Solar Carbo-Thermal and Methano-Thermal Reduction of MgO and ZnO for Metallic Powder and Syngas Production by Green Extractive Metallurgy
Abstract
:1. Introduction
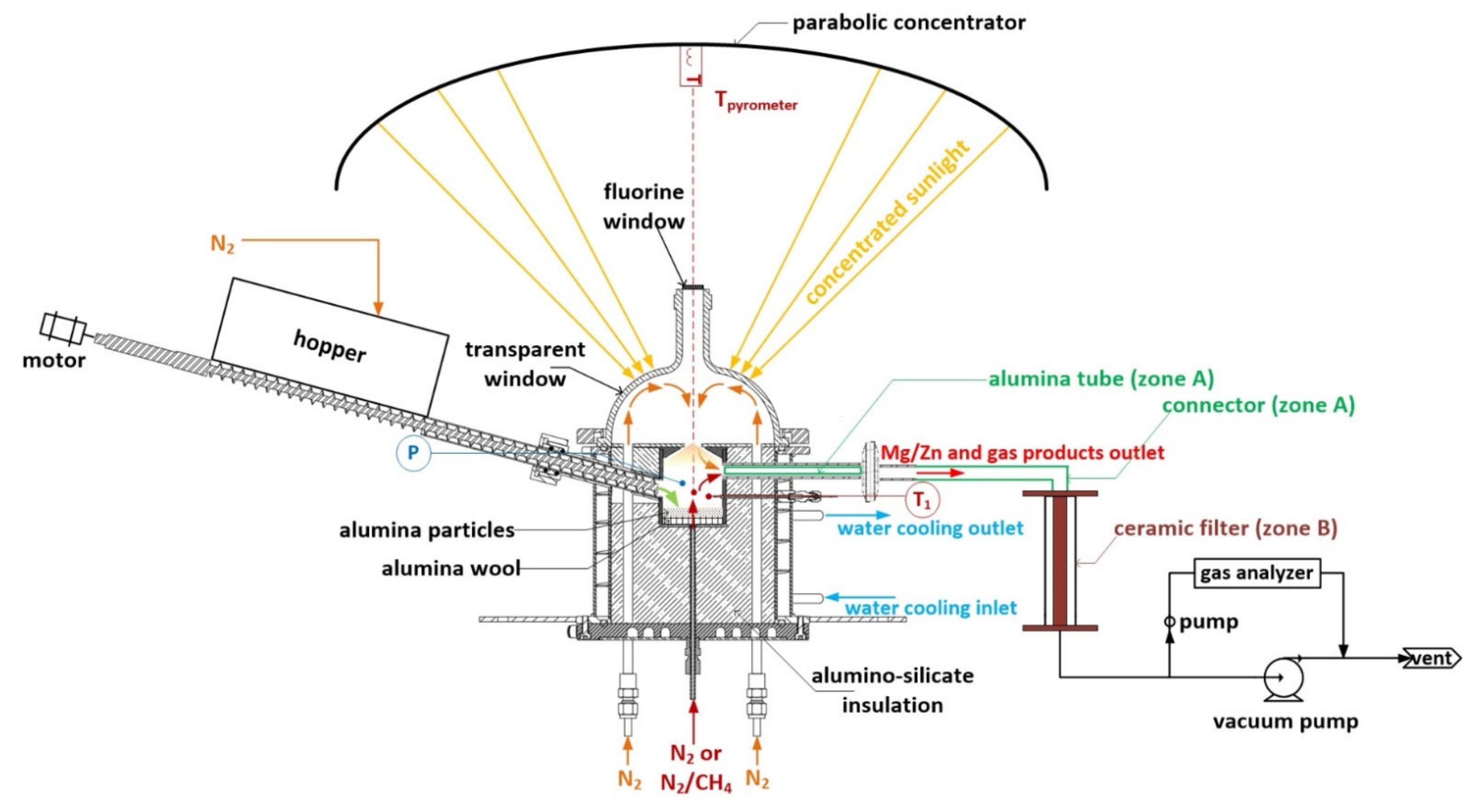
2. Materials and Methods
3. Results and Discussion
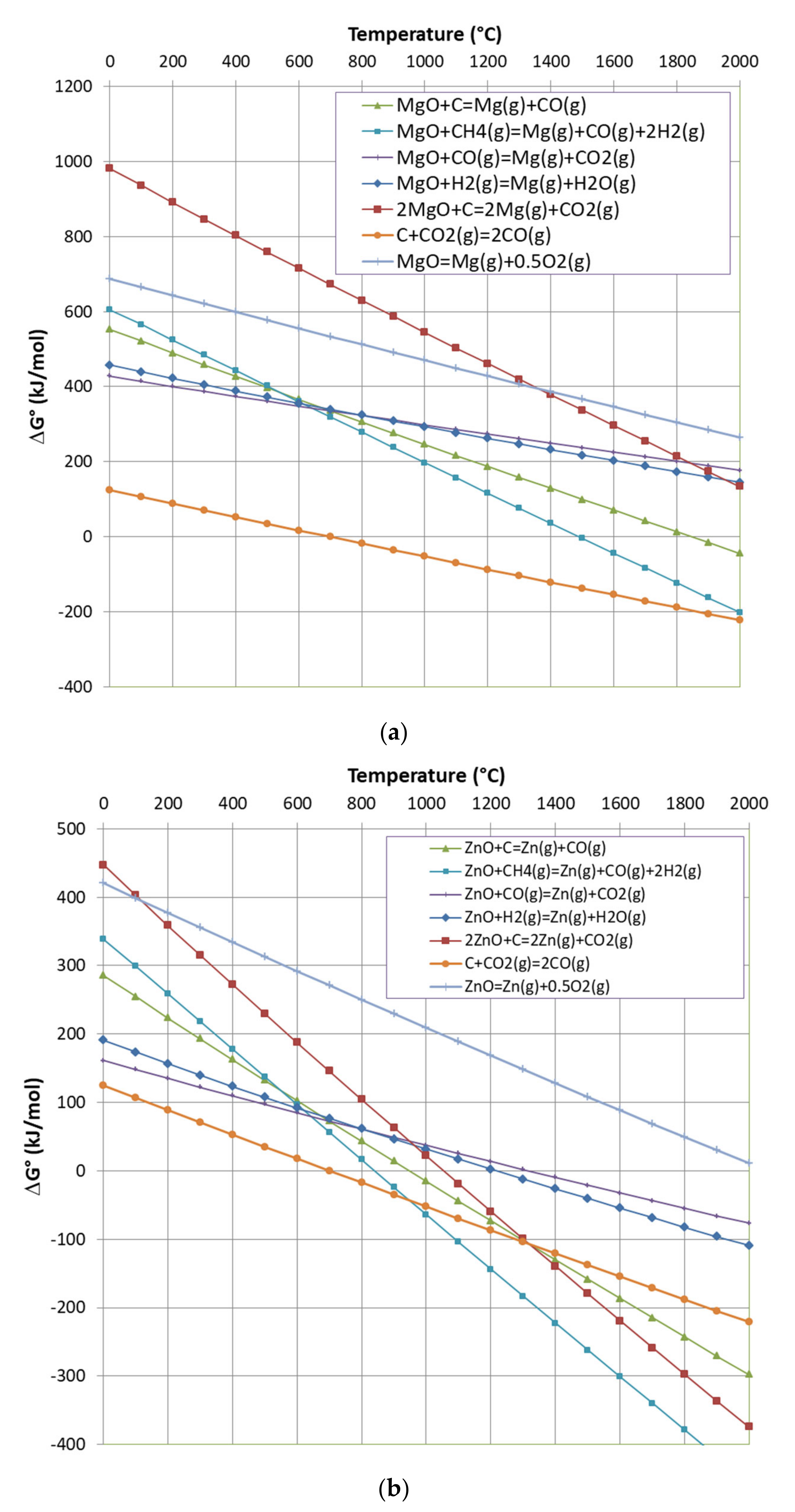
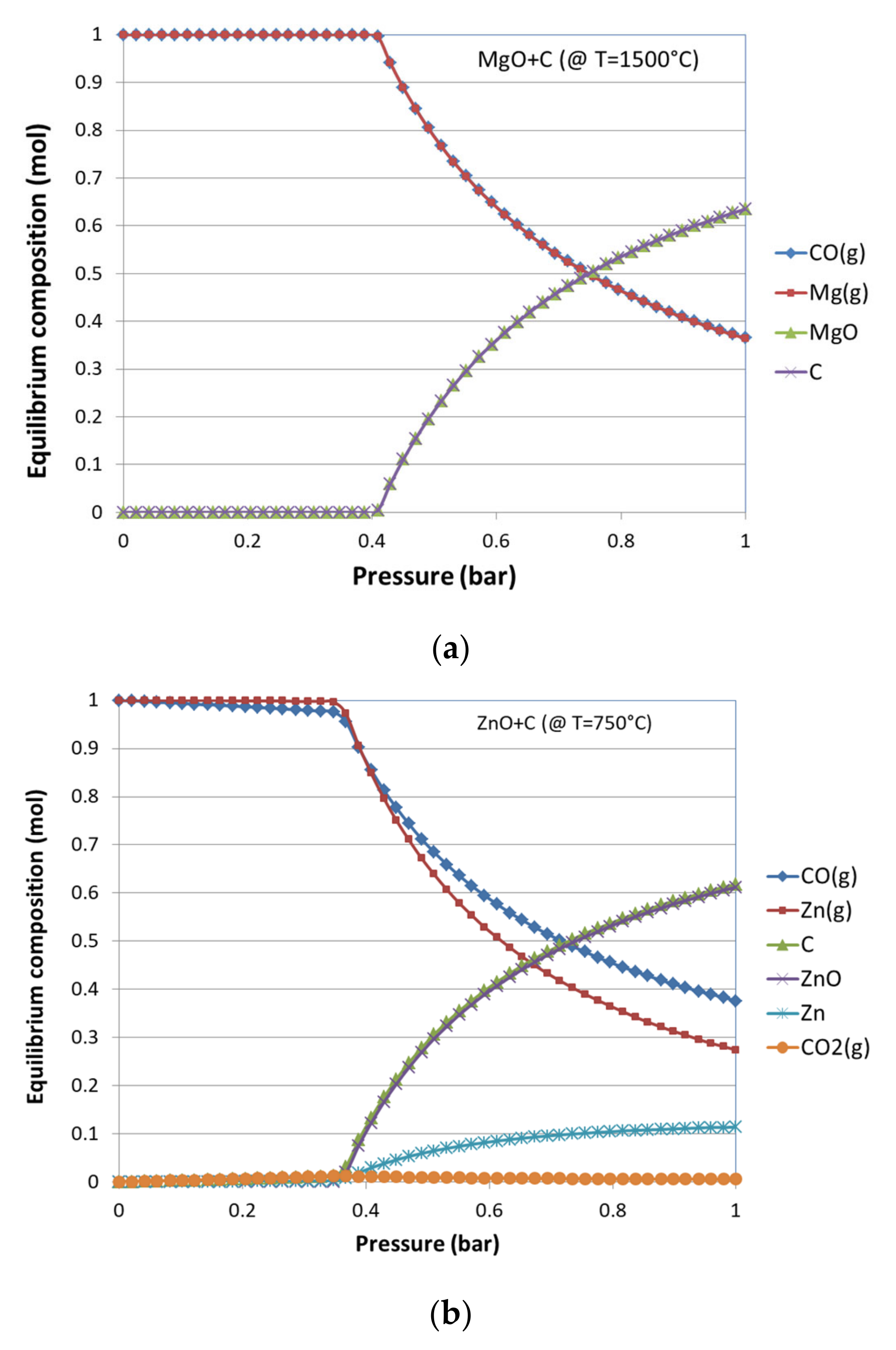
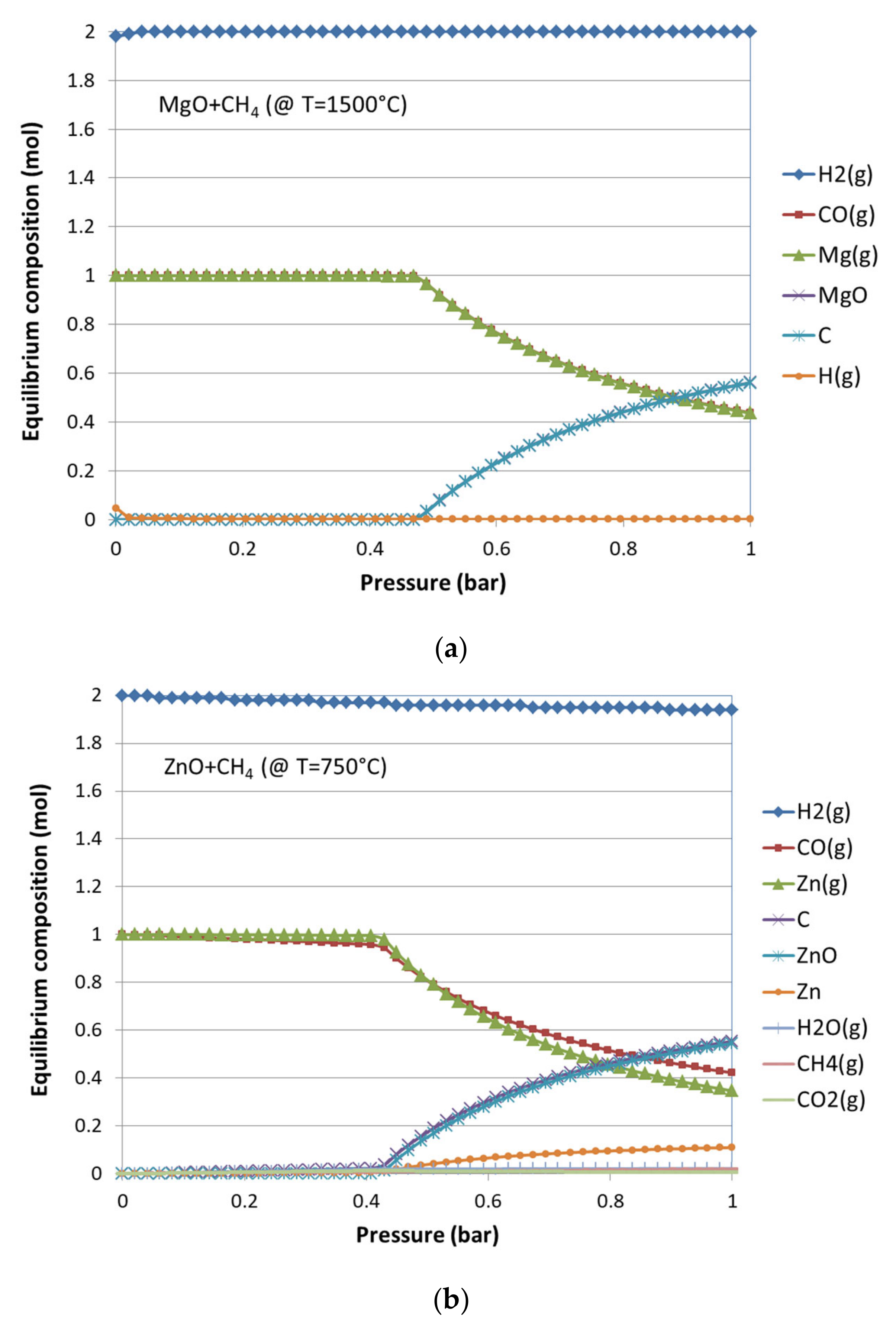
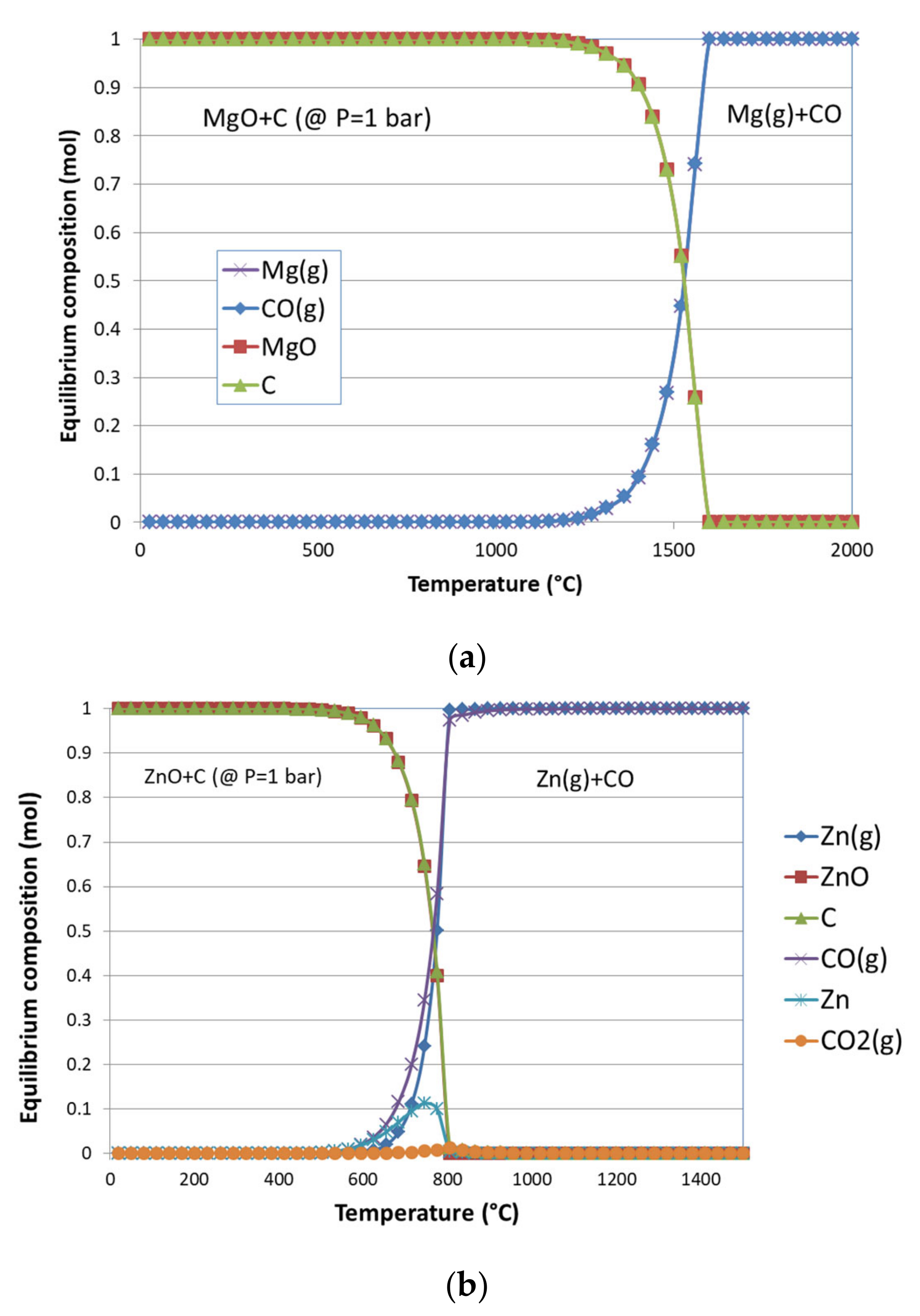
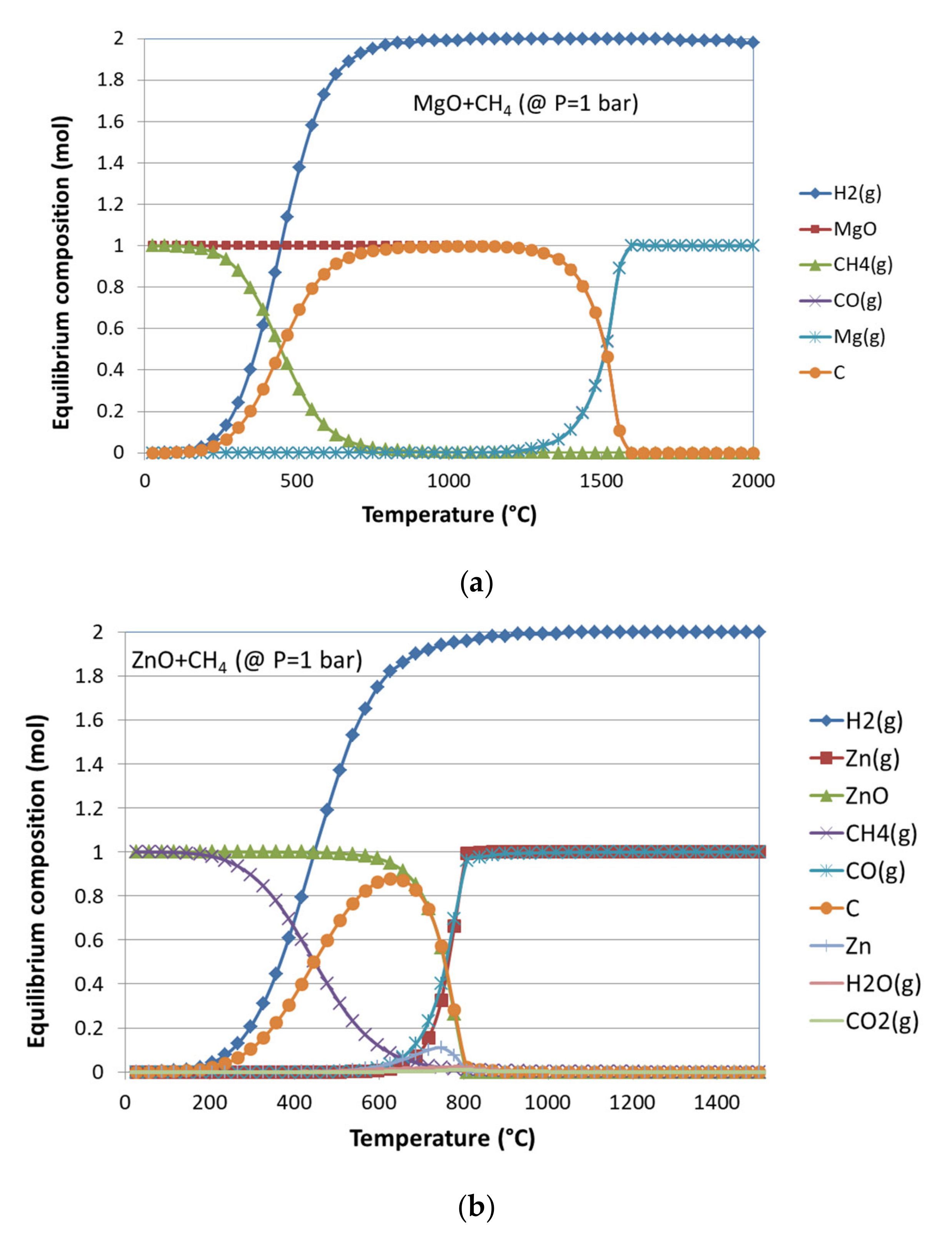
3.1. Thermodynamic Equilibrium Analysis
3.2. Solar Reactor Performace Evaluation
3.3. Batch Carbo-Thermal/Methano-Thermal Reduction of ZnO and MgO
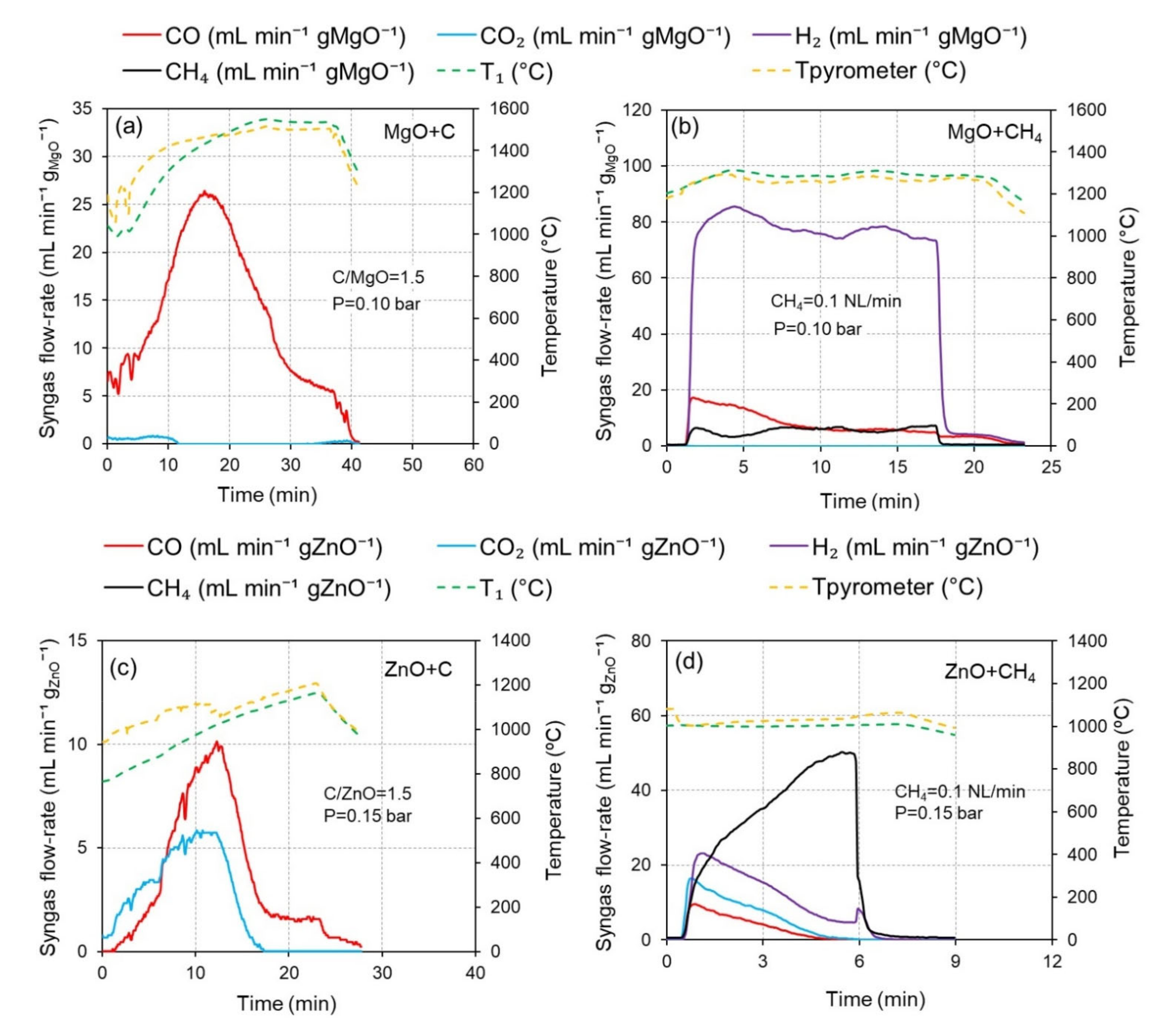
3.3.1. Syngas Production Rate
3.3.2. Gas Species Production Yield
3.4. Continuous Carbothemal Reduction
3.5. Solid Product Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chuayboon, S.; Abanades, S. An overview of solar decarbonization processes, reacting oxide materials, and thermochemical reactors for hydrogen and syngas production. Int. J. Hydrog. Energy 2020, 45, 25783–25810. [Google Scholar] [CrossRef]
- Rodat, S.; Abanades, S.; Boujjat, H.; Chuayboon, S. On the Path toward day and night continuous solar high temperature thermochemical processes: A review. Renew. Sustain. Energy Rev. 2020, 132, 110061. [Google Scholar] [CrossRef]
- Boujjat, H.; Rodat, S.; Chuayboon, S.; Abanades, S. Experimental and CFD investigation of inert bed materials effects in a high-temperature conical cavity-type reactor for continuous solar-driven steam gasification of biomass. Chem. Eng. Sci. 2020, 228, 115970. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Thermodynamic and experimental investigation of solar-driven biomass pyro-gasification using H2O, CO2, or ZnO oxidants for clean syngas and metallurgical Zn production. Processes 2021, 9, 687. [Google Scholar] [CrossRef]
