Microbial Prospection for Bioherbicide Production and Evaluation of Methodologies for Maximizing Phytotoxic Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microbial Prospecting and Isolation of Microorganisms
2.3. Submerged Fermentation
2.4. Strategies to Increase the Herbicidal Activity of Fermentation Broth
2.4.1. Co-Cultivation Medium
2.4.2. Post-Fermentation Ultrasound-Assisted Extraction (PF-UAE)
2.4.3. Co-Cultivation with PF-UAE
2.5. Application in Plant Species
2.5.1. Bioherbicidal Activity in C. sativus
2.5.2. Bioherbicidal Activity on Weeds
2.6. Identification of Microorganisms
2.7. Statistical Performance
3. Results
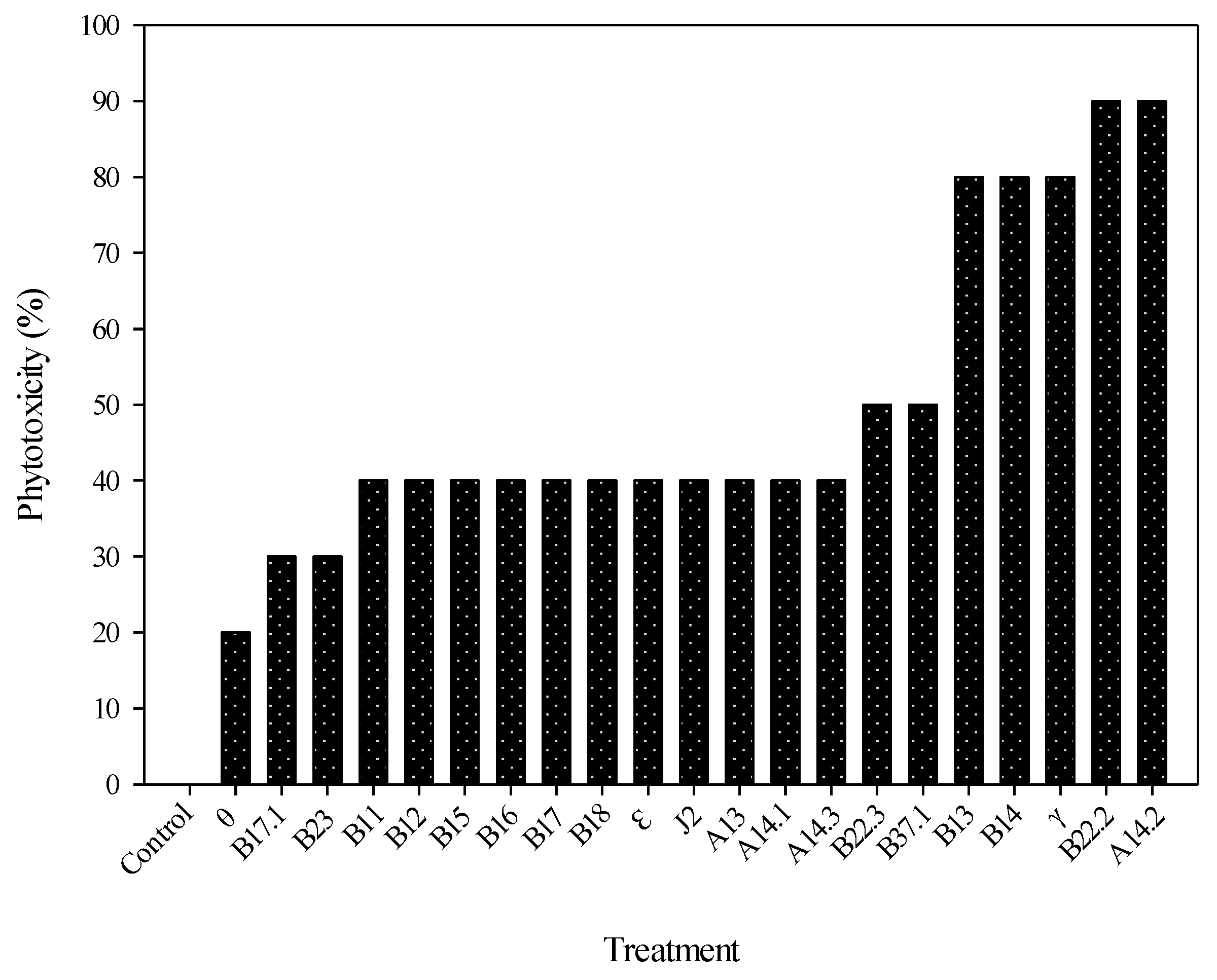
3.1. Initial Bioprospecting
3.2. Inhibition Potential on the Studied Weeds
3.3. Strategies to Increase the Herbicidal Activity of the Fermented Broth
3.4. Triple Dose Application
3.5. Post-Fermentation Ultrasound-Assisted Extraction (PF-UAE) of the Raw Broth
3.6. Co-Cultivation Medium
3.7. Post-Fermentation Ultrasound-Assisted Extraction (PF-UAE) of the Co-Cultivation Broth
3.8. Microbial Identification
4. Discussion
4.1. Initial Bioprospecting
4.2. Inhibition Potential on the Studied Weeds
4.3. Strategies to Increase the Herbicidal Activity of the Fermented Broth
4.4. Triple Dose Application
4.5. Post-Fermentation Ultrasound-Assisted Extraction (PF-UAE) of the Raw Broth
4.6. Co-Cultivation Medium
4.7. Post-Fermentation Ultrasound-Assisted Extraction (PF-UAE) of the Co-Cultivation Broth
4.8. Microbial Identification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klaic, R.; Sallet, D.; Foletto, E.L.; Jacques, R.J.S.; Guedes, J.V.C.; Kuhn, R.C.; Mazutti, M.A. Optimization of solid-state fermentation for bioherbicide production by Phoma sp. Braz. J. Chem. Eng. 2017, 34, 377–384. [Google Scholar] [CrossRef]
- Vasilakoglou, I.B.; Eleftherohorinos, I.; Dhima, K.V. Propanil-Resistant Barnyardgrass (Echinochloa crus-galli) Biotypes Found in Greece 1. Weed Technol. 2000, 14, 524–529. [Google Scholar] [CrossRef]
- Mathur, M.; Gehlot, P. Recruit the Plant Pathogen for Weed Management: Bioherbicide a Sustainable Strategy. In Fungi and their Role in Sustainable Development: Current Perspective; Springer: Singapore, 2018; pp. 159–181. ISBN 9789811303937. [Google Scholar]
- Heap, I.; Duke, S.O. Overview of glyphosate-resistant weeds worldwide. Pest Manag. Sci. 2018, 74, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 21 April 2022).
