Fundamental Study on Hydrogen Low-NOx Combustion Using Exhaust Gas Self-Recirculation
Abstract
:1. Introduction
2. Experiments and Methods
3. Results and Discussion
3.1. Combustion Stability
3.2. Temperature Distribution
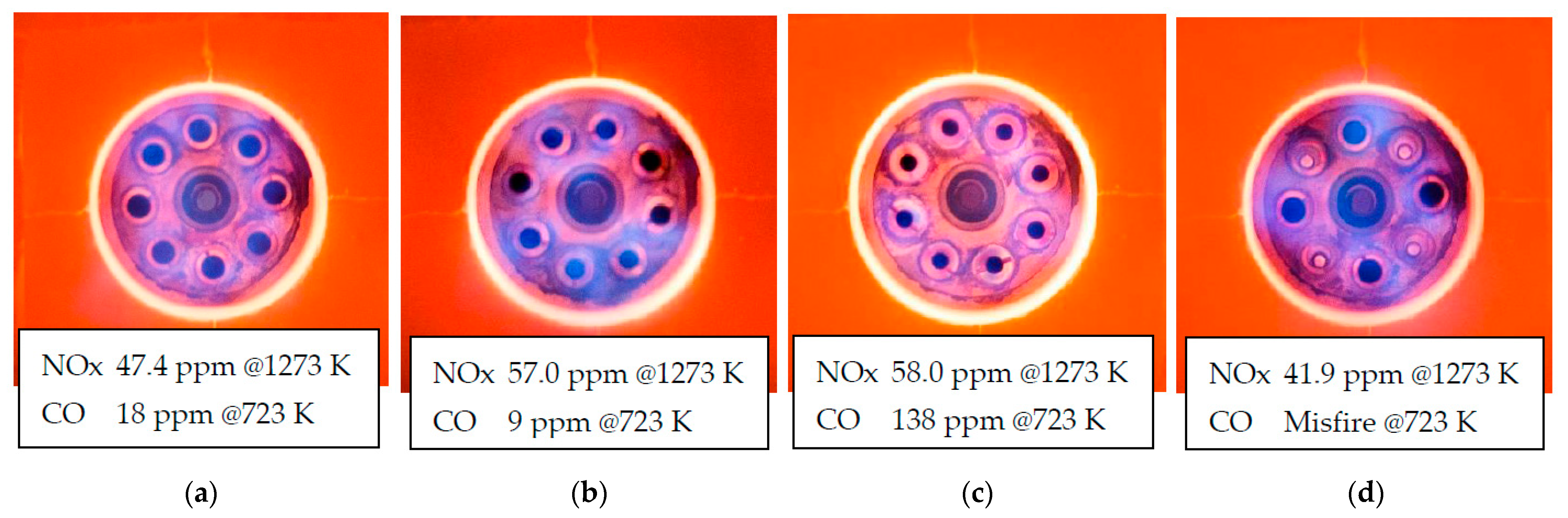
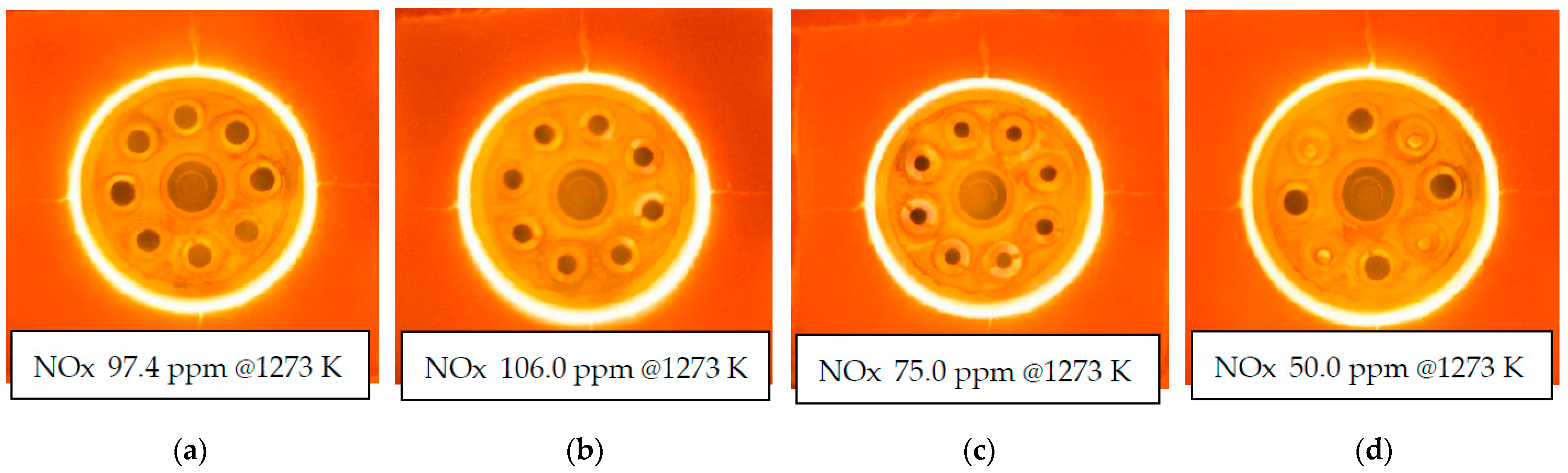
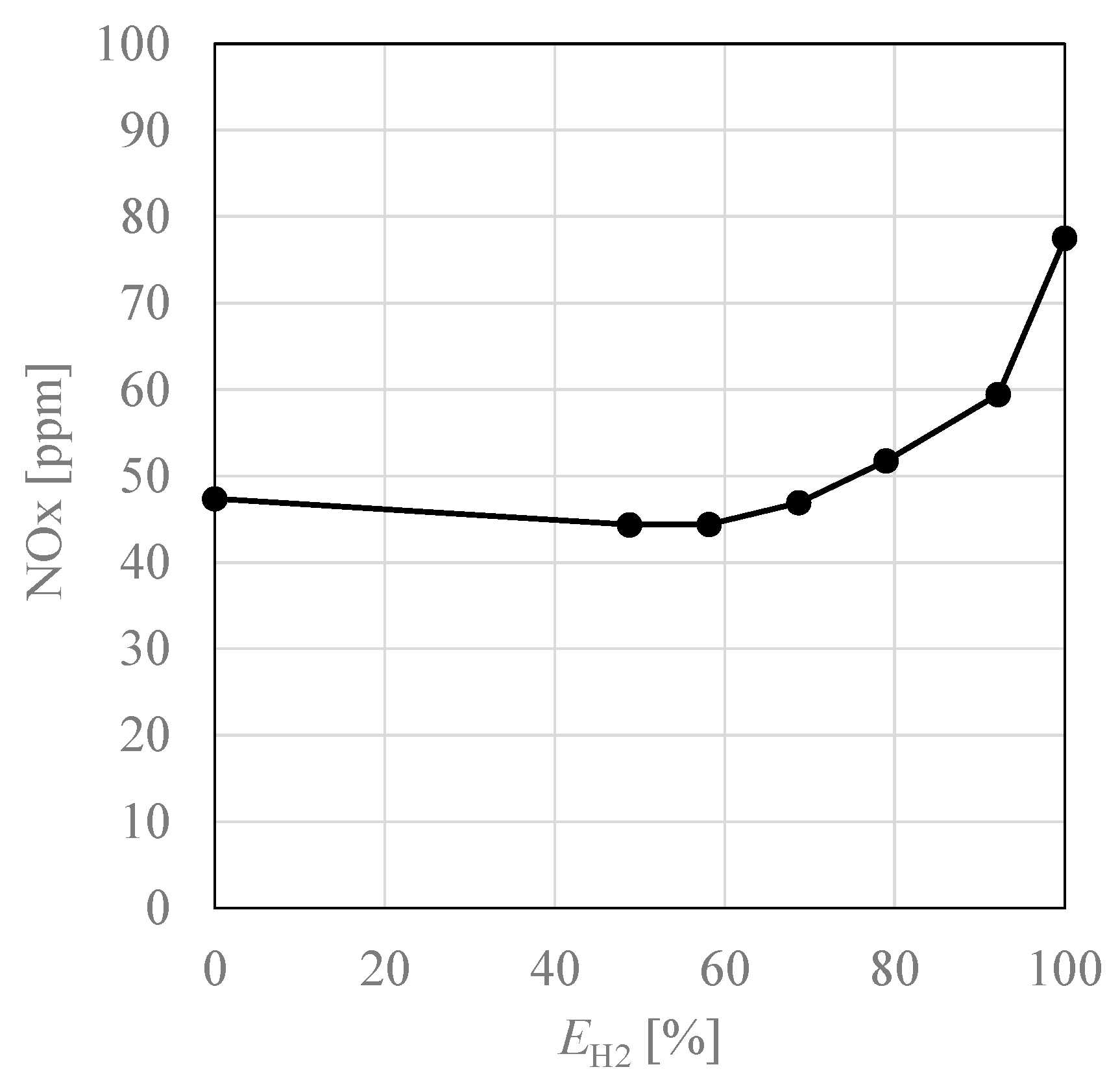
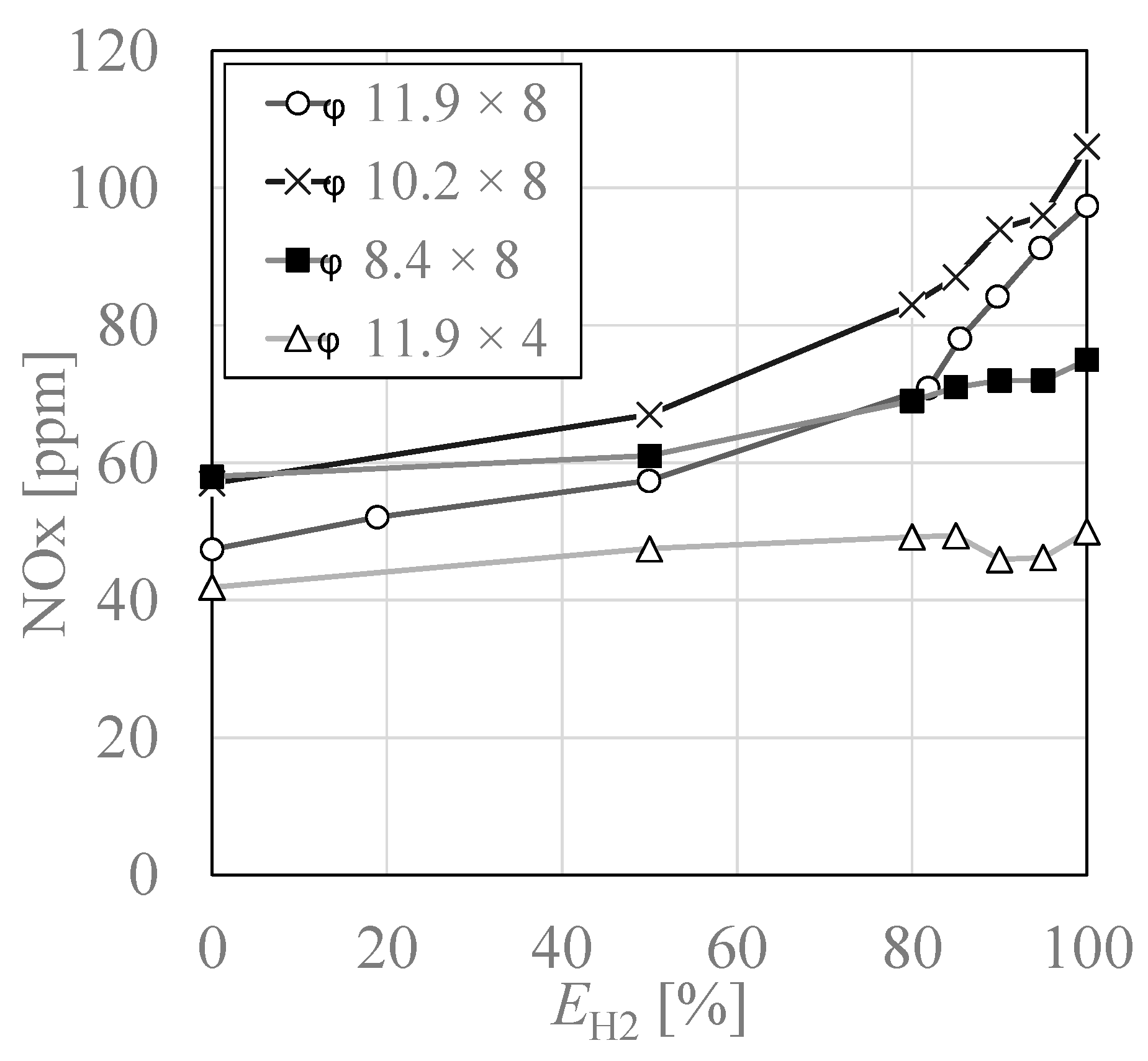
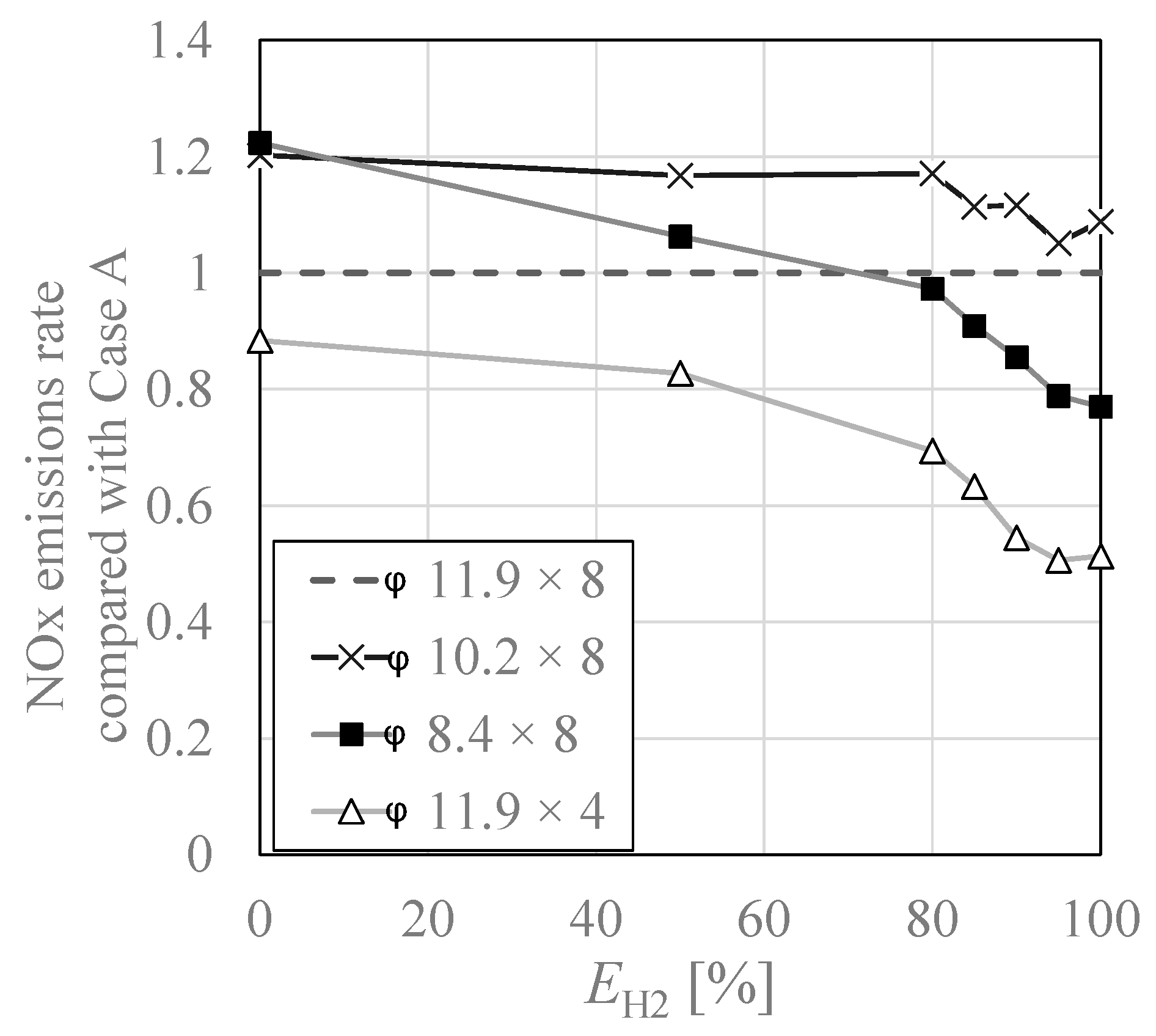
3.3. NOx Emissions Characteristics