- Boujjat, H.; Rodat, S.; Chuayboon, S.; Abanades, S. Experimental and numerical study of a directly irradiated hybrid solar/combustion spouted bed reactor for continuous steam gasification of biomass. Energy 2019, 189, 116118. [Google Scholar] [CrossRef]
- Boujjat, H.; Rodat, S.; Chuayboon, S.; Abanades, S. Numerical simulation of reactive gas-particle flow in a solar jet spouted bed reactor for continuous biomass gasification. Int. J. Heat Mass Transf. 2019, 144, 118572. [Google Scholar] [CrossRef]
- Osinga, T.; Olalde, G.; Steinfeld, A. Solar carbothermal reduction of ZnO: Shrinking packed-bed reactor modeling and experimental validation. Ind. Eng. Chem. Res. 2004, 43, 7981–7988. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Solar chemical looping reforming of methane combined with isothermal H2O/CO2 splitting using ceria oxygen carrier for syngas production. J. Energy Chem. 2020, 41, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Villafán-Vidales, H.I.; Abanades, S.; Montiel-González, M.; Romero-Paredes-Rubio, H. Carbo- and methanothermal reduction of tungsten trioxide into metallic tungsten for thermochemical production of solar fuels. Energy Technol. 2017, 5, 692–702. [Google Scholar] [CrossRef]
- Gokon, N.; Mataga, T.; Kondo, N.; Kodama, T. Thermochemical two-step water splitting by internally circulating fluidized bed of NiFe2O4 particles: Successive reaction of thermal-reduction and water-decomposition steps. Int. J. Hydrog. Energy 2011, 36, 4757–4767. