In Vitro Digestion and Fecal Fermentation of Polysaccharides from Hawthorn and Its Impacts on Human Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. In Vitro Digestion of HPS
2.2.1. Simulated Saliva–Gastrointestinal Digestion
2.2.2. Determination of the Content of Reducing Sugars
2.2.3. Determination of Free Monosaccharide
2.2.4. Infrared Spectroscopy Analysis of Digestion Products
2.2.5. Scanning Electron Microscope (SEM) Analysis
2.3. In Vitro Fermentation of HPS
2.3.1. In Vitro Fermentation Using Human Fecal Inoculum
2.3.2. Determination of pH, CR and Carbohydrate Content during In Vitro Fermentation
2.3.3. Determination of Monosaccharide
2.3.4. Determination of SCFAs
2.3.5. Gut Microbiota Analysis during In Vitro Fermentation
2.4. Statistical Analysis
3. Results and Discussion
3.1. Dynamic Changes in HPS during In Vitro Digestion
3.1.1. Changes in CR in HPS
3.1.2. Changes in Free Monosaccharides in HPS
3.1.3. Changes in FT-IR Spectra
3.1.4. Surface Structure of HPS during In Vitro Digestion
3.2. Dynamic Changes in HPS during In Vitro Fermentation
3.2.1. Changes in pH, CR, and Residual Carbohydrates in HPS
3.2.2. Changes in Free Monosaccharides in HPS
3.2.3. Effects of HPS on SCFAs Production
3.2.4. Effects of HPS on Gut Microbiota
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Q.; Du, J.; Jiang, Y.; Goff, H.D.; Cui, S.W. Pectic polysaccharides from hawthorn: Physicochemical and partial structural characterization. Food Hydrocoll. 2019, 90, 146–153. [Google Scholar] [CrossRef]
- Chai, W.M.; Chen, C.M.; Gao, Y.S.; Feng, H.L.; Ding, Y.M.; Shi, Y.; Zhou, H.T.; Chen, Q.X. Structural analysis of proanthocyanidins isolated from fruit stone of Chinese hawthorn with potent antityrosinase and antioxidant activity. J. Agric. Food Chem. 2014, 62, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, L.; Xing, Y.; Yang, S.; Li, H.; Cao, Y. Roles and Mechanisms of Hawthorn and Its Extracts on Atherosclerosis: A Review. Front. Pharm. 2020, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Ritchie, H.E.; Brown-Woodman, P.D. A reproductive screening test of hawthorn. J. Ethnopharmacol. 