechanistic Study of the Anti-Cancer Effect of Gynostemma pentaphyllum Saponins in the Apc Min/+ Mouse Model. Proteomics 2016, 16, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; He, W.; He, Y. Gleditsia Saponin C Induces A549 Cell Apoptosis via Caspase-Dependent Cascade and Suppresses Tumor Growth on Xenografts Tumor Animal Model. Front. Pharmacol. 2018, 8, 988. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.-L.; Huang, X.-Y.; Qiu, H.-C.; Gan, R.-Z.; Zhou, H.; Zhu, H.-Q.; Zhang, X.-X.; Lu, G.-D.; Liang, G. SSPH I, a Novel Anti-Cancer Saponin, Inhibits Autophagy and Induces Apoptosis via ROS Accumulation and ERK1/2 Signaling Pathway in Hepatocellular Carcinoma Cells. Onco. Targets Ther. 2020, 13, 5979–5991. [Google Scholar] [CrossRef]
- Chen, P.-S.; Shih, Y.-W.; Huang, H.-C.; Cheng, H.-W. Diosgenin, a Steroidal Saponin, Inhibits Migration and Invasion of Human Prostate Cancer PC-3 Cells by Reducing Matrix Metalloproteinases Expression. PLoS ONE 2011, 6, e20164. [Google Scholar] [CrossRef] [Green Version]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The Effects of Ultrasound Assisted Extraction on Yield, Antioxidant, Anticancer and Antimicrobial Activity of Polyphenol Extracts: A Review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Marti-Quijal, F.J.; Barba, F.J.; Altintas, Z. Current Emerging Trends in Antitumor Activities of Polysaccharides Extracted by Microwave- and Ultrasound-Assisted Methods. Int. J. Biol. Macromol. 2022, 202, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Loan, N.T.T.; Long, D.T.; Yen, P.N.D.; Hanh, T.T.M.; Pham, T.N.; Pham, D.T.N. Purification Process of Mangiferin from Mangifera Indica, L. Leaves and Evaluation of Its Bioactivities. Processes 2021, 9, 852. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Ma, Y.; Wang, Z.; Wang, T.; Xie, Y. Optimization of Taxol Extraction Process Using Response Surface Methodology and Investigation of Temporal and Spatial Distribution of Taxol in Taxus Mairei. Molecules 2021, 26, 5485. [Google Scholar] [CrossRef]
- Pham, D.-C.; Nguyen, H.-C.; Nguyen, T.-H.L.; Ho, H.-L.; Trinh, T.-K.; Riyaphan, J.; Weng, C.-F. Optimization of Ultrasound-Assisted Extraction of Flavonoids from Celastrus Hindsii Leaves Using Response Surface Methodology and Evaluation of Their Antioxidant and Antitumor Activities. BioMed Res. Int. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Detti, C.; dos Santos Nascimento, L.B.; Brunetti, C.; Ferrini, F.; Gori, A. Optimization of a Green Ultrasound-Assisted Extraction of Different Polyphenols from Pistacia lentiscus, L. Leaves Using a Response Surface Methodology. Plants 2020, 9, 1482. [Google Scholar] [CrossRef]
- Tlili, H.; Macovei, A.; Buonocore, D.; Lanzafame, M.; Najjaa, H.; Lombardi, A.; Pagano, A.; Dossena, M.; Verri, M.; Arfa, A.B.; et al. The Polyphenol/Saponin-Rich Rhus Tripartita Extract Has an Apoptotic Effect on THP-1 Cells through the PI3K/AKT/MTOR Signaling Pathway. BMC Complement. Med. Ther. 2021, 21, 153. [Google Scholar] [CrossRef]
- Hasballah, K.; Sarong, M.; Rusly, R.; Fitria, H.; Maida, D.R.; Iqhrammullah, M. Antiproliferative Activity of Triterpenoid and Steroid Compounds from Ethyl Acetate Extract of Calotropis Gigantea Root Bark against P388 Murine Leukemia Cell Lines. Sci. Pharm. 2021, 89, 21. [Google Scholar] [CrossRef]
- Park, K.-R.; Leem, H.H.; Lee, J.; Kwon, I.K.; Hong, J.T.; Yun, H.-M. Anti-Cancer Effects of Hederoside C, a Pentacyclic Triterpene Saponin, through the Intrinsic Apoptosis and STAT3 Signaling Pathways in Osteosarcoma. Am. J. Cancer Res. 2021, 11, 4541–4550. [Google Scholar] [PubMed]
