Exploring the Molecular Mechanism of Zhi Bai Di Huang Wan in the Treatment of Systemic Lupus Erythematosus Based on Network Pharmacology and Molecular Docking Techniques
Abstract
:1. Materials and Methods
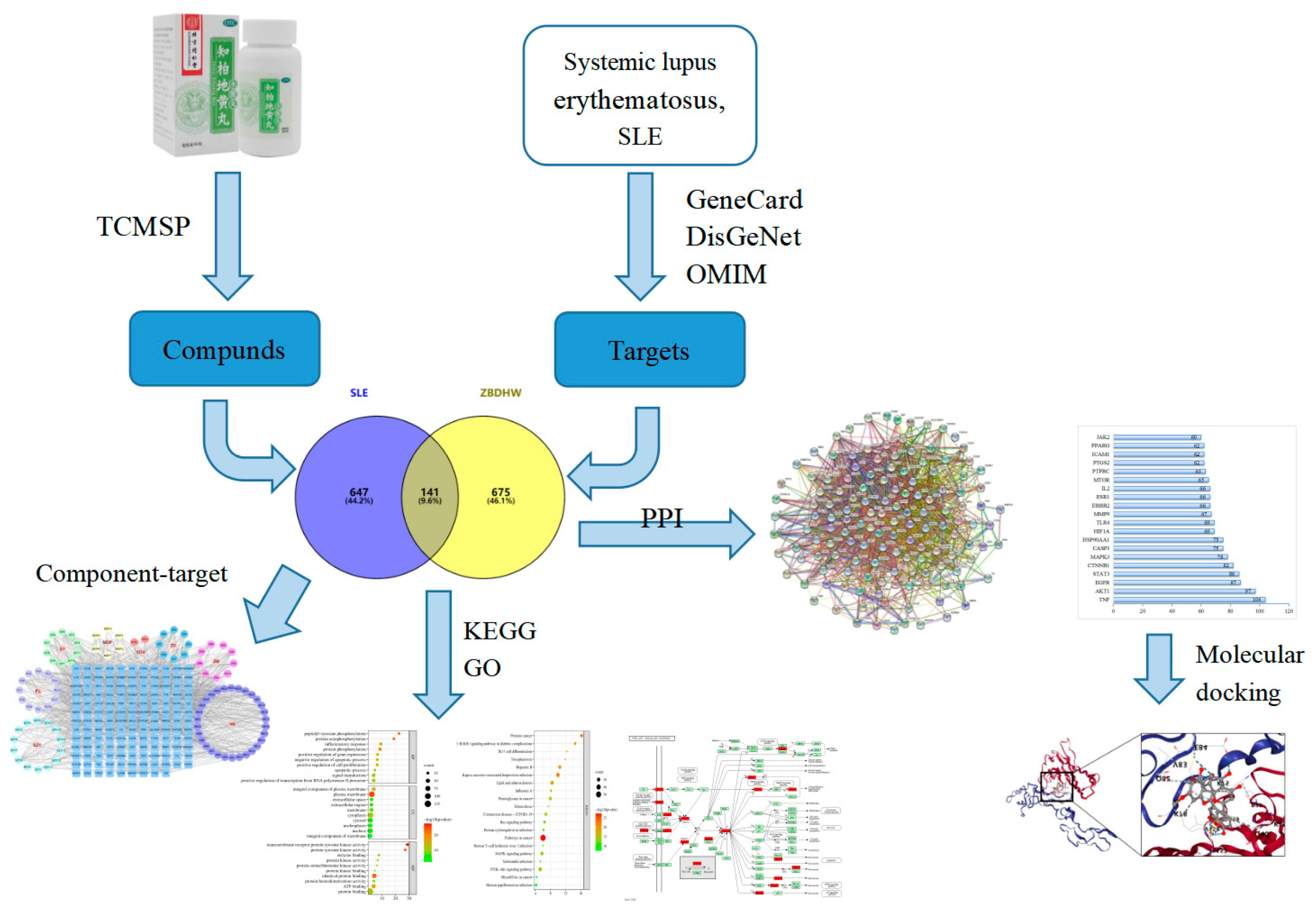
1.1. Collection of Potential Active Ingredients and Relevant Targets of ZBDHP
1.2. Collecting Targets for SLE and Constructing Veny Diagrams
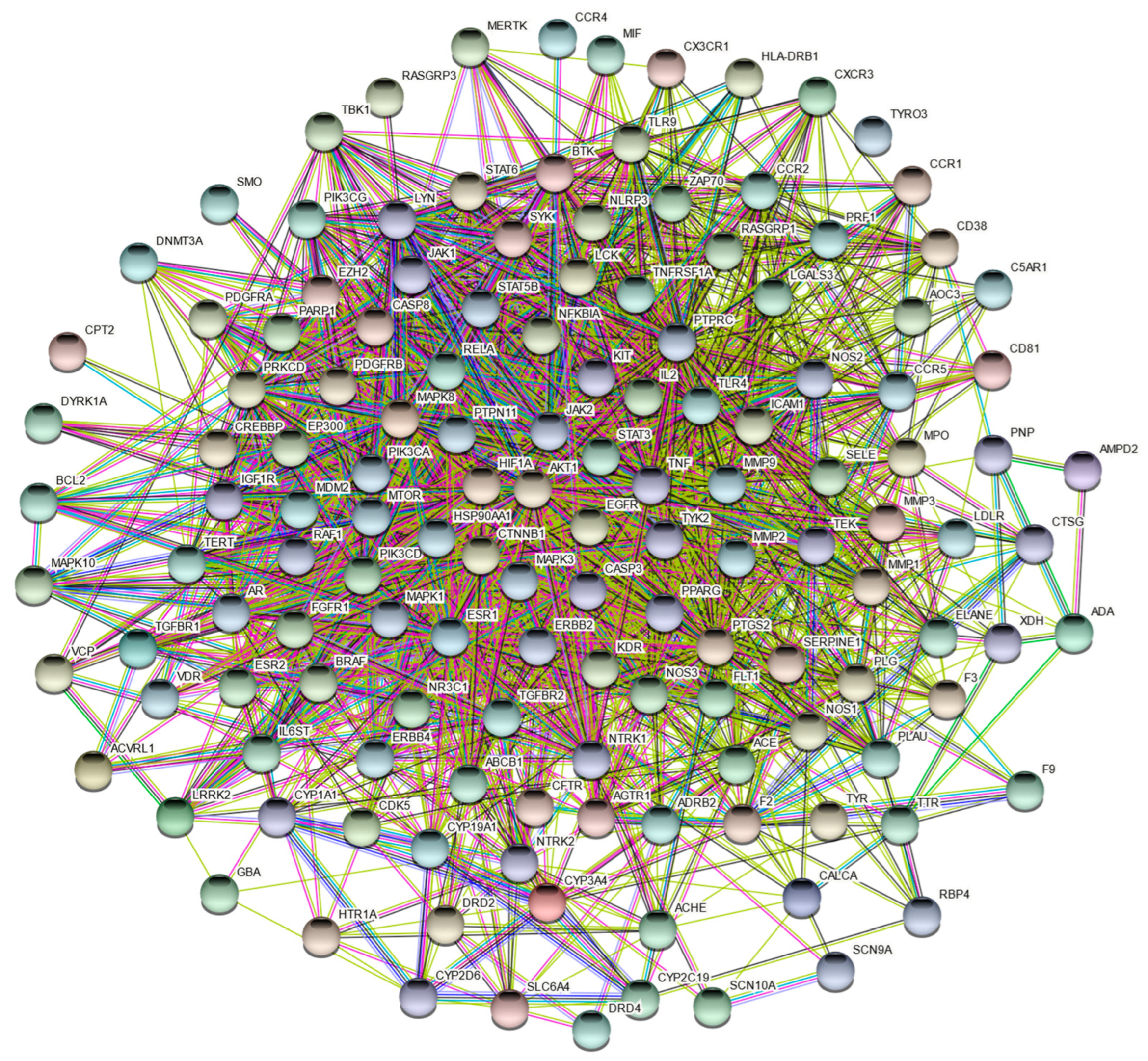
1.3. Construction of Protein Interaction Networks
1.4. Construction and Drug-Component-Common Target Gene Interaction Network
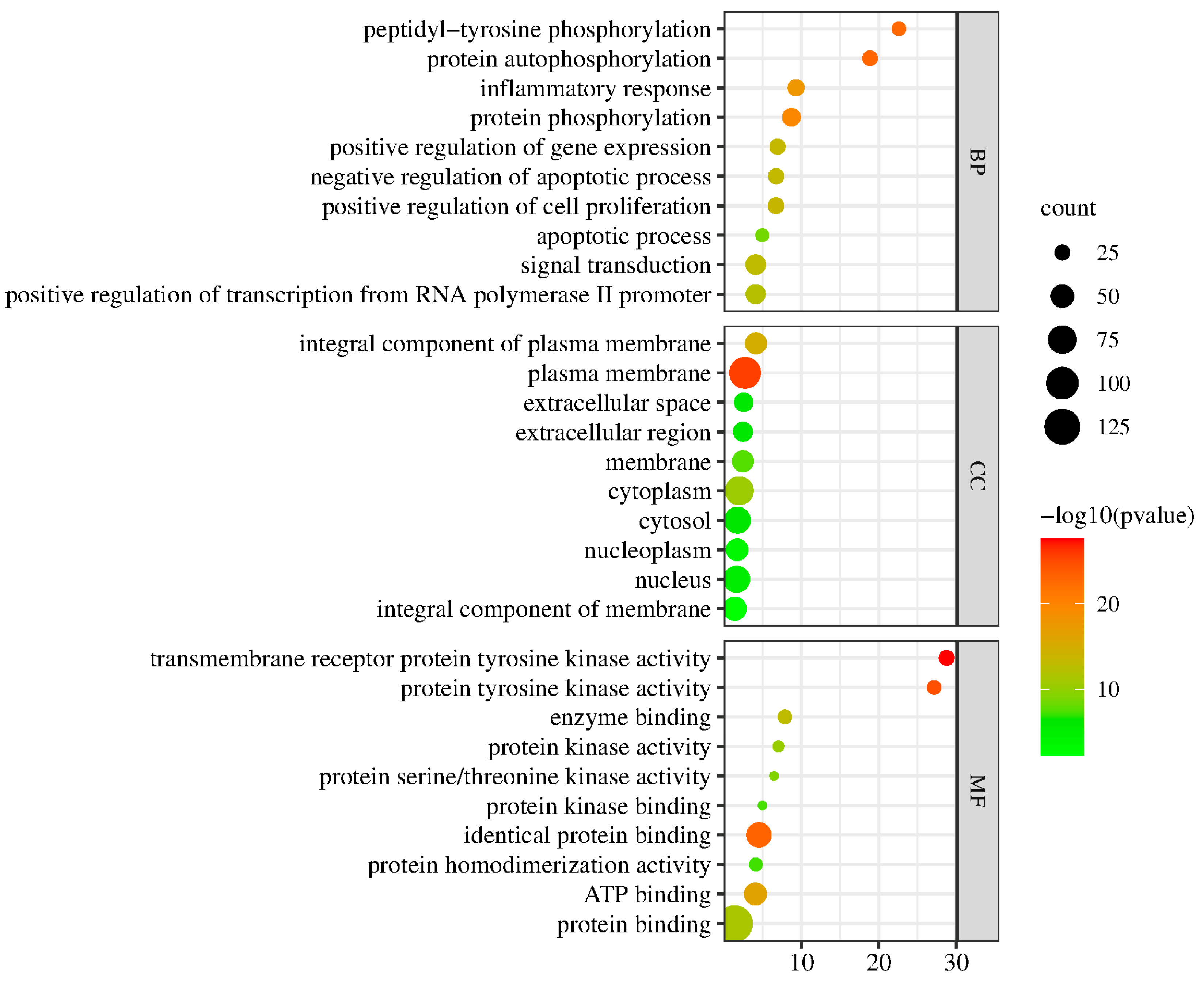
1.5. GO Functional Analysis and KEGG Pathway Enrichment Analysis
1.6. Molecular Docking Validation
2. Results
2.1. Active Ingredients and Targets of ZBDHP
2.2. Collecting Common Targets of Components and Diseases
2.3. Constructing the Interaction Network of Chinese Herbal Medicine—Active Ingredient—Intersection Target
2.4. Construction and Analysis of PPI Network
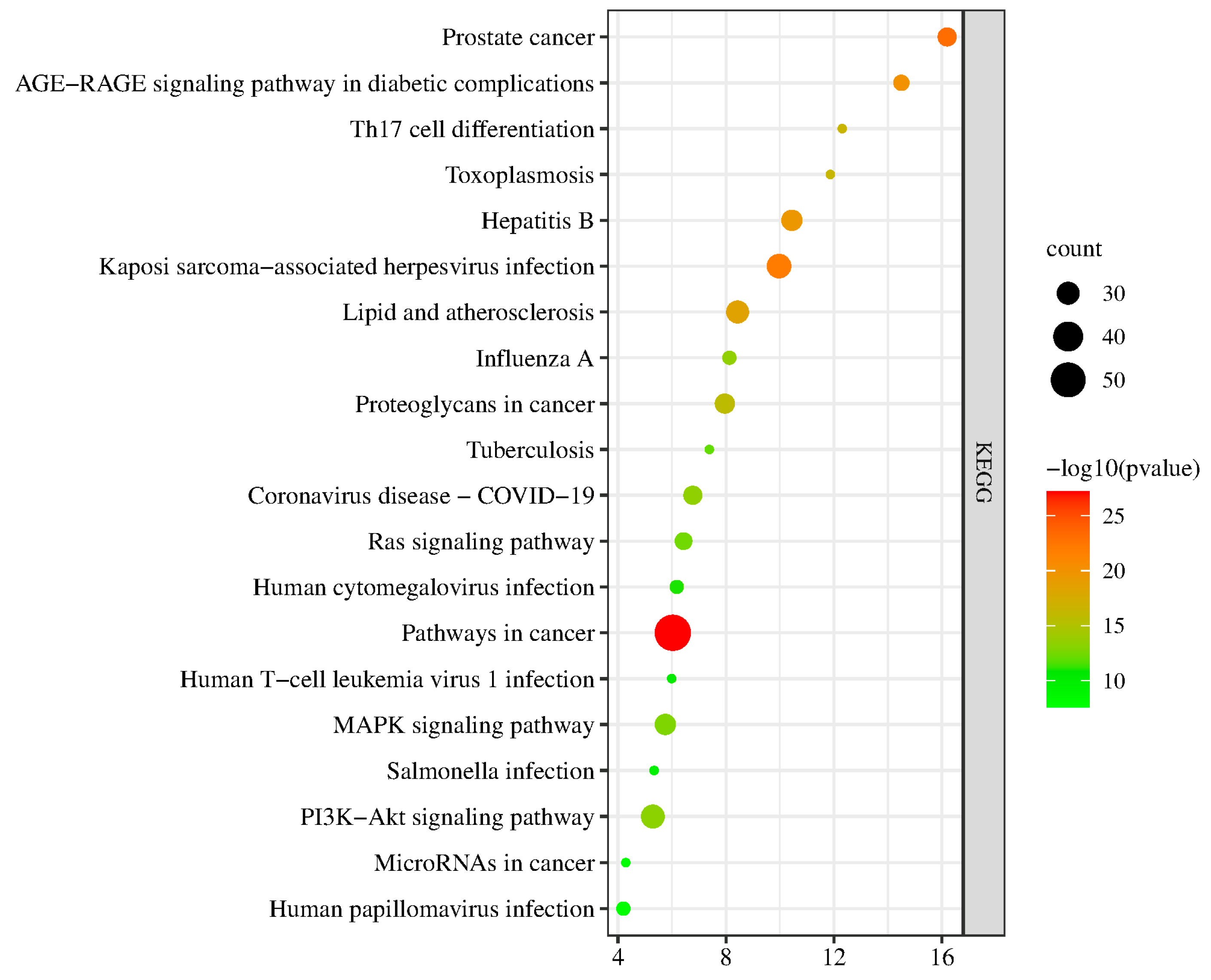
2.5. GO and KEGG Enrichment Analysis
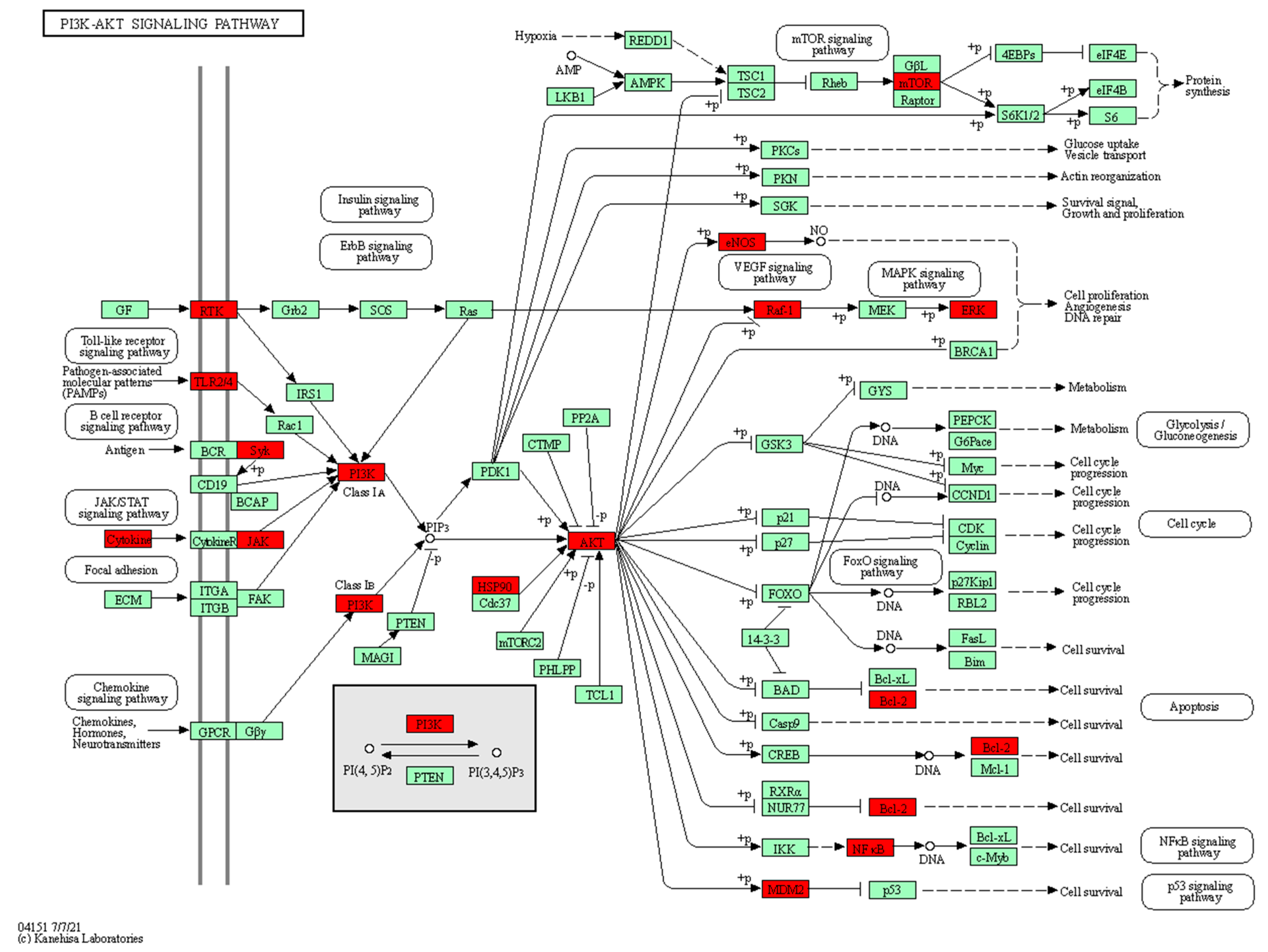
2.5.1. KEGG Enrichment Results
2.5.2. Go Analysis
2.6. Molecular Docking Validation
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crispin, J.C.; Liossis, S.N.; Kis-Toth, K.; Lieberman, L.A.; Kyttaris, V.C.; Juang, Y.T.; Tsokos, G.C. Pathogenesis of human systemic lupus erythe matosus:recent advances. Trends Mol. Med. 2010, 16, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Koo, M. Systemic Lupus Erythematosus Research: A Bibliometric Analysis over a 50-Year Period. Int. J. Environ. Res. Public Health 2021, 18, 7095. [Google Scholar] [CrossRef] [PubMed]
- Yeh, K.W.; Yu, C.H.; Chan, P.C.; Horng, J.T.; Huang, J.L. Burden of systemic lupus erythematosus in Taiwan: A population-based survey. Rheumatol. Int. 2013, 33, 1805–1811. [Google Scholar] [CrossRef]
- Li, M.T.; Zhao, Y.; Zhang, Z.Y.; Huang, C.B.; Liu, Y.; Gu, J.R.; Zhang, X.; Xu, H.J.; Li, X.F.; Wu, L.J.; et al. 2020 Chinese guidelines for the diagnosis and treatment of systemic lupus erythematosus. Rheumatol. Immunol. Res. 2020, 1, 5–23. [Google Scholar] [CrossRef]
