Abstract
The aim of the present study was to determine how thermal stimulation via electromagnetic microwave radiation impacts the yields of biogas and methane produced by methane fermentation of five selected energy crop species in anaerobic reactors. The resultant performance was compared with that of reactors with conventional temperature control. The highest biogas production capacity was achieved for maize silage and Virginia mallow silage (i.e., 680 ± 28 dm3N/kgVS and 506 ± 16 dm3N/kgVS, respectively). Microwave radiation as a method of heating anaerobic reactors provided a statistically-significantly boost in methane production from maize silage (18% increase). Biomethane production from maize silage rose from 361 ± 12 dm3N/kgVS to 426 ± 14 dm3N/kgVS. In the other experimental variants, the differences between methane concentrations in the biogas were non-significant.
1. Introduction
The limited reserves, increasing consumption, and rising prices of energy carriers have prompted research into developing biotechnological methods for biofuel production [1]. Energy crops used to this end are quick to grow, highly resistant to pests/diseases, and have low soil quality requirements [2]. Certain species of these crops accumulate pollutants in the root system, which means that their cultivation can also be a way to utilize barren agricultural land or degraded land [3,4]. Among the many species of energy crops that can be grown under temperate climate, the most commonly recommended are maize (Zea maize), giant miscanthus (Miscanthus x giganteus), and Virginia mallow (Sida hermaphrodita (L.) Rusby) [5].
Technologies for biogas production from energy crops have been developed since the 1930s. Methane fermentation has become a mature, well-established, and commonly deployed technology, but research is still ongoing to uncover effective, low-cost, and innovative solutions for the design, implementation, and improvement of biochemical methane generation from energy crops [6,7]. Particularly important are the economic parameters of biogas plants, considered in tandem with environmental indicators such as energy or water consumption [8,9]. Biomass from dedicated solid energy crops can be a substrate not only for the production of biogas, but can also be used as a good substitute for carbon in the production of high-quality and environmentally friendly solid fuels in the torrefaction and briquetting processes [10,11].
The present paper introduces an innovative method for MW (microwave) heating of anaerobic reactors for producing biogas from energy crop biomass. This solution, incorporated into full-scale installations, could eliminate the technological hurdles associated with heat exchangers, water jackets, or steam injectors. Microwave radiation energy can be directed to a mixture of anaerobic sludge and processed biomass, which means that less energy is wasted due to absorption by structural components of the reactor. This means that MW heating can be used to save energy.
In a typical biogas plant technological systems, the biogas is used to feed a cogeneration system where the potential energy of methane is converted into electricity and heat [12]. Heat is usually difficult to valorize and is often lost or partly used for heating fermenters [13]. In such an operating system of agricultural biogas plants, the use of MW is not sensible according to economic reasons. However, it should be emphasized that the model of biogas energy for cogeneration is often regarded as unjustified in economic and ecological terms [14]. At present, other methods are considered to be more prospective for the effective use of potential biogas energy and reduction in GHG emissions [15]. These methods are mainly based on the production of biomethane and its injection into the gas grid or its use to power engines in agricultural machinery or public transport [16]. In these cases, heat for the biogas plant must be supplied from external sources and is part of the operational cost of this bioenergetic system [17].
The research conducted so far has proven that the use of MW can be a justified method of ensuring appropriate thermal conditions in fermentation bioreactors. Braguglia et al. (2018) argue that MW can serve as an attractive alternative to convection heating due to the lower energy demand as a result of the lack of convection or conduction heat loss and the breaking of hydrogen bonds by polarization chains of microparticles [18]. In studies on the use of MW to support the fermentation of expired food products, a positive balance of the use of this physical factor has been proven. The power of the used magnetron was 300 W. The energy demand was 90 Wh·d−1, while the potential energy of the obtained biogas was 99.2 Wh·d−1. In this variant, a positive net energy balance of 9.2 Wh·d−1 was obtained. In the variant in which conventional heating was used, a negative energy balance was obtained [19]. Literature reports also indicate that microwave radiation can have non-thermal effects (i.e., those not linked to temperature increases) on biochemical systems [20]. There is scientific basis to believe that microwave radiation promotes the activity of anaerobic sludge microorganisms and affects microbial population patterns [21,22,23].
The aim of the present study was to determine how electromagnetic microwave radiation used as a thermal stimulant impacts the yields of biogas and methane produced by methane fermentation of the five selected energy crop species in anaerobic reactors. The resultant performance was compared against that of reactors with conventional temperature control.
