Evaluating the Potential of Glycyrrhiza uralensis (Licorice) in Treating Alcoholic Liver Injury: A Network Pharmacology and Molecular Docking Analysis Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening and Application of the Main Active Ingredients and Targets of G. uralensis
2.2. Screening of Alcoholic Liver Injury Targets
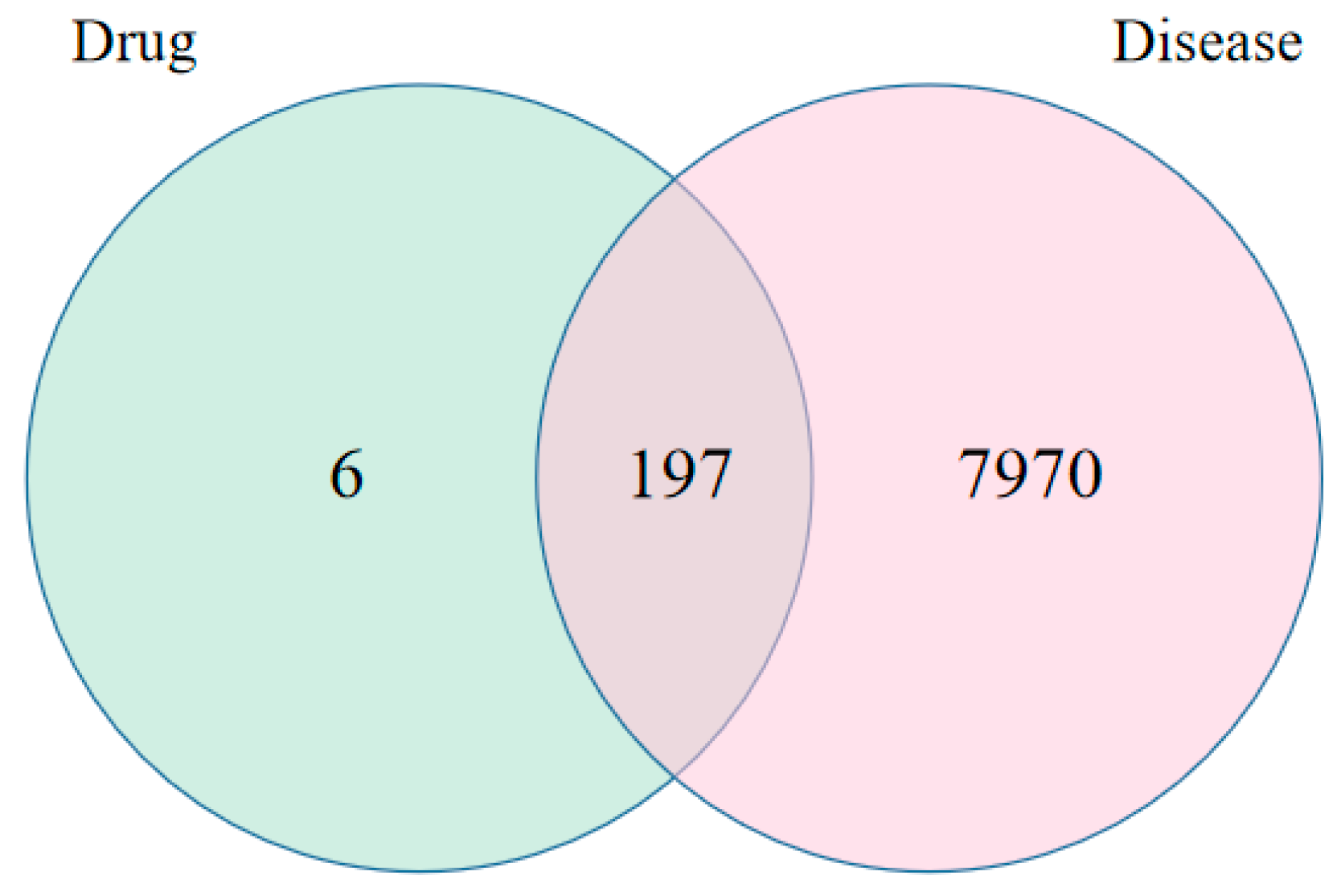
2.3. Selection of Intersecting Targets and Venn Diagram Creation for G. uralensis Treatment and Alcoholic Liver Injury
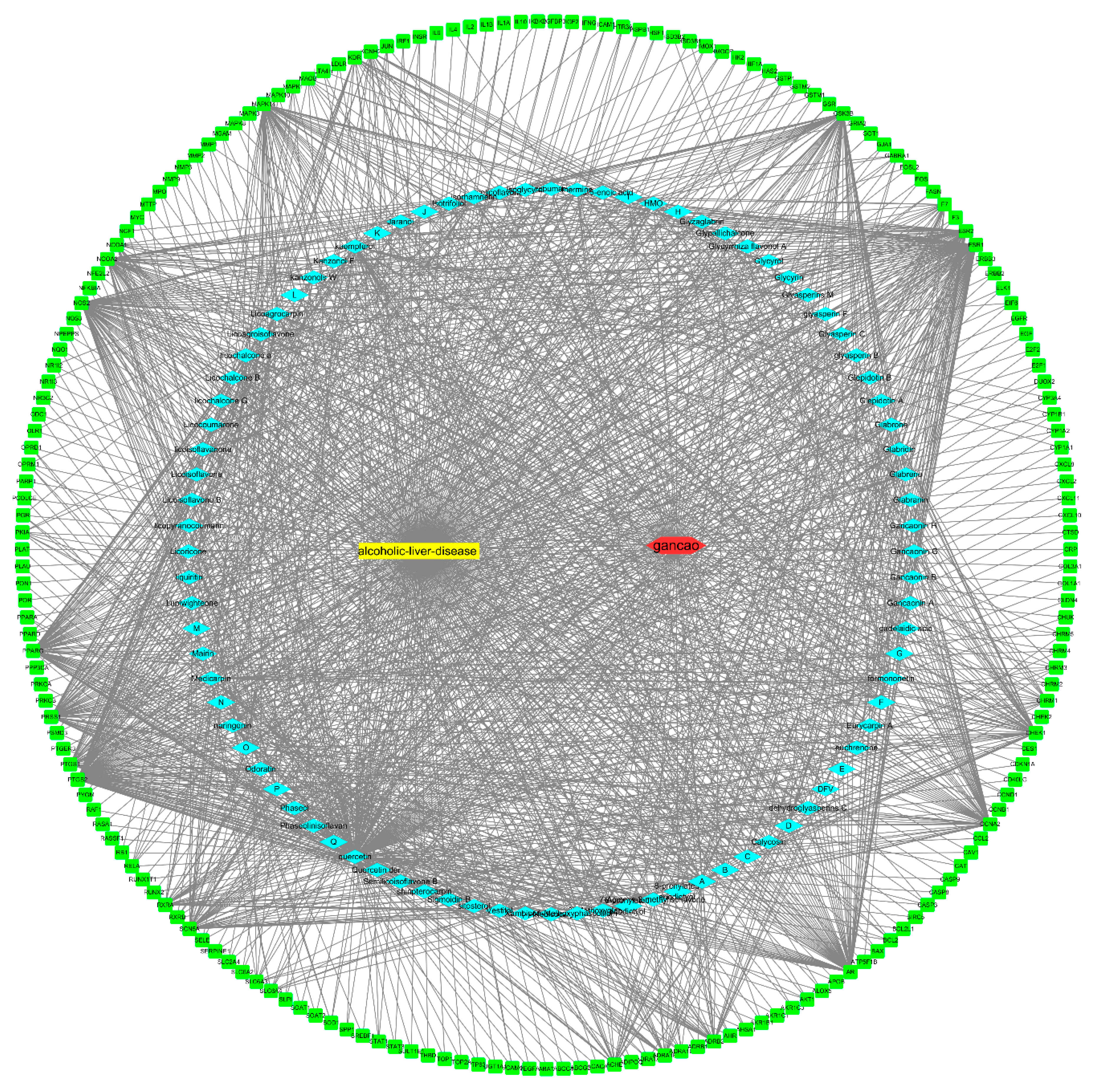
2.4. Construction of a Component–Target Network of G. uralensis and Alcoholic Liver Injury
2.5. Protein–Protein Interaction (PPI) Network Construction
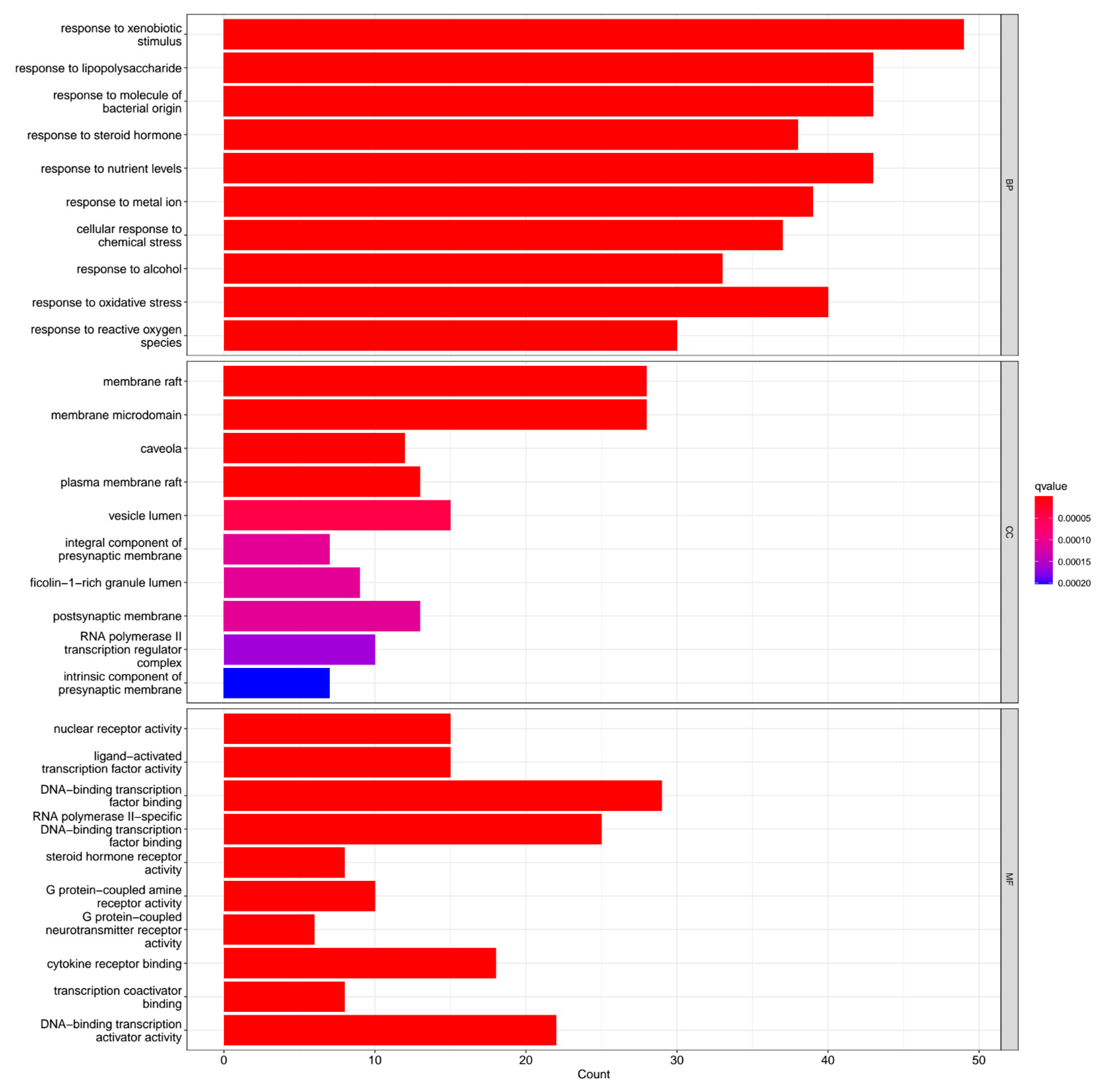
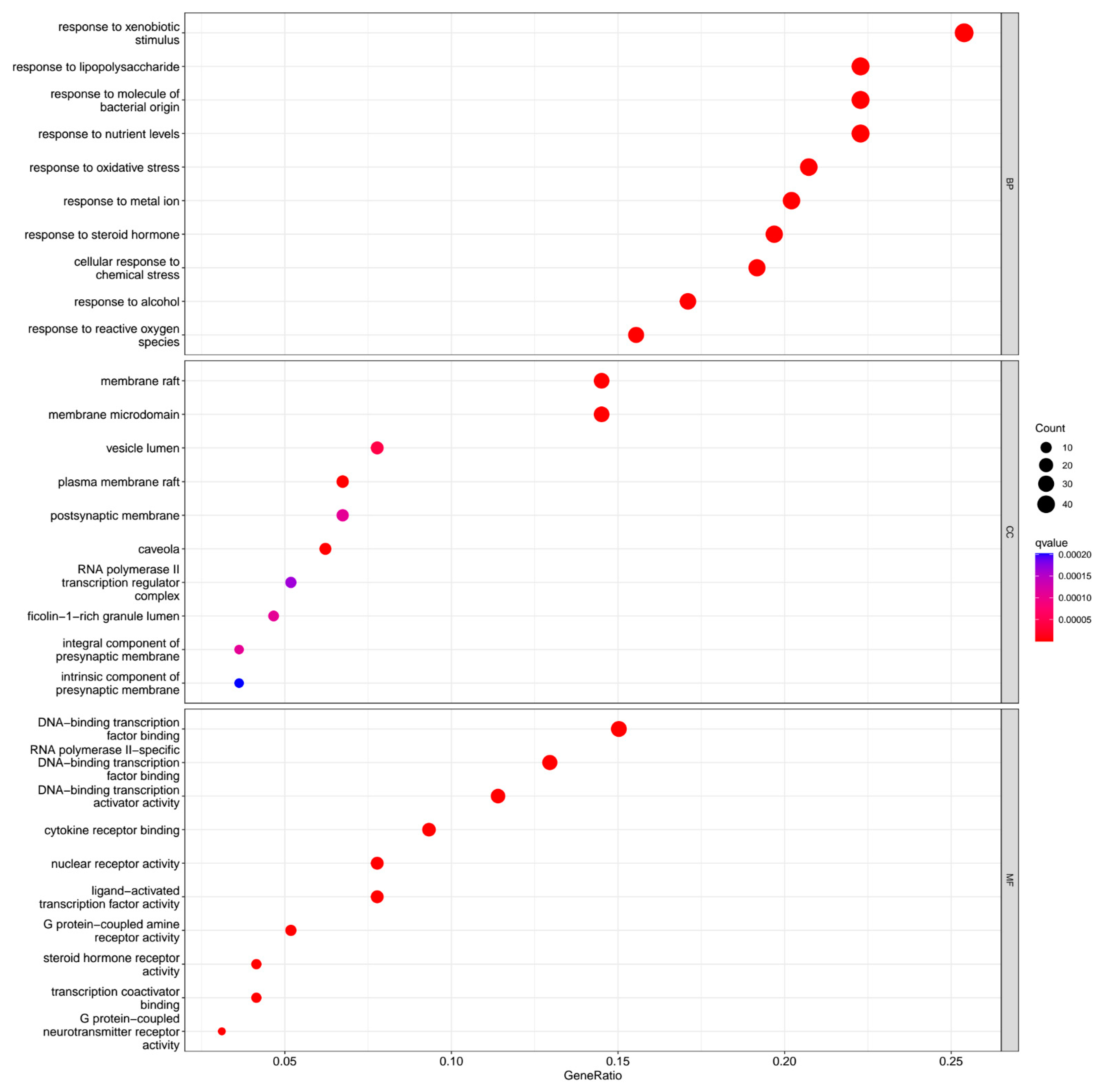
2.6. Gene Ontology (GO) Analysis
2.7. Kyoto Encyclopedia of Genes and Genomes (KEGG) Analysis
2.8. Molecular Docking
3. Results
3.1. Collection and Screening of Licorice Active Ingredients
3.2. Screening of Genes Related to Alcoholic Liver Injury
3.3. Target Screening of Licorice Ingredients and Alcoholic Liver Injury Targets
3.4. Construction of the Component–Target Network of G. uralensis and Alcoholic Liver Injury
3.5. Construction of the PPI Network
3.6. Enrichment of GO Functional Data
3.7. KEGG Pathway Enrichment
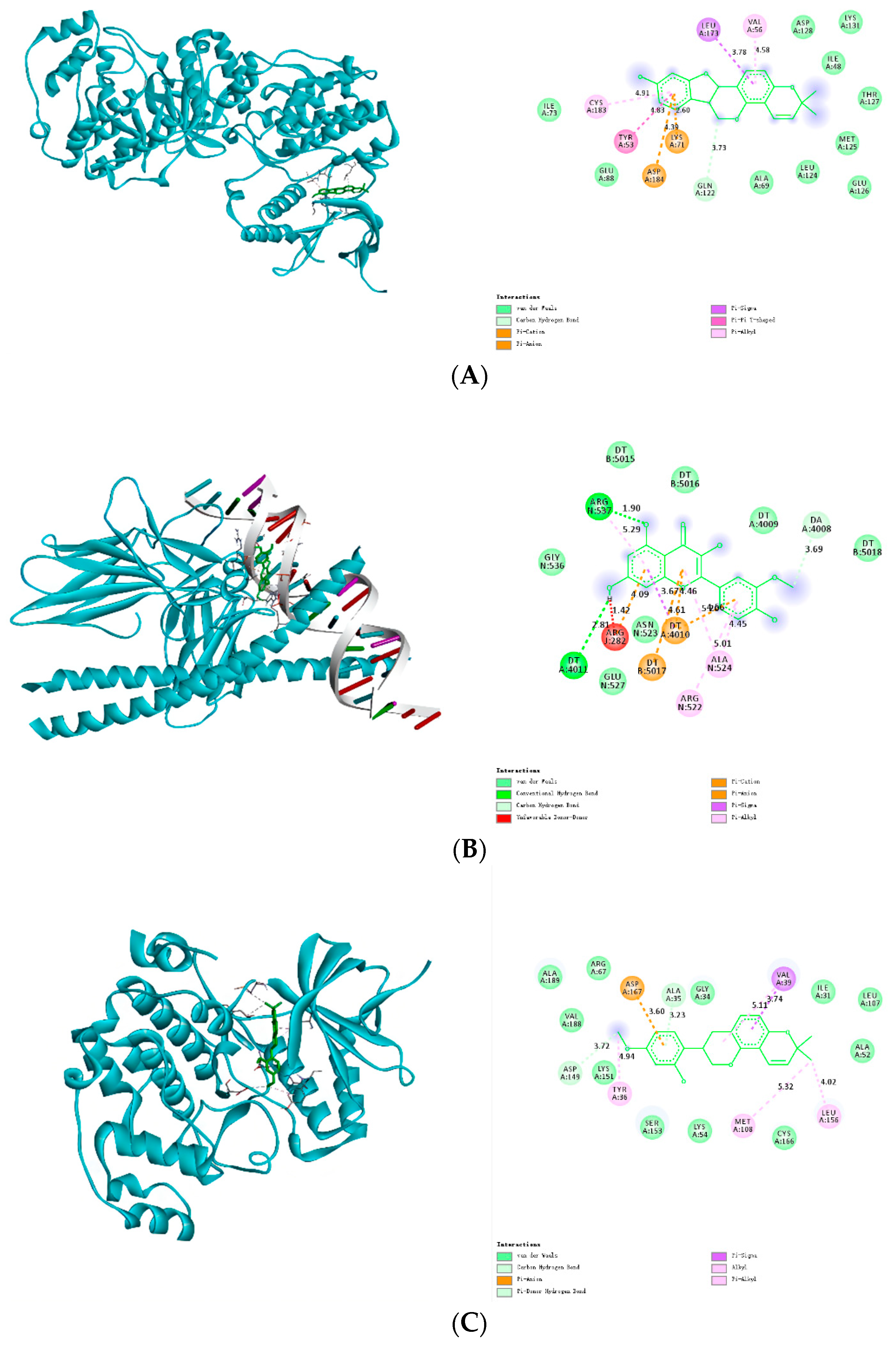
