Machine Learning for Identifying Medication-Associated Acute Kidney Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Design Process and Participants
2.2. Study Design and Setting
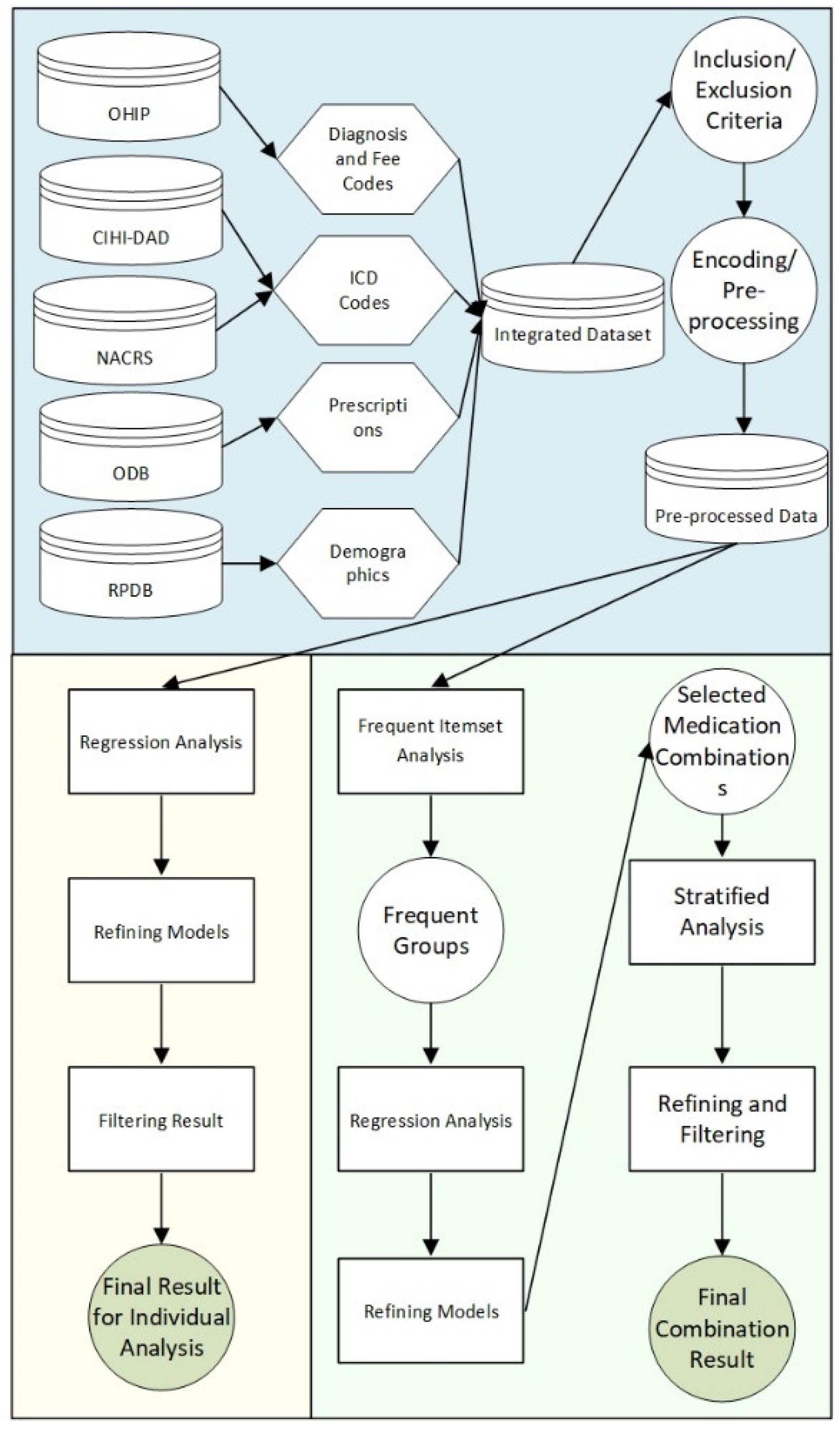
2.3. Workflow
2.4. Data Sources
2.5. Cohort Entry Criteria
2.6. Input Features
2.7. Outcome
2.8. Cohort Characteristics
2.9. Individual Medication Analysis
2.10. Medication Combination Analysis
2.11. Tools and Technologies
3. Results
3.1. Individual Medications and AKI
3.2. Medication Combinations and AKI
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Data Source | Description | Study Purpose |
|---|---|---|
| Canadian Institute for Health Information Discharge Abstract Database and National Ambulatory Care Reporting System | The Canadian Institute for Health Information Discharge Abstract Database and National Ambulatory Care Reporting System collect diagnostic and procedural variables for inpatient stays and ED visits, respectively. Diagnostic and inpatient procedural coding use the 10th version of the Canadian Modified International Classification of Disease system 10th Revision (after 2002). | Cohort creation, description, exposure, and outcome estimation |
| Ontario Drug Benefits | The Ontario Drug Benefits database includes a wide range of outpatient prescription medications available to all Ontario citizens over the age of 65. The error rate in the Ontario Drug Benefits database is less than 1%. | Medication prescriptions, description, and exposure |
| Registered Persons Database | The Registered Persons Database captures demographic (sex, date of birth, postal code) and vital status information on all Ontario residents. Relative to the Canadian Institute for Health Information Discharge Abstract Database in-hospital death flag, the Registered Persons Database has a sensitivity of 94% and a positive predictive value of 100%. | Cohort creation, description, and exposure |
| Ontario Health Insurance Plan | The Ontario Health Insurance Plan database contains information on Ontario physician billing claims for medical services using fee and diagnosis codes outlined in the Ontario Health Insurance Plan Schedule of Benefits. These codes capture information on outpatient, inpatient, and laboratory services rendered to a patient. | Cohort creation, stratification, description, exposure, and outcome |
| Variable | Database | Code | Set Code |
|---|---|---|---|
| Major cancer | Canadian Institute for Health Information Discharge Abstract Database | International Classification of Diseases 9th Revision | 150, 154, 155, 157, 162, 174, 175, 185, 203, 204, 205, 206, 207, 208, 2303, 2304, 2307, 2330, 2312, 2334 |
| International Classification of Diseases 10th Revision | 971, 980, 982, 984, 985, 986, 987, 988, 989, 990, 991, 993, C15, C18, C19, C20, C22, C25, C34, C50, C56, C61, C82, C83, C85, C91, C92, C93, C94, C95, D00, D010, D011, D012, D022, D075, D05 | ||
| Ontario Health Insurance Plan | Diagnosis | 203, 204, 205, 206, 207, 208, 150, 154, 155, 157, 162, 174, 175, 183, 185 | |
| Chronic liver disease | Canadian Institute for Health Information Discharge Abstract Database | International Classification of Diseases 9th Revision | 4561, 4562, 070, 5722, 5723, 5724, 5728, 573, 7824, V026, 571, 2750, 2751, 7891, 7895 |
| International Classification of Diseases 10th Revision | B16, B17, B18, B19, I85, R17, R18, R160, R162, B942, Z225, E831, E830, K70, K713, K714, K715, K717, K721, K729, K73, K74, K753, K754, K758, K759, K76, K77 | ||
| Ontario Health Insurance Plan | Diagnosis | 571, 573, 070 | |
| Fee code | Z551, Z554 | ||
| Coronary artery disease (excluding angina) | Canadian Institute for Health Information Discharge Abstract Database | Canadian Classification of Diagnostic, Therapeutic and Surgical Procedures | 4801, 4802, 4803, 4804, 4805, 481, 482, 483 |
| Canadian Classification of Health Interventions | 1IJ50, 1IJ76 | ||
| International Classification of Diseases 9th Revision | 412, 410, 411 | ||
| International Classification of Diseases 10th Revision | I21, I22, Z955, T822 | ||
| Ontario Health Insurance Plan | Diagnosis | 410, 412 | |
| Fee code | R741, R742, R743, G298, E646, E651, E652, E654, E655, Z434, Z448 | ||
| Diabetes | Canadian Institute for Health Information Discharge Abstract Database | International Classification of Diseases 9th Revision | 250 |
| International Classification of Diseases 10th Revision | E10, E11, E13, E14 | ||
| Ontario Health Insurance Plan | Diagnosis | 250 | |
| Fee code | Q040, K029, K030, K045, K046 | ||
| Heart failure | Canadian Institute for Health Information Discharge Abstract Database | Canadian Classification of Diagnostic, Therapeutic and Surgical Procedures | 4961, 4962, 4963, 4964 |
| Canadian Classification of Health Interventions | 1HP53, 1HP55, 1HZ53GRFR, 1HZ53LAFR, 1HZ53SYFR | ||
| International Classification of Diseases 9th Revision | I500, I501, I509, I255, J81 | ||
| International Classification of Diseases 10th Revision | I21, I22, Z955, T822 | ||
| Ontario Health Insurance Plan | Diagnosis | 428 | |
| Fee code | R701, R702, Z429 | ||
| Hypertension | Canadian Institute for Health Information Discharge Abstract Database | International Classification of Diseases 9th Revision | 401, 402, 403, 404, 405 |
| International Classification of Diseases 10th Revision | I10, I11, I12, I13, I15 | ||
| Ontario Health Insurance Plan | Diagnosis | 401, 402, 403 | |
| Kidney stones | Canadian Institute for Health Information Discharge Abstract Database | International Classification of Diseases 9th Revision | 5920, 5921, 5929, 5940, 5941, 5942, 5948, 5949, 27411 |
| International Classification of Diseases 10th Revision | N200, N201, N202, N209, N210, N211, N218, N219, N220, N228 | ||