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.-P. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]
- Daniel, J.; Brun, T.; Todero, I.; Almeida, T.C.; Confortin, T.C.; Kuhn, K.R. Association of Adjuvants with Culture Filtrate from Fusarium fujikuroi for Increasing the Control of Conyza sp. Biointerface Res. Appl. Chem. 2020, 10, 6481–6487. [Google Scholar] [CrossRef]
- Green, J.M. Current state of herbicides in herbicide-resistant crops. Pest Manag. Sci. 2014, 70, 1351–1357. [Google Scholar] [CrossRef]
- Duarte, L.L.; Santos, F.M.C.; Barreto, R.W. Mycobiota of the weed Conyza canadensis (Asteraceae) in Brazil. Fungal Biol. 2016, 120, 1118–1134. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, Y.; Guo, Q.; Xu, B. Biological weed control using Trichoderma polysporum strain HZ-31. Crop Prot. 2020, 134, 105161. [Google Scholar] [CrossRef]
- Bo, A.B.; Kim, J.D.; Kim, Y.S.; Sin, H.T.; Kim, H.J.; Khaitov, B.; Ko, Y.K.; Park, K.W.; Choi, J.S. Isolation, identification and characterization of Streptomyces metabolites as a potential bioherbicide. PLoS ONE 2019, 14, e0222933. [Google Scholar] [CrossRef]
- Triolet, M.; Guillemin, J.; Andre, O.; Steinberg, C. Fungal-based bioherbicides for weed control: A myth or a reality? Weed Res. 2019, 60, 60–77. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad-Hamdani, M.; Rosli, A.; Hamdan, H. Bioherbicides: An Eco-Friendly Tool for Sustainable Weed Management. Plants 2021, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczyk, M. The effect of living mulches and conventional methods of weed control on weed infestation and potato yield. Sci. Hortic. 2015, 191, 127–133. [Google Scholar] [CrossRef]
- Abbas, T.; Zahir, Z.A.; Naveed, M.; Kremer, R.J. Limitations of Existing Weed Control Practices Necessitate Development of Alternative Techniques Based on Biological Approaches. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 147, pp. 239–280. [Google Scholar] [CrossRef]
- Harding, D.P.; Raizada, M.N. Controlling weeds with fungi, bacteria and viruses: A review. Front. Plant Sci. 2015, 6, 659. [Google Scholar] [CrossRef] [PubMed]
- Aneja, K.R.; Kumar, V.; Jiloha, P.; Kaur, M.; Sharma, C.; Surain, P.; Dhiman, R.; Aneja, A. Potential Bioherbicides: Indian Perspectives. In Biotechnology: Prospects and Applications; Springer: New Delhi, India, 2013; Volume 9788132216834, pp. 197–215. [Google Scholar]
- Stefanski, F.S.; Camargo, A.F.; Scapini, T.; Bonatto, C.; Venturin, B.; Weirich, S.N.; Ulkovski, C.; Carezia, C.; Ulrich, A.; Michelon, W.; et al. Potential Use of Biological Herbicides in a Circular Economy Context: A Sustainable Approach. Front. Sustain. Food Syst. 2020, 4, 195. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Jensen, D.F.; Jonsson, M.; et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J. Pest Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd_Allah, E.F. Bioherbicides: Current knowledge on weed control mechanism. Ecotoxicol. Environ. Saf. 2018, 158, 131–138. [Google Scholar] [CrossRef]
- Neto, J.R.C.; dos Santos, M.S.N.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Phoma dimorpha phytotoxic activity potentialization for bioherbicide production. Biocatal. Agric. Biotechnol. 2021, 33, 101986. [Google Scholar] [CrossRef]
- Todero, I.; Confortin, T.C.; Luft, L.; Seibel, J.; Kuhn, R.C.; Tres, M.V.; Zabot, G.L.; Mazutti, M.A. Concentration of exopolysaccharides produced by Fusarium fujikuroi and application of bioproduct as an effective bioherbicide. Environ. Technol. 2019, 41, 2742–2749. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Z.; Zhuang, X.; Xu, L.; Chen, S.; Li, M. Research progress on microbial herbicides. Crop Prot. 2003, 22, 247–252. [Google Scholar] [CrossRef]
- Xu, D.; Xue, M.; Shen, Z.; Jia, X.; Hou, X.; Lai, D.; Zhou, L. Phytotoxic Secondary Metabolites from Fungi. Toxins 2021, 13, 261. [Google Scholar] [CrossRef]
- Todero, I.; Confortin, T.C.; Luft, L.; Brun, T.; Ugalde, G.A.; de Almeida, T.C.; Arnemann, J.A.; Zabot, G.L.; Mazutti, M.A. Formulation of a bioherbicide with metabolites from Phoma sp. Sci. Hortic. 2018, 241, 285–292. [Google Scholar] [CrossRef]
