The Use of Novel, Rapid Analytical Tools in the Assessment of the Stability of Tablets—A Pilot Analysis of Expired and Unexpired Tablets Containing Nifuroxazide
Abstract
:1. Introduction
2. Methods
2.1. Evaluation of Selected Preparations
2.2. Analysis of Directional Hemispherical Reflectance
2.3. Hyperspectral Analysis
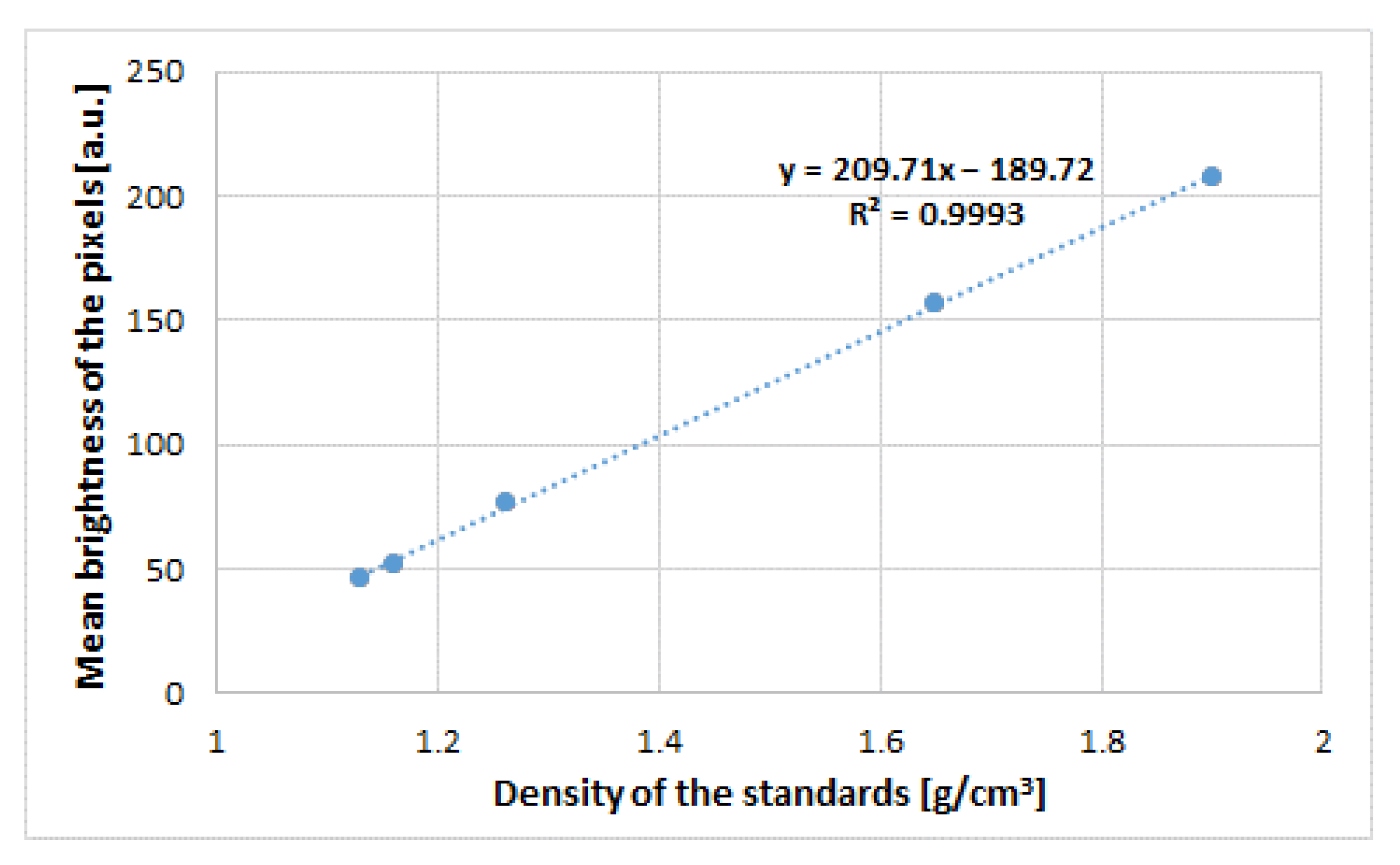
2.4. X-ray Computer Microtomography
2.5. Statistical Analysis
3. Results
3.1. Analysis of Total Directional Hemispherical Reflectance (THR)
3.2. Microtomograhic Analysis
3.2.1. Analysis of the Homogeneity of the Tested Tablets
3.2.2. Analysis of Coating Thickness of the Tested Tablets
3.3. Hyperspectral Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Ktash, M.; Stefanakis, M.; Boldrini, B.; Ostertag, E.; Brecht, M. Characterization of Pharmaceutical Tablets Using UV Hyperspectral Imaging as a Rapid In-Line Analysis Tool. Sensors 2021, 21, 4436. [Google Scholar] [CrossRef] [PubMed]
- Sarecka-Hujar, B.; Balwierz, R.; Ostróżka-Cieślik, A.; Dyja, R.; Łukowiec, D.; Jankowski, A. Scanning electron microscopy and X-ray energy dispersive spectroscopy—Useful tools in the analysis of pharmaceutical products. J. Phys. Conf. Ser. 2017, 931, 1–5. [Google Scholar] [CrossRef]
- Buzalewicz, I.; Podbielska, H. Biomedical Optics. Selected Issues; Oficyna Wydawnicza Politechniki Wrocławskiej: Wrocław, Porland, 2011; pp. 70–76. (In Polish) [Google Scholar]
- Schaepman-Strub, G.; Schaepman, M.E.; Painter, T.H.; Dangel, S.; Martonchik, J.V. Reflectance quantities in optical remote sensing—Definitions and case studies. Remote Sens. Environ. 2006, 103, 27–42. [Google Scholar] [CrossRef]
- Meisner, M.; Szulc-Musioł, B.; Sarecka-Hujar, B. Preliminary analysis of the hemispherical directional reflectance of Alugastrin tablets after 14 days of storage at 40 °C. Eur. J. Med. Technol. 2022, 1, 15–22. [Google Scholar]
- Jaglarz, J.; Kapłonek, W.; Lipiński, W.; Pawełczak, M.; Synak., R. Comparative Investigations of Smooth Surfaces Parameters using Light Scattering Methods. Pr. Naukowo-Badaw. IMM 2012, 2, 9–23. (In Polish) [Google Scholar]
- Feng, L.; Zhu, S.; Liu, F.; He, Y.; Bao, Y.; Zhang, C. Hyperspectral imaging for seed quality and safety inspection: A review. Plant Methods 2019, 15, 91. [Google Scholar] [CrossRef]
- Huang, H.; Qureshi, J.U.; Liu, S.; Sun, Z.; Zhang, C.; Wang, H. Hyperspectral Imaging as a Potential Online Detection Method of Microplastics. Bull. Environ. Contam. Toxicol. 2021, 107, 754–763. [Google Scholar] [CrossRef] [PubMed]
- da Silva, Í.B. X-ray Computed Microtomography technique applied for cementitious materials: A review. Micron 2018, 107, 1–8. [Google Scholar] [CrossRef]