| Peripheral vascular disease | Canadian Institute for Health Information Discharge Abstract Database | Canadian Classification of Diagnostic, Therapeutic and Surgical Procedures | 5125, 5129, 5014, 5016, 5018, 5028, 5038, 5126, 5159 |
| Canadian Classification of Health Interventions | 1KA76, 1KA50, 1KE76, 1KG50, 1KG57, 1KG76MI, 1KG87, 1IA87LA, 1IB87LA, 1IC87LA, 1ID87LA, 1KA87LA, 1KE57 | ||
| International Classification of Diseases 9th Revision | 4402, 4408, 4409, 5571, 4439, 444 | ||
| International Classification of Diseases 10th Revision | I700, I702, I708, I709, I731, I738, I739, K551 | ||
| Ontario Health Insurance Plan | Fee code | R787, R780, R797, R804, R809, R875, R815, R936, R783, R784, R785, E626, R814, R786, R937, R860, R861, R855, R856, R933, R934, R791, E672, R794, R813, R867, E649 | |
| Cerebrovascular disease (stroke or transient ischemic attack) | Canadian Institute for Health Information Discharge Abstract Database | International Classification of Diseases 9th Revision | 430, 431, 432, 4340, 4341, 4349, 435, 436, 3623 |
| International Classification of Diseases 10th Revision | I62, I630, I631, I632, I633, I634, I635, I638, I639, I64, H341, I600, I601, I602, I603, I604, I605, I606, I607, I609, I61, G450, G451, G452, G453, G458, G459, H340 | ||
| Chronic kidney disease | Canadian Institute for Health Information Discharge Abstract Database | International Classification of Diseases 9th Revision | 4030, 4031, 4039, 4040, 4041, 4049, 585, 586, 5888, 5889, 2504 |
| International Classification of Diseases 10th Revision | E102, E112, E132, E142, I12, I13, N08, N18, N19 | ||
| Ontario Health Insurance Plan | Diagnosis | 403, 585 |
| Variable | Database | Code Set | Code |
|---|---|---|---|
| Dialysis | Canadian Institute for Health Information Discharge Abstract Database | Canadian Classification of Diagnostic, Therapeutic and Surgical Procedures | 5127, 5142, 5143, 5195, 6698 |
| Canadian Classification of Health Interventions | 1PZ21, 1OT53DATS, 1OT53HATS, 1OT53LATS, 1SY55LAFT, 7SC59QD, 1KY76, 1KG76MZXXA, 1KG76MZXXN, 1JM76NC, 1JM76NCXXN | ||
| International Classification of Diseases 9th Revision | V451, V560, V568, 99673 | ||
| International Classification of Diseases 10th Revision | T824, Y602, Y612, Y622, Y841, Z49, Z992 | ||
| Ontario Health Insurance Plan | Fee code | R850, G324, G336, G327, G862, G865, G099, R825, R826, R827, R833, R840, R841, R843, R848, R851, R946, R943, R944, R945, R941, R942, Z450, Z451, Z452, G864, R852, R853, R854, R885, G333, H540, H740, R849, G323, G325, G326, G860, G863, G866, G330, G331, G332, G861, G082, G083, G085, G090, G091, G092, G093, G094, G095, G096, G294, G295 | |
| Kidney transplant | Canadian Institute for Health Information Discharge Abstract Database | Canadian Classification of Health Interventions | 1PC85 |
| Ontario Health Insurance Plan | Fee code | S435, S434 |
References
- Selby, N.M.; Crowley, L.; Fluck, R.J.; McIntyre, C.W.; Monaghan, J.; Lawson, N.; Kolhe, N.V. Use of Electronic Results Reporting to Diagnose and Monitor AKI in Hospitalized Patients. Clin. J. Am. Soc. Nephrol. 2012, 7, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.J.; Juurlink, I.; Bisset, L.H.; Bavakunji, R.; Mehta, R.L.; Devonald, M.A.J. A real-time electronic alert to improve detection of acute kidney injury in a large teaching hospital. Nephrol. Dial. Transpl. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2014, 29, 1888–1893. [Google Scholar] [CrossRef] [PubMed]
- Collister, D.; Pannu, N.; Ye, F.; James, M.; Hemmelgarn, B.; Chui, B.; Manns, B.; Klarenbach, S. Alberta Kidney Disease Network Health Care Costs Associated with AKI. Clin. J. Am. Soc. Nephrol. CJASN 2017, 12, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, T. Drug-induced renal disorders. Nippon Rinsho. Jpn. J. Clin. Med. 1978, 4, 2320–2321. [Google Scholar] [CrossRef]
- Kaufman, J.; Dhakal, M.; Patel, B.; Hamburger, R. Community-Acquired Acute Renal Failure. Am. J. Kidney Dis. 1991, 17, 191–198. [Google Scholar] [CrossRef]
- Gandhi, T.K.; Burstin, H.R.; Cook, E.F.; Puopolo, A.L.; Haas, J.S.; Brennan, T.A.; Bates, D.W. Drug complications in outpatients. J. Gen. Intern. Med. 2000, 15, 149–154. [Google Scholar] [CrossRef]
- Nash, K.; Hafeez, A.; Hou, S. Hospital-acquired renal insufficiency. Am. J. Kidney Dis. 2002, 39, 930–936. [Google Scholar] [CrossRef]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef]
- Choudhury, D.; Ahmed, Z. Drug-associated renal dysfunction and injury. Nat. Clin. Pract. Nephrol. 2006, 2, 80–91. [Google Scholar] [CrossRef]
- Morgan, S.G.; Hunt, J.; Rioux, J.; Proulx, J.; Weymann, D.; Tannenbaum, C. Frequency and cost of potentially inappropriate prescribing for older adults: A cross-sectional study. CMAJ Open 2016, 4, E346–E351. [Google Scholar] [CrossRef]
- Mehrabadi, A.; Liu, S.; Bartholomew, S.; Hutcheon, J.A.; Magee, L.A.; Kramer, M.S.; Liston, R.M.; Joseph, K.S. Canadian Perinatal Surveillance System Public Health Agency of Canada Hypertensive disorders of pregnancy and the recent increase in obstetric acute renal failure in Canada: Population based retrospective cohort study. BMJ Clin. Res. Ed. 2014, 349, g4731. [Google Scholar] [CrossRef]
- Liu, S.; Joseph, K.S.; Bartholomew, S.; Fahey, J.; Lee, L.; Allen, A.C.; Kramer, M.S.; Sauve, R.; Young, D.C.; Liston, R.M.; et al. Temporal Trends and Regional Variations in Severe Maternal Morbidity in Canada, 2003 to 2007. J. Obs. Gynaecol. Can. 2010, 32, 847–855. [Google Scholar] [CrossRef]
- Nadkarni, G.N.; Patel, A.A.; Ahuja, Y.; Annapureddy, N.; Agarwal, S.K.; Simoes, P.K.; Konstantinidis, I.; Kamat, S.; Archdeacon, M.; Thakar, C.V. Incidence, Risk Factors, and Outcome Trends of Acute Kidney Injury in Elective Total Hip and Knee Arthroplasty. Am. J. Orthop. 2016, 45, E12–E19. [Google Scholar]