- Daniel, J.J.; Zabot, G.L.; Tres, M.V.; Harakava, R.; Kuhn, R.C.; Mazutti, M.A. Fusarium fujikuroi: A novel source of metabolites with herbicidal activity. Biocatal. Agric. Biotechnol. 2018, 14, 314–320. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Oloke, J.K.; Bello, O.M.; Pradeep, M.; Jolly, R.S. Isolation, structural elucidation and bioherbicidal activity of an eco-friendly bioactive 2-(hydroxymethyl) phenol, from Pseudomonas aeruginosa (C1501) and its ecotoxicological evaluation on soil. Environ. Technol. Innov. 2019, 13, 304–317. [Google Scholar] [CrossRef]
- Capasso, R.; Evidente, A.; Cutignano, A.; Vurro, M.; Zonno, M.C.; Bottalico, A. Fusaric and 9,10-dehydrofusaric acids and their methyl esters from Fusarium nygamai. Phytochemistry 1996, 41, 1035–1039. [Google Scholar] [CrossRef]
- Luo, J.; Fang, Z.; Smith, R.L., Jr. Ultrasound-enhanced conversion of biomass to biofuels. Prog. Energy Combust. Sci. 2014, 41, 56–93. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.; Mohee, R. Ultrasound-assisted biological conversion of biomass and waste materials to biofuels: A review. Ultrason. Sonochemistry 2018, 40, 298–313. [Google Scholar] [CrossRef]
- Boruta, T.; Milczarek, I.; Bizukojc, M. Evaluating the outcomes of submerged co-cultivation: Production of lovastatin and other secondary metabolites by Aspergillus terreus in fungal co-cultures. Appl. Microbiol. Biotechnol. 2019, 103, 5593–5605. [Google Scholar] [CrossRef]
- Marmann, A.; Aly, A.H.; Lin, W.; Wang, B.; Proksch, P. Co-Cultivation—A Powerful Emerging Tool for Enhancing the Chemical Diversity of Microorganisms. Mar. Drugs 2014, 12, 1043–1065. [Google Scholar] [CrossRef]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef]
- Ohl, S.-W.; Klaseboer, E.; Khoo, B.C. Bubbles with shock waves and ultrasound: A review. Interface Focus 2015, 5, 20150019. [Google Scholar] [CrossRef]
- Cochran, S.A.; Prausnitz, M.R. Sonoluminescence as an Indicator of Cell Membrane Disruption by Acoustic Cavitation. In Ultrasound in Medicine & Biology; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Capocelli, M.; Prisciandaro, M.; Lancia, A.; Musmarra, D. Comparison between hydrodynamic and acoustic cavitation in microbial cell disruption. Chem. Eng. Trans. 2014, 38, 13–18. [Google Scholar] [CrossRef]
- Save, S.; Pandit, A.; Joshi, J. Microbial cell disruption: Role of cavitation. Chem. Eng. J. Biochem. Eng. J. 1994, 55, B67–B72. [Google Scholar] [CrossRef]
- Jadhav, A.; Holkar, C.; Goswami, A.D.; Pandit, A.B.; Pinjari, D.V. Acoustic Cavitation as a Novel Approach for Extraction of Oil from Waste Date Seeds. ACS Sustain. Chem. Eng. 2016, 4, 4256–4263. [Google Scholar] [CrossRef]
- Gehlot, P.; Singh, J. Fungi and Their Role in Sustainable Development: Current Perspectives; Springer: Singapore, 2018; pp. 383–411. ISBN 9789811303937. [Google Scholar]
- Sari, M.; Kusharyoto, W.; Brazia, A.C. Profiling Bioactive Compounds in Secondary Metabolites from Co-Cultivation between Actinomycetes and Pathogenic Bacteria. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2020; Volume 439. [Google Scholar] [CrossRef]
- Bertrand, S.; Bohni, N.; Schnee, S.; Schumpp, O.; Gindro, K.; Wolfender, J.-L. Metabolite induction via microorganism co-culture: A potential way to enhance chemical diversity for drug discovery. Biotechnol. Adv. 2014, 32, 1180–1204. [Google Scholar] [CrossRef] [PubMed]
- Harwani, D.; Begani, J.; Lakhani, J. Co-Cultivation Strategies to Induce De Novo Synthesis of Novel Chemical Scaffolds from Cryptic Secondary Metabolite Gene Clusters. In Fungi and their Role in Sustainable Development: Current Perspectives; Springer: Singapore, 2018; pp. 617–631. [Google Scholar] [CrossRef]
- Sandland, G.J.; Rodgers, J.K.; Minchella, D.J. Interspecific antagonism and virulence in hosts exposed to two parasite species. J. Invertebr. Pathol. 2007, 96, 43–47. [Google Scholar] [CrossRef]
- Júnior, F.W.R.; Scariot, M.A.; Forte, C.T.; Pandolfi, L.; Dil, J.M.; Weirich, S.; Carezia, C.; Mulinari, J.; Mazutti, M.A.; Fongaro, G.; et al. New perspectives for weeds control using autochthonous fungi with selective bioherbicide potential. Heliyon 2019, 5, e01676. [Google Scholar] [CrossRef]
- Carollo, E.M.; Santos Filho, H.P. Manual Básico de Técnicas Fitopatológicas. Embrapa Mandioca Frutic. 2016, 109. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/148757/1/Cartilha-ManualFito-215-14-Hermes.pdf (accessed on 9 August 2022).