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Tailoring hybrid nonstoichiometric ceria redox cycle for combined solar methane reforming and thermochemical conversion of H2O/CO2. Energy Fuels 2016, 30, 6050–6058. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Stepwise solar methane reforming and water-splitting via lattice oxygen transfer in iron and cerium oxides. Energy Technol. 2020, 8, 1900415. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Experimental screening of perovskite oxides as efficient redox materials for solar thermochemical CO2 conversion. Sustain. Energy Fuels 2018, 2, 843–854. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Julbe, A. Non-stoichiometric redox active perovskite materials for solar thermochemical fuel production: A review. Catalysts 2018, 8, 611. [Google Scholar] [CrossRef] [Green Version]
- Chuayboon, S.; Abanades, S.; Rodat, S. High-purity and clean syngas and hydrogen production from two-step CH4 reforming and H2O splitting through isothermal ceria redox cycle using concentrated sunlight. Front. Energy Res. 2020, 8, 128. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Beche, E.; Lemont, F.; Flamant, G. Hydrogen production from mixed cerium oxides via three-step water-splitting cycles. Solid State Ion. 2009, 180, 1003–1010. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Drobek, M.; Ayral, A.; Julbe, A. Recent progress on ceria doping and shaping strategies for solar thermochemical water and CO2 splitting cycles. AIMS Mater. Sci. 2019, 6, 657–684. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Julbe, A.; Jouannaux, J.; Cartoixa, B. Two-step CO2 and H2O splitting using perovskite-coated ceria foam for enhanced green fuel production in a porous volumetric solar reactor. J. CO2 Util. 2020, 41, 101257. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Syngas production via solar-driven chemical looping methane reforming from redox cycling of ceria porous foam in a volumetric solar reactor. Chem. Eng. J. 2019, 356, 756–770. [Google Scholar] [CrossRef]
- Warren, K.J.; Reim, J.; Randhir, K.; Greek, B.; Carrillo, R.; Hahn, D.W.; Scheffe, J.R. Theoretical and experimental investigation of solar methane reforming through the nonstoichiometric ceria redox cycle. Energy Technol. 2017, 5, 2138–2149. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S. Investigation of thermal and carbothermal reduction of volatile oxides (ZnO, SnO2, GeO2, and MgO) via solar-driven vacuum thermogravimetry for thermochemical production of solar fuels. Thermochimica Acta 2015, 605, 86–94. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Solar-driven chemical looping methane reforming using ZnO oxygen carrier for syngas and Zn production in a cavity-type solar reactor. Catalysts 2020, 10, 1356. [Google Scholar] [CrossRef]
- Chubukov, B.A.; Rowe, S.C.; Palumbo, A.W.; Wallace, M.A.; Weimer, A.W. Investigation of continuous carbothermal reduction of magnesia by magnesium vapor condensation onto a moving bed of solid particles. Powder Technol. 2019, 365, 2–11. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S.; Jumas, J.-C.; Olivier-Fourcade, J. Characterization of two-step tin-based redox system for thermochemical fuel production from solar-driven CO2 and H2O splitting cycle. Ind. Eng. Chem. Res. 2014, 53, 5668–5677. [Google Scholar] [CrossRef]