- Xue, J.L.; Daniels, F.; Star, R.A.; Kimmel, P.L.; Eggers, P.W.; Molitoris, B.A.; Himmelfarb, J.; Collins, A.J. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J. Am. Soc. Nephrol. JASN 2006, 17, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Kolhe, N.V.; Muirhead, A.W.; Wilkes, S.R.; Fluck, R.J.; Taal, M.W. The epidemiology of hospitalised acute kidney injury not requiring dialysis in England from 1998 to 2013: Retrospective analysis of hospital episode statistics. Int. J. Clin. Pract. 2016, 70, 330–339. [Google Scholar] [CrossRef]
- Siddiqui, N.F.; Coca, S.G.; Devereaux, P.J.; Jain, A.K.; Li, L.; Luo, J.; Parikh, C.R.; Paterson, M.; Thiessen Philbrook, H.; Wald, R.; et al. Secular trends in acute dialysis after elective major surgery—1995 to 2009. CMAJ 2012, 184, 1237–1245. [Google Scholar] [CrossRef]
- Mehta, R.L.; Pascual, M.T.; Soroko, S.; Savage, B.R.; Himmelfarb, J.; Ikizler, T.A.; Paganini, E.P.; Chertow, G.M. Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int. 2004, 66, 1613–1621. [Google Scholar] [CrossRef]
- Waikar, S.S.; Curhan, G.C.; Wald, R.; McCarthy, E.P.; Chertow, G.M. Declining mortality in patients with acute renal failure, 1988 to 2002. J. Am. Soc. Nephrol. JASN 2006, 17, 1143–1150. [Google Scholar] [CrossRef]
- Zulman, D.M.; Asch, S.M.; Martins, S.B.; Kerr, E.A.; Hoffman, B.B.; Goldstein, M.K. Quality of care for patients with multiple chronic conditions: The role of comorbidity interrelatedness. J. Gen. Intern. Med. 2014, 29, 529–537. [Google Scholar] [CrossRef]
- Pannu, N.; Nadim, M.K. An overview of drug-induced acute kidney injury. Crit. Care Med. 2008, 36, S216–S223. [Google Scholar] [CrossRef]
- Moffett, B.S.; Goldstei, S.L. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-Ill children. Clin. J. Am. Soc. Nephrol. 2011, 6, 856–863. [Google Scholar] [CrossRef]
- Rivosecchi, R.M.; Kellum, J.A.; Dasta, J.F.; Armahizer, M.J.; Bolesta, S.; Buckley, M.S.; Dzierba, A.L.; Frazee, E.N.; Johnson, H.J.; Kim, C.; et al. Drug Class Combination-Associated Acute Kidney Injury. Ann. Pharm. 2016, 50, 953–972. [Google Scholar] [CrossRef]
- Alexander, T.; McArthur, E.; Jandoc, R.; Welk, B.; Hayward, J.S.; Jain, A.K.; Braam, B.; Flockerzi, V.; Garg, A.X.; Quinn, R.R. Antihypertensive medications and the risk of kidney stones in older adults: A retrospective cohort study. Hypertens. Res. 2017, 40, 837–842. [Google Scholar] [CrossRef]
- Schetz, M.; Dasta, J.; Goldstein, S.; Golper, T. Drug-induced acute kidney injury. Curr. Opin. Crit. Care 2005, 11, 555–565. [Google Scholar] [CrossRef]
- Cartin-Ceba, R.; Kashiouris, M.; Plataki, M.; Kor, D.J.; Gajic, O.; Casey, E.T. Risk factors for development of acute kidney injury in critically ill patients: A systematic review and meta-analysis of observational studies. Crit. Care Res. Pract. 2012, 2012, 691013. [Google Scholar] [CrossRef]
- Zitnik, M.; Agrawal, M.; Leskovec, J. Modeling polypharmacy side effects with graph convolutional networks. In Proceedings of the Bioinformatics; Oxford University Press: Oxford, UK, 2018; Volume 34, pp. i457–i466. [Google Scholar]
- Han, J.; Kamber, M. Data Mining: Concepts and Techniques; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef]
- Kandasamy, K.; Chuah, J.K.C.; Su, R.; Huang, P.; Eng, K.G.; Xiong, S.; Li, Y.; Chia, C.S.; Loo, L.-H.; Zink, D. Prediction of drug-induced nephrotoxicity and injury mechanisms with human induced pluripotent stem cell-derived cells and machine learning methods. Sci. Rep. 2015, 5, 12337. [Google Scholar] [CrossRef]
- Dey, S.; Luo, H.; Fokoue, A.; Hu, J.; Zhang, P. Predicting adverse drug reactions through interpretable deep learning framework. BMC Bioinform. 2018, 19, 476. [Google Scholar] [CrossRef]
- Lysenko, A.; Sharma, A.; Boroevich, K.A.; Tsunoda, T. An integrative machine learning approach for prediction of toxicity-related drug safety. Life Sci. Alliance 2018, 1, 6. [Google Scholar] [CrossRef]
- Schmider, J.; Kumar, K.; LaForest, C.; Swankoski, B.; Naim, K.; Caubel, P.M. Innovation in Pharmacovigilance: Use of Artificial Intelligence in Adverse Event Case Processing. Clin. Pharm. Ther. 2019, 105, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, S.S.; Rostamzadeh, N.; Sedig, K.; Garg, A.X.; McArthur, E. Multiple Regression Analysis and Frequent Itemset Mining of Electronic Medical Records: A Visual Analytics Approach Using VISA_M3R3. Data 2020, 5, 33. [Google Scholar] [CrossRef]
- Cho, H.; Berger, B.; Peng, J. Compact Integration of Multi-Network Topology for Functional Analysis of Genes. Cell Syst. 2016, 3, 540–548.e5. [Google Scholar] [CrossRef]
- Siew, E.D.; Parr, S.K.; Abdel-Kader, K.; Eden, S.K.; Peterson, J.F.; Bansal, N.; Hung, A.M.; Fly, J.; Speroff, T.; Ikizler, T.A.; et al. Predictors of Recurrent AKI. J. Am. Soc. Nephrol. JASN 2016, 27, 1190–1200. [Google Scholar] [CrossRef]
- Liu, K.D.; Yang, J.; Tan, T.C.; Glidden, D.V.; Zheng, S.; Pravoverov, L.; Hsu, C.-Y.; Go, A.S. Risk Factors for Recurrent Acute Kidney Injury in a Large Population-Based Cohort. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2019, 73, 163–173. [Google Scholar] [CrossRef]
- Muller, M. Participatory Design; CRC Press: Boca Raton, FL, USA, 2007; pp. 1061–1081. [Google Scholar]
- Levy, A.R.; O’Brien, B.J.; Sellors, C.; Grootendorst, P.; Willison, D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can. J. Clin. Pharm. 2003, 10, 67–71. [Google Scholar]
- Williams, D.A.; McCullagh, P.; Nelder, J.A. Generalized Linear Models. Biometrics 1984, 40, 566. [Google Scholar] [CrossRef]
- Agrawal, R.; Swami, A.; Imielinski, T. Database Mining: A Performance Perspective. Ieee Trans. Knowl. Data Eng. 1993, 5, 914–925. [Google Scholar] [CrossRef]
- SAS Enterprise BI Server. Available online: https://www.sas.com/en_ca/software/enterprise-bi-server.html (accessed on 19 February 2020).