- Rabinovitch, L.; de Oliveira, E.J. Coletânea de Procedimentos Técnicos e Metodologias Empregadas Para o Estudo de Bacillus e Gêneros Esporulados Aeróbios Correlatos, 1st ed.; Montenegro Comunicação: Rio de Janeiro, Brazil, 2015; ISBN 9788567506043. [Google Scholar]
- Leber, A.L. Processing, Isolation, Detection, and Interpretation of Aerobic Bacteriology Cultures. In Clinical Microbiology Procedures Handbook; ASM Press: Washington, DC, USA, 2016; pp. 3.3.1.1–3.3.2.15. [Google Scholar]
- Schmaltz, S.; Aita, B.C.; Alves, E.A.; Fochi, A.; Bolson, V.F.; Navarro-Díaz, H.J.; Kuhn, R.C.; Mazutti, M.A. Ultrasound-assisted fermentation for production of β-1,3-glucanase and chitinase by Beauveria bassiana. J. Chem. Technol. Biotechnol. 2020, 96, 88–98. [Google Scholar] [CrossRef]
- Frans, R.; Crowley, H. Experimental Design and Techniques for Measuring and Analyzing Plant Responses to Weed Control Practices. In Southern Weed Science Society, Research Methods in Weed Science, 3rd ed.; Camper, N.D., Ed.; WSSA: Champaign, IL, USA, 1986; pp. 29–46. [Google Scholar]
- de Oliveira, C.T.; Alves, E.A.; Todero, I.; Kuhn, R.C.; de Oliveira, D.; Mazutti, M.A. Production of cutinase by solid-state fermentation and its use as adjuvant in bioherbicide formulation. Bioprocess Biosyst. Eng. 2019, 42, 829–838. [Google Scholar] [CrossRef]
- Brun, T.; Rabuske, J.E.; Confortin, T.C.; Luft, L.; Todero, I.; Fischer, M.; Zabot, G.L.; Mazutti, M.A. Weed control by metabolites produced from Diaporthe schini. Environ. Technol. 2020, 43, 139–148. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. Available online: https://webpages.charlotte.edu/~jweller2/pages/BINF8350f2011/BINF8350_Readings/Doyle_plantDNAextractCTAB_1987.pdf (accessed on 8 September 2022).
- Aveskamp, M.M.; Verkley, G.J.; de Gruyter, J.; Murace, M.A.; Perelló, A.; Woudenberg, J.H.; Groenewald, J.Z.; Crous, P.W. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 2009, 101, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.; Riesner, D. Purification of nucleic acids by selective precipitation with polyethylene glycol 6000. Anal. Biochem. 2006, 354, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- StatSoft Inc. Statistica (Data Analysis Software System); Version 8; StatSoft Inc.: Tulsa, OK, USA, 2008. [Google Scholar]
- De Souza, A.R.C.; Baldoni, D.B.; Lima, J.; Porto, V.; Marcuz, C.; Machado, C.; Ferraz, R.C.; Kuhn, R.C.; Jacques, R.J.S.; Guedes, J.V.; et al. Selection, isolation, and identification of fungi for bioherbicide production. Braz. J. Microbiol. 2016, 48, 101–108. [Google Scholar] [CrossRef]
- Manalil, S.; Coast, O.; Werth, J.; Chauhan, B.S. Weed management in cotton (Gossypium hirsutum L.) through weed-crop competition: A review. Crop Prot. 2017, 95, 53–59. [Google Scholar] [CrossRef]
- Kong, C.-H.; Hu, F.; Wang, P.; Wu, J.-L. Effect of allelopathic rice varieties combined with cultural management options on paddy field weeds. Pest Manag. Sci. 2008, 64, 276–282. [Google Scholar] [CrossRef]
- Cochavi, A.; Cohen, I.H.; Rachmilevitch, S. The role of different root orders in nutrient uptake. Environ. Exp. Bot. 2020, 179, 104212. [Google Scholar] [CrossRef]
- Anten, N.P.; Hirose, T. Limitations on photosynthesis of competing individuals in stands and the consequences for canopy structure. Oecologia 2001, 129, 186–196. [Google Scholar] [CrossRef]
- Wang, L.-W.; Showalter, A.M.; Ungar, I.A. Effects of intraspecific competition on growth and photosynthesis of Atriplex prostrata. Aquat. Bot. 2005, 83, 187–192. [Google Scholar] [CrossRef]