2. Materials and Methods
2.1. Experimental Design
The experiment was divided into two stages with different types of digester heating. Conventional and microwave heating were used in stages 1 and 2, respectively. Each stage was performed in five variants, with the plant species used as methane fermentation feedstock being the differentiating factor. The productivity of biogas via methane fermentation was tested for five plant feedstocks: maize silage (Zea maize), alfalfa silage (Medicago L.), Virginia mallow silage (Sida hermaphrodita (L.) Rusby), giant miscanthus silage (Miscanthus x giganteus), and haylage.
2.2. Materials
The energy crop silage was sourced from the Teaching & Research Station of the University of Warmia and Mazury in Bałdy (Warmińsko-Mazurskie Voivodeship, Poland). The characteristics of the different silage types are presented in Table 1.

Table 1.
Characteristics of the energy crop silage used in the experiment.
The plant feedstock was first ground and homogenized using a ROBO 3000 cutting mill. Approximately 100 g of fresh substrate was fed into the mill at a time, then crushed for 5 min to obtain an average particle size of 2.0–3.0 mm. This pre-treated feedstock was mixed with 200 cm3 of inoculum and excited for 30 min in a laboratory shaker at 160 rpm (the shaking duration was 20 min).
The anaerobic sludge (inoculum), with the characteristics given in Table 2, was harvested from a model digester with an active volume of 300 dm3. The digested feedstock was a mixture of cattle slurry and plant biomass. The reactor operated with a load of 2.0 kgVS/m3·d. Before being fed into the anaerobic reactors, the digestate used as inoculum was fermented over a period of 10 days, during which it was mixed and thermostated under mesophilic conditions (35 °C). No supplementation was provided. The characteristics of the feedstock-inoculum mixture are given in Table 3.

Table 2.
Characteristics of anaerobic sludge used as the inoculum of anaerobic reactors.

Table 3.
Characteristics of the anaerobic sludge–plant feedstock mixture.
2.3. Experimental Setup
The biomass feedstock, mixed with inoculum, was fed into respirometric reactors made up of reaction chambers with an active volume of 0.5 dm3. The organic load rate was 5.0 g VS/dm3 in all of the processing variants. The digesters were purged with nitrogen prior to the measurements to remove air and ensure anaerobic conditions inside. Respirometric reactors were placed in an incubator (stage 1) or a microwave chamber (stage 2). The experiment was conducted at 35 °C (mesophilic fermentation) with a measurement time of 40 days. The runs were conducted in triplicate.
The radiation in the microwave chamber was provided by a Plazmotronika microwave generator with a power range of 0 to 600 W and a frequency of 2.45 GHz. The radiation was supplied until the target temperature was reached. Afterward, the microwave generator was activated intermittently to maintain the target temperature within the measurement vessels. The microwave chamber design was such that only glass vessels could be placed within. The biogas meter was located above the microwave heating zone.
2.4. Calculation Methods
The biogas production rate (r) was determined for each experimental variant. The reaction rate constants (k) were determined based on the obtained experimental data by non-linear regression using Statistica 13.1 PL (Statistica 13.1 software, StatSoft Inc., Tulsa, OK, USA, 2016). The iterative method was applied in which the function is replaced in each iterative step with a linear differential with respect to the parameters determined. The coefficient of convergence φ2 was adopted as the measure of the curve’s fit (with the determined parameters) to the experimental data. This coefficient is the ratio of the sum square of the deviations of the values (calculated based on the constructed function) from the experimental values to the sum square of the deviations of the experimental values from the mean. The lower the value of the φ2 coefficient, the higher the convergence. The model was fitted to the experimental points so that the convergence coefficient did not exceed 0.2.
The fermentation rate coefficient (i.e., the percentage ratio of the VS load removed in the reactor to the VS load fed into the reactor) (1) and the VS removal rate (efficiency) coefficient (2) were determined using the following equations:
where is the fermentation rate [%]; is the VS removal rate [%]; is the VS levels in the influent [g/kg]; is the VS levels in the digested sludge [g/kg]; is the influent density [kg/dm3]; and is the digested sludge density [kg/dm3].