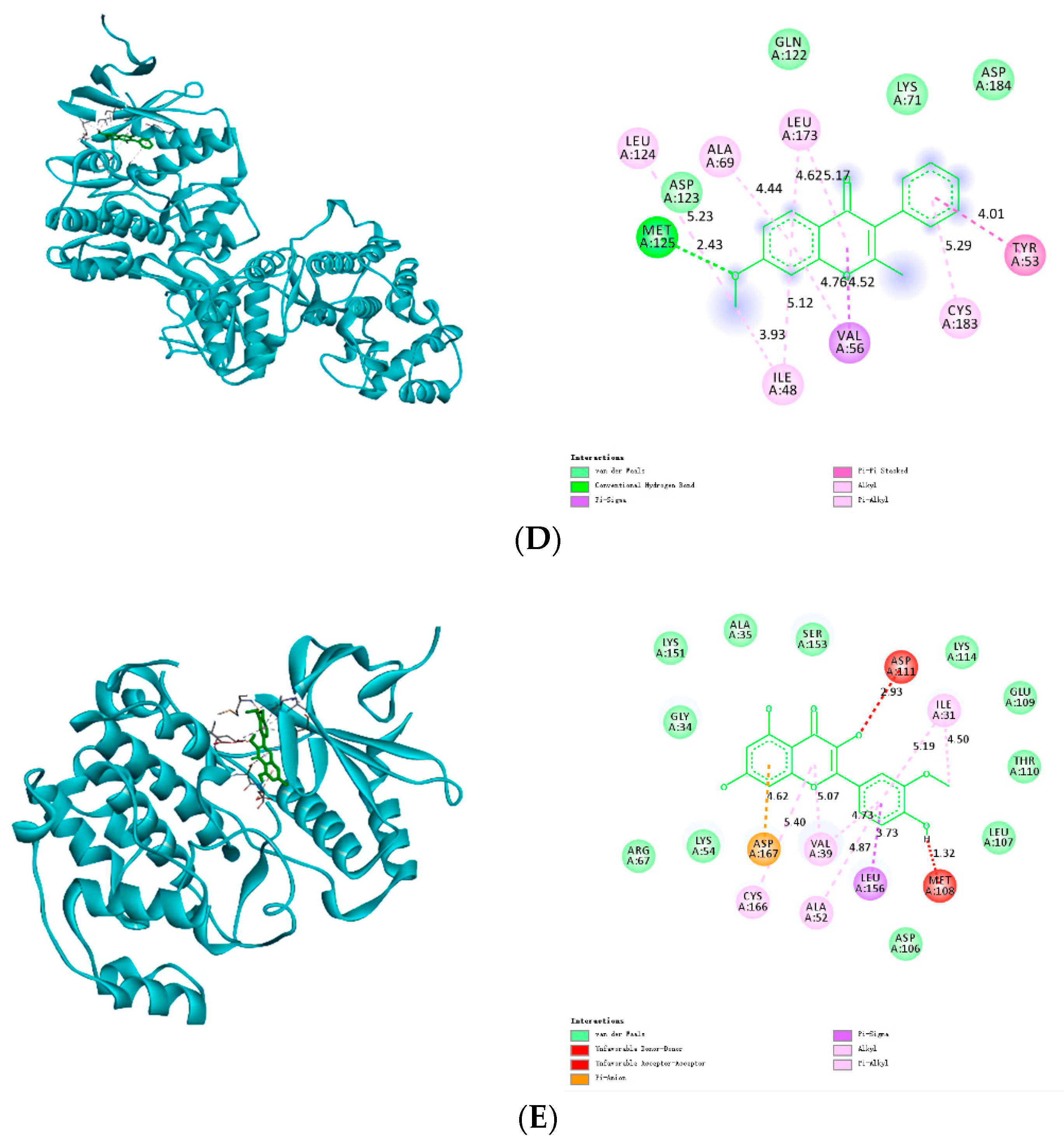
3.8. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Luo, H.Y.; Wang, M.N.; Xu, K.; Peng, Q.Y.; Zou, B.; Yin, S.; Yu, C.; Ren, L.Y.; Li, P.; Tang, L.; et al. Effect of Fushengong Decoction on PTEN/PI3K/AKT/NF-kappa B Pathway in Rats with Chronic Renal Failure via Dual-Dimension Network Pharmacology Strategy. Front. Pharmacol. 2022, 13, 807651. [Google Scholar] [CrossRef]
- An, Y.P.; Chen, P.; Chen, Y.; Wang, X.L.; Wang, Y.X.; Ding, T.X.; Yao, G.Z. Study on the mechanism of guanxinning tablet in the treatment of heart failure based on network pharmacology and molecular docking. New Tradit. Chin. Med. 2022, 12, 9–15. [Google Scholar] [CrossRef]
- Zhu, D.J.; Piao, B.K.; Yang, C.; Bai, J.Q.; Qin, T.; Zhu, H.W.; Zhang, P. Study on the mechanism of guben Jiedu prescription in the treatment of lung cancer based on network pharmacology, molecular docking and experimental verification. Chin. J. Chin. Med. Inf. 2022, 6, 1–9. [Google Scholar] [CrossRef]
- Yang, L.B.; Mi, Y.F.; Zou, C.Y.; Wang, Z.C. Study on the mechanism of Huoxue Shugan Decoction promoting accelerated rehabilitation after knee arthroplasty based on network pharmacology-molecular connection. Chin. Med. Bull. 2022, 21, 40–45. [Google Scholar] [CrossRef]