- Hemanth, K.M.; Sunil, K.J.; Spandana, V.; Sandeep, B.P. Anticancer Activity of Terpenoid Saponin Extract of Psidium Guajava on MCF-7 Cancer Cell Line Using DAPI and MTT Assays. Afr. J. Pharm. Pharmacol. 2021, 15, 206–211. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken Design: An Alternative for the Optimization of Analytical Methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Zhaobao, X.; Chunhong, T.; Gang, C.; Zhisong, S. Studied on corlorimetric determination of oleanolic acid in Chinese quince. Nat. Prod. Res. Dev. 2001, 13, 23–26. [Google Scholar]
- Tran, Q.T.; Pham, T.D.; Nguyen, T.D.; Luu, V.H.; Nghi Do, H.; Duy Le, X.; Nguyen, P.H.; Nguyen, M.C.; Luu, V.C.; Pham, M.Q.; et al. Optimization of Murrayafoline A Ethanol Extraction Process from the Roots of Glycosmis Stenocarpa, and Evaluation of Its Tumorigenesis Inhibition Activity on Hep-G2 Cells. Open Chem. 2021, 19, 830–842. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, T.; Wang, S.; Shen, C.; Yan, S.; Song, Z.; Liu, Y.; Yang, F.; Hai, P.; Li, Y. Separation of a New Triterpenoid Saponin Together with Six Known Ones from Clematis Tangutica (Maxim.) Korsh and Evaluation of Their Cytotoxic Activities. Nat. Prod. Res. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated Solvent Extraction: A Technique for Sample Preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Anh, T.T.; Thanh Huyen, N.T.; Lam, T.D. Effect of Extracting Process on Saponin Content and Antioxidant Activity of Gleditschia fera (Lour.) Merr Dried Fruit Extract. IOP Conf. Ser. Mater. Sci. Eng. 2019, 544, 012026. [Google Scholar] [CrossRef]
- Muhammad, U.; Zhu, X.; Lu, Z.; Han, J.; Sun, J.; Tayyaba, S.; Abbasi, B.; Ali Siyal, F.; Dhama, K.; Saqib, J. Effects of Extraction Variables on Pharmacological Activities of Vine Tea Extract (Ampelopsis Grossedentata). Int. J. Pharmacol. 2018, 14, 495–505. [Google Scholar] [CrossRef]
- Yan, F.; Fan, K.; He, J.; Gao, M. Ultrasonic-Assisted Solvent Extraction of Carotenoids from Rapeseed Meal: Optimization Using Response Surface Methodology: Extraction of Carotenoids. J. Food Qual. 2015, 38, 377–386. [Google Scholar] [CrossRef]
- Almusallam, I.A.; Mohamed Ahmed, I.A.; Babiker, E.E.; Al Juhaimi, F.Y.; Fadimu, G.J.; Osman, M.A.; Al Maiman, S.A.; Ghafoor, K.; Alqah, H.A.S. Optimization of Ultrasound-Assisted Extraction of Bioactive Properties from Date Palm (Phoenix dactylifera, L.) Spikelets Using Response Surface Methodology. LWT 2021, 140, 110816. [Google Scholar] [CrossRef]
- Toan, T.Q.; Phong, T.D.; Tien, D.D.; Linh, N.M.; Mai Anh, N.T.; Hong Minh, P.T.; Duy, L.X.; Nghi, D.H.; Pham Thi, H.H.; Nhut, P.T.; et al. Optimization of Microwave-Assisted Extraction of Phlorotannin from Sargassum Swartzii (Turn.) C. Ag. with Ethanol/Water. Nat. Prod. Commun. 2021, 16, 1934578X2199618. [Google Scholar] [CrossRef]
- Kuete, V.; Tankeo, S.B.; Saeed, M.E.M.; Wiench, B.; Tane, P.; Efferth, T. Cytotoxicity and Modes of Action of Five Cameroonian Medicinal Plants against Multi-Factorial Drug Resistance of Tumor Cells. J. Ethnopharmacol. 2014, 153, 207–219. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Schilling, J.K.; Miller, J.S.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G. New Cytotoxic Oleanane Saponins from the Infructescences of Polyscias Amplifolia from the Madagascar Rainforest. Planta Med. 2003, 69, 440–444. [Google Scholar] [CrossRef] [PubMed]



| Independent Variables | Codes | Variable Range (Δ) | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | 1 | |||
| Z1: Extraction temperature (°C) | A | 10 | 45 | 55 | 65 |
| Z2: Extraction time (min) | B | 20 | 40 | 60 | 80 |
| Z3: Ultrasonic power (W) | C | 50 | 100 | 150 | 200 |