2.5. Analytical Methods
Dry matter content in biomass, anaerobic sludge, and in the plant feedstock–sludge mixture was determined gravimetrically at the start of the process and after 40 days of methane fermentation. Samples dried at 105 °C were also assayed for total carbon (TC), total organic carbon (TOC), and total nitrogen (TN) using a Flash 2000 analyzer (Thermo Scientific, Delft, The Netherlands). The pH value of the aqueous homogenized solutions was measured with a pH meter (1000 L, VWR International, Radnor, PA, USA). COD in the filtrate was determined with a DR 2800 spectrophotometer (HACH Lange, Düsseldorf, Germany). The composition of the biogas obtained was assayed using a GMF 430 analyzer (Gas Data) and a gas chromatograph (GC) (7890A, Agilent, Santa Clara, CA, USA). The gas chromatograph was equipped with two Hayesep Q columns (80/100), two molecular sieve columns (60/80), and a Porapak Q column (80/100) working at 70 °C. The temperature at the injection port and the detector port was 150 °C and 250 °C, respectively. Helium and argon were used as carrier gases (flow rate: 15 mL/min).
2.6. Statistical Methods
The statistical analysis of the experimental results was conducted using STATISTICA 13.1 PL (Statistica 13.1 software, StatSoft Inc., Tulsa, OK, USA, 2016). The hypothesis of the normality of distribution of each analyzed variable was verified using the W Shapiro–Wilk test. One-way analysis of variance (ANOVA) was used to determine differences between variables. Homogeneity of variance in groups was determined using a Levene test. Significant differences between the variables were determined via Tukey’s HSD test. Results were considered significant at α = 0.05.
3. Results
3.1. Biogas Production and Composition
Conventional heating produced the best results when maize silage and Virginia mallow silage were used as fermentation feedstock (Table 4, Figure 1). Average biogas yields from conventionally-heated reactors were 680 ± 28 dm3N/kg VS for the former and 506 ± 16 dm3N/kgVS for the latter (p < 0.05) (Table 4, Figure 1). No significant variations in biogas yields were detected between the variants where miscanthus silage and haylage were used (p > 0.05). Methane fermentation of alfalfa silage produced the lowest biogas yields at 331 ± 13 dm3N/kgVS (Table 4, Figure 1). The largest methane fraction in the post-fermentation gas was obtained from alfalfa silage (54.9 ± 1.8% CH4), whereas the lowest levels were noted for haylage (51.6 ± 1.5% CH4). Based on the biogas production efficiency and methane content, the methane yield in stage 1 was determined to be the highest after maize silage fermentation (361 ± 12 dm3N CH4/kgVS), and lowest for alfalfa silage (181 ± 8 dm3N CH4/kgVS) (Table 4). No significant differences in methane yields were detected in any of the variants where miscanthus silage and haylage were used as fermentation substrates (p > 0.05). The biogas production rate was the highest for fermentation of maize silage and Virginia mallow silage. Alfalfa silage, on the other hand, performed the worst in terms of production rate (Table 5). The biogas production rate constant was the same for all fermentation substrates (Table 5). The highest values of ηF and ηVS were recorded for maize silage (i.e., 92.60 ± 1.1% (Figure 2a) and 96.40 ± 1.2% (Figure 2b), respectively). Conversely, the lowest values were obtained for alfalfa silage (i.e., 76.44 ± 1.2% ηF (Figure 2a) and 88.99 ± 1.2% ηVS (Figure 2b)).

Table 4.
Efficiency of biogas and methane production and biogas composition from methane fermentation of different energy crops.
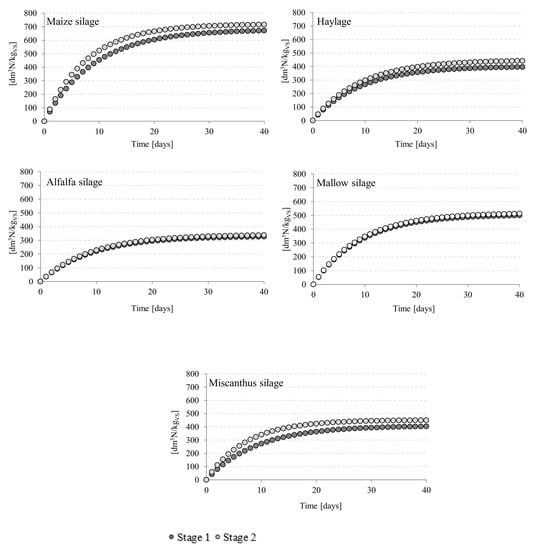
Figure 1.
Details of the biogas production process in different experimental variants.

Table 5.
Reaction kinetics (k is the rate constant, r is the rate coefficient) of biogas production from energy crops.
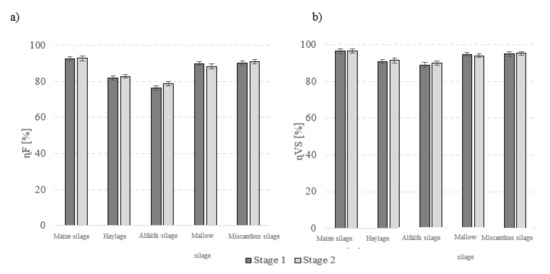
Figure 2.