- Landis, E.N.; Keane, D.T. X-ray microtomography. Mater. Charact. 2010, 61, 1305–1316. [Google Scholar] [CrossRef]
- Vanhoof, R.; Hubrechts, J.M.; Roebben, E.; Nyssen, H.J.; Nulens, E.; Leger, J.; De Schepper, N. The comparative activity of pefloxacin, enoxacin, ciprofloxacin and 13 other antimicrobial agents against enteropathogenic microorganisms. Infection 1986, 14, 294–298. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, M.; El-Sayed, R.M.; Helal, M.A.; Ibrahiem, A.T.; Elmahdi, H.S.; Eladl, M.A.; Bilay, S.E.; Alshahrani, A.M.; Tawfik, M.K.; Hamed, Z.E. Nifuroxazide Mitigates Angiogenesis in Ehlrich’s Solid Carcinoma: Molecular Docking, Bioinformatic and Experimental Studies on Inhibition of Il-6/Jak2/Stat3 Signaling. Molecules 2021, 13, 6858. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Zhang, Y.C.; Xie, R.R.; Song, L.N.; Yang, W.L.; Xin, Z.; Cao, X.; Yang, J.K. Nifuroxazide improves insulin secretion and attenuates high glucose-induced inflammation and apoptosis in INS-1 cells. Eur. J. Pharmacol. 2021, 15, 174042. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ye, T.; Yu, X.; Lei, Q.; Yang, F.; Xia, Y.; Song, X.; Liu, L.; Deng, H.; Gao, T.; et al. Nifuroxazide exerts potent anti-tumor and anti-metastasis activity in melanoma. Sci. Rep. 2016, 6, 20253. [Google Scholar] [CrossRef] [PubMed]
- Wilczyński, S.; Koprowski, R.; Stolecka-Warzecha, A.; Duda, P.; Deda, A.; Ivanova, D.; Kiselova-Kaneva, Y.; Błońska-Fajfrowska, B. The use of microtomographic imaging in the identification of counterfeit medicines. Talanta 2019, 195, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Wilczyński, S.; Koprowski, R.; Błońska-Fajfrowska, B. Directional reflectance analysis for identifying counterfeit drugs: Preliminary study. J. Pharm. Biomed. Anal. 2016, 124, 341–346. [Google Scholar] [CrossRef]
- Thosar, S.S.; Forbess, R.A.; Ebube, N.K.; Chen, Y.; Rubinovitz, R.L.; Kemper, M.S.; Reier, G.E.; Wheatley, T.A.; Shukla, A.J. A comparison of reflectance and transmittance near-infrared spectroscopic techniques in determining drug content in intact tablets. Pharm. Dev. Technol. 2001, 6, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Donoso, M.; Kildsig, D.O.; Ghaly, E.S. Prediction of tablet hardness and porosity using near-infrared diffuse reflectance spectroscopy as a nondestructive method. Pharm. Dev. Technol. 2003, 8, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Markl, D.; Wahl, P.; Pichler, H.; Sacher, S.; Khinast, J.G. Characterization of the coating and tablet core roughness by means of 3D optical coherence tomography. Int. J. Pharm. 2018, 536, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.E.; Crossman, E. The influence of tablet shape and pan speed on intra-tablet film coating uniformity. Drug Dev. Ind. Pharm. 1997, 23, 1239–1243. [Google Scholar] [CrossRef]