- Hu, X.H.; Tang, Z.S.; Wu, Q.J.; Song, Z.X.; Hongbo, X. Study on the molecular mechanism of FHD in the prevention and treatment of alcoholic liver disease. Clin. Pharmacol. New Drug Chin. Med. 2021, 32, 1309–1320. [Google Scholar] [CrossRef]
- Liu, W.B.; Zhong, J.B.; Wang, H. Pharmacology study based on network stream the mechanism of action of yellow grass in treatment of alcoholic liver injury. J. Food Sci. Technol. Lancet 2022, 6, 9–17. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhang, K.; Gao, X.J.; Guo, X.L.; Xu, X.J.; Li, G.Y.; Wang, H.Y.; Wang, J.H. Protective effect and mechanism of Hugan Buzur granule on alcohol-induced acute liver injury in mice. J. Shihezi Univ. Nat. Sci. Ed. 2022, 40, 106–112. [Google Scholar] [CrossRef]
- Liu, C.; Ding, J.X.; Zhou, Y.; Yin, Z.G.; Luo, H.T.; Kong, W.T. Protective effect of Honeysuckle on acute alcoholic liver injury in rats based on network pharmacology. Chin. J. Tradit. Chin. Med. 2021, 17, 4531–4540. [Google Scholar] [CrossRef]
- Yang, X.Y.; Su, X.L. Glycyrrhetinic acid protect liver function of access mechanism. Chin. J. Histochem. Cytochem. 2019, 28, 66–70. [Google Scholar] [CrossRef]
- Chu, X.L.; Hou, C.Z.; Mao, Y.; Cai, Y.F.; Jiang, P.Y.; Zhu, S.J. Peony liquorice decoction in treatment of cancer pain mechanism of network pharmacology study. J. Hainan Med. Coll. 2019, 25, 1686–1692. [Google Scholar] [CrossRef]