AD performance indicators (a) ηF coefficient of fermentation and (b) η removal efficiency.
Microwave radiation was found to improve biogas yields in the maize silage, haylage, or miscanthus silage runs (p < 0.05). Using microwave radiation to control temperature conditions in anaerobic reactors for producing biogas from maize silage increased the yield by 5.78% compared with stage 1 at 720 ± 27 dm3N/kgVS (p < 0.05) (Table 4, Figure 1). The biogas yield per unit of miscanthus silage organic mass in the microwave-heated reactors was 10.52% higher than that in stage 1, reaching 452 ± 12 dm3N/kgVS (Table 4, Figure 1). The highest gains in biogas production (11.38% increase; 448 ± 13 dm3N/kgVS) were observed for haylage. Biogas produced through methane fermentation of maize silage in microwave-heated reactors had 6.13% more methane than that produced in conventionally-heated reactors (p < 0.05). In the other experimental variants, the differences between the methane content in the biogas were non-significant (p > 0.05). Nevertheless, more methane was produced in microwave-heated reactors when the fermentation substrate was maize silage (an increase of 18% compared with stage 1), haylage (14.44% increase), or miscanthus silage (10.81% increase). The rate of biogas production from maize silage, haylage, and miscanthus silage was higher than that obtained in stage 1 (Table 5). The methane yield in stage 2 was the highest (426 ± 14 dm3N CH4/kgVS) when maize silage was used as the fermentation substrate, whereas the lowest values (188 ± 9 dm3N CH4/kgVS) were recorded for alfalfa silage (Table 4). The post-fermentation methane yields were similar between Virginia mallow silage, haylage, and giant miscanthus silage (Table 4). As with conventional heating, the best methane fermentation performance was obtained using maize silage at ηF 92.80 ± 1.3% and ηVS 96.56 ± 1.1%.
3.2. Characteristics of Digestate
Digestate assays indicate that the process of anaerobic decomposition resulted in reduced TC, TOC, and COD in the filtrate in all of the experimental series (Table 6). The lowest TOCs at 166 ± 12 mg/g and 171 ± 12 mg/g were observed for giant miscanthus silage and maize silage digestate (respectively) in microwave-heated reactors. The digested miscanthus silage filtrate also had the lowest COD at 7.7 ± 1.0 g O2/dm3 (Table 6). On the other hand, the type of digester heating did not affect the characteristics of Virginia mallow silage and alfalfa silage digestates (p > 0.05).

Table 6.
Characteristics of the digestate in different experimental variants.
4. Discussion
The experiment tested the efficiency of biogas and methane production from selected energy crops including maize silage, which is the most popular choice for agricultural biogas plants [24]. An innovative method for heating digesters with microwaves was employed to increase the biogas production efficiency.
Conventional heating ensured the best biogas-production performance (680 ± 28 dm3N/kgVS) when maize silage was used as fermentation feedstock. The biogas itself contained 53.0 ± 1.2% methane, which translates into a biomethane yield of 361 ± 12 dm3N CH4/kgVS (Table 4). The only other variant to reach similarly high biomethane yield was the Virginia mallow silage (263 ± 10 dm3N CH4/kgVS). For the other energy crops, the recorded biomethane production was more than 40% lower than that produced through maize silage fermentation (Table 4). The literature data indicates that the Virginia mallow may be a promising alternative to conventional energy crops for biogas production [25,26]. It has very low requirements in terms of soil conditions and can grow even on sandy or rocky soils [27]. Virginia mallow biomass yields (10–25 t TS/ha) are similar to those of maize [3,25], and have been shown by multiple biogas production studies to have a substantial methanogenic capacity of over 400 dm3N/kgTS [28,29]—a property also corroborated by the present study.
Using microwave radiation to control temperature conditions in anaerobic reactor chambers boosted methane production from maize silage, haylage, and giant miscanthus silage, but had no effect on yields from alfalfa silage or Virginia mallow silage. The highest gains in biogas production—11.38%—were observed for haylage. The most pronounced improvement in terms of methane content of biogas (6.13% increase) was obtained for maize silage fermentation, producing 18% more biomethane than in the conventional heating stage.