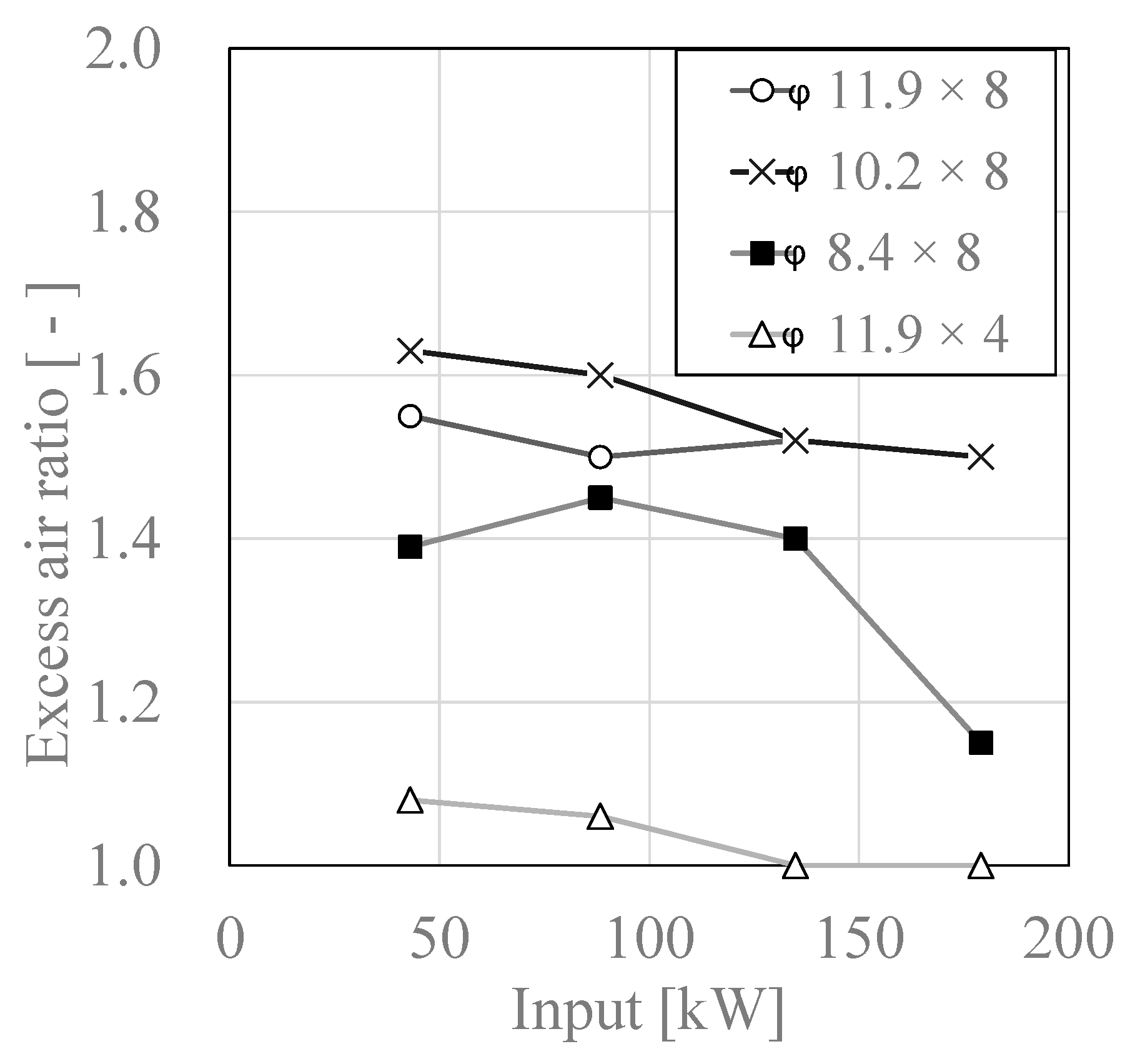
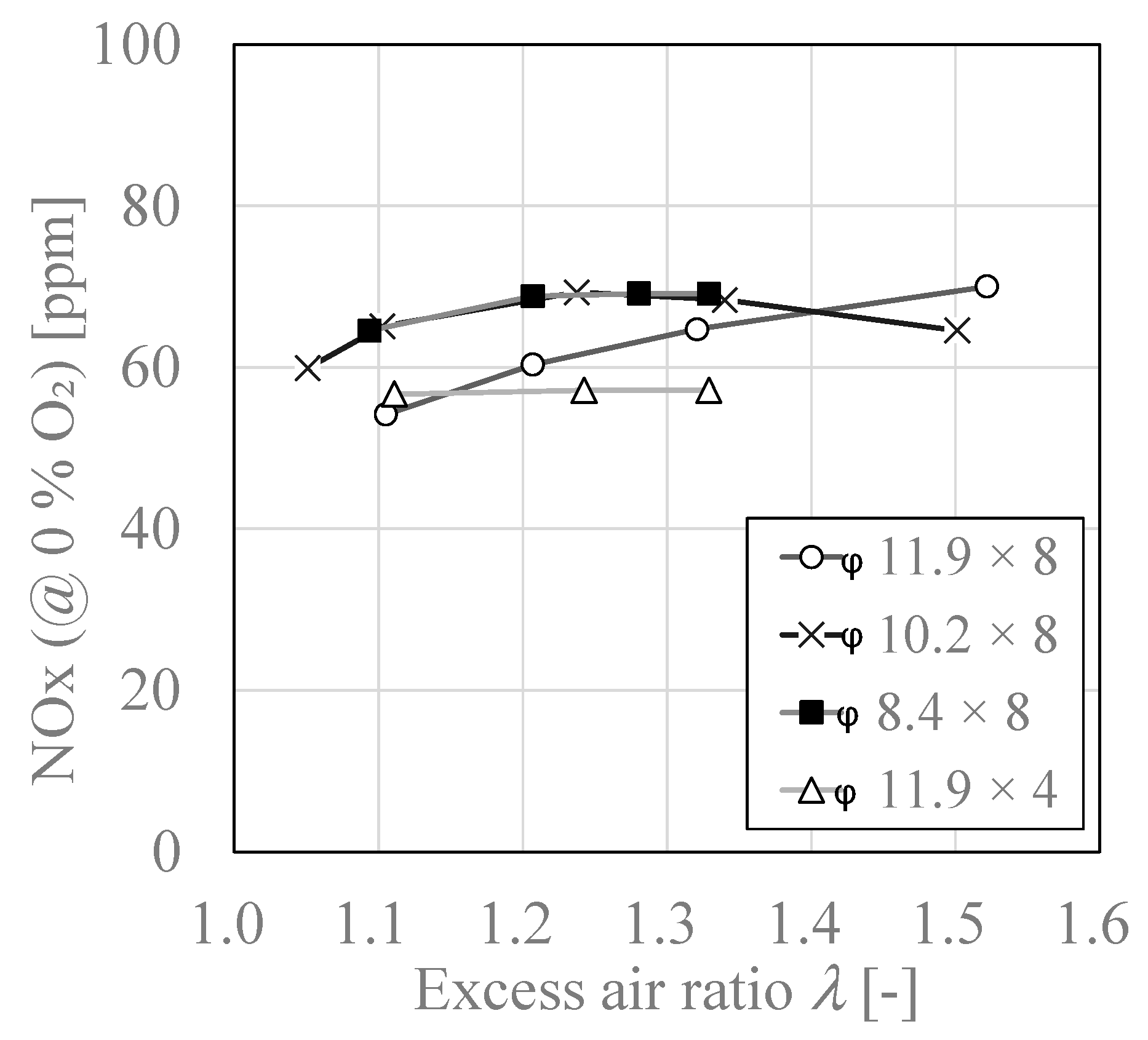
3.4. Relationship between Excess Air Ratio “λ” and NOx
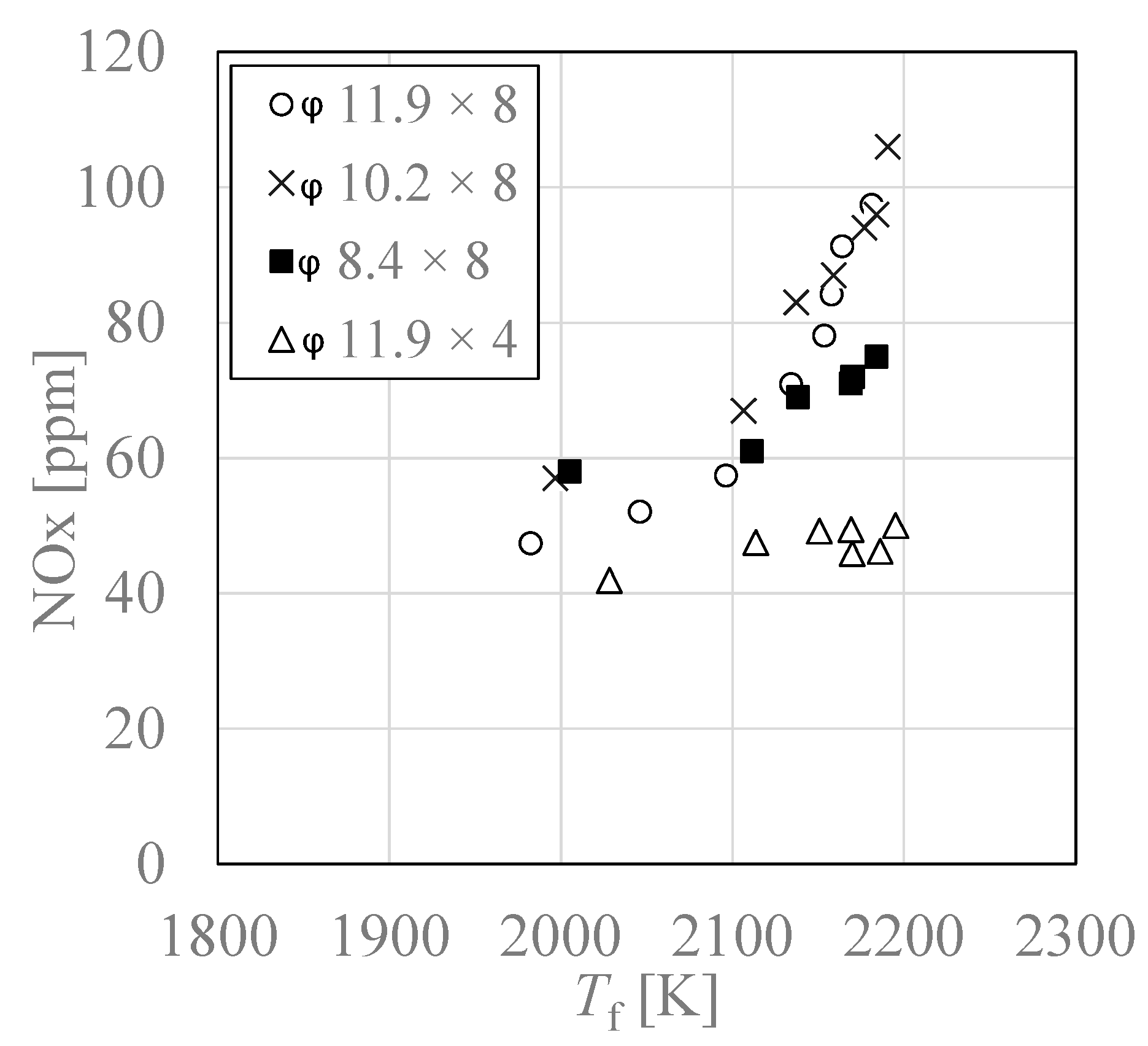
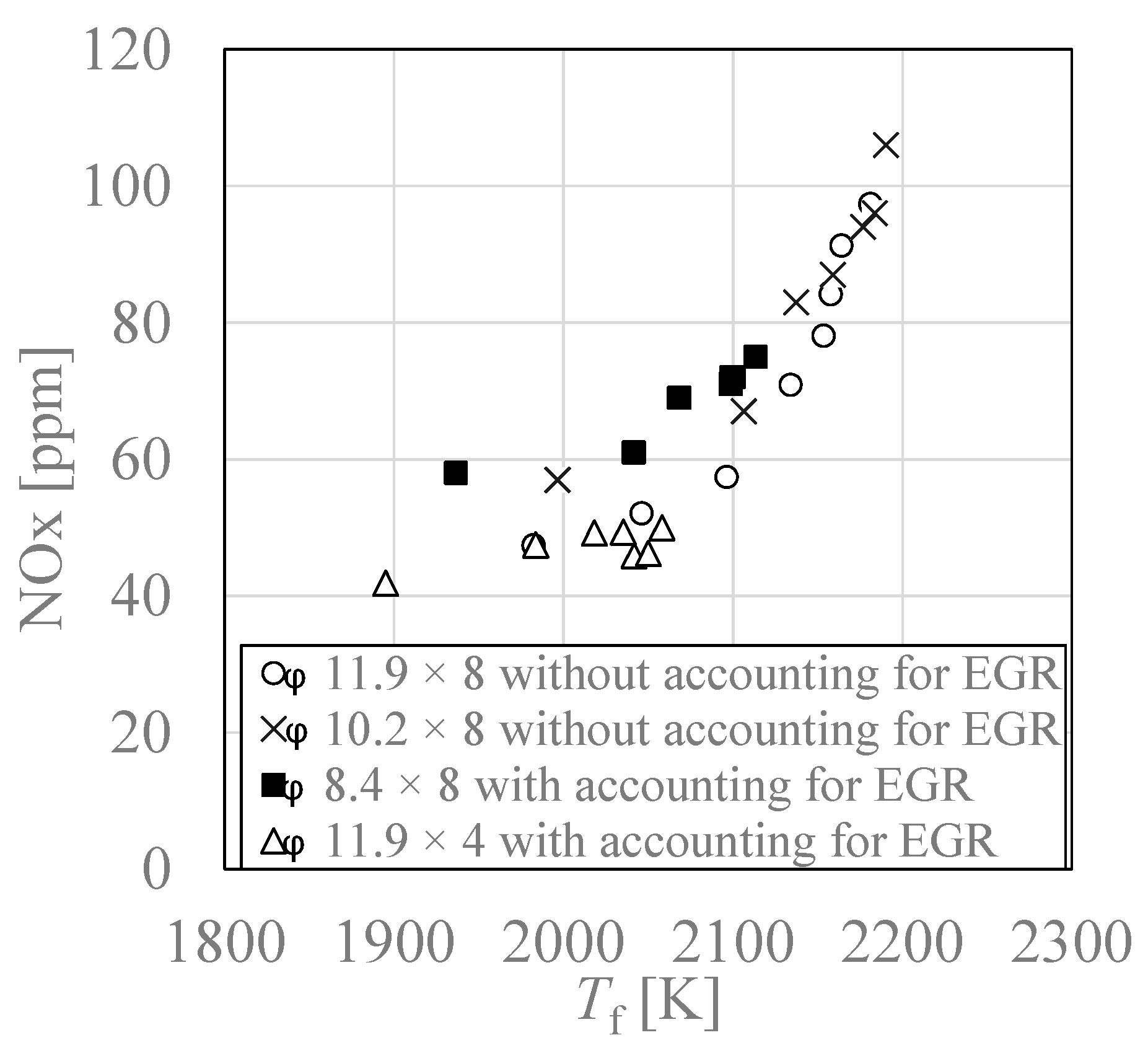
3.5. Relationship between Adiabatic Flame Temperature “Tf” and NOx
4. Conclusions
- (1)
- When hydrogen was burned using a burner with city gas specifications, NOx increased twice as much due to the increase in flame temperature.