2008, 118, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, G.B.; Tang, X.; Zhang, C.; Zhao, W.; Wang, J.; Chen, H. Anti-cancer potential of polysaccharide extracted from hawthorn (Crataegus.) on human colon cancer cell line HCT116 via cell cycle arrest and apoptosis. J. Funct. Foods 2020, 64, 103677. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Huang, Z.; Xie, W.; Tong, C.; Bao, R.; Sun, X.; Li, W.; Li, S. Pectin oligosaccharide from hawthorn fruit ameliorates hepatic inflammation via NF-κB inactivation in high-fat diet fed mice. J. Funct. Foods 2019, 57, 345–350. [Google Scholar] [CrossRef]
- Yu, Z.; Song, G.; Liu, J.; Wang, J.; Zhang, P.; Chen, K. Beneficial effects of extracellular polysaccharide from Rhizopus nigricans on the intestinal immunity of colorectal cancer mice. Int. J. Biol. Macromol. 2018, 115, 718–726. [Google Scholar] [CrossRef]
- Li, X.; Guo, R.; Wu, X.; Liu, X.; Ai, L.; Sheng, Y.; Song, Z.; Wu, Y. Dynamic digestion of tamarind seed polysaccharide: Indigestibility in gastrointestinal simulations and gut microbiota changes in vitro. Carbohydr. Polym. 2020, 239, 116194. [Google Scholar] [CrossRef]
- Carnachan, S.M.; Bootten, T.J.; Mishra, S.; Monro, J.A.; Sims, I.M. Effects of simulated digestion in vitro on cell wall polysaccharides from kiwifruit (Actinidia spp.). Food Chem. 2012, 133, 132–139. [Google Scholar] [CrossRef]
- Thondre, P.S.; Monro, J.A.; Mishra, S.; Henry, C.J.K. High molecular weight barley β-glucan decreases particle breakdown in chapattis (Indian flat breads) during in vitro digestion. Food Res. Int. 2010, 43, 1476–1481. [Google Scholar] [CrossRef]
- Yun, L.; Li, D.; Yang, L.; Zhang, M. Hot water extraction and artificial simulated gastrointestinal digestion of wheat germ polysaccharide. Int. J. Biol. Macromol. 2019, 123, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shao, G.; Yang, J.; Zhao, H.; Qu, D.; Zhang, D.; Zhu, D.; He, Y.; Liu, H. Contribution of soybean polysaccharides in digestion of oil-in-water emulsion-based delivery system in an in vitro gastric environment. Food Sci. Technol. Int. 2020, 26, 444–452. [Google Scholar] [CrossRef]