The efficiency of biogas production from biomass is influenced by many factors characterizing organic substrates including the level of hydration, the content of lignin, cellulose, and hemicellulose, the properties of lignocellulosic complexes, toxic compounds, difficult to decompose, and not susceptible to biodegradation in an anaerobic environment [30]. However, the main factor that directly determines the amount and composition of the biogas is the concentration of organic compounds [31,32]. In the presented studies, the highest content of organic substances at the level of 94.6 ± 1.1% was observed in maize silage biomass, while the lowest of 88.0 ± 1.1% was seen in alfalfa silage (Table 1), which directly correlated with both the rate of biogas production (Table 5) and its final production (Table 4). A significant difference in the content of organic compounds was also visible in the TOC values of the tested biomass of energy plants. In the case of this parameter, maize silage was characterized by a value of 417 ± 14 mg/g, while for alfalfa silage, it was only 352 ± 10 mg/g (Table 1). The research also proved that the concentration of readily available organic substances translates into the values of the fermentation rate (ηF) and VS removal rate (ηVS). The highest values of these parameters characterizing the course of methane fermentation, above 90%, were obtained for maize silage, while the lowest for alfalfa silage (Figure 2).
Microwave-based heating technologies have multiple advantages: they offer high heating efficiency, have selective and consistent effects, and can be activated/terminated immediately. The temperature increase of the exposed object is primarily affected by the vibration of dipole molecules and, to a lesser extent, ion migration (in solutions only) [33]. Microwave energy is dispersed as heat from the internal resistance of the rotation. The excited dipole molecules release the microwave-donated energy as heat through friction, thus producing a temperature increase. However, biological systems have been shown to exhibit interactions that hint at other, non-thermal effects of microwave exposure [34,35]. Microwave energy separates polar bonds and accelerates chemical/physical processes. It can easily penetrate inside molecules, increasing ion speed and inducing collisions with other molecules. A number of non-thermal effects of microwave radiation have been reported in the literature including changes in the structure and function of biofilms, and have also been shown to affect cell membrane transport [36]. Electromagnetic fields can also affect cell membranes by directly or indirectly triggering changes in the properties of ligand-binding receptors (e.g., Ca2+), neurotransmitters, or hormones [37,38]. Microwaves have also been noted to affect the enzymatic activity of in vitro cultures [39,40]. Parker et al. [41] found that the rate of enzymatic reaction under microwave heating can be up to two or three times higher than in a conventionally-heated system. Absorption of microwave radiation by DNA has been observed to induce changes in living organisms [42,43], but the exact mechanism by which microwaves interfere with DNA function is unknown. Exposure of DNA and protein molecules to microwave radiation does not change their structure, but can, under certain conditions, affect their chemical bonds [44]. Experiments conducted by Shamis et al. [45] have demonstrated that exposing E. coli cells to microwave radiation under sublethal temperature conditions causes the cell membrane to undergo a reversible, electrokinetic induction without any bactericidal effects. A study by Zieliński et al. [23], examining the effect of microwave radiation on biofilm activity in immobilized-biomass reactors, showed that MW exposure can highly stimulate microbial activity, even when all other process parameters are the same. Performance improvements were particularly pronounced in terms of nitrifying activity. Another study used an innovative multi-section hybrid anaerobic reactor (M-SHAR) design with microwave heating for methane fermentation of liquid dairy waste (LDW) largely composed of acid whey. This experiment also showed improved performance in terms of organic compound removal and biogas production over a conventionally-heated reactor [46]. The nature of non-thermal effects of microwaves has yet to be fully explained [47]. Nevertheless, the present study indicates that they can be used to stimulate biochemical processes in anaerobic reactors, thus increasing biogas and methane yields from energy crops.
5. Conclusions
The study showed that the highest biogas production capacity of 680 ± 28 dm3N/kgVS and 506 ± 16 dm3N/kgVS could be achieved using maize silage and Virginia mallow silage. Conversely, alfalfa silage produced the lowest biogas yield (331 ± 13 dm3N/kgVS). Microwave radiation as a method for heating anaerobic reactors significantly boosted methane generation from maize silage (18% increase) (i.e., from 361 ± 12 dm3N/kgVS to 426 ± 14 dm3N/kgVS). The differences between the methane content in the biogas were non-significant in the other experimental variants (p > 0.05). Though the presented technology did not produce a statistically significant increase in methane fermentation efficiency for most of the tested substrates, it does eliminate the technological hurdles encountered when using heat exchangers, water jackets, or steam injectors, while also saving energy.