- (2)
- When the air nozzles diameter was reduced and the air flow velocity was increased by a factor of 1.4, the amount of fuel gas drawn into the air nozzle increased and the local air ratio decreased, resulting in an increase in NOx.
- (3)
- When the combustion air nozzle diameter was reduced and the combustion air flow velocity was doubled, NOx increased under low hydrogen mixing ratio conditions as same as mentioned above, but NOx reduced under high hydrogen mixing ratio conditions due to exhaust gas recirculation.
- (4)
- When the number of air nozzles was reduced to half instead of the air nozzles diameter and the combustion air velocity was doubled, NOx was reduced at all co-firing rates, and a 50% reduction was successfully achieved in hydrogen combustion. And the NOx in this case was almost equal to the NOx in the case of city gas combustion in the city gas model.
- (5)
- By reducing the number of air nozzles, the distance between air nozzles became larger, resulting in a larger EGR ratio. As a result, misfire occurred in hydrocarbon combustion, but in hydrogen combustion, stable combustion could be continued without abnormal combustion.
- (6)
- When the number of air nozzles was reduced to half, the exhaust gas recirculation rate was about 10%, which was assumed from the adiabatic flame temperature simulated by the equilibrium calculation of chemical kinetics simulator software.
- (7)
- It was confirmed that low-NOx combustion by exhaust gas recirculation is possible in hydrogen combustion.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verhage, A.J.; Coolegem, J.F.; Mulder, M.J.; Yildirim, M.H.; de Bruijn, F.A. 30,000 h operation of a 70 kW stationary PEM fuel cell system using hydrogen from a chlorine factory. Int. J. Hydrogen Energy 2013, 38, 4714–4724. [Google Scholar] [CrossRef]
- Haneda, T.; Akisawa, A. Technological assessment of PEFC power generation system using by-product hydrogen produced from a caustic soda plant. Int. J. Hydrogen Energy 2017, 42, 3240–3249. [Google Scholar] [CrossRef]
- Pareja, J.; Burbano, H.J.; Amell, A.; Carvajal, J. Laminar burning velocities and flame stability analysis of hydrogen/air premixed flames at low pressure. Int. J. Hydrogen Energy 2011, 36, 6317–6324. [Google Scholar] [CrossRef]
- Takizawa, K.; Takahashi, A.; Tokuhashi, K.; Kondo, S.; Sekiya, A. Burning velocity measurements of nitrogen-containing compounds. J. Hazard. Mater. 2008, 155, 144–152. [Google Scholar] [CrossRef]
- Ono, R.; Nifuku, M.; Fujiwara, S.; Horiguchi, S.; Oda, T. Minimum ignition energy of hydrogen–air mixture: Effects of humidity and spark duration. J. Electrost. 2007, 65, 87–93. [Google Scholar] [CrossRef]
- Sadaghiani, M.S.; Arami-Niya, A.; Zhang, D.; Tsuji, T.; Tanaka, Y.; Seiki, Y.; May, E.F. Minimum ignition energies and laminar burning velocities of ammonia, HFO-1234yf, HFC-32 and their mixtures with carbon dioxide, HFC-125 and HFC-134a. J. Hazard. Mater. 2020, 407, 124781. [Google Scholar] [CrossRef] [PubMed]
- Choudhuri, A. Characteristics of hydrogen–hydrocarbon composite fuel turbulent jet flames. Int. J. Hydrogen Energy 2003, 28, 445–454. [Google Scholar] [CrossRef]
- Choudhuri, A.R.; Gollahalli, S.R. Computational and Experimental Study of the Structure of Hydrocarbon-Hydrogen Turbulent Jet Flame. In Proceedings of the 41st Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 6–9 January 2003; p. AIAA 2003-335. [Google Scholar] [CrossRef]
- Taguchi, S.; Takahashi, T.; Taniyama, K.; Nakagaki, H. Development of hydrogen combustion technology in industrial burners. In Proceedings of the Symposium (Japanese) on Combustion, Osaka, Japan, 14–16 November 2018; p. C342. [Google Scholar]
- Toyota Develops World’s First General-Purpose Hydrogen Burner for Industrial Use. Available online: https://global.toyota/en/newsroom/corporate/25260001.html (accessed on 25 October 2021).
- Yamaguchi, M.; Haneji, T.; Nakabayashi, H.; Hagihara, Y. Evaluation of NOx emission from Hydrogen-Oxygen Com-bustion in the Industrial Furnace. In Proceedings of the Symposium (Japanese) on Combustion, Online, 22–24 November 2021; p. B333. [Google Scholar]
- Konnov, A. On the relative importance of different routes forming NO in hydrogen flames. Combust. Flame 2003, 134, 421–424. [Google Scholar] [CrossRef]
- Frassoldati, A.; Faravelli, T.; Ranzi, E. A wide range modeling study of NOx formation and nitrogen chemistry in hydrogen combustion. Int. J. Hydrogen Energy 2006, 31, 2310–2328. [Google Scholar] [CrossRef]
- Skottene, M.; Rian, K.E. A study of NOx formation in hydrogen flames. Int. J. Hydrogen Energy 2007, 32, 3572–3585. [Google Scholar] [CrossRef]
- Purohit, A.L.; Nalbandyan, A.; Malte, P.C.; Novosselov, I.V. NNH mechanism in low-NOx hydrogen combustion: Experimental and numerical analysis of formation pathways. Fuel 2021, 292, 120186. [Google Scholar] [CrossRef]
- Zeldovich, Y.B. The Oxidation of Nitrogen in Combustion and Explosions. Acta Physicochim. USSR 1946, 21, 577–628. [Google Scholar]
- Nakamachi, I.; Takei, M.; Hase, T.; Moriyama, T. Gas Nensho No Riron to Jissai, 2nd ed.; The Energy Conservation Center: Tokyo, Japan, 2012. (In Japanese) [Google Scholar]
- Delabroy, O.; Haile, E.; Lacas, F.; Candel, S.; Pollard, A.; Sobiesiak, A.; Becker, H. Passive and active control of NOx in industrial burners. Exp. Therm. Fluid Sci. 1998, 16, 64–75. [Google Scholar] [CrossRef]
- Van Der Drift, A.; Tjeng, S.; Beckers, G.; Beesteheerde, J. Low-NOx hydrogen burner. Int. J. Hydrogen Energy 1996, 21, 445–449. [Google Scholar] [CrossRef]
- Rørtveit, G.J.; Zepter, K.; Skreiberg, Ø.; Fossum, M.; Hustad, J.E. A comparison of low-NOx burners for combustion of methane and hydrogen mixtures. Proc. Combust. Inst. 2002, 29, 1123–1129. [Google Scholar] [CrossRef]
- Heffel, J.W. NOx emission reduction in a hydrogen fueled internal combustion engine at 3000 rpm using exhaust gas recirculation. Int. J. Hydrogen Energy 2003, 28, 1285–1292. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, L.; Liu, H.; Liu, Z.; Zhen, H. Numerical Investigation on the Flame Structure and CO/NO Formations of the Laminar Premixed Biogas–Hydrogen Impinging Flame in the Wall Vicinity. Energies 2021, 14, 7308. [Google Scholar] [CrossRef]
- Rao, A.; Wu, Z.; Mehra, R.K.; Duan, H.; Ma, F. Effect of hydrogen addition on combustion, performance and emission of stoichiometric compressed natural gas fueled internal combustion engine along with exhaust gas recirculation at low, half and high load conditions. Fuel 2021, 304, 121358. [Google Scholar] [CrossRef]
- Guo, H.; Zhou, S.; Zou, J.; Shreka, M. A Numerical Investigation on De-NOx Technology and Abnormal Combustion Control for a Hydrogen Engine with EGR System. Processes 2020, 8, 1178. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, K.; Zhang, Y.; Zeng, F.; He, X.; Takyi, S.A.; Tontiwachwuthikul, P. Numerical Simulation of Combustion of Natural Gas Mixed with Hydrogen in Gas Boilers. Energies 2021, 14, 6883. [Google Scholar] [CrossRef]
- The Japan Society of Mechanical Engineers. JSME Combustion Handbook; The Japan Society of Mechanical Engineers: Tokyo, Japan, 1995; pp. 158–160. [Google Scholar]
- Tokyo Gas Co., Ltd. Heating Value of Tokyo Gas. Available online: https://home.tokyo-gas.co.jp/gas/userguide/shurui.html (accessed on 21 November 2021).