- Chambon, M.; Abanades, S.; Flamant, G. Solar Thermal reduction of ZnO and SnO2: Characterization of the recombination reaction with O2. Chem. Eng. Sci. 2010, 65, 3671–3680. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S. Thermodynamic and kinetic study of the carbothermal reduction of SnO 2 for solar thermochemical fuel generation. Energy Fuels 2014, 28, 1396–1405. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Solar metallurgy for sustainable Zn and Mg production in a vacuum reactor using concentrated sunlight. Sustainability 2020, 12, 6709. [Google Scholar] [CrossRef]
- Perkins, C.; Lichty, P.R.; Weimer, A.W. Thermal ZnO dissociation in a rapid aerosol reactor as part of a solar hydrogen production cycle. Int. J. Hydrog. Energy 2008, 33, 499–510. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Clean magnesium production using concentrated solar heat in a high-temperature cavity-type thermochemical reactor. J. Clean. Prod. 2019, 232, 784–795. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Combined ZnO Reduction and methane reforming for co-production of pure zn and syngas in a prototype solar thermochemical reactor. Fuel Process. Technol. 2021, 211, 106572. [Google Scholar] [CrossRef]
- Abanades, S.; Flamant, G. Hydrogen production from solar thermal dissociation of methane in a high-temperature fluid-wall chemical reactor. Chem. Eng. Process. Process Intensif. 2008, 47, 490–498. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Thermochemical performance assessment of solar continuous methane-driven ZnO reduction for co-production of pure zinc and hydrogen-rich syngas. Chem. Eng. J. 2021, 429, 132356. [Google Scholar] [CrossRef]