- RStudio|Open Source & Professional Software for Data Science Teams. Available online: https://rstudio.com/ (accessed on 19 February 2020).
- Kohli, H.S.; Bhaskaran, M.C.; Muthukumar, T.; Thennarasu, K.; Sud, K.; Jha, V.; Gupta, K.L.; Sakhuja, V. Treatment-related acute renal failure in the elderly: A hospital-based prospective study. Nephrol. Dial. Transpl. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2000, 15, 212–217. [Google Scholar] [CrossRef]
- Peres, L.A.B.; da Cunha, A.D. Acute nephrotoxicity of cisplatin: Molecular mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef]
- Pierson-Marchandise, M.; Gras, V.; Moragny, J.; Micallef, J.; Gaboriau, L.; Picard, S.; Choukroun, G.; Masmoudi, K.; Liabeuf, S.; French National Network of Pharmacovigilance Centres. The drugs that mostly frequently induce acute kidney injury: A case- noncase study of a pharmacovigilance database. Br. J. Clin. Pharm. 2017, 83, 1341–1349. [Google Scholar] [CrossRef]
- Bove, T.; Belletti, A.; Putzu, A.; Pappacena, S.; Denaro, G.; Landoni, G.; Bagshaw, S.M.; Zangrillo, A. Intermittent furosemide administration in patients with or at risk for acute kidney injury: Meta-analysis of randomized trials. PLoS ONE 2018, 13, e0196088. [Google Scholar] [CrossRef]
- Ho, K.M.; Power, B.M. Benefits and risks of furosemide in acute kidney injury. Anaesthesia 2010, 65, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Sari, A. Nephrotoxic Effects of Drugs. Poisoning Mod. World New Tricks Old Dog 2019. [Google Scholar] [CrossRef]
- Alirezaei, A.; Argani, H.; Asgharpour, M.; Bahadorimonfared, A.; Bakhtiyari, M. An update on allopurinol and kidney failure; new trend for an old drug. J. Renal Injury Prevent. 2017, 6, 297–302. [Google Scholar] [CrossRef]
- Perez-Ruiz, F. Treatment with Allopurinol is Associated with Lower Risk of Acute Kidney Injury in Patients with Gout: A Retrospective Analysis of a Nested Cohort. Rheumatol. Ther. 2017, 4, 419–425. [Google Scholar] [CrossRef]
- Aamdal, S. Can ondansetron hydrochloride (Zofran) enhance the nephrotoxic potential of other drugs? Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1992, 3, 774. [Google Scholar] [CrossRef]
- Saruta, T.; Kanno, Y.; Hayashi, K.; Suzuki, H. Renal effects of amlodipine. J. Hum. Hypertens. 1995, 9, S11–S16. [Google Scholar]
- Liu, J.; Sun, G.; He, Y.; Song, F.; Chen, S.; Guo, Z.; Liu, B.; Lei, L.; He, L.; Chen, J. Early β-blockers administration might be associated with a reduced risk of contrast-induced acute kidney injury in patients with acute myocardial infarction. J. Thorac. Dis. 2019, 11, 1589. [Google Scholar] [CrossRef]
- Leaf, D.E.; Swinkels, D.W. Catalytic iron and acute kidney injury. Am. J. Physiol. Ren. Physiol. 2016, 311, F871–F876. [Google Scholar] [CrossRef]
- Neyra, J.A.; Rocha, N.A.; Bhargava, R.; Vaidya, O.U.; Hendricks, A.R.; Rodan, A.R. Rhabdomyolysis-induced acute kidney injury in a cancer patient exposed to denosumab and abiraterone: A case report. BMC Nephrol. 2015, 16, 118. [Google Scholar] [CrossRef]
- Juncos, L.A.; Juncos, L.I. Mineralocorticoid receptor antagonism in AKI: A new hope? Am. Soc. Nephrol. 2016, 27, 335–337. [Google Scholar] [CrossRef]
- Jha, P.K.; Vankalakunti, M.; Siddini, V.; Bonu, R.; Prakash, G.K.; Babu, K.; Ballal, H.S. Sunitinib induced nephrotic syndrome and thrombotic microangiopathy. Indian J. Nephrol. 2013, 23, 67. [Google Scholar]
- Dupont, A.G. Carvedilol and the kidney. Clin. Investig. 1992, 70, S127–S131. [Google Scholar] [CrossRef]
- Fleet, J.L.; Weir, M.A.; McArthur, E.; Ozair, S.; Devereaux, P.J.; Roberts, M.A.; Jain, A.K.; Garg, A.X. Kidney function and population-based outcomes of initiating oral atenolol versus metoprolol tartrate in older adults. Am. J. Kidney Dis. 2014, 64, 883–891. [Google Scholar] [CrossRef]
- Erbayraktar, Z.; Evlice, A.; Yener, G.; Ulusu, N.N. Effects of donepezil on liver and kidney functions for the treatment of Alzheimer’s disease. J. Integr. Neurosci. 2017, 16, 335–346. [Google Scholar] [CrossRef]
- Lopau, K.; Hefner, L.; Bender, G.; Heidbreder, E.; Wanner, C. Haemodynamic effects of valsartan in acute renal ischaemia/reperfusion injury. Nephrol. Dial. Transpl. 2001, 16, 1592–1597. [Google Scholar] [CrossRef][Green Version]
- Dev, V.; Dixon, S.N.; Fleet, J.L.; Gandhi, S.; Gomes, T.; Harel, Z.; Jain, A.K.; Shariff, S.Z.; Tawadrous, D.; Weir, M.A.; et al. Higher anti-depressant dose and major adverse outcomes in moderate chronic kidney disease: A retrospective population-based study. BMC Nephrol. 2014, 15, 79. [Google Scholar] [CrossRef]
- Mackowski, A.; Chen, H.-K.; Levitt, M. Successful management of chronic high-output ileostomy with high dose loperamide. BMJ Case Rep. 2015, 2015, bcr2015209411. [Google Scholar] [CrossRef]
- Mishima, E.; Maruyama, K.; Nakazawa, T.; Abe, T.; Ito, S. Acute kidney injury from excessive potentiation of calcium-channel blocker via synergistic CYP3A4 inhibition by clarithromycin plus voriconazole. Intern. Med. 2017, 56, 1687–1690. [Google Scholar] [CrossRef]
- Mori, Y.; Matsubara, H.; Nose, A.; Shibasaki, Y.; Masaki, H.; Kosaki, A.; Okigaki, M.; Fujiyama, S.; Tanaka-Uchiyama, Y.; Hasegawa, T.; et al. Safety and availability of doxazosin in treating hypertensive patients with chronic renal failure. Hypertens. Res. 2001, 24, 359–363. [Google Scholar] [CrossRef]