- Wei, F.Z.; Song, Y.T.; Gong, A.M.; Pan, C.D.; Zhuang, Y.P.; Zhang, X.; Zeng, M.Y. Investigating the molecular mechanism of Xijiao Dihuang decoction for the treatment of SLE based on network pharmacology and molecular docking analysis. Bio Med Res. Int. 2022, 2022, 5882346. [Google Scholar] [CrossRef] [PubMed]
- Vanessa, O.P.; Ivana, N.A.; Cañas, C.A.; Gabriel, J.T. Mortality in systemic lupus erythematosus: Causes, predictors and interventions. Expert Rev. Clin. Immunol. 2018, 14, 1043–1053. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Shen, C.Y.; Liao, H.T.; Li, K.J.; Lee, H.T.; Lu, C.S.; Wu, C.H.; Kuo, Y.M.; Hsieh, S.C.; Yu, C.L. Molecular and Cellular Bases of Immunosenescence, Inflammation, and Cardiovascular Complications Mimicking “Inflammaging” in Patients with Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2019, 20, 3878. [Google Scholar] [CrossRef]
- Rees, F.; Doherty, M.; Grainge, M.; Lanyon, P.; Davenport, G.; Zhang, W. Mortality in Systemic Lupus Erythematosus in the UK 1999–2012; Oxford University Press: Oxford, UK, 2015; Volume 54, p. 64. [Google Scholar]
- Nguyen, N.T.; Nguyen, T.H.; Pham, T.N.H.; Huy, N.T.; Bay, M.V.; Pham, M.Q.; Nam, P.C.; Vu, V.V.; Ngo, S.T. Autodock Vina adopts more accurate binding poses but Autodock4 forms better binding affinity. J. Chem. Inf. Modeling 2020, 60, 204–211. [Google Scholar] [CrossRef]
- Wong, F.; Krishnan, A.; Zheng, E.J.; Stärk, H.; Manson, A.L.; Earl, A.M.; Jaakkola, T.; Collins, J.J. Benchmarking AlphaFold-enabled molecular docking predictions for antibiotic discovery. Mol. Syst. Biol. 2022, 18, e11081. [Google Scholar] [CrossRef]
- Sciascia, S.; Mompean, E.; Radin, M.; Roccatello, D.J.; Cuadrado, M. Rate of Adverse Effects of Medium- to High-Dose Glucocorticoid Therapy in Systemic Lupus Erythematosus: A Systematic Review of Randomized Control Trials. Clin. Drug Investig. 2017, 37, 519–524. [Google Scholar] [CrossRef]
- Guillermo, R.I.; Manel, R.C.; Pilar, B.Z.; Munthera, K.M. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: A systematic review. Ann. Rheum. Dis. 2010, 69, 20–28. [Google Scholar] [CrossRef]