- Zhang, Q.; Gladden, L.F.; Avalle, P.; Zeitler, J.A.; Mantle, M.D. Terahertz pulsed imaging and magnetic resonance imaging as tools to probe formulation stability. Pharmaceutics 2013, 5, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Sacher, S.; Peter, A.; Khinast, J.G. Feasibility of In-line monitoring of critical coating quality attributes via OCT: Thickness, variability, film homogeneity and roughness. Int. J. Pharm. X 2020, 3, 100067. [Google Scholar] [CrossRef] [PubMed]
- Razuc, M.; Grafia, A.; Gallo, L.; Ramírez-Rigo, M.V.; Roma?ach, R.J. Near-infrared spectroscopic applications in pharmaceutical particle technology. Drug Dev. Ind. Pharm. 2019, 45, 1565–1589. [Google Scholar] [CrossRef] [PubMed]
- Galata, D.L.; Mészáros, L.A.; Kállai-Szabó, N.; Szabó, E.; Pataki, H.; Marosi, G.; Nagy, Z.K. Applications of machine vision in pharmaceutical technology: A review. Eur. J. Pharm. Sci. 2021, 159, 105717. [Google Scholar] [CrossRef] [PubMed]
- Barimani, S.; Kleinebudde, P. Monitoring of tablet coating processes with colored coatings. Talanta 2018, 178, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Vilímová, P.; Peikertová, P.; Kulhánková, L.; Řeháčková, L.; Tokarský, J. Polyaniline as a Precursor of Multi-Layer Graphene: Microscopic and Microspectroscopic Study. J. Nanosci. Nanotechnol. 2019, 19, 7736–7747. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.S.; Bajracharya, R.; Lee, S.H.; Han, H.K. Pharmaceutical Application of Tablet Film Coating. Pharmaceutics 2020, 12, 853. [Google Scholar] [CrossRef]
- Mészáros, L.A.; Galata, D.L.; Madarász, L.; Köte, Á.; Csorba, K.; Dávid, Á.Z.; Domokos, A.; Szabó, E.; Nagy, B.; Marosi, G.; et al. Digital UV/VIS imaging: A rapid PAT tool for crushing strength, drug content and particle size distribution determination in tablets. Int. J. Pharm. 2020, 578, 119174. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Dong, Y.; Markl, D.; Williams, B.M.; Zheng, Y.; Shen, Y.; Zeitler, J.A. Measurement of the Intertablet Coating Uniformity of a Pharmaceutical Pan Coating Process With Combined Terahertz and Optical Coherence Tomography In-Line Sensing. J. Pharm. Sci. 2017, 106, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- May, R.K.; Evans, M.J.; Zhong, S.; Warr, I.; Gladden, L.F.; Shen, Y.; Zeitler, J.A. Terahertz in-line sensor for direct coating thickness measurement of individual tablets during film coating in real-time. J. Pharm. Sci. 2011, 100, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Oman Kadunc, N.; Sibanc, R.; Dreu, R.; Likar, B.; Tomaževič, D. In-line monitoring of pellet coating thickness growth by means of visual imaging. Int. J. Pharm. 