- Mizutani, Y. Nensho Kougaku; Morikita Publishing Co., Ltd.: Tokyo, Japan, 1977; p. 194. (In Japanese) [Google Scholar]
- Cho, E.-S.; Chung, S.H. Numerical evaluation of NOx mechanisms in methane-air counterflow premixed flames. J. Mech. Sci. Technol. 2009, 23, 659–666. [Google Scholar] [CrossRef]
- Fujimine, T.; Nakamachi, I.; Hayakawa, A. Combustion Stabilization of Gas Burners. J. Combust. Soc. Jpn. 2009, 51, 85–93. (In Japanese) [Google Scholar] [CrossRef]
- Mi, J.; Li, P.; Zheng, C. Impact of injection conditions on flame characteristics from a parallel multi-jet burner. Energy 2011, 36, 6583–6595. [Google Scholar] [CrossRef]
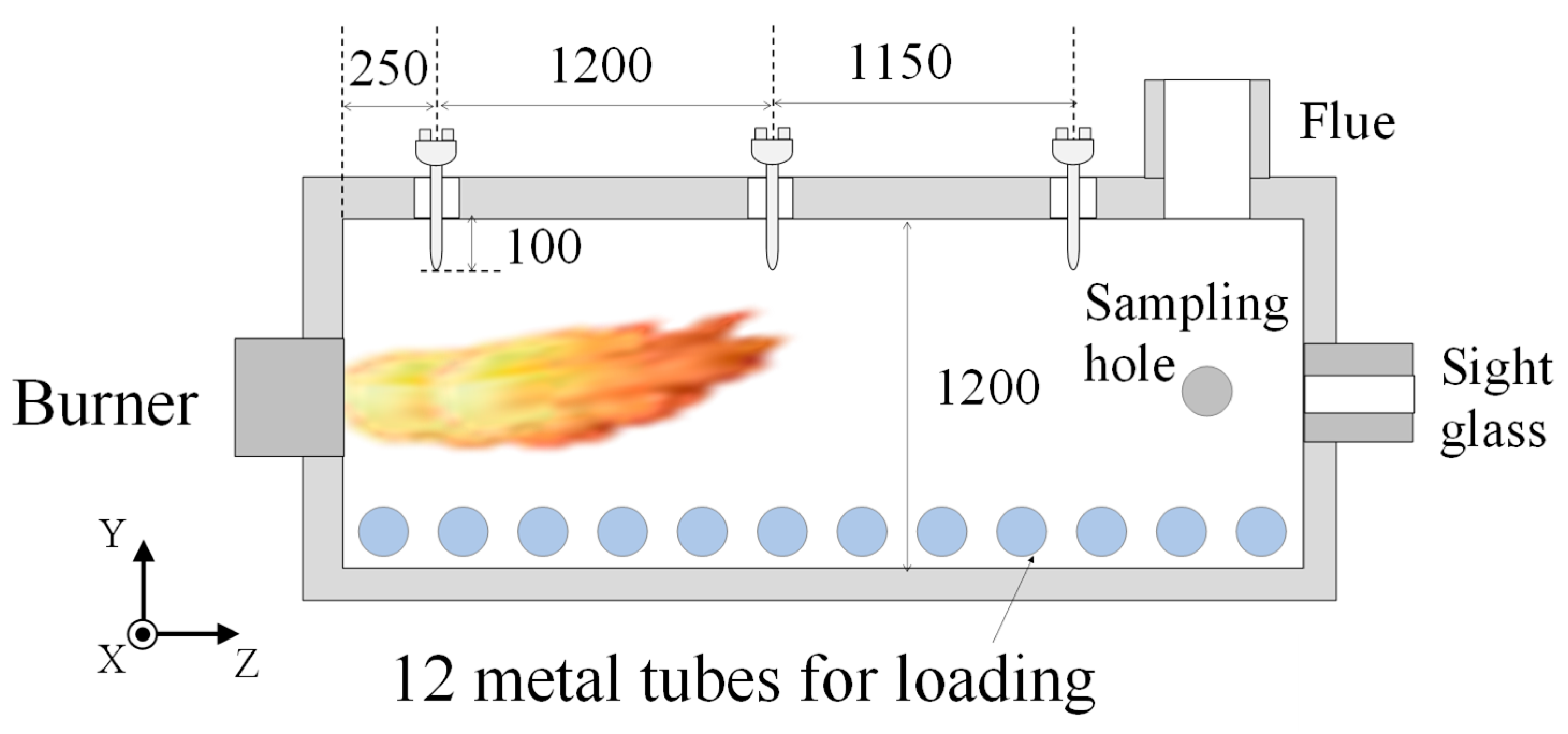
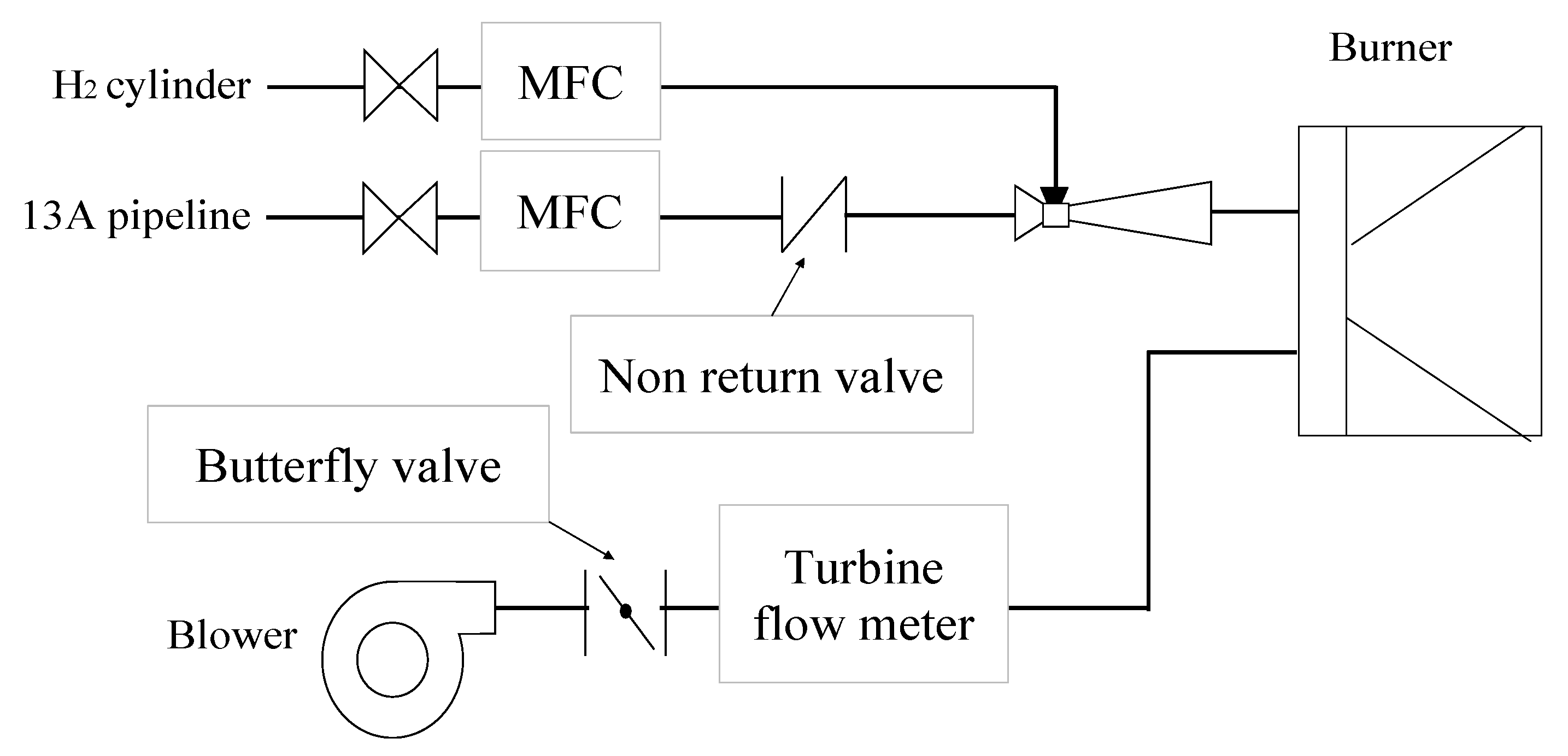
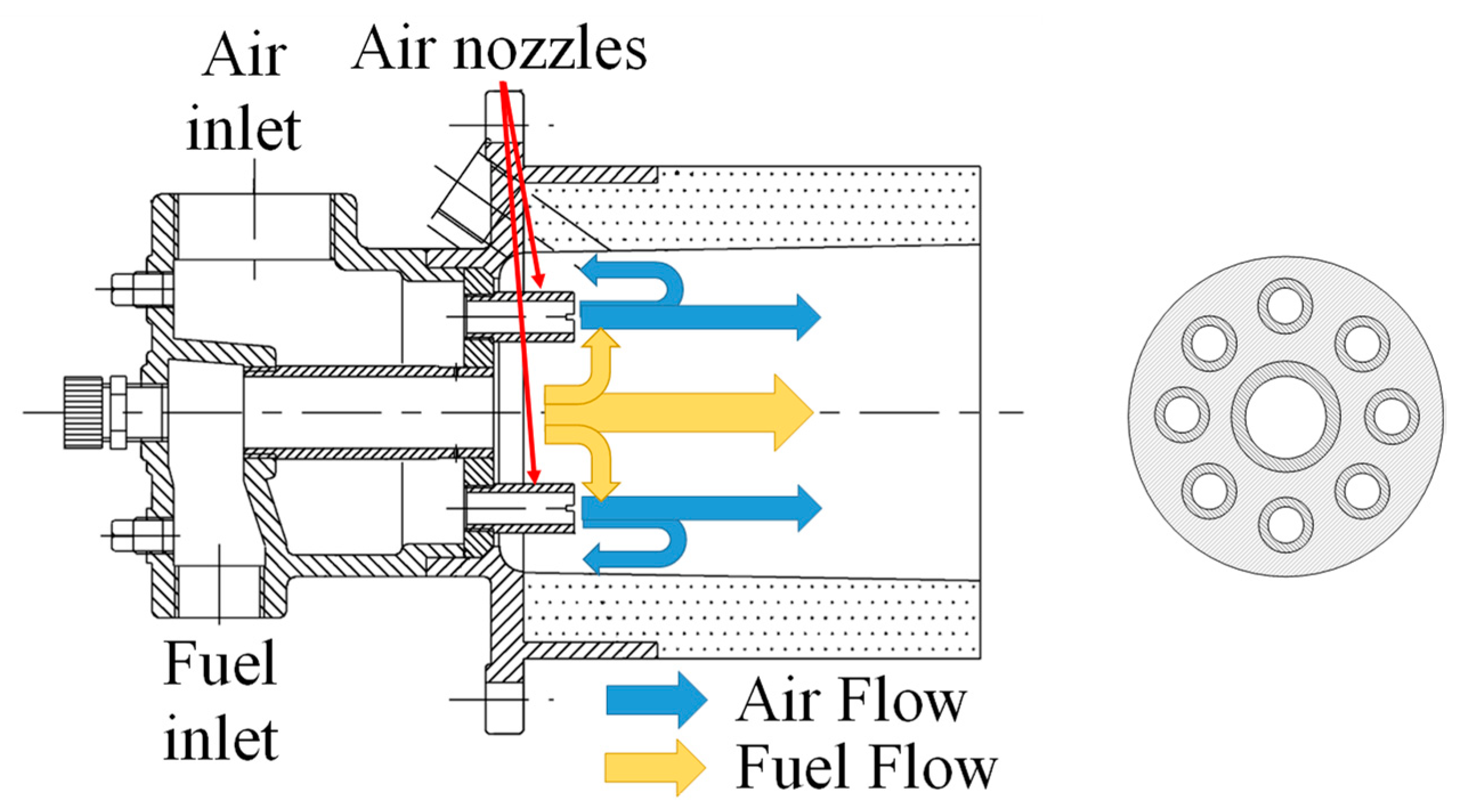


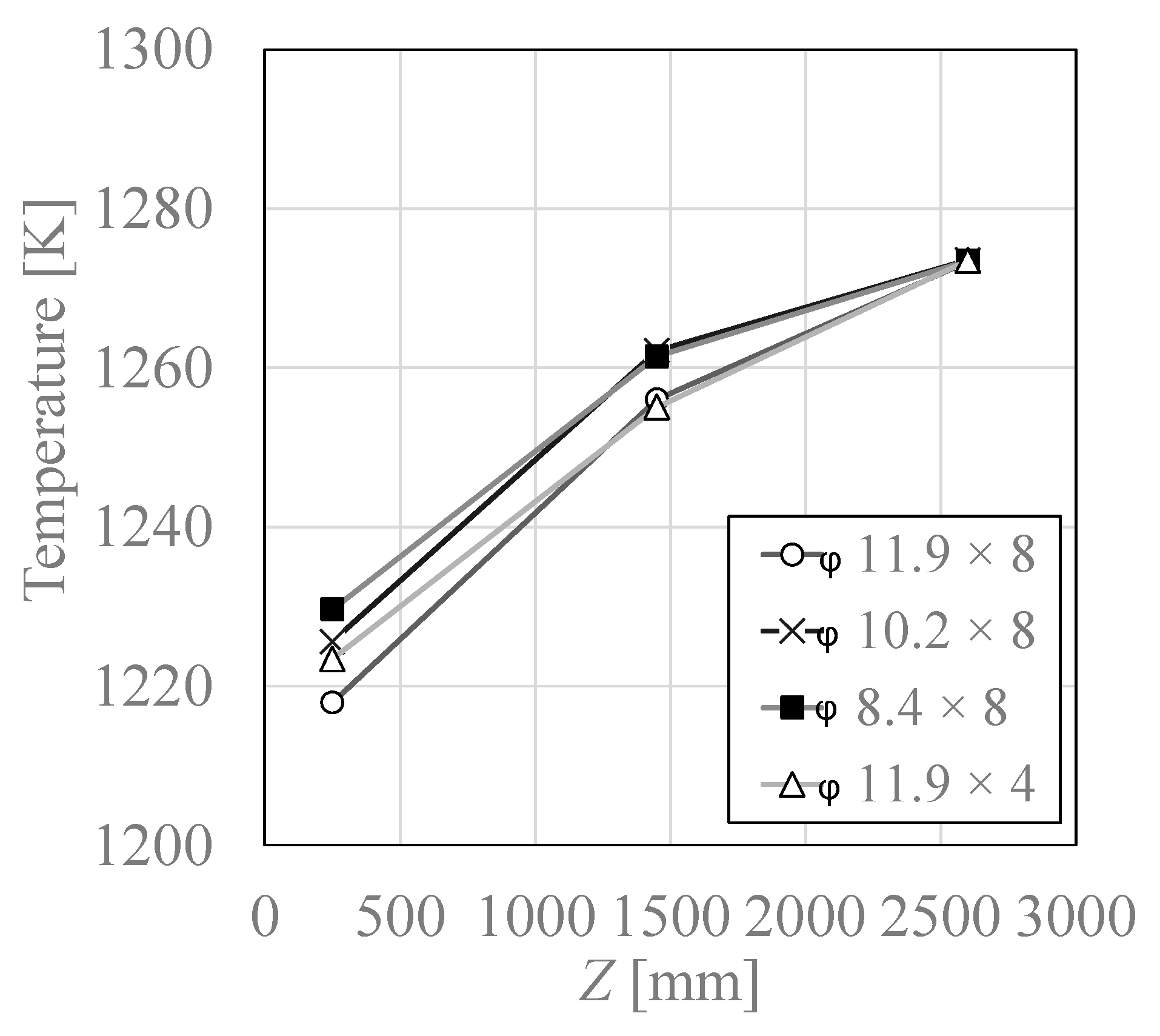

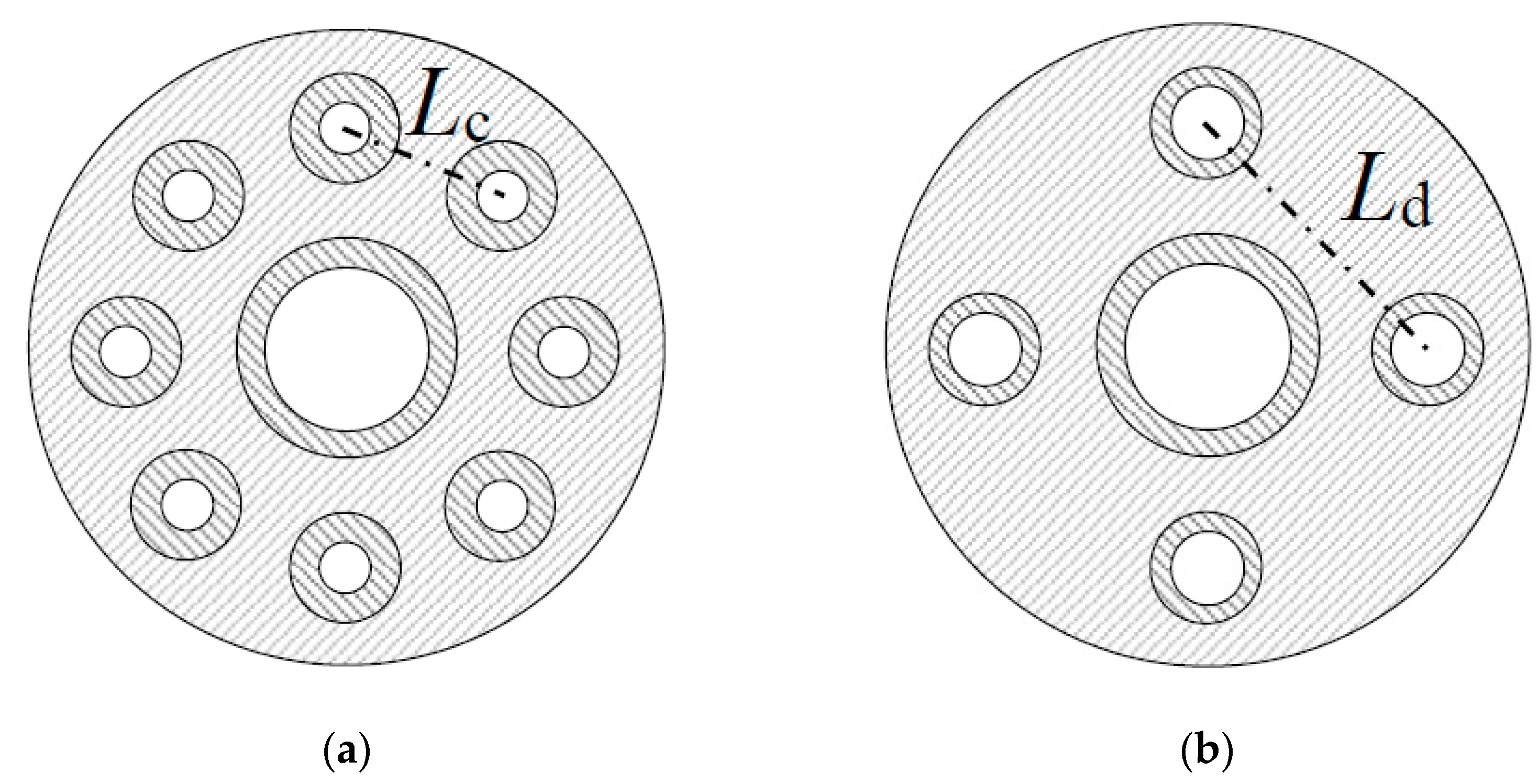
| No. | Case | Air Nozzles Diameter Da [mm] | Number of Air Nozzles N | Gas Velocity Ratio Va/Vf |
|---|---|---|---|---|
| 1 | φ11.9 × 8 | 11.9 | 8 | 2.36 |
| 2 | φ10.2 × 8 | 10.2 | 8 | 3.21 |
| 3 | φ8.4 × 8 | 8.4 | 8 | 4.74 |
| 4 | φ11.9 × 4 | 11.9 | 4 | 4.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikuchi, K.; Hori, T.; Akamatsu, F. Fundamental Study on Hydrogen Low-NOx Combustion Using Exhaust Gas Self-Recirculation. Processes 2022, 10, 130. https://doi.org/10.3390/pr10010130
Kikuchi K, Hori T, Akamatsu F. Fundamental Study on Hydrogen Low-NOx Combustion Using Exhaust Gas Self-Recirculation. Processes. 2022; 10(1):130. https://doi.org/10.3390/pr10010130
Chicago/Turabian StyleKikuchi, Kenta, Tsukasa Hori, and Fumiteru Akamatsu. 2022. "Fundamental Study on Hydrogen Low-NOx Combustion Using Exhaust Gas Self-Recirculation" Processes 10, no. 1: 130. https://doi.org/10.3390/pr10010130
APA StyleKikuchi, K., Hori, T., & Akamatsu, F. (2022). Fundamental Study on Hydrogen Low-NOx Combustion Using Exhaust Gas Self-Recirculation. Processes, 10(1), 130. https://doi.org/10.3390/pr10010130