- Ghaleba, R.M.; Fahmy, K.A. Premature ovarian failure in systemic lupus erythematosus patients: Is it related to cyclophosphamide treatment? Egypt. Rheumatol. Rehabil. 2019, 46, 85–91. [Google Scholar] [CrossRef]
- Wang, Y.; Han, M.; Pedigo, C.E.; Xie, Z.M.; Wang, W.J.; Liu, J.P. Chinese Herbal Medicine for Systemic Lupus Erythematosus: A Systematic Review and Meta-analysis of Randomized, Placebo- Controlled Trials. Evid. Based Integr. Med. 2021, 27, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.C.; Lin, C.C.; Li, C.I.; Chiang, J.H.; Li, T.C.; Lin, J.G. Traditional Chinese medicine therapy improves the survival of systemic lupus erythematosus patients. Semin. Arthritis Rheum. 2016, 45, 596–603. [Google Scholar] [CrossRef]
- Sun, Y.W.; Bao, Y.G.; Yu, H.; Chen, Q.J.; Lu, F.; Zhai, S.; Chun, F.; Zhang, F.L.; Wang, C.Z.; Yuan, C.S. Anti-rheumatoid arthritis effects of flavonoids from Daphne genkwa. Int. Immunopharmacol. 2020, 83, 106384. [Google Scholar] [CrossRef]
- Leu, Y.L.; Wang, T.H.; Wu, C.C.; Huang, K.Y.; Jiang, Y.W.; Hsu, Y.C.; Chen, C.Y. Hydroxygenkwanin Suppresses Non-Small Cell Lung Cancer Progression by Enhancing EGFR Degradation. Molecules 2020, 25, 941. [Google Scholar] [CrossRef]
- Lee, M.Y.; Park, B.Y.; Kwon, O.K.; Yuk, J.E.; Oh, S.R.; Kim, H.S.; Lee, H.K.; Ahn, K.S. Anti- inflammatory activity of (-)-aptosimon isolated from Daphne genkwa in RAW264.7 cells. Int. Immunopharmacol. 2009, 9, 878–885. [Google Scholar] [CrossRef]
- Zhang, C.F.; Zhang, S.L.; He, X.; Yang, X.L.; Wu, H.T.; Lin, B.Q.; Jiang, C.P.; Wang, J.; Yu, C.H.; Yang, Z.L.; et al. Antioxidant effects of Genkwa flos flavonoids on Freund’s adjuvant- induced rheumatoid arthritis in rats. J. Ethnopharmacol. 2014, 153, 793–800. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, J.H.; Zhang, Y.; An, R.B.; Min, B.S.; Joung, H.; Lee, H.K. Anti-complementary activity of protostane type triterpenes from Alismatis Rhizoma. Arch. Pharm. Res. 2003, 26, 463–465. [Google Scholar] [CrossRef]
- Zhang, R.F.; Wan, J.X.; Xu, Y.F. Alisol B inhibited complement 3a-induced human renal tubular epithelial to mesenchymal transition. Zhongguo Zhong Xi Yi Jie He Za Zhi 2012, 32, 1407–1412. [Google Scholar]