- Zhang, X.R.; Lin, T.; Wang, X.L.; Wang, X.J.; Gu, H. Preparation of salvianolic acid B, tanshinone II_(A) and glycyrrhetinic acid oral emulsions and their protective effects on acetaminophen-induced acute liver injury. Chin. J. Tradit. Chin. Med. TCM 2022, 7, 1–12. [Google Scholar] [CrossRef]
- Ma, Z.J.; Dong, J.M.; Yu, X.H.; Li, M.; Chen, X.; Zhao, K.J.; Rao, Q.; Zhang, C.E. Effect of Glycyrrhiza licorice on reducing hepatotoxicity of Tripterygium wilfordii based on HMGB1 inflammatory pathway. Hosp. Drug Use Eval. Anal. China 2022, 22, 644–647+651. [Google Scholar] [CrossRef]
- Zhang, F. Clinical efficacy and safety of silibinin meglumine tablets combined with compound glycyrrhizin tablets in the treatment of fatty liver. Syst. Med. 2022, 7, 91–94. [Google Scholar] [CrossRef]
- Mou, W.Q.; Xu, G.; Zhao, J.; Chen, Y.Y.; Bai, Z.F.; Xiao, X.H. Licorice active ingredient to antidepressant drug induced liver injury prevention and cure function. Chin. J. Tradit. Chin. Med. TCM. 2022, 6, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y. Exploring the pharmacological mechanism of Fu Bin in the treatment of active RA based on data mining and network pharmacology. Tianjin Univ. Chin. Med. 2021, 3, 35–41. [Google Scholar] [CrossRef]
- Ding, S.S.; Kang, J.; Huang, Y.Q.; Chen, H.Q.; Gan, H.J. Based on network pharmacology Chen Shang different disease pathogenesis mechanism study. J. Fujian TCM 2020, 6, 51–56. [Google Scholar] [CrossRef]
