Variations in Using Diagnosis Codes for Defining Age-Related Macular Degeneration Cohorts
Abstract
1. Introduction
2. Methods
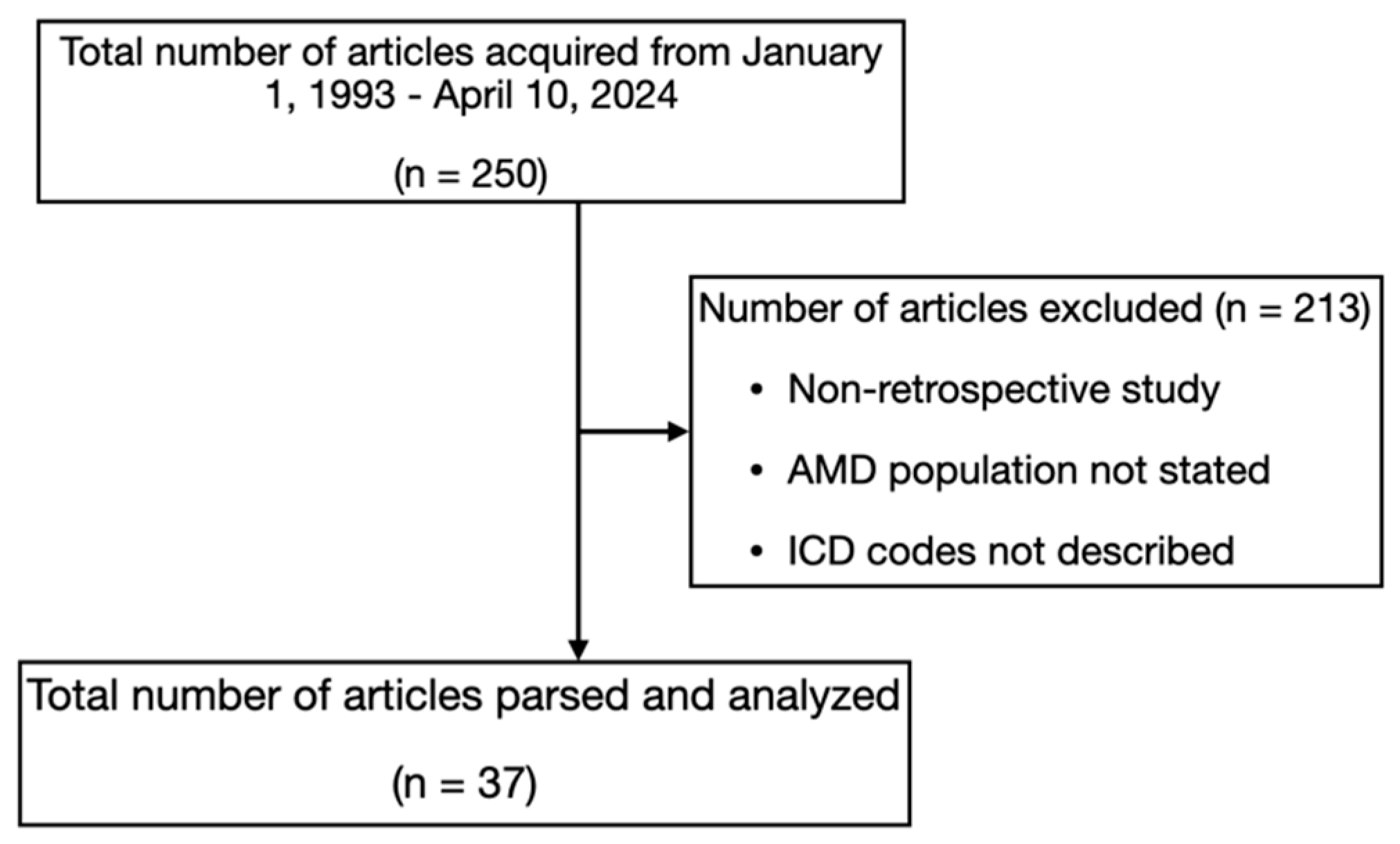
2.1. Article Search and Review
2.2. Article Parsing
2.3. Statistical Analysis
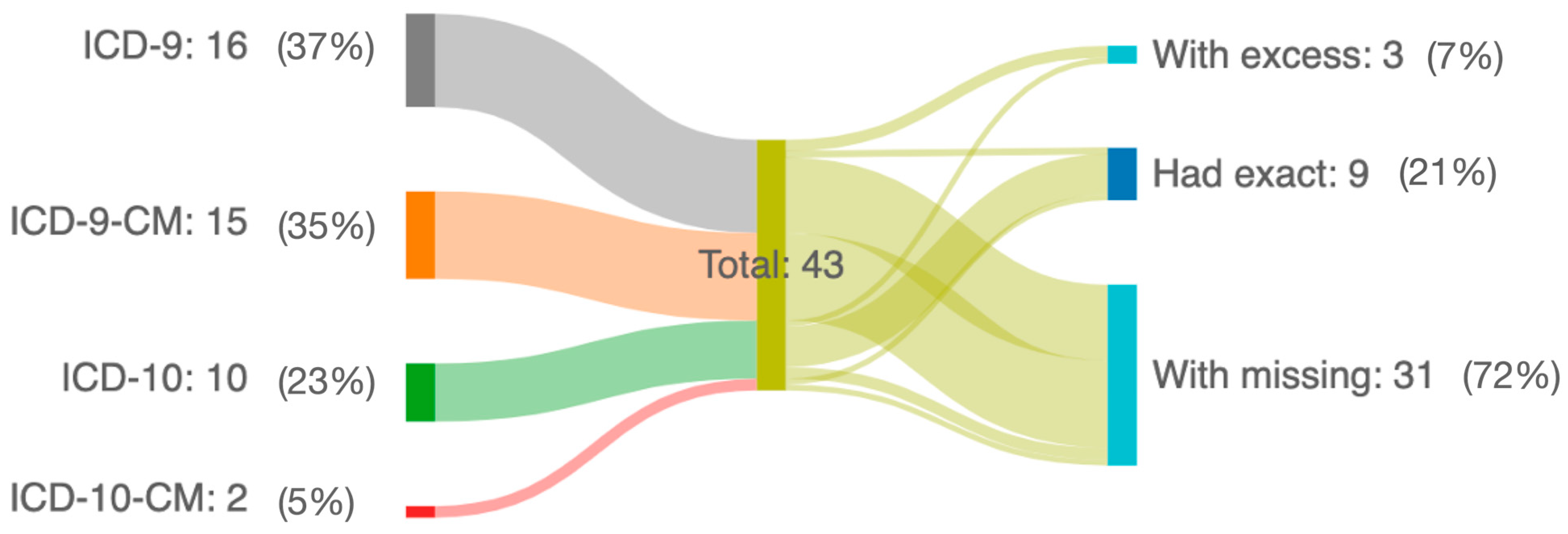
3. Results
EHR—Electronic Health Record
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalaw, F.G.P.; Alex, V.; Walker, E.; Bartsch, D.U.; Freeman, W.R.; Borooah, S. Inner Retinal Thickness and Vasculature in Patients with Reticular Pseudodrusen. Ophthalmic Res. 2023, 66, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, M.V.; Rabiolo, A.; Sacconi, R.; Carnevali, A.; Querques, L.; Bandello, F.; Querques, G. coherence tomography angiography in dry age-related macular degeneration. Surv. Ophthalmol. 2018, 63, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L.; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Clinical Classification of Age-related Macular Degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Finocchio, L.; Zeppieri, M.; Gabai, A.; Toneatto, G.; Spadea, L.; Salati, C. Recent Developments in Gene Therapy for Neovascular Age-Related Macular Degeneration: A Review. Biomedicines 2023, 11, 3221. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Malik, I.A.; Shariq, F.; Afridi, E.K.; Taha, M.; Raufi, N.; Naveed, A.K.; Iqbal, J.; Habte, A. Advancements in the treatment of geographic atrophy: Focus on pegcetacoplan in age-related macular degeneration. Ann. Med. Surg. 2023, 85, 6067–6077. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.K.; Ferdi, A.C.; Gillies, M.C.; Watson, S.L. Clinical Registries in Ophthalmology. Ophthalmology 2019, 126, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.F.; Sommer, A.; Rich, W.L.; Lum, F.; Parke, D.W. The 2016 American Academy of Ophthalmology IRIS® Registry (Intelligent Research in Sight) Database. Ophthalmology 2018, 125, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- The All of Us Research Program Investigators. The “All of Us” Research Program. N. Engl. J. Med. 2019, 381, 668–676. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2023. Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 12 November 2023).