2014, 470, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Možina, M.; Tomaževič, D.; Pernuš, F.; Likar, B. Automated visual inspection of imprint quality of pharmaceutical tablets. Mach. Vis. Appl. 2013, 24, 63–73. [Google Scholar] [CrossRef]
- Lin, H.; Dong, Y.; Markl, D.; Zhang, Z.; Shen, Y.; Zeitler, J.A. Pharmaceutical Film Coating Catalog for Spectral Domain Optical Coherence Tomography. J. Pharm. Sci. 2017, 106, 3171–3176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möltgen, C.V.; Herdling, T.; Reich, G. A novel multivariate approach using science-based calibration for direct coating thickness determination in real-time NIR process monitoring. Eur. J. Pharm. Biopharm. 2013, 85, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Wahl, P.R.; Peter, A.; Wolfgang, M.; Khinast, J.G. How to measure coating thickness of tablets: Method comparison of optical coherence tomography, near-infrared spectroscopy and weight-, height- and diameter gain. Eur. J. Pharm. Biopharm. 2019, 142, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Radtke, J.; Rehbaum, H.; Kleinebudde, P. Raman Spectroscopy as a PAT-Tool for Film-Coating Processes: In-Line Predictions using one PLS Model for Different Cores. Pharmaceutics 2020, 12, 796. [Google Scholar] [CrossRef]
- Kim, B.; Woo, Y.A. Coating process optimization through in-line monitoring for coating weight gain using Raman spectroscopy and design of experiments. J. Pharm. Biomed. Anal. 2018, 154, 278–284. [Google Scholar] [CrossRef]
- Shi, G.; Lin, L.; Liu, Y.; Chen, G.; Luo, Y.; Wu, Y.; Li, H. Pharmaceutical application of multivariate modelling techniques: A review on the manufacturing of tablets. RSC Adv. 2021, 11, 8323–8345. [Google Scholar] [CrossRef]
- Mukharya, A.; Patel, P.U.; Chaudhary, S. Effect assessment of “film coating and packaging” on the photo-stability of highly photo-labile antihypertensive products. Int. J. Pharm. Investig. 2013, 3, 77–87. [Google Scholar] [CrossRef] [Green Version]





| λ Range (nm) | 335–380 | 400–540 | 480–600 | 590–720 | 700–1100 | 1000–1700 | 1700–2500 |
|---|---|---|---|---|---|---|---|
| Type of Tablet | THR (a.u.), M ± SD | ||||||
| Unexpired | 0.135 ± 0.027 | 0.499 ± 0.013 | 0.784 ± 0.016 | 0.941 ± 0.026 | 1.002 ± 0.012 | 0.849 ± 0.011 | 0.511 ± 0.015 |