- Yu, M.; Xiao, B.; Hao, X.; Tan, J.; Gu, J.; Wang, G.; Wang, W.; Zhang, Y. Pumpkin polysaccharide preparation, simulated gastrointestinal digestion, and in vivo biodistribution. Int. J. Biol. Macromol. 2019, 141, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.R.; Liu, W.; Su, Y.; Han, Q.H.; Zhao, L.; Zhang, Q.; Lin, D.R.; et al. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Duan, X.Y.; Duan, S.Q.; Li, C.; Hu, B.; Liu, A.P.; Wu, Y.L.; Wu, H.J.; Chen, H.; Wu, W.J. Effects ofin vitrodigestion and fecal fermentation on the stability and metabolic behavior of polysaccharides from Craterellus cornucopioides. Food Funct. 2020, 11, 6899–6910. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.; He, X.; Wang, S.; Liu, F.; Huang, Q.; Fu, X.; Chen, T.; Zhang, B. Cell Wall Integrity of Pulse Modulates the in Vitro Fecal Fermentation Rate and Microbiota Composition. J. Agric. Food Chem. 2020, 68, 1091–1100. [Google Scholar] [CrossRef]
- Wu, D.-T.; Yuan, Q.; Guo, H.; Fu, Y.; Li, F.; Wang, S.-P.; Gan, R.-Y. Dynamic changes of structural characteristics of snow chrysanthemum polysaccharides during in vitro digestion and fecal fermentation and related impacts on gut microbiota. Food Res. Int. 2021, 141, 109888. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; He, Y.; Xiang, P.Y.; Wang, S.P.; Cao, Z.W.; Gou, T.; Shen, M.M.; Zhao, L.; Qin, W.; Gan, R.Y.; et al. Effects of simulated saliva-gastrointestinal digestion on the physicochemical properties and bioactivities of okra polysaccharides. Carbohydr. Polym. 2020, 238, 116183. [Google Scholar] [CrossRef]
- Wu, D.T.; Fu, Y.; Guo, H.; Yuan, Q.; Nie, X.R.; Wang, S.P.; Gan, R.Y. In vitro simulated digestion and fecal fermentation of polysaccharides from loquat leaves: Dynamic changes in physicochemical properties and impacts on human gut microbiota. Int. J. Biol. Macromol. 2021, 168, 733–742. [Google Scholar] [CrossRef]
- Han, X.; Bai, B.; Zhou, Q.; Niu, J.; Yuan, J.; Zhang, H.; Jia, J.; Zhao, W.; Chen, H. Dietary supplementation with polysaccharides from Ziziphus Jujuba cv. Pozao intervenes in immune response via regulating peripheral immunity and intestinal barrier function in cyclophosphamide-induced mice. Food Funct. 2020, 11, 5992–6006. [Google Scholar] [CrossRef]