- Shan, J.L. Effects of inflammatory signaling pathway on chronic alcoholic liver injury and acute gouty arthritis in rabbits. Jiangxi Univ. Tradit. Chin. Med. 2021, 5, 21–30. [Google Scholar] [CrossRef]
- Huang, Y.X.; Guo, J.Y.; Chen, Q.W.; Zhou, Y.Q.; Yang, C.; Du, Y.K. Study on the molecular mechanism of Baihua Dan in the treatment of osteoarthritis based on network pharmacology and molecular connection. Hunan Chin. Med. J. 2022, 38, 164–173. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, M.J.; Lang, J.; Shi, Y.P.; Guo, J.S.; Zhang, S.G. Study on molecular mechanism of Pharmacological action of Pudilan Xiaoyan Oral Liquid and its relationship with disease based on network pharmacology. New Tradit. Chin. Med. 2022, 8, 1–9. [Google Scholar] [CrossRef]
- Li, W.S. Based on the network pharmacology to protect liver slice of alcoholic liver injury prevention mechanism research. China Med. Univ. 2022, 2, 16–20. [Google Scholar] [CrossRef]
- Chen, J.J. Study on pharmacological mechanism of anti-osteoporosis Chinese medicine combination and drug pair based on network pharmacology. Huazhong Univ. Sci. Technol. 2020, 3, 6–10. [Google Scholar] [CrossRef]
- Zhang, J.X.; Chao, L.Q.; Wang, F. Study on the mechanism of Liuwei Dihuang pill in the treatment of depression based on network pharmacology and molecular connection technique. J. Tradit. Chin. Med. 2022, 5, 1719–1726. [Google Scholar] [CrossRef]