- Vanderperren, B.; Rizzo, M.; Angenot, L.; Haufroid, V.; Jadoul, M.; Hantson, P. Acute Liver Failure with Renal Impairment Related to the Abuse of Senna Anthraquinone Glycosides. Ann. Pharm. 2005, 39, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Katsumori, Y.; Kawano, M.; Mori, S.; Takeshige, R.; Mukai, J.; Imada, H.; Shimoura, H.; Takahashi, H.; Horai, T. Quetiapine-related Acute Kidney Injury Requiring Transient Continuous Hemodiafiltration. Intern. Med. 2018, 57, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Boccanfuso, J.A.; Hutton, M.; McAllister, B. The effects of megestrol acetate on nutritional parameters in a dialysis population. J. Ren. Nutr. 2000, 10, 36–43. [Google Scholar] [CrossRef]
- Rammohan, M.; Kalantar-Zadeh, K.; Liang, A.; Ghossein, C. Megestrol Acetate in a Moderate Dose for the Treatment of Malnutrition-Inflammation Complex in Maintenance Dialysis Patients. J. Ren. Nutr. 2005, 15, 345–355. [Google Scholar] [CrossRef]
- Miller, A.; Price, G. Gabapentin toxicity in renal failure: The importance of dose adjustment. Pain Med. 2009, 10, 190–192. [Google Scholar] [CrossRef]
- Dawlilng, S.; Lynn, K.; Rosser, R.; Braithwaite, R. The pharmacokinetics of nortriptyline in patients with chronic renal failure. Br. J. Clin. Pharm. 1981, 12, 39–45. [Google Scholar] [CrossRef]
- Nandikanti, D.K.; Gosmanova, E.O.; Gosmanov, A.R. Acute kidney injury associated with linagliptin. Case Rep. Endocrinol. 2016, 2016, 2. [Google Scholar] [CrossRef]
- McCoy, A.B.; Waitman, L.R.; Gadd, C.S.; Danciu, I.; Smith, J.P.; Lewis, J.B.; Schildcrout, J.S.; Peterson, J.F. A computerized provider order entry intervention for medication safety during acute kidney injury: A quality improvement report. Am. J. Kidney Dis. 2010, 56, 832–841. [Google Scholar] [CrossRef]
- Lipson, E.J.; Huff, C.A.; Holanda, D.G.; McDevitt, M.A.; Fine, D.M. Lenalidomide-induced acute interstitial nephritis. Oncology 2010, 15, 961. [Google Scholar] [CrossRef]
- Georgaki-Angelaki, E.; Stergiou, N.; Naoum, E.; Papassotiriou, I.; Anagnostakou, M. Olmesartan medoxomil-induced acute renal failure in a premature newborn following maternal exposure during pregnancy: A case report and review of the literature. NDT Plus 2009, 2, 295–297. [Google Scholar] [CrossRef]
- Gupta, A.; Puri, V.; Sharma, R.; Puri, S. Folic acid induces acute renal failure (ARF) by enhancing renal prooxidant state. Exp. Toxicol. Pathol. 2012, 64, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Peskoe, S.T.; McMillan, J.H.; Lorch, A.; Sussman, H.; Ozawa, T. Reversible acute renal failure associated with chlorthalidone therapy: Possible drug-induced interstitial nephritis. J. Med. Assoc. GA 1978, 67, 17–18. [Google Scholar] [PubMed]
- Allison, S.J. Effect of perioperative aspirin and clonidine on AKI. Nat. Rev. Nephrol. 2015, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Puri, N.; Mohey, V.; Singh, M.; Kaur, T.; Pathak, D.; Buttar, H.S.; Singh, A.P. Dipyridamole attenuates ischemia reperfusion induced acute kidney injury through adenosinergic A1 and A2A receptor agonism in rats. Naunyn-Schmiedeberg’s Arch. Pharm. 2016, 389, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Dixon, S.N.; Reiss, J.P.; Wald, R.; Parikh, C.R.; Gandhi, S.; Shariff, S.Z.; Pannu, N.; Nash, D.M.; Rehman, F. Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: A population-based cohort study. Ann. Intern. Med. 2014, 161, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Perazella, M.A. The Urine Sediment as a Biomarker of Kidney Disease. Am. J. Kidney Dis. 2015, 66, 748–755. [Google Scholar] [CrossRef]
- Watanabe, R.; Takahashi, K.; Tada, K.; Ishimura, A. A case of acute kidney injury associated with the use of oseltamivir and clarithromycin that was treated by hemodialysis. Nihon Toseki Igakkai Zasshi 2014, 47, 755–759. [Google Scholar] [CrossRef]
- Rp, B. Metolazone and Furosemide Combination in Cardiorenal Syndrome: Short-Term Safety and Efficacy Among Admitted Patients in a Tertiary Hospital. JOJ Urol. Nephrol. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Shulenberger, C.E.; Jiang, A.; Devabhakthuni, S.; Ivaturi, V.; Liu, T.; Reed, B.N. Efficacy and Safety of Intravenous Chlorothiazide versus Oral Metolazone in Patients with Acute Decompensated Heart Failure and Loop Diuretic Resistance. Pharmacother. J. Hum. Pharm. Drug Ther. 2016, 36, 852–860. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Delaney, A.; Jones, D.; Ronco, C.; Bellomo, R. Diuretics in the Management of Acute Kidney Injury: A Multinational Survey. Acute Kidney Inj. 2007, 156, 236–249. [Google Scholar] [CrossRef]
- Bennett, W.M.; Pulliam, J.P. Cyclosporine nephrotoxicity. Ann. Intern. Med. 1983, 99, 851–854. [Google Scholar] [CrossRef]
- Jacob, K.A.; Leaf, D.E.; Dieleman, J.M.; van Dijk, D.; Nierich, A.P.; Rosseel, P.M.; van der Maaten, J.M.; Hofland, J.; Diephuis, J.C.; de Lange, F.; et al. Intraoperative High-Dose Dexamethasone and Severe AKI after Cardiac Surgery. JASN 2015, 26, 2947–2951. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Allen, D.A.; Kieswich, J.E.; Patel, N.S.A.; Harwood, S.; Mazzon, E.; Cuzzocrea, S.; Raftery, M.J.; Thiemermann, C.; Yaqoob, M.M. Dexamethasone Ameliorates Renal Ischemia-Reperfusion Injury. JASN 2009, 20, 2412–2425. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.T.; Etminan, M.; Brophy, J.M.; Hartzema, A.G.; Delaney, J.A. Risk of acute kidney injury associated with the use of fluoroquinolones. CMAJ Can. Med. Asoc. J. 2013, 185, E475–E482. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-P.; Huang, S.-K.; Tao, P.; Chien, C.-W. A population-based study on the association between acute renal failure (ARF) and the duration of polypharmacy. BMC Nephrol. 2012, 13, 96. [Google Scholar] [CrossRef]