- Adetunji, C.O.; Oloke, J.K.; Osemwegie, O.O. Environmental fate and effects of granular pesta formulation from strains of Pseudomonas aeruginosa C1501 and Lasiodiplodia pseudotheobromae C1136 on soil activity and weeds. Chemosphere 2018, 195, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.R.C.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Bioherbicidal action of Phoma dimorpha fermented broth on seeds and plants of Senna obtusifolia. Pesqui. Agropecu. Trop. 2020, 50, e56894. [Google Scholar] [CrossRef]
- Maiquel, P.P.; Marcio, A.M.; Thiago, C.A.; Luis, E.C.; Adriano, A.M.; Jerson, V.C.G.; Raquel, C.K. Bioherbicide based on Diaporthe sp. secondary metabolites in the control of three tough weeds. Afr. J. Agric. Res. 2016, 11, 4242–4249. [Google Scholar] [CrossRef]
- Todero, I.; Confortin, T.C.; Soares, J.F.; Brun, T.; Luft, L.; Rabuske, J.E.; Kuhn, R.C.; Tres, M.V.; Zabot, G.L.; Mazutti, M.A. Concentration of metabolites from Phoma sp. using microfiltration membrane for increasing bioherbicidal activity. Environ. Technol. 2018, 40, 2364–2372. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, R.; Baur, P. O-TEQ®, a Formulation Concept That Overcomes the Incompatibility between Water and Oil. Pflanzenschutz-Nachr. Bayer 2007, 60, 7–26. [Google Scholar]
- Gasic, S.; Tanovic, B. Biopesticide formulations, possibility of application and future trends. Pestic. Fitomedicina 2013, 28, 97–102. [Google Scholar] [CrossRef]
- Taban, A.; Saharkhiz, M.J.; Khorram, M. Formulation and assessment of nano-encapsulated bioherbicides based on biopolymers and essential oil. Ind. Crops Prod. 2020, 149, 112348. [Google Scholar] [CrossRef]
- Haarmann, J.A.; Young, B.G.; Johnson, W.G. Control of Palmer amaranth (Amaranthus palmeri) regrowth following failed applications of glufosinate and fomesafen. Weed Technol. 2021, 35, 464–470. [Google Scholar] [CrossRef]
- Syahri, R.; Widaryanto, E.; Wicaksono, K.P. Bioactive compound from mangoes leaves extract as potential soil bioherbicide to control amaranth weed (Amaranthus spinosus Linn.). J. Degraded Min. Lands Manag. 2017, 4, 829–836. [Google Scholar] [CrossRef]
- Tanaka, S.; Sawaya, M.R.; Yeates, T.O. Structure and Mechanisms of a Protein-Based Organelle in Escherichia coli. Science 2010, 327, 81–84. [Google Scholar] [CrossRef]
- Yeates, T.O.; Kerfeld, C.A.; Heinhorst, S.; Cannon, G.C.; Shively, J.M. Protein-based organelles in bacteria: Carboxysomes and related microcompartments. Nat. Rev. Genet. 2008, 6, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.; Pitt, W.; Falk, S.; Derby, J. The effects of Phoma macrostoma on nontarget plant and target weed species. Biol. Control 2011, 58, 379–386. [Google Scholar] [CrossRef]
- Ehgartner, D.; Herwig, C.; Fricke, J. Morphological analysis of the filamentous fungus Penicillium chrysogenum using flow cytometry—The fast alternative to microscopic image analysis. Appl. Microbiol. Biotechnol. 2017, 101, 7675–7688. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Veiter, L.; Rajamanickam, V.; Herwig, C. The filamentous fungal pellet—Relationship between morphology and productivity. Appl. Microbiol. Biotechnol. 2018, 102, 2997–3006. [Google Scholar] [CrossRef]
- Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2003, 22, 189–259. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Kane, P.M. The yeast lysosome-like vacuole: Endpoint and crossroads. Biochim. Biophys. Acta 2009, 1793, 650–663. [Google Scholar] [CrossRef] [PubMed]