- Lee, J.W.; Kobayashi, Y.; Nakamichi, Y.; Udagawa, N.; Takahashi, N.; Im, N.K.; Seo, H.J.; Jeon, W.B.; Yonezawa, T.; Cha, B.Y.; et al. Alisol-B, a novel phyto-steroid, suppresses the RANKL-induced osteoclast formation and prevents bone loss in mice. Biochem. Pharmacol. 2010, 80, 352–361. [Google Scholar] [CrossRef]
- Muszynska, B.; Kala, K.; Firlej, A.; Sulkowska-Ziaja, K. “Cantharellus cibarius”: Culinary-medicinal mushroom content and biological activity. Acta Pol. Pharm. Drug Res. 2016, 73, 589–598. [Google Scholar]
- Aringer, M.; Feierl, E.; Steiner, G.; Stummvoll, G.H.; Höfler, E.; Steiner, C.W.; Radda, I.; Smolen, J.S.; Graninger, W.B. Increased bioactive TNF in human systemic lupus erythematosus: Associations with cell death. Lupus 2002, 11, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Vizcaya, A.; Isenberg, D.A. The use of anti-TNF-alpha therapies for patients with Systemic Lupus Erythematosus. Where are we now? Expert Opin. Biol. Ther. 2020, 21, 639–647. [Google Scholar] [CrossRef]
- Zhu, L.J.; Yang, X.; Chen, W.Y.; Li, X.Y.; Ji, Y.L.; Mao, H.P.; Nie, J.; Yu, X.Q. Decreased expressions of the TNF-alpha signaling adapters in peripheral blood mononuclear cells (PBMCs) are correlated with disease activity in patients with systemic lupus erythematosus. Clin. Rheumatol. 2007, 26, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Idborg, H.; Eketjäll, S.; Pettersson, S.; Gustafsson, J.T.; Zickert, A.; Kvarnström, M.; Oke, V.; Jakobsson, P.J.; Gunnarsson, I.; Svenungsson, E. TNF-α and plasma albumin as biomarkers of disease activity in systemic lupus erythematosus. Lupus Sci. Med. 2018, 5, e000260. [Google Scholar] [CrossRef]
- Adhya, Z.; El Anbari, M.; Anwar, S.; Mortimer, A.; Marr, N.; Karim, M.Y. Soluble TNF-R1, VEGF and other cytokines as markers of disease activity in systemic lupus erythematosus and lupus nephritis. Lupus 2019, 28, 713–721. [Google Scholar] [CrossRef]
- Martin, A. Inflammatory markers in systemic lupus erythematosus. J. Autoimmun. 2019, 110, 102374. [Google Scholar] [CrossRef]
- Aringer, M.; Graninger, W.B.; Steiner, G.; Smolen, J.S. Safety and efficacy of TNF α blockade in systemic lupus erythematosus–an open label study. Arthritis Rheum. 2004, 50, 3161–31619. [Google Scholar] [CrossRef]