- Zhang, K.B.; Li, H.; Wang, S.G. Study on the mechanism of Wuling SAN in the treatment of prostatic hyperplasia based on network pharmacology and molecular docking technology. J. Tradit. Chin. Med. 2022, 5, 1727–1733. [Google Scholar] [CrossRef]
- Bai, T.Y.; Wu, S.H. Study on mechanism of action of Mubai SAN in treatment of peptic ulcer based on network pharmacology combined with GEO data. J. Tradit. Chin. Med. 2022, 5, 1734–1741. [Google Scholar] [CrossRef]
- Kang, N.; Duan, C.C.; Zhang, Q.Y.; Yue, J.Y.; Liu, Y.J.; Li, Q. Study on the potential mechanism of salvia miltiorrhiza in the treatment of habitual abortion based on network pharmacology and molecular docking technology. J. Tradit. Chin. Med. 2022, 5, 1711–1718. [Google Scholar] [CrossRef]
- Yu, S.W.; Qi, Y. Pharmacokinetics of Achyranthe bidentata and Uncaria chinensis in the treatment of diabetes mellitus with hypertension. Henan Tradit. Chin. Med. 2022, 8, 1206–1213. [Google Scholar] [CrossRef]
- Jia, H.Y.; Lv, N.; Feng, Z.H. Study on the mechanism of Dioscorea xanthophyll-Prunella schisandrae in the treatment of subacute thyroiditis based on network pharmacology and molecular connection. Henan Tradit. Chin. Med. 2022, 8, 1199–1205. [Google Scholar] [CrossRef]