- Chao, C.-T.; Tsai, H.-B.; Wu, C.-Y.; Lin, Y.-F.; Hsu, N.-C.; Chen, J.-S.; Hung, K.-Y. Cumulative Cardiovascular Polypharmacy Is Associated with the Risk of Acute Kidney Injury in Elderly Patients. Medicine 2015, 94, e1251. [Google Scholar] [CrossRef]
- Loboz, K.K.; Shenfield, G.M. Drug combinations and impaired renal function—The “triple whammy”. Br. J. Clin. Pharm. 2005, 59, 239–243. [Google Scholar] [CrossRef]
- Fournier, J.-P.; Lapeyre-Mestre, M.; Sommet, A.; Dupouy, J.; Poutrain, J.-C.; Montastruc, J.-L. Laboratory monitoring of patients treated with antihypertensive drugs and newly exposed to non steroidal anti-inflammatory drugs: A cohort study. PLoS ONE 2012, 7, e34187. [Google Scholar] [CrossRef]
- Adhiyaman, V.; Asghar, M.; Oke, A.; White, A.D.; Shah, I.U. Nephrotoxicity in the elderly due to co-prescription of angiotensin converting enzyme inhibitors and nonsteroidal anti-inflammatory drugs. J. R. Soc. Med. 2001, 94, 512–514. [Google Scholar] [CrossRef]
- Fournier, J.-P.; Sommet, A.; Durrieu, G.; Poutrain, J.-C.; Lapeyre-Mestre, M.; Montastruc, J.-L. French Network of Regional Pharmacovigilance Centres Drug interactions between antihypertensive drugs and non-steroidal anti-inflammatory agents: A descriptive study using the French Pharmacovigilance database. Fundam. Clin. Pharm. 2014, 28, 230–235. [Google Scholar] [CrossRef]
- Audia, P.; Feinfeld, D.A.; Dubrow, A.; Winchester, J.F. Metformin-induced lactic acidosis and acute pancreatitis precipitated by diuretic, celecoxib, and candesartan-associated acute kidney dysfunction. Clin. Toxicol. 2008, 46, 164–166. [Google Scholar] [CrossRef]
- Steinhäuslin, F.; Munafo, A.; Buclin, T.; Macciocchi, A.; Biollaz, J. Renal effects of nimesulide in furosemide-treated subjects. Drugs 1993, 46 (Suppl. 1), 257–262. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Ren, H.; Chen, X.; Xie, J.; Chen, N. Diuretics associated acute kidney injury: Clinical and pathological analysis. Ren. Fail. 2014, 36, 1051–5105. [Google Scholar] [CrossRef]
- Gois, P.H.F.; Canale, D.; Volpini, R.A.; Ferreira, D.; Veras, M.M.; Andrade-Oliveira, V.; Câmara, N.O.S.; Shimizu, M.H.M.; Seguro, A.C. Allopurinol attenuates rhabdomyolysis-associated acute kidney injury: Renal and muscular protection. Free Radic. Biol. Med. 2016, 101, 176–189. [Google Scholar] [CrossRef]
- Jiang, Y.; McCombs, J.S.; Park, S.H. A Retrospective Cohort Study of Acute Kidney Injury Risk Associated with Antipsychotics. CNS Drugs 2017, 31, 319–326. [Google Scholar] [CrossRef]
- Zhang, X.; Donnan, P.T.; Bell, S.; Guthrie, B. Non-steroidal anti-inflammatory drug induced acute kidney injury in the community dwelling general population and people with chronic kidney disease: Systematic review and meta-analysis. BMC Nephrol. 2017, 18, 256. [Google Scholar] [CrossRef]
- Dixit, M.; Doan, T.; Kirschner, R.; Dixit, N. Significant Acute Kidney Injury Due to Non-steroidal Anti-inflammatory Drugs: Inpatient Setting. Pharmaceuticals 2010, 3, 1279–1285. [Google Scholar] [CrossRef]
- Karajala, V.; Mansour, W.; Kellum, J.A. Diuretics in acute kidney injury. Minerva Anestesiol. 2009, 75, 251–257. [Google Scholar]
| Characteristics | Patients Admitted to Hospital or Visited ED | ||
|---|---|---|---|
| Total Patients | AKI | No AKI | |
| Cohort size | 924,533 | 25,084 (3%) | 899,449 (97%) |
| Age, yr, Mean (SD) | |||
| 65 to <70 | 192,678 | 2522 (1.3%) | 190,156 (98.7%) |
| 70 to <80 | 382,989 | 7946 (2.1%) | 375,043 (97.9%) |
| 80 to <90 | 274,842 | 10,370 (3.8%) | 264,472 (96.2%) |
| ≥90 | 74,024 | 4246 (5.7%) | 69,778 (94.3%) |
| Women | 516,175 | 12,139 (2.4%) | 504,036 (97.6%) |
| Year of Cohort Entry (index date) | |||
| 2014–2015 | 605,244 | 16,689 (2.8%) | 588,555 (97.2%) |
| 2015–2016 | 319,289 | 8395 (2.6%) | 310,894 (97.4%) |
| Rural residence | 151,323 | 2097 (1.4%) | 149,226 (98.6%) |
| Long-term care | 43,351 | 3118 (7.2%) | 40,233 (92.8%) |
| Income Quintile | |||
| 1 | 180,227 | 5466 (3%) | 5466 (3%) |
| 2 | 192,686 | 5515 (2.9%) | 5515 (2.9%) |
| 3 | 182,957 | 4909 (2.7%) | 4909 (2.7%) |
| 4 | 186,407 | 4829 (2.6%) | 4829 (2.6%) |
| 5 | 182,256 | 4365 (2.4%) | 4365 (2.4%) |
| Comorbid Conditions | |||
| Hypertension | 814,604 | 24,209 (3%) | 790,395 (97%) |
| Diabetes | 358,472 | 13,837 (3.9%) | 344,635 (96.1%) |
| Heart failure | 125,136 | 7623 (6.1%) | 117,513 (93.9%) |
| Coronary artery disease | 239,437 | 8392 (3.5%) | 231,045 (96.5%) |
| Chronic liver disease | 33,359 | 1245 (3.7%) | 32,114 (96.3%) |
| Cancer | 145,286 | 4253 (2.9%) | 141,033 (97.1%) |
| Chronic kidney disease | 86,442 | 7759 (9%) | 78,683 (91%) |
| Kidney stones | 12,457 | 391 (3.1%) | 12,066 (96.9%) |
| Peripheral vascular disease | 13,197 | 660 (5%) | 12,537 (95%) |
| Cerebrovascular disease | 25,835 | 1180 (4.6%) | 24,655 (95.4%) |
| Medication | p-Value | Odds Ratio (OR) | Std. Error | OR’s 95% CI |
|---|---|---|---|---|
| Sunitinib Malate (Antineoplastic Miscellaneous) | 1.6 × 10−9 | 4.59 | 0.25 | 2.72–7.37 |
| Lenalidomide (Immunosuppressive Agents) | 9.4 × 10−17 | 3.58 | 0.15 | 2.62–4.79 |