- Feng, X.; Bie, N.; Li, J.; Zhang, M.; Feng, Y.; Ding, T.; Zhao, Y.; Wang, C. Effect of in vitro simulated gastrointestinal digestion on the antioxidant activity, molecular weight, and microstructure of polysaccharides from Chinese yam. Int. J. Biol. Macromol. 2022, 207, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Cao, C.; Ren, B.; Zhang, B.; Huang, Q.; Li, C. Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota. Carbohydr. Polym. 2018, 183, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.B.; Ye, G.Y.; Li, G.C.; Cao, H.; Wang, Z.H.; Ji, S.G. RID serve as a more appropriate measure than phenol sulfuric acid method for natural water-soluble polysaccharides quantification. Carbohydr. Polym. 2022, 278, 12. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.-R.; Fu, Y.; Wu, D.-T.; Huang, T.-T.; Jiang, Q.; Zhao, L.; Zhang, Q.; Lin, D.-R.; Chen, H.; Qin, W. Ultrasonic-Assisted Extraction, Structural Characterization, Chain Conformation, and Biological Activities of a Pectic-Polysaccharide from Okra (Abelmoschus esculentus). Molecules 2020, 25, 1155. [Google Scholar] [CrossRef]
- Wu, D.T.; Liu, W.; Han, Q.H.; Du, G.; Li, H.Y.; Yuan, Q.; Fu, Y.; Zhao, L.; Zhang, Q.; Li, S.Q.; et al. Physicochemical characteristics and antioxidant activities of non-starch polysaccharides from different kiwifruits. Int. J. Biol. Macromol. 2019, 136, 891–900. [Google Scholar] [CrossRef]
- Wu, D.-T.; Nie, X.-R.; Gan, R.-Y.; Guo, H.; Fu, Y.; Yuan, Q.; Zhang, Q.; Qin, W. In vitro digestion and fecal fermentation behaviors of a pectic polysaccharide from okra (Abelmoschus esculentus) and its impacts on human gut microbiota. Food Hydrocoll. 2021, 114, 106577. [Google Scholar] [CrossRef]
- Li, W.; Wang, C.; Yuan, G.; Pan, Y.; Chen, H. Physicochemical characterisation and α-amylase inhibitory activity of tea polysaccharides under simulated salivary, gastric and intestinal conditions. Int. J. Food Sci. Tech. 2018, 53, 423–429. [Google Scholar] [CrossRef]