- Aringer, M.; Smolen, J.S. Efficacy and safety of TNF-blocker therapy in systemic lupus erythematosus. Drug Saf. 2008, 7, 411–419. [Google Scholar] [CrossRef]
- Zhu, L.J.; Yang, X.; Yu, X.Q. Anti-TNF-α Therapies in Systemic Lupus Erythematosus. J. Biomed. Biotechnol. 2010, 2010, 465898. [Google Scholar] [CrossRef] [PubMed]
- Taher, E.T.; Kaushal, P.; Fabian, F.B.; Salvinia, M.; David, A.I.; Maikel, P.P.; Rizgar, A.M. Protein phosphorylation and kinome profiling reveal altered regulation of multiple signaling pathways in B lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2010, 62, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Sonia, G.R.; Jose-Luis, C.R.; Norberto, O.C.; Esther, Z.; Raquel, R.F.; Salvador, A.S.; Pilar, N.; Jaime, S.; Mercedes, Z. Altered AKT1 and MAPK1 Gene Expression on Peripheral Blood Mononuclear Cells and Correlation with T-Helper-Transcription Factors in Systemic Lupus Erythematosus Patients. Mediat. Inflamm. 2012, 2012, 495934. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Qin, H.; Lin, J.; Xie, L.; Chen, S.; Liang, J.; Xu, J. Downregulation of miR-633 activated AKT/mTOR pathway by targeting AKT1 in lupus CD4 T cells. Lupus 2019, 28, 510–519. [Google Scholar] [CrossRef]
- Hefny, H.M.; Abualfadl, E.M.; Youssef, E.A.M.; Ismail, M.A.; Soliman, T.M.; Ahmed, R.H.A.; Abozaid, H.S.M. Urinary epidermal growth factor as a marker for lupus nephritis: Clinical, laboratory, and histopathological study. Egypt. Rheumatol. Rehabil. 2021, 48, 13. [Google Scholar] [CrossRef]
- Ayati, A.; Moghimi, S.; Salarinejad, S.; Safavi, M.; Pouramiri, B.; Foroumadi, A. A review on progression of epidermal growth factor receptor (EGFR) inhibitors as an efficient approach in cancer targeted therapy. Bioorganic Chem. 2020, 99, 103811. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Jerusalem, G.; Laurentiis, M.D.; Im, S.; Petrakova, K.; Valeria Bianchi, G.; Martín, M.; Nusch, A.; et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: Updated overall survival. Ann. Oncol. 2021, 32, 1015–1024. [Google Scholar] [CrossRef]