| Expired | 0.099 ± 0.021 | 0.483 ± 0.018 | 0.768 ± 0.021 | 0.920 ± 0.022 | 0.994 ± 0.014 | 0.849 ± 0.013 | 0.509 ± 0.016 |
| Stressed | 0.130 ± 0.032 | 0.511 ± 0.005 | 0.787 ± 0.007 | 0.951 ± 0.009 | 1.004 ± 0.010 | 0.856 ± 0.009 | 0.523 ± 0.017 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | 0.021 | 0.023 | 0.006 |
| Type of Tablet | TIS, M ± SD | p | σ, M ± SD | p |
|---|---|---|---|---|
| Unexpired | 0.944 ± 0.197 | 0.129 | 76.071 ± 49.817 | 0.716 |
| Expired | 1.073 ± 0.254 | 77.939 ± 47.761 | ||
| Stressed | 0.914 ± 0.234 | 76.701 ± 50.707 |
| Type of Tablet | Emissivity (ε), M ± SD | p |
|---|---|---|
| Unexpired | 0.326 ± 0.286 | 0.103 |
| Expired | 0.340 ± 0.293 | |
| Stressed | 0.319 ± 0.299 |
| Type of Tablet | Density (g/cm3), M ± SD | p |
|---|---|---|
| Unexpired | 1.193 ± 0.003 | <0.001 * |
| Expired | 1.191 ± 0.002 | |
| Stressed | 1.180 ± 0.003 |
| Type of Tablet | Bottom Surface (µm), M ± SD | p | Side Surface (µm), M ± SD | p |
|---|---|---|---|---|
| Unexpired | 42.000 ± 8.052 | <0.001 * | 29.700 ± 8.571 | <0.001 ** |
| Expired | 30.367 ± 7.261 | 25.633 ± 6.960 | ||
| Stressed | 22.345 ± 4.328 | 20.429 ± 6.131 |
| Type of Tablet | M ± SD | p |
|---|---|---|
| Unexpired | 0.327 ± 0.114 | <0.001 * |
| Expired | 0.304 ± 0.101 | |
| Stressed | 0.254 ± 0.070 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarecka-Hujar, B.; Szulc-Musioł, B.; Meisner, M.; Duda, P. The Use of Novel, Rapid Analytical Tools in the Assessment of the Stability of Tablets—A Pilot Analysis of Expired and Unexpired Tablets Containing Nifuroxazide. Processes 2022, 10, 1934. https://doi.org/10.3390/pr10101934
Sarecka-Hujar B, Szulc-Musioł B, Meisner M, Duda P. The Use of Novel, Rapid Analytical Tools in the Assessment of the Stability of Tablets—A Pilot Analysis of Expired and Unexpired Tablets Containing Nifuroxazide. Processes. 2022; 10(10):1934. https://doi.org/10.3390/pr10101934
Chicago/Turabian StyleSarecka-Hujar, Beata, Beata Szulc-Musioł, Michał Meisner, and Piotr Duda. 2022. "The Use of Novel, Rapid Analytical Tools in the Assessment of the Stability of Tablets—A Pilot Analysis of Expired and Unexpired Tablets Containing Nifuroxazide" Processes 10, no. 10: 1934. https://doi.org/10.3390/pr10101934
APA StyleSarecka-Hujar, B., Szulc-Musioł, B., Meisner, M., & Duda, P. (2022). The Use of Novel, Rapid Analytical Tools in the Assessment of the Stability of Tablets—A Pilot Analysis of Expired and Unexpired Tablets Containing Nifuroxazide. Processes, 10(10), 1934. https://doi.org/10.3390/pr10101934