- Guo, D.; Lei, J.; He, C.; Peng, Z.; Liu, R.; Pan, X.; Meng, J.; Feng, C.; Xu, L.; Cheng, Y.; et al. In vitro digestion and fermentation by human fecal microbiota of polysaccharides from Clitocybe squamulose. Int. J. Biol. Macromol. 2022, 208, 343–355. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Cheng, Y.; Guo, Y.; Hu, B.; Yao, W.; Zhu, X.; Qian, H. Study on fecal fermentation characteristics of aloe polysaccharides in vitro and their predictive modeling. Carbohydr. Polym. 2021, 256, 117571. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X.; Liu, R.H. In vitro digestibility and prebiotic potential of a novel polysaccharide from Rosa roxburghii Tratt fruit. J. Funct. Foods 2019, 52, 408–417. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Huang, F.; Yang, C.; Huang, Q. In Vitro Digestion and Fermentation by Human Fecal Microbiota of Polysaccharides from Flaxseed. Molecules 2020, 25, 4354. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.M.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; Francois, P.; de Vos, W.M.; et al. Responses of Gut Microbiota and Glucose and Lipid Metabolism to Prebiotics in Genetic Obese and Diet-Induced Leptin-Resistant Mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, J.; Ge, X.; Xu, W.; Chen, Y.; Li, F.; Cheng, D.; Shao, R. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from Helicteres angustifolia L. Int. J. Biol. Macromol. 2019, 141, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Aweya, J.J.; Huang, Z.-X.; Kang, Z.-Y.; Bai, Z.-H.; Li, K.-H.; He, X.-T.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydr. Polym. 2020, 234, 115894. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, P.; Ma, C.; Tang, J.; Zhang, X. Gut microbiota, host health, and polysaccharides. Biotechnol. Adv. 2013, 31, 318–337. [Google Scholar] [CrossRef]
- Kimura, I. Host Energy Regulation via SCFAs Receptors, as Dietary Nutrition Sensors, by Gut Microbiota. Yakugaku Zasshi 2014, 134, 1037–1042. [Google Scholar] [CrossRef]
- Ferchaud-Roucher, V.; Pouteau, E.; Piloquet, H.; Zair, Y.; Krempf, M. Colonic fermentation from lactulose inhibits lipolysis in overweight subjects. Am. J. Physiol.-Endocrinol. Metab. 2005, 289, E716–E720. [Google Scholar] [CrossRef]