- Zhou, X.R.; Zhou, Z.F.; Liu, X.; Ma, L.; Shen, L. Study on the mechanism of rhizoma coptidis in the treatment of diabetic peripheral neuropathy based on network pharmacology and molecular docking. Liaoning Tradit. Chin. Med. Mag. 2022, 6, 1–14. Available online: http://kns.cnki.net/kcms/detail/21.1128.R.20220614.1627.164.html (accessed on 4 June 2022).
- Zhang, C.W.L.; Liu, L.; Du, X.Q.; Liang, Y.N.; Lv, X.L. Study on the molecular mechanism of Guchang Zhixie pill in the treatment of ulcerative colitis based on network pharmacology and molecular docking technology. Liaoning Tradit. Chin. Med. Mag. 2022, 6, 1–18. Available online: http://kns.cnki.net/kcms/detail/21.1128.R.20220614.1625.162.htm (accessed on 4 June 2022).
- Zhang, H.; Zhao, Y.X.; Yang, H.R.; Dong, X. Effects of formononetin combined with down-regulation of Mir-4326 on the growth and invasion of hepatocellular carcinoma cells HCCLM3. Propr. Chin. Med. 2022, 44, 1636–1640. [Google Scholar]
- Zhou, Y.M.; Shen, L.; Tian, Y.; Sun, X. Effect of glabridin on proliferation of hepatocellular carcinoma Hep3B cells. Cent. South Pharm. 2020, 18, 1483–1487. [Google Scholar] [CrossRef]