- El-Gendi, H.; Saleh, A.K.; Badierah, R.; Redwan, E.M.; El-Maradny, Y.A.; El-Fakharany, E.M. A Comprehensive Insight into Fungal Enzymes: Structure, Classification, and Their Role in Mankind’s Challenges. J. Fungi 2021, 8, 23. [Google Scholar] [CrossRef]
- Ogawa, M.; García-Martínez, T.; Bisson, L.; Mauricio, J.C.; Moreno, J.; Moreno-García, J. Mapping the intracellular metabolome of yeast biocapsules—Spherical structures of yeast attached to fungal pellets. New Biotechnol. 2020, 58, 55–60. [Google Scholar] [CrossRef]
- Caudal, F.; Tapissier-Bontemps, N.; Edrada-Ebel, R.A. Impact of Co-Culture on the Metabolism of Marine Microorganisms. Mar. Drugs 2022, 20, 153. [Google Scholar] [CrossRef]
- Tranel, P.J. Herbicide resistance in Amaranthus tuberculatus. Pest Manag. Sci. 2020, 77, 43–54. [Google Scholar] [CrossRef]
- Ye, M.; Tang, X.; Yang, R.; Zhang, H.; Li, F.; Tao, F.; Li, F.; Wang, Z. Characteristics and Application of a Novel Species of Bacillus: Bacillus velezensis. ACS Chem. Biol. 2018, 13, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pandey, L.M. Prospective of fungal pathogen-based bioherbicides for the control of water hyacinth: A review. J. Basic Microbiol. 2022, 62, 415–427. [Google Scholar] [CrossRef] [PubMed]
| Region | Geographical Coordinates | Collected Species | Number of Isolated Strains |
|---|---|---|---|
| Santa Maria, Brazil | −29.6914 S, −53.8008 W | Conyza sp. And Contaminations * | 31 |
| Cerro Largo, Brazil | −28.1506 S, −54.7386 W | Conyza sp. And B. pilosa | 6 |
| Marcelino Ramos, Brazil | −27.4673 S, −519085 W | Conyza sp., Spermacoce latifolia, and E. crusgalli | 26 |
| PH (cm) | RL (cm) | SFM (g) | SDM (g) | RFM (g) | RDM (g) | |
|---|---|---|---|---|---|---|
| E. crusgalli | ||||||
| B13 | 37.0 ± 5.4 | 32.5 ± 2.5 G | 12.482 ± 3.787 I | 2.572 ± 0.118 I | 7.275 ± 2.443 | 3.316 ± 0.972 |
| B14 | 31.0 ± 1.4 I | 29.0 ± 7.0 | 8.462 ± 3.684 I | 1.931 ± 0.885 I | 5.060 ± 2.101 I | 2.261 ± 0.854 I |
| A14.2 | 31.0 ± 4.3 I | 28.9 ± 7.8 | 12.151 ± 3.546 I | 3.524 ± 1.030 | 7.862 ± 3.759 | 3.207 ± 1.170 |
| B22.2 | 37.5 ± 3.5 | 25.4 ± 4.3 | 9.370 ± 2.088 I | 2.589 ± 0.353 I | 6.733 ± 2.293 | 3.119 ± 0.669 |
| γ | 34.1 ± 1.4 I | 24.0 ± 1.8 | 16.871 ± 4.150 I | 3.880 ± 0.877 | 15.662 ± 9.624 | 4.801 ± 0.847 |
| Control | 38.3 ± 2.3 | 24.0 ± 1.8 | 25.733 ± 1.872 | 4.700 ± 0.529 | 10.633 ± 2.669 | 4.200 ± 0.557 |
| A. hybridus | ||||||
| B13 | 18.0 ± 5.6 I | 17.5 ± 2.1 | 2.465 ± 1.085 I | 0.902 ± 0.386I | 1.174 ± 0.734 | 0.607 ± 0.390 |
| B14 | 29.5 ± 1.7 | 22.0 ± 2.4 | 5.233 ± 1.062 I | 2.542 ± 0.539 | 2.490 ± 0.662 | 1.680 ± 0.355 |
| A14.2 | 24.8 ± 0.5 I | 18.3 ± 2.6 | 7.254 ± 0.528 | 2.221 ± 0.164 I | 1.497 ± 0.397 | 0.545 ± 0.169 |
| B22.2 | 21.9 ± 11.5 | 18.1 ± 2.7 | 4.720 ± 3.684 | 1.460 ± 1.194 I | 1.928 ± 1.211 | 0.854 ± 0.525 |
| γ | 24.9 ± 8.1 | 17.3 ± 6.8 | 3.987 ± 2.894 I | 1.318 ± 0.953 I | 1.470 ± 1.171 | 0.697 ± 0.580 |
| Control | 31.0 ± 1.5 | 22.5 ± 5.8 | 8.355 ± 1.062 | 3.337 ± 0.043 | 2.450 ± 1.343 | 1.247 ± 0.769 |
| B. pilosa | ||||||
| B13 | 22.9 ± 2.4 | 27.3 ± 4.1 | 6.478 ± 2.115 | 1.855 ± 0.425 | 1.431 ± 0.460 | 0.875 ± 0.037 G |
| B14 | 22.0 ± 2.9 | 28.8 ± 3.0 | 4.859 ± 1.745 | 1.632 ± 0.796 | 1.112 ± 0.405 | 0.793 ± 0.144 |
| A14.2 | 23.4 ± 2.0 | 29.0 ± 0.0 | 4.137 ± 0.867 | 1.781 ± 0.394 | 0.964 ± 0.273 | 0.763 ± 0.198 |
| B22.2 | 16.5 ± 5.1I | 26.3 ± 5.6 | 2.572 ± 1.441 | 0.854 ± 0.562 | 0.660 ± 0.474 | 0.389 ± 0.268 |