- Alam, M.E.; Han, S.; Nguyen, Q.B.; Salem Hamouda, A.M.; Gupta, M. Development of new magnesium based alloys and their nanocomposites. J. Alloy. Compd. 2011, 509, 8522–8529. [Google Scholar] [CrossRef]
- Abanades, S. Metal oxides applied to thermochemical water-splitting for hydrogen production using concentrated solar energy. ChemEngineering 2019, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Abanades, S. Thermogravimetry analysis of CO2 and H2O reduction from solar nanosized Zn powder for thermochemical fuel production. Ind. Eng. Chem. Res. 2012, 51, 741–750. [Google Scholar] [CrossRef]
- Abu-Hamed, T.; Karni, J.; Epstein, M. The use of boron for thermochemical storage and distribution of solar energy. Solar Energy 2007, 81, 93–101. [Google Scholar] [CrossRef]
- Schwab, B.; Ruh, A.; Manthey, J.; Drosik, M. Zinc. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Villasmil, W.; Brkic, M.; Wuillemin, D.; Meier, A.; Steinfeld, A. Pilot scale demonstration of a 100-KWth solar thermochemical plant for the thermal dissociation of ZnO. J. Solar Energy Eng. 2013, 136, 11011–11016. [Google Scholar] [CrossRef]
- Halmann, M.; Frei, A.; Steinfeld, A. Vacuum carbothermic reduction of Al2O3, BeO, MgO-CaO, TiO2, ZrO2, HfO2 + ZrO2, SiO2, SiO2 + Fe2O3, and GeO2 to the metals. A thermodynamic study. Mineral Process. Extr. Metall. Rev. 2011, 32, 247–266. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, B.; Yang, C.; Yang, B.; Qu, T.; Liu, H.; Dai, Y.; Liu, D. Analysis of magnesia carbothermic reduction process in vacuum. Metall. Mater. Trans. B 2014, 45, 1936–1941. [Google Scholar] [CrossRef]