- Rodriguez-Cabezas, M.E.; Galvez, J.; Lorente, M.D.; Concha, A.; Camuesco, D.; Azzouz, S.; Osuna, A.; Redondo, L.; Zarzuelo, A. Dietary fiber down-regulates colonic tumor necrosis factor alpha and nitric oxide production in trinitrobenzenesulfonic acid-induced colitic rats. J. Nutr. 2002, 132, 3263–3271. [Google Scholar] [CrossRef]
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Xu, S.Y.; Chen, X.Q.; Liu, Y.; Cheong, K.L. Ultrasonic/microwave-assisted extraction, simulated digestion, and fermentation in vitro by human intestinal flora of polysaccharides from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 152, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yan, Y.; Peng, Y.; Chen, D.; Mi, J.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. In vitro digestion under simulated saliva, gastric and small intestinal conditions and fermentation by human gut microbiota of polysaccharides from the fruits of Lycium barbarum. Int. J. Biol. Macromol. 2019, 125, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Yasukawa, Z.; Inoue, R.; Ozeki, M.; Okubo, T.; Takagi, T.; Honda, A.; Naito, Y. Effect of Repeated Consumption of Partially Hydrolyzed Guar Gum on Fecal Characteristics and Gut Microbiota: A Randomized, Double-Blind, Placebo-Controlled, and Parallel-Group Clinical Trial. Nutrients 2019, 11, 2170. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Qian, C.; Li, N.; Fang, Z.; Zhao, J.; Zhang, H.; Chen, W.; Liu, Z.; Lu, W. Protein diets with the role of immune and gut microbial regulation alleviate DSS-induced chronic ulcerative colitis. Food Sci. Nutr. 2021, 9, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Tsoi, H.; Yu, J. Fusobacterium and Escherichia: Models of colorectal cancer driven by microbiota and the utility of microbiota in colorectal cancer screening. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 651–657. [Google Scholar] [CrossRef] [PubMed]

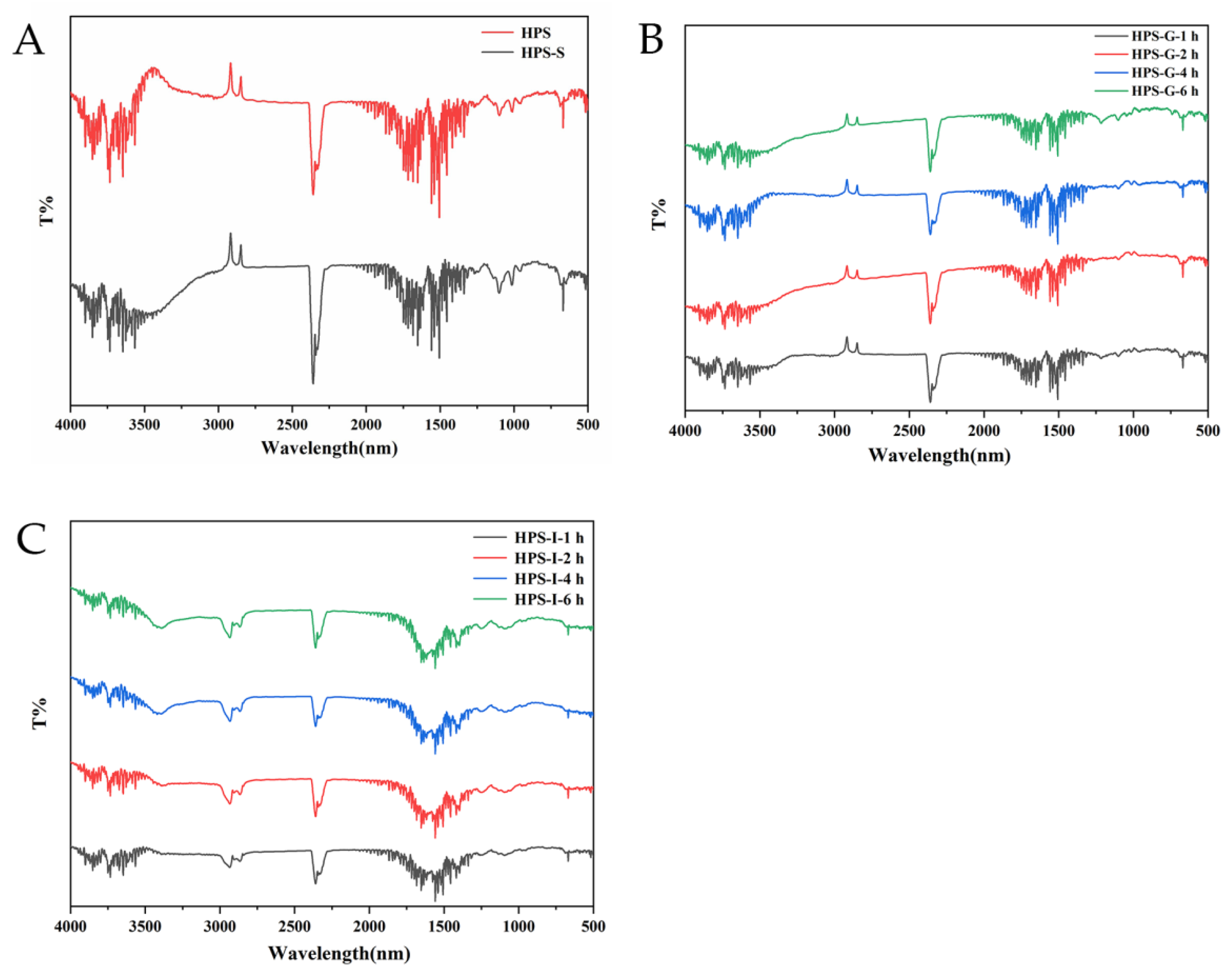


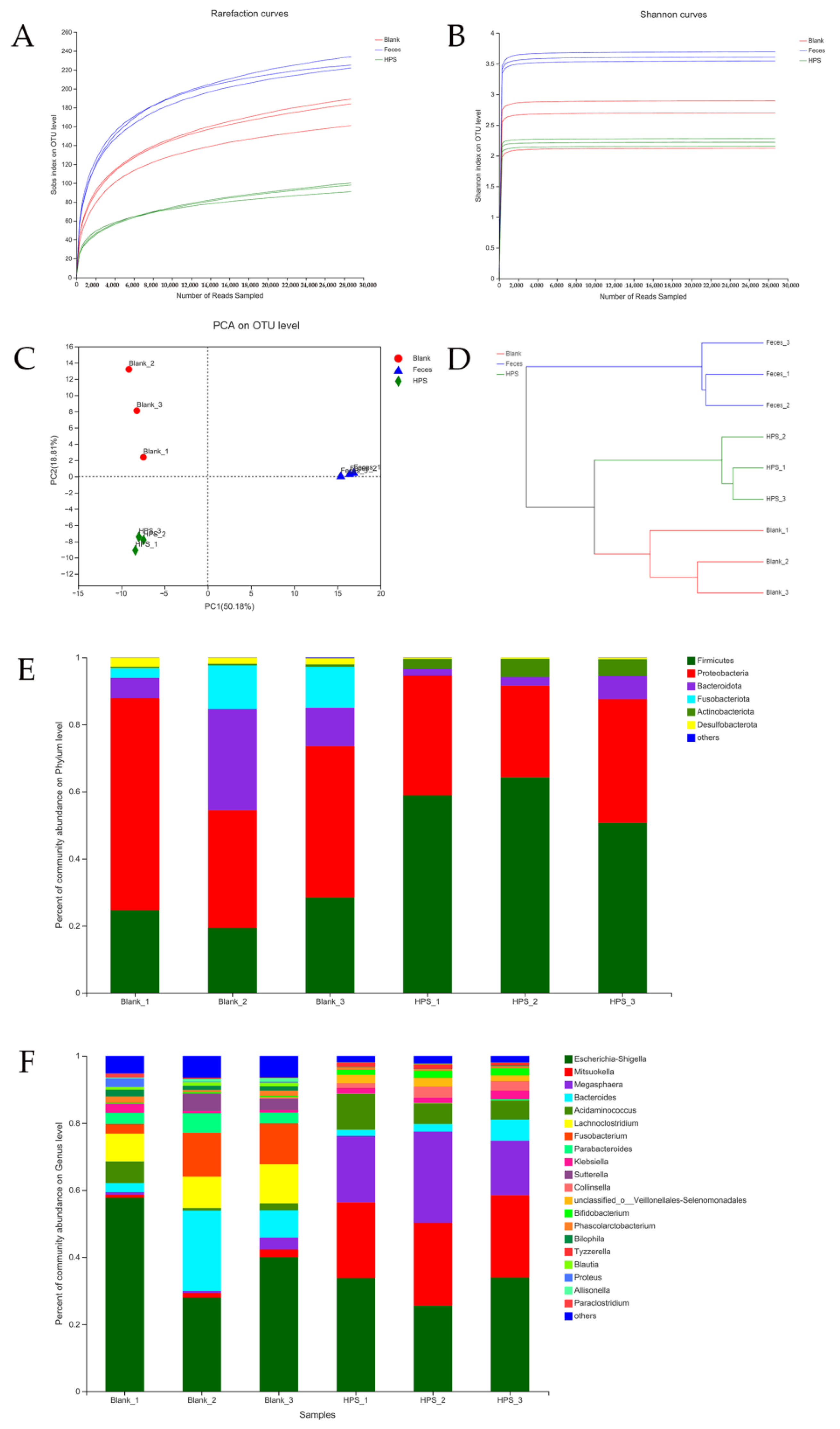
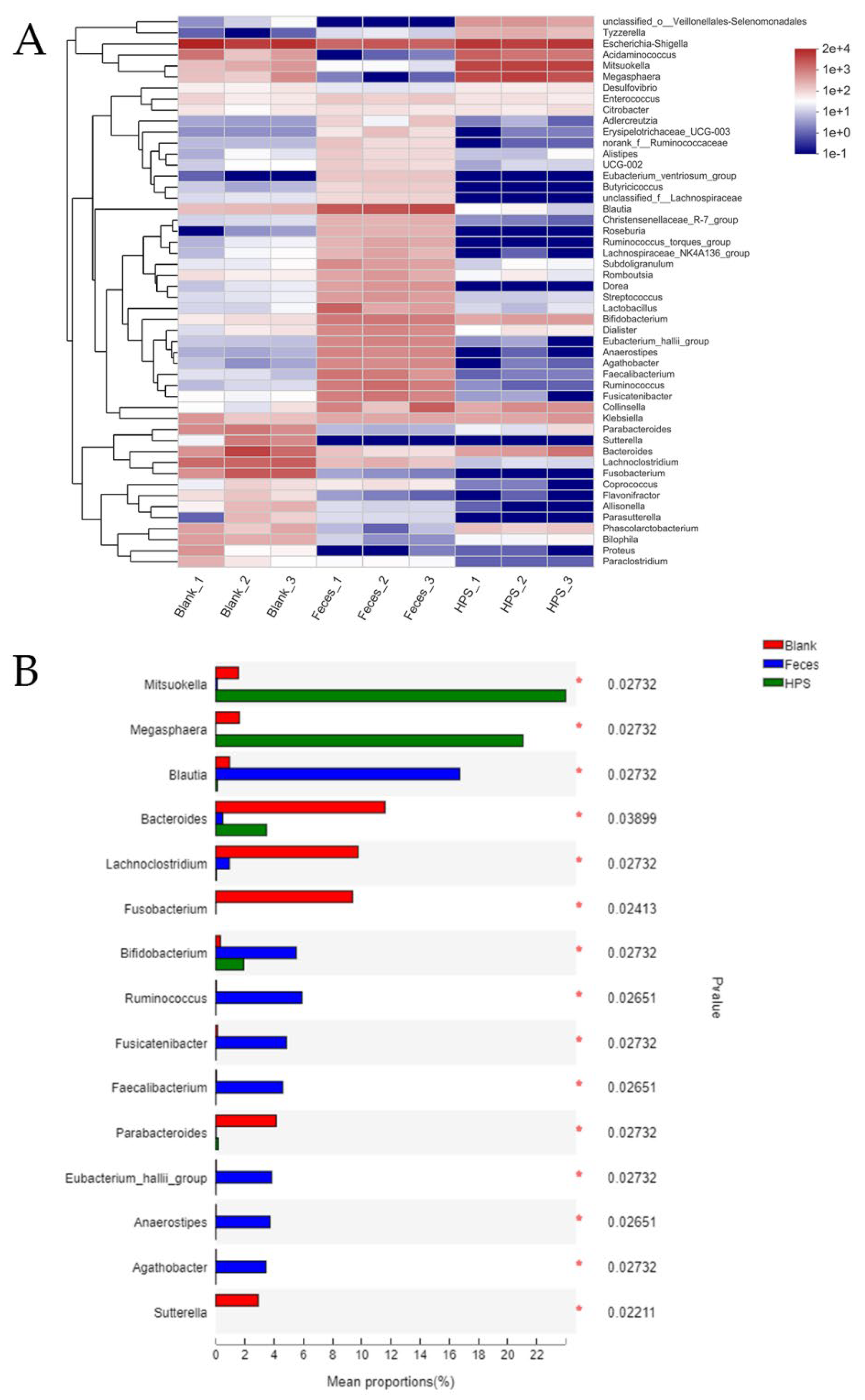