- Magnuson, J.A.; Dixon, B.E. Public Health Informatics and Information Systems, 3rd ed.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/nchs/icd/icd-10-cm.htm (accessed on 12 November 2023).
- Fung, K.W.; Xu, J.; McConnell-Lamptey, S.; Pickett, D.; Bodenreider, O. A practical strategy to use the ICD-11 for morbidity coding in the United States without a clinical modification. J. Am. Med. Inform. Assoc. 2023, 30, 1614–1621. [Google Scholar] [CrossRef]
- Duan, Y.; Mo, J.; Klein, R.; Scott, I.U.; Lin, H.-M.; Caulfield, J.; Patel, M.; Liao, D. Age-Related Macular Degeneration Is Associated with Incident Myocardial Infarction among Elderly Americans. Ophthalmology 2007, 114, 732–737. [Google Scholar] [CrossRef]
- Sloan, F.A.; Brown, D.S.; Carlisle, E.S.; Picone, G.A.; Lee, P.P. Monitoring Visual Status: Why Patients Do or Do Not Comply with Practice Guidelines. Health Serv. Res. 2004, 39, 1429–1448. [Google Scholar] [CrossRef]
- Halpern, M.T.; Schmier, J.K.; Covert, D.; Venkataraman, K. Resource Utilization and Costs of Age-Related Macular Degeneration. Health Care Financ. Rev. 2006, 27, 37–47. [Google Scholar] [PubMed]
- Zlateva, G.P.; Javitt, J.C.; Shah, S.N.; Zhou, Z.; Murphy, J.G. Comparison of comorbid conditions between neovascular age–related macular degeneration patients and a control cohort in the medicare population. Retina 2007, 27, 1292–1299. [Google Scholar] [CrossRef]
- Swanson, M.W.; McGwin, G.J. Anti-Inflammatory Drug Use and Age-Related Macular Degeneration. Optom. Vis. Sci. 2008, 85, 947–950. [Google Scholar] [CrossRef]
- Liao, D.; Mo, J.; Duan, Y.; Klein, R.; Scott, I.U.; Huang, K.A.; Zhou, H. Is Age-Related Macular Degeneration Associated with Stroke Among Elderly Americans?§. Open Ophthalmol. J. 2008, 2, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Day, S.; Acquah, K.; Mruthyunjaya, P.; Grossman, D.S.; Lee, P.P.; Sloan, F.A. Ocular Complications After Anti–Vascular Endothelial Growth Factor Therapy in Medicare Patients With Age-Related Macular Degeneration. Am. J. Ophthalmol. 2011, 152, 266–272. [Google Scholar] [CrossRef]
- Latkany, P.; Duggal, M.; Goulet, J.; Paek, H.; Rambo, M.; Palmisano, P.; Levin, W.; Erdos, J.; Justice, A.; Brandt, C. The need for validation of large administrative databases: Veterans Health Administration ICD-9CM coding of exudative age-related macular degeneration and ranibizumab usage. J. Ocul. Biol. Dis. Inform. 2010, 3, 30–34. [Google Scholar] [CrossRef]
- Day, S.; Acquah, K.; Lee, P.P.; Mruthyunjaya, P.; Sloan, F.A. Medicare Costs for Neovascular Age-Related Macular Degeneration, 1994–2007. Am. J. Ophthalmol. 2011, 152, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.D.; VanderBeek, B.L.; Talwar, N.; Nan, B.; Musch, D.C.; Zacks, D.N. Rates of Nonexudative and Exudative Age-Related Macular Degeneration among Asian American Ethnic Groups. Investig. Opthalmol. Vis. Sci. 2011, 52, 6842. [Google Scholar] [CrossRef]
- French, D.D.; Margo, C.E. Age-Related Macular Degeneration, Anti–Vascular Endothelial Growth Factor Agents, and Short-term Mortality: A Postmarketing Medication Safety and Surveillance Study. Retina 2011, 31, 1036–1042. [Google Scholar] [CrossRef]