| Run | A | B | C | Y1 (%) | Y2 (mg/g) |
|---|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 7.68 ± 0.17 | 29.90 ± 0.19 |
| 2 | +1 | −1 | 0 | 12.79 ± 0.13 | 34.85 ± 0.22 |
| 3 | −1 | +1 | 0 | 10.92 ± 0.18 | 32.30 ± 0.19 |
| 4 | +1 | +1 | 0 | 13.72 ± 0.15 | 36.68 ± 0.18 |
| 5 | −1 | 0 | −1 | 8.83 ± 0.21 | 36.66 ± 0.23 |
| 6 | +1 | 0 | −1 | 12.52 ± 0.22 | 37.57 ± 0.24 |
| 7 | −1 | 0 | +1 | 9.55 ± 0.16 | 38.85 ± 0.21 |
| 8 | +1 | 0 | +1 | 13.88 ± 0.11 | 41.51 ± 0.27 |
| 9 | 0 | −1 | −1 | 9.83 ± 0.12 | 35.05 ± 0.22 |
| 10 | 0 | +1 | −1 | 13.41 ± 0.19 | 37.39 ± 0.26 |
| 11 | 0 | −1 | +1 | 10.95 ± 0.14 | 39.00 ± 0.28 |
| 12 | 0 | +1 | +1 | 14.05 ± 0.15 | 40.63 ± 0.22 |
| 13 | 0 | 0 | 0 | 13.80 ± 0.22 | 41.66 ± 0.25 |
| 14 | 0 | 0 | 0 | 13.88 ± 0.14 | 41.52 ± 0.27 |
| 15 | 0 | 0 | 0 | 14.50 ± 0.17 | 40.35 ± 0.29 |
| 16 | 0 | 0 | 0 | 14.44 ± 0.22 | 39.37 ± 0.28 |
| 17 | 0 | 0 | 0 | 14.36 ± 0.18 | 40.17 ± 0.24 |
| Source | Y1 | Y2 | ||
|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | |
| Model | 48.37 | <0.0001 * | 16.63 | 0.0006 * |
| A | 179.13 | <0.0001 * | 18.18 | 0.0037 * |
| B | 83.02 | <0.0001 * | 7.35 | 0.0302 * |
| C | 10.50 | 0.0142 * | 19.37 | 0.0032 * |
| AB | 7.58 | 0.0284 * | 0.07 | 0.7970 NS |
| AC | 0.58 | 0.4704 NS | 0.67 | 0.4411 NS |
| BC | 0.32 | 0.5891 NS | 0.11 | 0.7514 NS |
| A2 | 85.21 | <0.0001 * | 39.48 | 0.0004 * |
| B2 | 25.02 | 0.0016 * | 56.19 | 0.0001 * |
| C2 | 29.33 | 0.0010 * | 6.31 | 0.0403 * |
| Lack of fit | 2.48 | 0.2008 NS | 1.53 | 0.3368 NS |
| R2 | 0.9842 | 0.9553 | ||
| Adj- R2 | 0.9636 | 0.8981 | ||
| Response | Model Equations | R2 | p-Value |
|---|---|---|---|
| Extraction yield | Y1 = 14.20 + 1.99A + 1.36B + 0.48C − 0.58AB − 1.89A2 − 1.03B2 − 1.11C2 (4) | 0.9842 | <0.0001 |
| TSC | Y2 = 40.61 + 1.61A + 1.02B + 1.66C − 3.27A2 − 3.91B2 + 1.31C2 (5) | 0.9553 | 0.0006 |
| Independent Variables | Real Variables | ||||
|---|---|---|---|---|---|
| A | B | C | Extraction Temperature (°C) | Extraction Time (min) | Ultrasonic Power (W) |
| 0.375 | 0.245 | 0.687 | 58.8 | 64.9 | 184.4 |
| Sample | IC50 | |||
|---|---|---|---|---|
| A549 | HepG2 | PC-3 | Hela | |
| P. fruticosa extract (mg/mL) | 18.5 | 14.3 | 20.4 | 25.2 |
| Paclitaxel a (ng/mL) | 3.5 | 3.8 | 3.7 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoang, L.T.; Huyen, H.T.T.; Chung, H.V.; Duy, L.X.; Toan, T.Q.; Bich, H.T.; Minh, P.T.H.; Pham, D.T.N.; Hien, T.T. Optimization of Total Saponin Extraction from Polyscias fruticosa Roots Using the Ultrasonic-Assisted Method and Response Surface Methodology. Processes 2022, 10, 2034. https://doi.org/10.3390/pr10102034
Khoang LT, Huyen HTT, Chung HV, Duy LX, Toan TQ, Bich HT, Minh PTH, Pham DTN, Hien TT. Optimization of Total Saponin Extraction from Polyscias fruticosa Roots Using the Ultrasonic-Assisted Method and Response Surface Methodology. Processes. 2022; 10(10):2034. https://doi.org/10.3390/pr10102034
Chicago/Turabian StyleKhoang, Le Trung, Hoang Thi Thu Huyen, Huynh Van Chung, Le Xuan Duy, Tran Quoc Toan, Hoang Thi Bich, Pham Thi Hong Minh, Dung Thuy Nguyen Pham, and Tran Thien Hien. 2022. "Optimization of Total Saponin Extraction from Polyscias fruticosa Roots Using the Ultrasonic-Assisted Method and Response Surface Methodology" Processes 10, no. 10: 2034. https://doi.org/10.3390/pr10102034
APA StyleKhoang, L. T., Huyen, H. T. T., Chung, H. V., Duy, L. X., Toan, T. Q., Bich, H. T., Minh, P. T. H., Pham, D. T. N., & Hien, T. T. (2022). Optimization of Total Saponin Extraction from Polyscias fruticosa Roots Using the Ultrasonic-Assisted Method and Response Surface Methodology. Processes, 10(10), 2034. https://doi.org/10.3390/pr10102034