- Huang, C.M.; Tsai, C.H.; Chen, C.L.; Chang, C.P.; Lai, C.C.; Tsai, F.J. Epidermal growth factor receptor (EGFR) gene Bsr I polymorphism is associated with systemic lupus erythematosus. Lupus 2004, 13, 773–776. [Google Scholar] [CrossRef]
- Bollee, G.; Flamant, M.; Schordan, S.; Fligny, C.; Rumpel, E.; Milon, M.; Schordan, E.; Sabaa, N.; Vandermeersch, S.; Galaup, A.; et al. Epidermal growth factor receptor promotes glomerular injury and renal failure in rapidly progressive crescentic glomerulonephritis. Nat. Med. 2011, 17, 1521. [Google Scholar] [CrossRef]
- Patrícia, C.R.; Russo, P.A.; Zhe, Z.; Lucrezia, C.; Kelly, M.; Stefania, G.; Schulz, S.W.; Kiani, A.N.; Michelle, P.; Sullivan, K.E. The Role of MicroRNAs and Human Epidermal Growth Factor Receptor 2 in Proliferative Lupus Nephritis. Arthritis Rheumatol. 2015, 67, 2415–2426. [Google Scholar] [CrossRef]
- Samantha, S.W.; Joel, M.G.; Eliza, F.C.; Chen, H.; Lu, R.F.; Macwana, S.; Bean, K.; Maecker, H.T.; Utz, P.J.; James, J.A. Mycophenolate mofetil reduces STAT3 phosphorylation in systemic lupus erythematosus patients. JCI Insight 2019, 4, e124575. [Google Scholar] [CrossRef]
- Chen, S.Y.; Liu, M.F.; Kuo, P.Y.; Wang, C.R. Upregulated expression of STAT3/IL-17 in patients with systemic lupus erythematosus. Clin. Rheumatol. 2019, 38, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Zhang, N.; Zhang, X.M.; Qin, M.T.; Dong, Y.D.; Lei, J. MiR-410 Down-Regulates the Expression of Interleukin-10 by Targeting STAT3 in the Pathogenesis of Systemic Lupus Erythematosus. Cell. Physiol. Biochem. 2016, 39, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Aleš, G.; Marija, H.; Tadej, A. The Role of STAT Signaling Pathways in the Pathogenesis of Systemic Lupus Erythematosus. Clin. Rev. Allergy Immunol. 2017, 52, 164–181. [Google Scholar] [CrossRef]
- Du, Y.; Du, L.J.; He, Z.X.; Zhou, J.; Wen, C.P.; Zhang, Y. Cryptotanshinone ameliorates the pathogenesis of systemic lupus erythematosus by blocking T cell proliferation. Int. Immunopharmacol. 2019, 74, 105677. [Google Scholar] [CrossRef]