| γ | 20.5 ± 1.3I | 14.0 ± 1.0 | 4.347 ± 2.081 | 1.213 ± 0.695 | 1.004 ± 0.628 | 0.438 ± 0.262 |
| Control | 24.6 ± 1.9 | 25.5 ± 10.7 | 4.460 ± 1.886 | 1.553 ± 0.374 | 1.089 ± 0.581 | 0.520 ± 0.184 |
| Conyza sp. | ||||||
| B13 | 21.0 ± 2.2 | 18.3 ± 2.5 | 9.695 ± 3.687 | 2.368 ± 0.545 | 8.285 ± 2.280 | 1.900 ± 0.466 G |
| B14 | 22.5 ± 3.0 | 23.5 ± 7.7 | 12.175 ± 4.413 | 2.515 ± 0.821 | 12.797 ± 0.476 G | 2.315 ± 1.007 G |
| A14.2 | 18.0 ± 5.0 | 18.5 ± 5.2 | 6.290 ± 3.038 | 1.515 ± 0.769 | 6.538 ± 2.354 | 1.510 ± 0.653 |
| B22.2 | 25.5 ± 5.2 | 24.0 ± 5.8 | 13.493 ± 6.620 | 2.710 ± 1.369 | 12.138 ± 6.080 | 2.265 ± 1.589 |
| γ | 19.3 ± 5.1 | 14.8 ± 4.5 | 10.775 ± 6.366 | 2.360 ± 1.329 | 8.238 ± 4.465 | 1.735 ± 1.052 |
| Control | 24.5 ± 4.7 | 19.0 ± 0.8 | 10.585 ± 4.826 | 2.010 ± 1.066 | 6.263 ± 2.333 | 0.850 ± 0.417 |
| PH (cm) | RL (cm) | SFM (g) | SDM (g) | RFM (g) | RDM (g) | |
|---|---|---|---|---|---|---|
| Raw broth–Triple dose | ||||||
| B13 | 53.9 ± 3.1 | 29.3 ± 3.6 I | 17.097 ± 4.014 I | 1.698 ± 0.313 I | 3.010 ± 0.901 | 0.305 ± 0.043 I |
| B14 | 55.6 ± 4.2 | 27.7 ± 1.1 I | 16.218 ± 4.909 I | 1.734 ± 0.380 I | 2.012 ± 0.536 I | 0.251 ± 0.079 I |
| B22.2 | 56.4 ± 3.2 | 31.3 ± 3.8 | 16.440 ± 3.366 I | 2.013 ± 0.278 I | 2.301 ± 0.318 | 0.435 ± 0.081 |
| γ | 55.0 ± 4.8 | 22.7 ± 2.4 I | 9.743 ± 2.005 I | 1.210 ± 0.378 I | 0.539 ± 0.176 I | 0.127 ± 0.052 I |
| A14.2 | 55.4 ± 2.7 | 32.9 ± 2.6 | 22.639 ± 2.683 | 2.286 ± 0.308 | 3.801 ± 0.859 G | 0.457 ± 0.092 |
| Raw broth–UAE | ||||||
| B13 | 61.4 ± 4.8 | 30.7 ± 4.4 | 22.394 ± 2.610 | 1.807 ± 0.235 I | 2.416 ± 0.344 | 0.247 ± 0.034 I |
| B14 | 55.9 ± 2.3 | 28.0 ± 2.5 I | 16.360 ± 5.431 I | 1.718 ± 0.441 I | 1.744 ± 0.665 I | 0.312 ± 0.126 I |
| B22.2 | 49.0 ± 3.9 I | 28.4 ± 2.2 I | 9.002 ± 2.591 I | 1.295 ± 0.189 I | 1.478 ± 0.513 I | 0.231 ± 0.055 I |
| γ | 61.6 ± 4.2 | 31.1 ± 4.3 | 22.933 ± 5.726 | 2.135 ± 0.549 | 2.683 ± 0.621 | 0.296 ± 0.088 I |
| A14.2 | 47.7 ± 1.9 I | 26.9 ± 2.7 I | 12.095 ± 1.870 I | 0.985 ± 0.245 I | 1.789 ± 0.266 I | 0.198 ± 0.079 I |
| Co-cultivation | ||||||
| A14.2/B22.2 | 53.4 ± 2.9 | 30.4 ± 4.4 | 15.171 ± 2.802 I | 1.702 ± 0.293 I | 1.414 ± 0.475 I | 0.272 ± 0.091 I |
| B13/B14 | 55.1 ± 3.0 | 34.4 ± 3.4 | 20.064 ± 4.061 | 2.027 ± 0.505 | 2.296 ± 0.566 | 0.371 ± 0.129 |
| B14/γ | 63.3 ± 3.9 G | 30.3 ± 4.0 | 25.727 ± 3.922 | 2.227 ± 0.290 | 2.941 ± 0.502 | 0.349 ± 0.073 |
| B22.2/γ | 57.7 ± 3.6 | 31.1 ± 3.4 | 23.787 ± 3.934 | 2.375 ± 0.371 | 3.223 ± 0.641 | 0.413 ± 0.111 |
| Co-cultivation and UAE | ||||||
| A14.2/B22.2 | 57.1 ± 4.4 | 29.6 ± 3.8 I | 12.992 ± 2.178 I | 1.438 ± 0.200 I | 1.426 ± 0.388 I | 0.194 ± 0.055 I |
| B13/B14 | 59.4 ± 3.4 | 32.9 ± 3.2 | 19.930 ± 5.065 | 2.038 ± 0.356 I | 2.430 ± 0.575 | 0.283 ± 0.068 I |
| B14/γ | 56.9 ± 3.6 | 27.1 ± 1.9 I | 15.890 ± 3.856 I | 1.697 ± 0.329 I | 0.917 ± 0.167 I | 0.203 ± 0.067 I |
| B22.2/γ | 59.9 ± 1.8 | 30.3 ± 1.8 I | 18.420 ± 4.003 I | 1.828 ± 0.407 I | 1.818 ± 0.640 I | 0.260 ± 0.112 I |
| Control | 57.4 ± 4.5 | 33.7 ± 2.9 | 22.298 ± 1.982 | 2.434 ± 0.228 | 2.769 ± 0.529 | 0.437 ± 0.081 |
| PH (cm) | RL (cm) | SFM (g) | SDM (g) | RFM (g) | RDM (g) | |
|---|---|---|---|---|---|---|
| Raw broth—Triple dose | ||||||
| B13 | 19.0 ± 3.0 | 32.1 ± 5.6 | 3.832 ± 1.284 G | 0.573 ± 0.136 G | 0.664 ± 0.237 G | 0.100 ± 0.039 G |
| B14 | 16.2 ± 2.0 I | 36.0 ± 6.9 G | 3.023 ± 0.698 | 0.476 ± 0.088 | 0.502 ± 0.149 G | 0.081 ± 0.027 |
| B22.2 | 19.0 ± 2.2 | 39.4 ± 5.7 G | 3.772 ± 0.888 G | 0.603 ± 0.129 G | 0.511 ± 0.177 G | 0.108 ± 0.036 G |