| Processes | Time (h) | CR (mg/mL) |
|---|---|---|
| Origin | - | 0.082 ± 0.0041 a |
| Saliva digestion | 0.05 | 0.083 ± 0.0023 a |
| Saliva–gastric digestion | 1 | 0.100 ± 0.0055 b |
| 2 | 0.102 ± 0.0082 b | |
| 4 | 0.103 ± 0.0010 b | |
| 6 | 0.104 ± 0.0077 c | |
| Saliva–gastrointestinal digestion | 1 | 0.105 ± 0.0061 c |
| 2 | 0.106 ± 0.0056 c | |
| 4 | 0.107 ± 0.0042 c | |
| 6 | 0.107 ± 0.0064 c |
| Groups | Time (h) | pH |
|---|---|---|
| Blank | 0 | 8.29 ± 0.046 a |
| 6 | 7.53 ± 0.044 b | |
| 12 | 7.37 ± 0.044 c | |
| 24 | 7.19 ± 0.026 c | |
| HPS | 0 | 7.37 ± 0.026 d |
| 6 | 6.54 ± 0.035 e | |
| 12 | 6.17 ± 0.026 f | |
| 24 | 5.37 ± 0.026 g |
| Content | Time (h) | HPS |
|---|---|---|
| CR (mg/mL) | 0 | 0.388 ± 0.0060 a |
| 6 | 0.437 ± 0.0040 b | |
| 12 | 0.301 ± 0.0079 c | |
| 24 | 0.300 ± 0.0080 c | |
| Residual carbohydrate (%) | 0 | 100.00 ± 0.0600 a |
| 6 | 97.09 ± 0.1967 b | |
| 12 | 96.51 ± 0.1808 c | |
| 24 | 94.77 ± 0.1374 d |
| Groups. | Chao1 | ACE | Shannon | Simpson |
|---|---|---|---|---|
| Blank | 230.27 ± 12.57 a | 211.52 ± 10.99 a | 2.57 ± 0.40 a | 0.179 ± 0.096 a |
| Original feces | 253.74 ± 12.05 a | 249.62 ± 10.50 b | 3.62 ± 0.07 b | 0.048 ± 0.006 b |
| HPS | 129.36 ± 14.99 b | 129.64 ± 13.11 c | 2.22 ± 0.06 a | 0.216 ± 0.115 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, K.; Zhou, Q.; Han, X.; Gao, Z.; Peng, R.; Lin, X.; Cheng, X.; Zhao, W. In Vitro Digestion and Fecal Fermentation of Polysaccharides from Hawthorn and Its Impacts on Human Gut Microbiota. Processes 2022, 10, 1922. https://doi.org/10.3390/pr10101922
Zhou K, Zhou Q, Han X, Gao Z, Peng R, Lin X, Cheng X, Zhao W. In Vitro Digestion and Fecal Fermentation of Polysaccharides from Hawthorn and Its Impacts on Human Gut Microbiota. Processes. 2022; 10(10):1922. https://doi.org/10.3390/pr10101922
Chicago/Turabian StyleZhou, Kaixuan, Qian Zhou, Xue Han, Zhe Gao, Ruyan Peng, Xuan Lin, Xinlong Cheng, and Wen Zhao. 2022. "In Vitro Digestion and Fecal Fermentation of Polysaccharides from Hawthorn and Its Impacts on Human Gut Microbiota" Processes 10, no. 10: 1922. https://doi.org/10.3390/pr10101922