Author Contributions
Conceptualization, M.Z. and M.D.; Methodology, M.D.; Validation, M.Z.; Formal analysis, M.Z. and M.D.; Investigation, M.Z., M.D. and J.K.; Resources, M.Z., M.D. and J.K.; Data curation, M.Z.; Writing—original draft preparation, M.D. and J.K.; Writing—review and editing, M.Z., M.D. and J.K.; Visualization, M.D. and J.K.; Supervision, M.Z.; Project administration, M.Z.; Funding acquisition, M.Z., M.D. and J.K. All authors have read and agreed to the published version of the manuscript.
Funding
The manuscript was supported by a project financially supported by the Minister of Education and Science in the range of the program entitled “Regional Initiative of Excellence” for the years 2019–2022, project no. 010/RID/2018/19, amount of funding: 12,000,000 PLN, and the work WZ/WB-IIŚ/2/2019, funded by the Minister of Education and Science.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grifoni, M.; Pedron, F.; Barbafieri, M.; Rosellini, I.; Petruzzelli, G.; Franchi, E. Sustainable Valorization of Biomass: From Assisted Phytoremediation to Green Energy Production. In Handbook on Assisted and Amendments Enhanced Sustainable Remediation Technology; Prasad, M.N.V., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Kollmann, T.; Nabel, M.; Damm, T.; Klose, H.; Müller, M.; Bläsing, M.; Seebold, S.; Krafft, S.; Kuperjans, I.; et al. Valorization of Sida (Sida hermaphrodita) biomass for multiple energy purposes. GCB Bioenergy 2017, 9, 202–214. [Google Scholar] [CrossRef]
- Nabel, M.; Temperton, V.M.; Poorter, H.; Lücke, A.; Jablonowski, N.D. Energizing marginal soils—The establishment of the energy crop Sida hermaphrodita as dependent on digestate fertilization, NPK, and legume intercropping. Biomass Bioenergy 2016, 87, 9–16. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dzienis, L. Giant miscanthus as a substrate for biogas production. J. Ecol. Eng. 2015, 16, 139–142. [Google Scholar] [CrossRef]
- Kacprzak, A.; Michalska, K.; Romanowska-Duda, Z.; Grzesik, M. Energy crops as a valuable raw material for biogas production. Cosm. Probl. Biol. Sci. 2012, 61, 281–293. [Google Scholar]
- Khan, M.U.; Lee, J.T.E.; Bashir, M.A.; Dissanayake, P.D.; Ok, Y.S.; Tong, Y.W.; Shariati, M.A.; Wu, S.; Ahring, B.K. Current status of biogas upgrading for direct biomethane use: A review. Renew. Sustain. Energy Rev. 2021, 149, 111343. [Google Scholar] [CrossRef]
- Markou, G.; Brule, M.; Balafoutis, A.; Kornaros, M.; Georgakakis, D.; Papadakis, G. Biogas production from energy crops in northern Greece: Economics of electricity generation associated with heat recovery in a greenhouse. Clean Technol. Environ. Policy 2017, 19, 1147–1167. [Google Scholar] [CrossRef]
- Cinar, S.; Cinar, S.O.; Wieczorek, N.; Sohoo, I.; Kuchta, K. Integration of Artificial Intelligence into Biogas Plant Operation. Processes 2021, 9, 85. [Google Scholar] [CrossRef]
- Lombardi, L.; Francini, G. Techno-economic and environmental assessment of the main biogas upgrading technologies. Renew. Energy 2020, 156, 440–458. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Ojo, O.T. Biomass Torrefaction for the Production of High-Grade Solid Biofuels: A Review. Bioenergy Res. 2020, 13, 999–1015. [Google Scholar] [CrossRef]
- Olugbade, T.; Ojo, O.; Mohammed, T. Influence of Binders on Combustion Properties of Biomass Briquettes: A Recent Review. Bioenergy Res. 2019, 12, 241–259. [Google Scholar] [CrossRef]
- Borek, K.; Romaniuk, W.; Roman, K.; Roman, M.; Kuboń, M. The Analysis of a Prototype Installation for Biogas Production from Chosen Agricultural Substrates. Energies 2021, 14, 2132. [Google Scholar] [CrossRef]
- Czekała, W.; Gawrych, K.; Smurzyńska, A.; Mazurkiewicz, J.; Pawlisiak, A.; Chełkowski, D.; Brzoski, M. The possibility of functioning micro-scale biogas plant in selected farm. J. Water Land Dev. 2017, 35, 19–25. [Google Scholar] [CrossRef][Green Version]
- Bourdin, S.; Raulin, F.; Josset, C. On the (un)successful deployment of renewable energies: Territorial context matters. A conceptual framework and an empirical analysis of biogas projects. Energy Stud. Rev. 2020, 24, 4088. [Google Scholar] [CrossRef]