| γ | 16.0 ± 2.4 I | 25.9 ± 7.5 | 2.466 ± 1.100 | 0.464 ± 0.116 | 0.312 ± 0.137 | 0.060 ± 0.027 |
| A14.2 | 23.0 ± 3.8 | 45.0 ± 8.2 G | 5.660 ± 1.396 G | 0.800 ± 0.104 G | 0.946 ± 0.302 G | 0.161 ± 0.036 G |
| Raw broth—UAE | ||||||
| B13 | 21.0 ± 2.8 | 30.4 ± 2.4 | 3.567 ± 0.833 G | 0.446 ± 0.201 | 0.516 ± 0.133 G | 0.091 ± 0.024 G |
| B14 | 18.4 ± 2.3 | 35.1 ± 9.4 | 2.734 ± 0.631 | 0.457 ± 0.082 | 0.292 ± 0.055 | 0.061 ± 0.021 |
| B22.2 | 18.6 ± 4.0 | 30.1 ± 6.0 | 3.367 ± 1.121 | 0.538 ± 0.124 G | 0.548 ± 0.228 G | 0.079 ± 0.040 |
| γ | 21.1 ± 2.3 | 18.1 ± 5.1 I | 0.843 ± 0.273 I | 0.198 ± 0.035 I | 0.083 ± 0.045 I | 0.018 ± 0.009 I |
| A14.2 | 11.6 ± 2.4 I | 36.4 ± 6.9 G | 4.571 ± 1.439 G | 0.694 ± 0.173 G | 0.788 ± 0.286 G | 0.132 ± 0.048 G |
| Co-cultivation | ||||||
| A14.2/B22.2 | 23.4 ± 3.4 | 24.9 ± 7.9 | 3.384 ± 1.024 | 0.468 ± 0.114 | 0.440 ± 0.263 | 0.065 ± 0.015 |
| B13/B14 | 19.0 ± 1.7 | 25.3 ± 4.3 | 2.662 ± 0.506 | 0.413 ± 0.061 | 0.312 ± 0.111 | 0.055 ± 0.021 |
| B14/γ | 16.3 ± 2.3 I | 20.1 ± 3.3 I | 1.420 ± 0.394 I | 0.271 ± 0.040 I | 0.129 ± 0.068 I | 0.031 ± 0.015 I |
| B22.2/γ | 17.4 ± 2.6 | 25.0 ± 2.9 | 2.139 ± 0.441 | 0.398 ± 0.042 | 0.226 ± 0.054 I | 0.038 ± 0.008 I |
| Co-cultivation and UAE | ||||||
| A14.2/B22.2 | 22.7 ± 5.6 | 37.0 ± 6.4 G | 4.972 ± 1.655 G | 0.679 ± 0.202 G | 0.896 ± 0.255 G | 0.138 ± 0.045 G |
| B13/B14 | 16.6 ± 4.1 | 27.4 ± 11.5 | 2.134 ± 1.000 | 0.425 ± 0.145 | 0.273 ± 0.059 | 0.058 ± 0.027 |
| B14/γ | 19.8 ± 2.6 | 31.1 ± 3.0 | 3.367 ± 0.651 G | 0.614 ± 0.152 G | 0.479 ± 0.141 G | 0.091 ± 0.025 G |
| B22.2/γ | 18.5 ± 3.9 | 27.3 ± 1.9 | 2.703 ± 0.709 | 0.457 ± 0.072 | 0.312 ± 0.086 | 0.050 ± 0.013 |
| Control | 20.2 ± 2.3 | 27.3 ± 5.2 | 2.567 ± 0.504 | 0.414 ± 0.053 | 0.311 ± 0.076 | 0.058 ± 0.011 |
| Code | γ | B22.2 | B14 | B13 | A14.2 |
|---|---|---|---|---|---|
| A14.2 | − + | + + | − + | − + | |
| B13 | − + | + − | + + | ||
| B14 | + + | + − | |||
| B22.2 | + + | ||||
| γ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schein, D.; Santos, M.S.N.; Schmaltz, S.; Nicola, L.E.P.; Bianchin, C.F.; Ninaus, R.G.; Menezes, B.B.d.; Santos, R.C.d.; Zabot, G.L.; Tres, M.V.; et al. Microbial Prospection for Bioherbicide Production and Evaluation of Methodologies for Maximizing Phytotoxic Activity. Processes 2022, 10, 2001. https://doi.org/10.3390/pr10102001
Schein D, Santos MSN, Schmaltz S, Nicola LEP, Bianchin CF, Ninaus RG, Menezes BBd, Santos RCd, Zabot GL, Tres MV, et al. Microbial Prospection for Bioherbicide Production and Evaluation of Methodologies for Maximizing Phytotoxic Activity. Processes. 2022; 10(10):2001. https://doi.org/10.3390/pr10102001
Chicago/Turabian StyleSchein, Dinalva, Maicon S. N. Santos, Silvana Schmaltz, Luiz E. P. Nicola, Cristiane F. Bianchin, Renata G. Ninaus, Bryan B. de Menezes, Ricardo C. dos Santos, Giovani Leone Zabot, Marcus V. Tres, and et al. 2022. "Microbial Prospection for Bioherbicide Production and Evaluation of Methodologies for Maximizing Phytotoxic Activity" Processes 10, no. 10: 2001. https://doi.org/10.3390/pr10102001
APA StyleSchein, D., Santos, M. S. N., Schmaltz, S., Nicola, L. E. P., Bianchin, C. F., Ninaus, R. G., Menezes, B. B. d., Santos, R. C. d., Zabot, G. L., Tres, M. V., & Mazutti, M. A. (2022). Microbial Prospection for Bioherbicide Production and Evaluation of Methodologies for Maximizing Phytotoxic Activity. Processes, 10(10), 2001. https://doi.org/10.3390/pr10102001