| Abiraterone Acetate (Not Identified) | 1.7 × 10−10 | 2.61 | 0.15 | 1.92–3.48 |
| Metolazone (Diuretics) | 1.3 × 10−60 | 2.38 | 0.05 | 2.14–2.63 |
| Cyclosporine (Immunosuppressive Agents) | 4.0 × 10−6 | 2.18 | 0.17 | 1.54–3 |
| Megestrol Acetate (Progesteron Analogues) | 2.6 × 10−7 | 2.08 | 0.14 | 1.56–2.72 |
| Lithium Carbonate (Antimanic Agents) | 4.7 × 10−12 | 2.04 | 0.1 | 1.66–2.48 |
| Atropine Sulfate & Diphenoxylate Hcl (Antidiarrhea) | 3.4 × 10−10 | 2.00 | 0.11 | 1.6–2.46 |
| Furosemide (Diuretics) | 2.6 × 10−133 | 1.93 | 0.02 | 1.87–2 |
| Prochlorperazine Maleate (Antiemetics & Antinauseants) | 9.1 × 10−26 | 1.93 | 0.06 | 1.7–2.17 |
| Spironolactone (Diuretics (Potassium-Sparing)) | 2.6 × 10−112 | 1.87 | 0.03 | 1.77–1.97 |
| Methyldopa (Centrally Acting Antiadrenergic) | 4.9 × 10-6 | 1.84 | 0.13 | 1.4–2.37 |
| Hydralazine Hcl (Vasodilator Antihypertensive Drugs) | 1.5 × 10−26 | 1.76 | 0.05 | 1.58–1.95 |
| Dexamethasone (Corticosteroids, Plain) | 2.4 × 10−19 | 1.74 | 0.06 | 1.54–1.96 |
| Ondansetron Hcl (Antiemetics & Antinauseants) | 9.1 × 10−13 | 1.69 | 0.07 | 1.46–1.94 |
| Clonidine Hcl (Centrally Acting Antiadrenergic) | 3.9 × 10-6 | 1.69 | 0.09 | 1.4–2.02 |
| Allopurinol (Xanthine Oxidase Inhibitor) | 1.2 × 10−81 | 1.51 | 0.02 | 1.45–1.57 |
| Linagliptin (Unclassified Therapeutic Agents) | 4.1 × 10−24 | 1.50 | 0.04 | 1.38–1.62 |
| Loperamide (Antidiarrhea) | 1.4 × 10-9 | 1.47 | 0.06 | 1.29–1.66 |
| Glyburide (Oral Anti-Glycemic) | 1.3 × 10−12 | 1.46 | 0.04 | 1.34–1.58 |
| Chlorthalidone (Diuretics) | 1.2 × 10−18 | 1.42 | 0.06 | 1.25–1.59 |
| Atenolol (Beta Blockers) | 1.23 × 10−8 | 1.40 | 0.02 | 1.06–1.17 |
| Acetylsalicylic Acid & Dipyridamole (Adenosine Diphosphate Inhibitors) | 2.9 × 10−7 | 1.36 | 0.06 | 1.21–1.53 |
| Olmesartan Medoxomil (Angiotensin Ii Antagonist) | 7.9 × 10−13 | 1.35 | 0.04 | 1.24–1.46 |
| Iron Ferrous Fumarate (Iron Preparations) | 1.9 × 10−39 | 1.34 | 0.02 | 1.29–1.4 |
| Quetiapine Fumarate (Antipsychotic Agents) | 4.4 × 10−6 | 1.34 | 0.03 | 1.26–1.43 |
| Nortriptyline Hcl (Tricyclic Antidepressant) | 7.2 × 10−19 | 1.34 | 0.06 | 1.18–1.51 |
| Mirtazapine (Antidepressants: Miscellaneous) | 2.1 × 10−15 | 1.33 | 0.04 | 1.24–1.43 |
| Iron Ferrous Gluconate (Iron Preparations) | 8.2 × 10−16 | 1.33 | 0.04 | 1.24–1.43 |
| Terazosin (Alpha Adrenergic Blocking Agents) | 1.5 × 10−7 | 1.33 | 0.05 | 1.19–1.48 |
| Olanzapine (Antipsychotic Agents) | 8.5 × 10−7 | 1.33 | 0.06 | 1.18–1.48 |
| Fenofibrate (Antilipemic: Fibrates) | 3.6 × 10−8 | 1.32 | 0.05 | 1.19–1.46 |
| Carvedilol (Beta-Blockers) | 5.1 × 10−9 | 1.31 | 0.05 | 1.19–1.43 |
| Doxazosin Mesylate (Alpha Adrenergic Blocking Agents) | 6.6 × 10−7 | 1.30 | 0.05 | 1.17–1.43 |
| Folic Acid (Vitamin B Complex) | 6.9 × 10−9 | 1.28 | 0.04 | 1.18–1.39 |
| Trimethoprim (Sulfonamides, Trimetroprim & Combination) | 8.5 × 10−12 | 1.27 | 0.03 | 1.19–1.36 |
| Indapamide (Diuretics) | 3.9 × 10−8 | 1.26 | 0.03 | 1.19–1.33 |
| Sulfamethoxazole (Anti-Bacterial Sulfonamide) | 2.1 × 10−14 | 1.26 | 0.03 | 1.18–1.35 |
| Moxifloxacin Hcl (Fluoroquinolones) | 1.8 × 10−10 | 1.24 | 0.04 | 1.15–1.34 |
| Nifedipine (Calcium Blockers) | 3.3 × 10−6 | 1.21 | 0.03 | 1.14–1.28 |
| Lisinopril (Ace Inhibitors) | 5.1 × 10−6 | 1.21 | 0.04 | 1.12–1.31 |
| Gabapentin (Gamma-Aminobutyric Acid (Gaba) Derivatives) | 7.6 × 10−9 | 1.20 | 0.03 | 1.13–1.27 |
| Oseltamivir Phosphate (Antiviral Agents-Influenza Virus Specific) | 1.1 × 10−10 | 1.20 | 0.04 | 1.11–1.3 |
| Metoprolol (Beta-Blockers) | 6.4 × 10−11 | 1.19 | 0.03 | 1.12–1.25 |
| Donepezil Hcl (Cholinesterase Inhibitors) | 1.9 × 10−8 | 1.18 | 0.03 | 1.11–1.25 |
| Gliclazide (Oral Anti-Glycemic) | 3.7 × 10−11 | 1.17 | 0.03 | 1.12–1.23 |
| Hydrochlorothiazide (Diuretics) | 1.9 × 10−18 | 1.16 | 0.02 | 1.12–1.2 |
| Metoprolol Tartrate (Beta-Blockers) | 1.7 × 10−21 | 1.16 | 0.02 | 1.11–1.21 |
| Amlodipine Besylate (Calcium Blockers) | 2.4 × 10−6 | 1.15 | 0.02 | 1.12–1.19 |
| Valsartan (Angiotensin Ii Antagonist) | 3.1 × 10−11 | 1.15 | 0.03 | 1.09–1.21 |
| Digoxin (Digitalis Preparations) | 1.85 × 10−6 | 1.15 | 0.03 | 1.09–1.22 |
| Bisoprolol Fumarate (Beta-Blockers) | 9.9 × 10−8 | 1.14 | 0.02 | 1.1–1.18 |
| Senna (Cathartics & Laxatives) | 1.7 × 10−9 | 1.14 | 0.02 | 1.08–1.19 |
| Ramipril (Ace Inhibitors) | 9.7 × 10−15 | 1.13 | 0.02 | 1.09–1.17 |
| Metformin Hcl (Oral Anti-Glycemic) | 1.8 × 10−11 | 1.10 | 0.02 | 1.06–1.14 |
| Base Medication | Other Medication in Comb. | Base Odds Ratio | Comb Odds Ratio | %Chg in Odds Ratio |
|---|---|---|---|---|
| Indapamide (Diuretics) | Clavulanic Acid Potassium (Penicillins) | 1.24 | 2.22 | 79.00 |
| Indapamide (Diuretics) | Amoxicillin (Penicillins) | 1.24 | 2.21 | 78.27 |
| Furosemide (Diuretics) | Alprazolam (Benzodiazepine Derivatives) | 1.86 | 3.27 | 75.86 |
| Donepezil Hcl (Cholinesterase Inhibitors) | Indapamide (Diuretics) | 1.16 | 2.00 | 72.77 |
| Mirtazapine (Antidepressants: Miscellaneous) | Celecoxib (Non-Steroidal Anti-Inflammatory: Non-Asa Base) | 1.31 | 2.27 | 72.41 |
| Quetiapine Fumarate (Antipsychotic Agents) | Celecoxib (Non-Steroidal Anti-Inflammatory: Non-Asa Base) | 1.32 | 2.26 | 70.79 |
| Nortriptyline Hcl (Tricyclic Antidepressant) | Acetaminophen & Oxycodone Hcl (Analgesics & Antipyretics: Miscellaneous) | 1.27 | 2.13 | 67.49 |
| Doxazosin Mesylate (Alpha Adrenergic Blocking Agents) | Perindopril Tert.Butylamine (Ace Inhibitors) | 1.22 | 2.02 | 66.03 |