- Xie, W.; Chen, J.; Wang, H.; Zhang, X.; Peng, X.; Yang, Y. Kinetics of magnesium preparation by vacuum-assisted carbothermic reduction method. Rare Metals 2016, 35, 192–197. [Google Scholar] [CrossRef]
- Yang, C.; Tian, Y.; Qu, T.; Yang, B.; Xu, B.; Dai, Y. Analysis of the behavior of magnesium and CO vapor in the carbothermic reduction of magnesia in a vacuum. J. Magnes. Alloy. 2014, 2, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Xiong, N.; Tian, Y.; Yang, B.; Xu, B.-Q.; Liu, D.-C.; Dai, Y.-N. Volatilization and condensation behaviours of mg under vacuum. Vacuum 2018, 156, 463–468. [Google Scholar] [CrossRef]
- Chubukov, B.A.; Palumbo, A.W.; Rowe, S.C.; Hischier, I.; Groehn, A.J.; Weimer, A.W. Pressure dependent kinetics of magnesium oxide carbothermal reduction. Thermochim. Acta 2016, 636, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Chubukov, B.A.; Palumbo, A.W.; Rowe, S.C.; Wallace, M.A.; Weimer, A.W. Enhancing the rate of magnesium oxide carbothermal reduction by catalysis, milling, and vacuum operation. Ind. Eng. Chem. Res. 2017, 56, 13602–13609. [Google Scholar] [CrossRef]
- Brkic, M.; Koepf, E.; Meier, A. Solar carbothermal reduction of aerosolized ZnO particles under vacuum: Modeling, experimentation, and characterization of a drop-tube reactor. Chem. Eng. J. 2017, 313, 435–449. [Google Scholar] [CrossRef]



| Run No. | Reaction | Mode | Metal Oxide Mass (g) | Reducer Flow Rate | Pressure (bar) | T1 max (°C) | CO (mmol/gmetal oxide) | CO2 (mmol/gmetal oxide) | H2 (mmol/gmetal oxide) | XMgO or XZnO (%) | XCH4 (%) | 𝜂solar-to-fuel (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MgO + 1.5AC | Batch | 2.0 | - | 0.10 | 1550 | 1.32 | 23.8 | 0.2 | - | 97.8 | - | 1.7 |