- Ardolino, F.; Cardamone, G.F.; Parrillo, F.; Arena, U. Biogas-to-biomethane upgrading: A comparative review and assessment in a life cycle perspective. Renew. Sustain. Energy Rev. 2021, 139, 110588. [Google Scholar] [CrossRef]
- Verbeeck, K.; Buelens, L.C.; Galvita, V.V.; Marin, G.B.; Van Geem, K.M.; Rabaey, K. Upgrading the value of anaerobic digestion via chemical production from grid injected biomethane. Energy Environ. Sci. 2018, 11, 1788–1802. [Google Scholar] [CrossRef]
- Aryal, N.; Kvist, T. Alternative of Biogas Injection into the Danish Gas Grid System—A Study from Demand Perspective. ChemEngineering 2018, 2, 43. [Google Scholar] [CrossRef]
- Braguglia, C.M.; Gallipoli, A.; Gianico, A.; Pagliaccia, P. Anaerobic bioconversion of food waste into energy: A critical review. Bioresour. Technol. 2018, 248, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Kazimierowicz, J.; Zieliński, M.; Dębowski, M. Influence of the Heating Method on the Efficiency of Biomethane Production from Expired Food Products. Fermentation 2021, 7, 12. [Google Scholar] [CrossRef]
- Kou, X.; Li, R.; Hou, L.; Zhang, L.; Wang, S. Identifying possible non-thermal effects of radio frequency energy on inactivating food microorganisms. Int. J. Food Microbiol. 2018, 269, 89–97. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Z.; Xiouras, C.; Stefanidis, G.D.; Li, X.; Gao, X. Fundamentals and applications of microwave heating to chemicals separation processes. Renew. Sustain. Energy Rev. 2019, 114, 109316. [Google Scholar] [CrossRef]
- Zielińska, M.; Cydzik-Kwiatkowska, A.; Zieliński, M.; Dębowski, M. Impact of temperature, microwave radiation and organic loading rate on methanogenic community and biogas production during fermentation of dairy wastewater. Bioresour. Technol. 2013, 129, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Ciesielski, S.; Cydzik-Kwiatkowska, A.; Turek, J.; Dębowski, M. Influence of microwave radiation on bacterial community structure in biofilm. Process Biochem. 2007, 42, 1250–1253. [Google Scholar] [CrossRef]
- Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Rudnicka, A.; Dudek, M.; Romanowska-Duda, Z.; Zieliński, M. The effects of Microalgae Biomass Co-Substrate on Biogas Production from the Common Agricultural Biogas Plants Feedstock. Energies 2020, 13, 2186. [Google Scholar] [CrossRef]
- Nahm, M.; Morhart, C. Virginia mallow (Sida hermaphrodita (L.) Rusby) as perennial multipurpose crop: Biomass yields, energetic valorization, utilization potentials, and management perspectives. GCB Bioenergy 2018, 10, 393–404. [Google Scholar] [CrossRef]
- Papamatthaiakis, N.; Laine, A.; Haapala, A.; Ikonen, R.; Kuittinen, S.; Pappinen, A.; Kolström, M.; Mola-Yudego, B. New energy crop alternatives for Northern Europe: Yield, chemical and physical properties of Giant knotweed (Fallopia sachalinensis var. ‘Igniscum’) and Virginia mallow (Sida hermaphrodita). Fuel 2021, 304, 121349. [Google Scholar] [CrossRef]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Jablonowski, N.D. Effects of digestate fertilization on Sida hermaphrodita: Boosting biomass yields on marginal soils by increasing soil fertility. Biomass Bioenergy 2017, 107, 207–213. [Google Scholar] [CrossRef]
- Cumplido-Marin, L.; Graves, A.R.; Burgess, P.J.; Morhart, C.; Paris, P.; Jablonowski, N.D.; Facciotto, G.; Bury, M.; Martens, R.; Nahm, M. Two Novel Energy Crops: Sida hermaphrodita (L.) Rusby and Silphium perfoliatum L.—State of Knowledge. Agronomy 2020, 10, 928. [Google Scholar] [CrossRef]
- Siwek, H.; Włodarczyk, M.; Mozdzer, E.; Bury, M.; Kitczak, T. Chemical composition and biogas formation potential of Sida hermaphrodita and Silphium perfoliatum. Appl. Sci. 2019, 9, 4016. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Ibelli-Bianco, C. Predictive regression models for biochemical methane potential tests of biomass samples: Pitfalls and challenges of laboratory measurements. Renew. Sustain. Energy Rev. 2020, 127, 109890. [Google Scholar] [CrossRef]