- Ding, C.L.; Chen, X.G.; Dascani, P.; Hu, X.L.; Bolli, R.; Zhang, H.G.; Mcleish, K.R.; Yan, J. STAT3 Signaling in B Cells Is Critical for Germinal Center Maintenance and Contributes to the Pathogenesis of Murine Models of Lupus. J. Immunol. Jimmunol. 2016, 196, 4477–4486. [Google Scholar] [CrossRef]
- Iacobas, D.; Wen, J.; Iacobas, S.; Schwartz, N.; Putterman, C. Remodeling of Neurotransmission, Chemokine, and PI3K-AKT Signaling Genomic Fabrics in Neuropsychiatric Systemic Lupus Erythematosus. Genes 2021, 12, 251. [Google Scholar] [CrossRef]
- He, J.J.; Ma, J.; Ren, B.R.; Liu, A.J. Advances in systemic lupus erythematosus pathogenesis via mTOR signaling pathway. Semin. Arthritis Rheum. 2019, 50, 314–320. [Google Scholar] [CrossRef]
- Li, M.F.; Yu, D.T.; Wang, Y.; Luo, N.; Han, G.M.; Yang, B. Interferon-α activates interleukin-1 receptor-associated kinase 1 to induce regulatory T-cell apoptosis in patients with systemic lupus erythematosus. J. Dermatol. 2021, 48, 1172–1185. [Google Scholar] [CrossRef]
- Mok, C.C. The Jakinibs in systemic lupus erythematosus: Progress and prospects. Expert Opin. Investig. Drugs 2019, 28, 85–92. [Google Scholar] [CrossRef]





| Mol ID | Molecule Name | Degree | OB (%) | DL | Type |
|---|---|---|---|---|---|
| MOL005530 | Hydroxygenkwanin | 28 | 36.47 | 0.27 | SZY |
| MOL000830 | Alisol B | 28 | 34.47 | 0.82 | ZX |
| MOL001677 | asperglaucide | 28 | 58.02 | 0.52 | ZM |
| MOL000279 | Cerevisterol | 27 | 37.96 | 0.77 | FL |
| MOL002660 | niloticin | 27 | 41.41 | 0.82 | HB |
| MOL000785 | palmatine | 27 | 64.6 | 0.65 | HB |
| MOL000285 | (2R)-2-[(5R,10S,13R,14R,16R,17R)-16-hydroxy-3-keto-4,4,10,13,14-pentamethyl-1,2,5,6,12,15,16,17-octahydrocyclopenta[a]phenanthren- 17-yl]-5-isopropyl-hex-5-enoic acid | 25 | 38.26 | 0.82 | FL |
| MOL000310 | Denudatin B | 25 | 61.47 | 0.38 | SY |
| MOL000322 | Kadsurenone | 25 | 54.72 | 0.38 | SY |
| MOL000422 | kaempferol | 25 | 41.88 | 0.24 | ZM, MDP |
| Dock | Molecule Name | Target | Binding Energy (kcal/mol) |
|---|---|---|---|
| MOL005530 | Hydroxygenkwanin | TNF | −6.8 |
| MOL000830 | Alisol B | TNF | −7.4 |
| MOL001677 | asperglaucide | TNF | −6.2 |
| MOL000279 | Cerevisterol | TNF | −7.7 |
| MOL002660 | niloticin | TNF | −7.5 |
| MOL005530 | Hydroxygenkwanin | AKT1 | −6.6 |