| 2 | MgO + CH4 | Batch | 2.0 | 0.1 NL/min | 0.10 | 1314 | 1.10 | 7.1 | 0.1 | 57.3 | 28.9 | 91.7 | 3.0 |
| 3 | ZnO + 1.5AC | Batch | 2.4 | - | 0.15 | 1167 | 0.82 | 4.2 | 2.6 | - | 76.5 | - | 1.1 |
| 4 | ZnO + CH4 | Batch | 2.0 | 0.1 NL/min | 0.15 | 1000 | 0.77 | 0.9 | 1.6 | 3.3 | 60.6 | 26.6 | 2.6 |
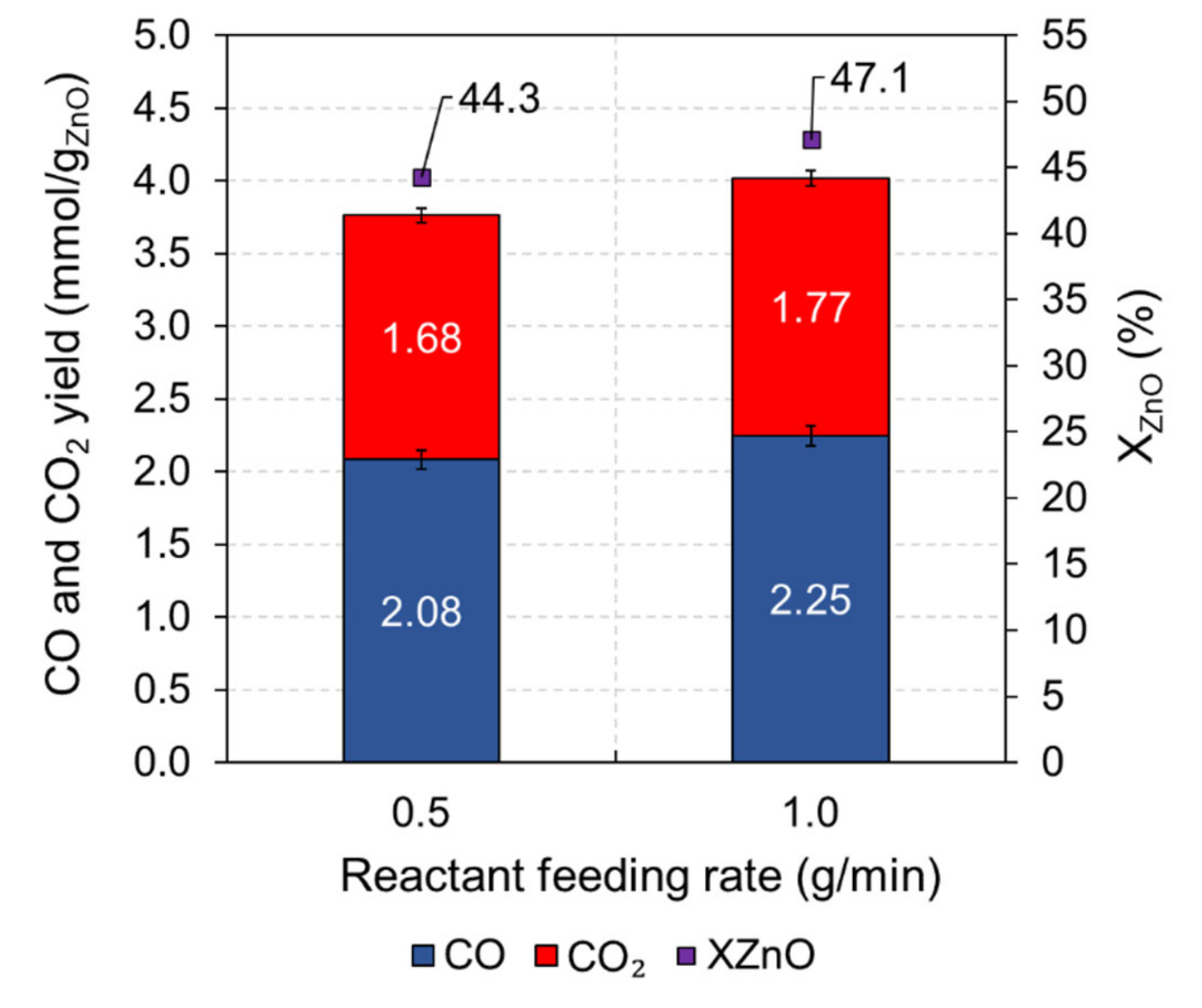
| 5 | ZnO + 1.5CB | Continuous | 10 | 0.5 g/min | 0.90 | 950 | 0.80 | 2.1 | 1.7 | - | 44.3 | - | 3.3 |
| 6 | ZnO + 1.5CB | Continuous | 10 | 1.0 g/min | 0.90 | 950 | 0.73 | 2.3 | 1.8 | - | 47.1 | - | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuayboon, S.; Abanades, S. Solar Carbo-Thermal and Methano-Thermal Reduction of MgO and ZnO for Metallic Powder and Syngas Production by Green Extractive Metallurgy. Processes 2022, 10, 154. https://doi.org/10.3390/pr10010154
Chuayboon S, Abanades S. Solar Carbo-Thermal and Methano-Thermal Reduction of MgO and ZnO for Metallic Powder and Syngas Production by Green Extractive Metallurgy. Processes. 2022; 10(1):154. https://doi.org/10.3390/pr10010154
Chicago/Turabian StyleChuayboon, Srirat, and Stéphane Abanades. 2022. "Solar Carbo-Thermal and Methano-Thermal Reduction of MgO and ZnO for Metallic Powder and Syngas Production by Green Extractive Metallurgy" Processes 10, no. 1: 154. https://doi.org/10.3390/pr10010154
APA StyleChuayboon, S., & Abanades, S. (2022). Solar Carbo-Thermal and Methano-Thermal Reduction of MgO and ZnO for Metallic Powder and Syngas Production by Green Extractive Metallurgy. Processes, 10(1), 154. https://doi.org/10.3390/pr10010154