- Das, S.; Das, I.; Ghangrekar, M.M. Role of applied potential on microbial electrosynthesis of organic compounds through carbon dioxide sequestration. J. Environ. Chem. Eng. 2020, 8, 104028. [Google Scholar] [CrossRef]
- Piechota, G. Multi-step biogas quality improving by adsorptive packed column system as application to biomethane upgrading. J. Environ. Chem. Eng. 2021, 9, 105944. [Google Scholar] [CrossRef]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Leong, T.S.; Gamlath, C.J.; Martin, G.J.; AshokKumar, M. Effects of High Pressure, Microwave and Ultrasound Processing on Proteins and Enzyme Activity in Dairy Systems—A Review. Innov. Food Sci. Emerg. Technol. 2019, 57, 102192. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kisielewska, M.; Kazimierowicz, J. Evaluation of Anaerobic Digestion of Dairy Wastewater in an Innovative Multi-Section Horizontal Flow Reactor. Energies 2020, 13, 2392. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Yang, S.; Zhang, A. Performance of a microwave radiation induced persulfate-hydrogen peroxide binary-oxidant process in treating dinitrodiazophenol wastewater. Sep. Purif. Technol. 2020, 236, 116253. [Google Scholar] [CrossRef]
- Pillet, F.; Gibot, L.; Catrain, A.; Kolosnjaj-Tabi, J.; Courtois, K.; Chretiennot, T.; Bellard, E.; Tarayre, J.; Golzio, M.; Vezinet, R. High power electromagnetic pulse applicators for evaluation of biological effects induced by electromagnetic radiation waves. RSC Adv. 2018, 8, 16319–16329. [Google Scholar] [CrossRef]
- Kulbacka, J.; Choromanska, A.; Rossowska, J.; Wezgowiec, J.; Saczko, J.; Rols, M.P. Cell membrane transport mechanisms: Ion channels and electrical properties of cell membranes. Adv. Anat. Embryol. Cell Biol. 2017, 227, 39–58. [Google Scholar] [CrossRef]
- Mertenes, B.; Knorr, D. Developments of nonthermal processes for food preservation. Food Technol. 1992, 5, 125–133. [Google Scholar]
- Horikoshi, S.; Nakamura, K.; Yashiro, M.; Kadomatsu, K.; Serpone, N. Probing the effect(s) of the microwaves’ electromagnetic fields in enzymatic reactions. Sci. Rep. 2019, 9, 8945. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-C.; Adams, D.M.; Amelkina, O.; White, K.K.; Amoretti, L.A.; Whitaker, M.G.; Comizzoli, P. Influence of microwave-assisted dehydration on morphological integrity and viability of cat ovarian tissues: First steps toward long-term preservation of complex biomaterials at supra-zero temperatures. PLoS ONE 2019, 14, e0225440. [Google Scholar] [CrossRef]
- Parker, M.C.; Besson, T.; Lamare, S.; Legoy, M.D. Microwave radiation can increase the rate of enzyme catalysed reaction in organic media. Tetrahedron Lett. 1996, 37, 8383–8386. [Google Scholar] [CrossRef]
- Lai, Y.F.; Wang, H.Y.; Peng, R.Y. Establishment of injury models in studies of biological effects induced by microwave radiation. Mil. Med. Res. 2021, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhi, W.J.; Wang, L.F.; Hu, X.J. Recent advances in the effects of microwave radiation on brains. Mil. Med. Res. 2017, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Svidlov, A.; Drobotenko, M.; Basov, A.; Gerasimenko, E.; Malyshko, V.; Elkina, A.; Baryshev, M.; Dzhimak, S. DNA Dynamics under Periodic Force Effects. Int. J. Mol. Sci. 2021, 22, 7873. [Google Scholar] [CrossRef] [PubMed]
- Shamis, Y.; Taube, A.; Mitik-Dineva, N.; Croft, R.; Crawford, R.J.; Ivanova, E.P. Specific electromagnetic effects of microwave radiation on Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. Microwave Radiation Influence on Dairy Waste Anaerobic Digestion in a Multi-Section Hybrid Anaerobic Reactor (M-SHAR). Processes 2021, 9, 1772. [Google Scholar] [CrossRef]
- Rougier, C.; Prorot, A.; Chazal, P.; Leveque, P.; Leprat, P. Thermal and Nonthermal Effects of Discontinuous Microwave Exposure (2.45 Gigahertz) on the Cell Membrane of Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 4832–4841. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).