- Lu, H.M.; Zhou, J.F.; Xiao, Q.F.; Feng, H.H.; Deng, X.M.; Ci, X.X. Licorice chalcone A upregulates Nrf2 signaling pathway against APAP-induced hepatotoxicity. In Proceedings of the 15th Conference of the Veterinary Pharmacology Toxicology Branch of China Animal Husbandry and Veterinary Institute Academic, Lanzhou, China, 13–16 October 2019; pp. 166–167. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Xie, S.; Geng, Z.; Yang, X.; Zhang, Q. Evaluating the Potential of Glycyrrhiza uralensis (Licorice) in Treating Alcoholic Liver Injury: A Network Pharmacology and Molecular Docking Analysis Approach. Processes 2022, 10, 1808. https://doi.org/10.3390/pr10091808
Zhu L, Xie S, Geng Z, Yang X, Zhang Q. Evaluating the Potential of Glycyrrhiza uralensis (Licorice) in Treating Alcoholic Liver Injury: A Network Pharmacology and Molecular Docking Analysis Approach. Processes. 2022; 10(9):1808. https://doi.org/10.3390/pr10091808
Chicago/Turabian StyleZhu, Lichun, Shuangquan Xie, Zhihua Geng, Xuhai Yang, and Qian Zhang. 2022. "Evaluating the Potential of Glycyrrhiza uralensis (Licorice) in Treating Alcoholic Liver Injury: A Network Pharmacology and Molecular Docking Analysis Approach" Processes 10, no. 9: 1808. https://doi.org/10.3390/pr10091808
APA StyleZhu, L., Xie, S., Geng, Z., Yang, X., & Zhang, Q. (2022). Evaluating the Potential of Glycyrrhiza uralensis (Licorice) in Treating Alcoholic Liver Injury: A Network Pharmacology and Molecular Docking Analysis Approach. Processes, 10(9), 1808. https://doi.org/10.3390/pr10091808