| Metoprolol Tartrate (Beta-Blockers) | Amitriptyline Hcl (Tricyclic Antidepressant) | 1.15 | 1.90 | 65.96 |
| Iron Ferrous Fumarate (Iron Preparations) | Bupropion Hcl (Antidepressants) | 1.33 | 2.21 | 65.63 |
| Nortriptyline Hcl (Tricyclic Antidepressant) | Lorazepam (Benzodiazepine Derivatives) | 1.25 | 2.06 | 64.85 |
| Furosemide (Diuretics) | Trandolapril (Ace Inhibitors) | 1.86 | 3.01 | 61.77 |
| Indapamide (Diuretics) | Donepezil Hcl (Cholinesterase Inhibitors) | 1.24 | 2.00 | 61.77 |
| Allopurinol (Xanthine Oxidase Inhibitor) | Venlafaxine Hcl (Selective Serotonin Reuptake Inhibitors-Other) | 1.49 | 2.41 | 61.65 |
| Terazosin (Alpha Adrenergic Blocking Agents) | Irbesartan (Angiotensin Ii Antagonist) | 1.27 | 2.03 | 59.84 |
| Fenofibrate (Antilipemic: Fibrates) | Candesartan Cilexetil (Angiotensin Ii Antagonist) | 1.27 | 2.02 | 58.17 |
| Terazosin (Alpha Adrenergic Blocking Agents) | Pantoprazole Sodium (Proton Pump Inhibitors) | 1.25 | 1.97 | 57.23 |
| Lithium Carbonate (Antimanic Agents) | Atorvastatin Calcium (Antilipemic: Statins) | 1.84 | 2.86 | 55.79 |
| Spironolactone (Diuretics (Potassium-Sparing)) | Clonazepam (Benzodiazepine Derivatives) | 1.85 | 2.81 | 52.26 |
| Allopurinol (Xanthine Oxidase Inhibitor) | Morphine Sulfate (Narcotics: Opiate Agonists) | 1.50 | 2.26 | 50.77 |
| Iron Ferrous Fumarate (Iron Preparations) | Meloxicam (Non-Steroidal Anti-Inflammatory: Non-Asa Base) | 1.33 | 2.01 | 50.75 |
| Folic Acid (Vitamin B Complex) | Hydrochlorothiazide (Diuretics) | 1.22 | 1.82 | 49.59 |
| Dexamethasone (Corticosteroids, Plain) | Gabapentin (Gamma-Aminobutyric Acid (Gaba) Derivatives) | 1.67 | 2.49 | 49.42 |
| Quetiapine Fumarate (Antipsychotic Agents) | Pregabalin (Anticonvulsants: Miscellaneous) | 1.32 | 1.95 | 47.67 |
| Dexamethasone (Corticosteroids, Plain) | Ramipril (Ace Inhibitors) | 1.69 | 2.48 | 46.97 |
| Metoprolol (Beta-Blockers) | Omeprazole (Proton Pump Inhibitors) | 1.17 | 1.71 | 46.70 |
| Ondansetron Hcl (Antiemetics & Antinauseants) | Ranitidine Hcl (Histamine H2 Receptor Antagonist) | 1.62 | 2.37 | 46.49 |
| Furosemide (Diuretics) | Metformin (Oral Anti-Glycemics) | 1.86 | 2.69 | 44.56 |
| Quetiapine Fumarate (Antipsychotic Agents) | Atenolol (Beta-Blockers) | 1.32 | 1.90 | 43.70 |
| Iron Ferrous Fumarate (Iron Preparations) | Metformin (Oral Anti-Glycemics) | 1.34 | 1.91 | 43.05 |
| Spironolactone (Diuretics (Potassium-Sparing)) | Candesartan Cilexetil (Angiotensin Ii Antagonist) | 1.84 | 2.62 | 42.47 |
| Spironolactone (Diuretics (Potassium-Sparing)) | Enalapril Sodium (Ace Inhibitors) | 1.86 | 2.61 | 40.83 |
| Furosemide (Diuretics) | Acetaminophen & Oxycodone Hcl (Analgesics & Antipyretics: Miscellaneous) | 1.85 | 2.60 | 40.35 |
| Spironolactone (Diuretics (Potassium-Sparing)) | Dabigatran Etexilate (Anticoagulants Miscellaneous) | 1.85 | 2.60 | 40.22 |
| Furosemide (Diuretics) | Clonidine Hcl (Centrally Acting Antiadrenergic) | 1.86 | 2.61 | 40.11 |
| Furosemide (Diuretics) | Cefuroxime Axetil (Cephalosporin) | 1.86 | 2.61 | 40.02 |
| Known | Likely-Confounded |
|---|---|
| Furosemide [46,47] | Hydralazine Hcl [48] |
| Allopurinol [49,50] | Ondansetron Hcl [51] |
| Amlodipine [45,52] | Lithium Carbonate [48] |
| Hydrochlorothiazide [45]. | Bisoprolol Fumarate [53] |
| Iron Ferrous Fumarate [54] | Abiraterone Acetate [55] |
| Spironolactone [56] | Sunitinib Malate [57] |
| Bisoprolol [45] | Carvedilol [58] |
| Atenolol [59] | Donepezil Hcl [60] |
| Metoprolol [59] | Acetylsalicylic Acid [48] |
| Valsartan [61] | Mirtazapine [62] |
| Indapamide [45] | Loperamide [63] |
| Nifedipine [64] | Doxazosin Mesylate [65] |
| Iron Ferrous Gluconate [54] | Senna [66] |
| Quetiapine [67] | Megestrol Acetate [68,69] |
| Gabapentin [70] | Nortriptyline [71] |
| Linagliptin [72] | Terazosin |
| Glyburide [73] | Prochlorperazine Maleate |
| Lenalidomide [74] | |
| Trimethoprim [45] | |
| Olmesartan Medoxomil [75] | |
| Ramipril [45] | |
| Gliclazide [45] | |
| Atropine Sulfate [45] | |
| Folic Acid [76] | |
| Chlorthalidone [77] | |
| Clonidine Hcl [78] | |
| Fenofibrate [45] | |
| Dipyridamole [79] | |
| Olanzapine [80] | |
| Digoxin [45] | |
| Lisinopril [45] | |
| Methyldopa [81] | |
| Oseltamivir Phosphate [82] | |
| Metolazone [83,84,85] | |
| Cyclosporine [86] | |
| Dexamethasone [87,88] | |
| Moxifloxacin Hcl [89] | |
| Sulfamethoxazole [45] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, S.S.; Rostamzadeh, N.; Sedig, K.; Lizotte, D.J.; Garg, A.X.; McArthur, E. Machine Learning for Identifying Medication-Associated Acute Kidney Injury. Informatics 2020, 7, 18. https://doi.org/10.3390/informatics7020018
Abdullah SS, Rostamzadeh N, Sedig K, Lizotte DJ, Garg AX, McArthur E. Machine Learning for Identifying Medication-Associated Acute Kidney Injury. Informatics. 2020; 7(2):18. https://doi.org/10.3390/informatics7020018
Chicago/Turabian StyleAbdullah, Sheikh S., Neda Rostamzadeh, Kamran Sedig, Daniel J. Lizotte, Amit X. Garg, and Eric McArthur. 2020. "Machine Learning for Identifying Medication-Associated Acute Kidney Injury" Informatics 7, no. 2: 18. https://doi.org/10.3390/informatics7020018
APA StyleAbdullah, S. S., Rostamzadeh, N., Sedig, K., Lizotte, D. J., Garg, A. X., & McArthur, E. (2020). Machine Learning for Identifying Medication-Associated Acute Kidney Injury. Informatics, 7(2), 18. https://doi.org/10.3390/informatics7020018