| MOL000830 | Alisol B | AKT1 | −6.6 |
| MOL001677 | asperglaucide | AKT1 | −6.0 |
| MOL000279 | Cerevisterol | AKT1 | −7.2 |
| MOL002660 | niloticin | AKT1 | −7.4 |
| MOL005530 | Hydroxygenkwanin | EGFR | −7.4 |
| MOL000830 | Alisol B | EGFR | −7.8 |
| MOL001677 | asperglaucide | EGFR | −6.6 |
| MOL000279 | Cerevisterol | EGFR | −8.3 |
| MOL002660 | niloticin | EGFR | −7.8 |
| MOL005530 | Hydroxygenkwanin | STAT3 | −7.5 |
| MOL000830 | Alisol B | STAT3 | −8.2 |
| MOL001677 | asperglaucide | STAT3 | −7.5 |
| MOL000279 | Cerevisterol | STAT3 | −7.7 |
| MOL002660 | niloticin | STAT3 | −7.4 |
| MOL005530 | Hydroxygenkwanin | CTNNB1 | −8.2 |
| MOL000830 | Alisol B | CTNNB1 | −7.4 |
| MOL001677 | asperglaucide | CTNNB1 | −8.5 |
| MOL000279 | Cerevisterol | CTNNB1 | −6.7 |
| MOL002660 | niloticin | CTNNB1 | −7.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, Y.; Zhang, X.; Luo, S.; Wei, F.; Song, Y.; Lin, G.; Yao, M.; Gong, A. Exploring the Molecular Mechanism of Zhi Bai Di Huang Wan in the Treatment of Systemic Lupus Erythematosus Based on Network Pharmacology and Molecular Docking Techniques. Processes 2022, 10, 1914. https://doi.org/10.3390/pr10101914
Zhuang Y, Zhang X, Luo S, Wei F, Song Y, Lin G, Yao M, Gong A. Exploring the Molecular Mechanism of Zhi Bai Di Huang Wan in the Treatment of Systemic Lupus Erythematosus Based on Network Pharmacology and Molecular Docking Techniques. Processes. 2022; 10(10):1914. https://doi.org/10.3390/pr10101914
Chicago/Turabian StyleZhuang, Yanping, Xuan Zhang, Simin Luo, Fangzhi Wei, Yitian Song, Guiling Lin, Minghui Yao, and Aimin Gong. 2022. "Exploring the Molecular Mechanism of Zhi Bai Di Huang Wan in the Treatment of Systemic Lupus Erythematosus Based on Network Pharmacology and Molecular Docking Techniques" Processes 10, no. 10: 1914. https://doi.org/10.3390/pr10101914
APA StyleZhuang, Y., Zhang, X., Luo, S., Wei, F., Song, Y., Lin, G., Yao, M., & Gong, A. (2022). Exploring the Molecular Mechanism of Zhi Bai Di Huang Wan in the Treatment of Systemic Lupus Erythematosus Based on Network Pharmacology and Molecular Docking Techniques. Processes, 10(10), 1914. https://doi.org/10.3390/pr10101914