- Stein, J.D.; Hanrahan, B.W.; Comer, G.M.; Sloan, F.A. Diffusion of Technologies for the Care of Older Adults with Exudative Age-Related Macular Degeneration. Am. J. Ophthalmol. 2013, 155, 688–696.e2. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.D.; Blachley, T.S.; Musch, D.C. Identification of Persons With Incident Ocular Diseases Using Health Care Claims Databases. Am. J. Ophthalmol. 2013, 156, 1169–1175.e3. [Google Scholar] [CrossRef] [PubMed]
- Sloan, F.A.; Yashkin, A.P.; Chen, Y. Gaps in Receipt of Regular Eye Examinations among Medicare Beneficiaries Diagnosed with Diabetes or Chronic Eye Diseases. Ophthalmology 2014, 121, 2452–2460. [Google Scholar] [CrossRef]
- Qualls, L.G.; Hammill, B.G.; Wang, F.; Lad, E.M.; Schulman, K.A.; Cousins, S.W.; Curtis, L.H. Costs of newly diagnosed neovascular age-related macular degeneration among medicare beneficiaries, 2004–2008. Retina 2013, 33, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Kume, A.; Ohshiro, T.; Sakurada, Y.; Kikushima, W.; Yoneyama, S.; Kashiwagi, K. Treatment Patterns and Health Care Costs for Age-Related Macular Degeneration in Japan. Ophthalmology 2016, 123, 1263–1268. [Google Scholar] [CrossRef]
- Leisy, H.B.; Rastogi, A.; Guevara, G.; Ahmad, M.; Smith, R.T. The association of geographic atrophy and decreased renal function in patients with age-related macular degeneration. Eye 2017, 31, 62–67. [Google Scholar] [CrossRef]
- Lee, W.J.A.; Cheng, C.L.; Lee, C.H.; Kao Yang, Y.H.; Lin, S.J.; Hsieh, C.Y. Risks of newly onset hemorrhagic stroke in patients with neovascular age-related macular degeneration. Pharmacoepidemiol. Drug Saf. 2017, 26, 1277–1285. [Google Scholar] [CrossRef]
- Gower, E.W.; Stein, J.D.; Shekhawat, N.S.; Mikkilineni, S.; Blachley, T.S.; Pajewski, N.M. Geographic and Demographic Variation in Use of Ranibizumab Versus Bevacizumab for Neovascular Age-related Macular Degeneration in the United States. Am. J. Ophthalmol. 2017, 184, 157–166. [Google Scholar] [CrossRef]
- Chiu, S.; Shaw, J.; Luong, T.; Fong, D.; Modjtahedi, B. Coding patterns used by ophthalmologists for hydroxychloroquine retinal toxicity. Clin. Ophthalmol. 2018, 12, 2261–2265. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Windsor, M.A.; Feuer, W.J.; Sun, S.J.; Frick, K.D.; Swanson, E.A.; Huang, D. Estimating Medicare and Patient Savings From the Use of Bevacizumab for the Treatment of Exudative Age-related Macular Degeneration. Am. J. Ophthalmol. 2018, 191, 135–139. [Google Scholar] [CrossRef]
- Halladay, C.W.; Hadi, T.; Anger, M.D.; Greenberg, P.B.; Sullivan, J.M.; Konicki, P.E.; Peachey, N.S.; Igo, R.P.; Iyengar, S.K.; Wu, W.-C.; et al. Genetically-guided algorithm development and sample size optimization for age-related macular degeneration cases and controls in electronic health records from the VA Million Veteran Program. AMIA Summits Transl. Sci. Proc. 2019, 2019, 153–162. [Google Scholar]
- Schnabolk, G.; Rohrer, B.; Simpson, K.N. Increased Nonexudative Age-Related Macular Degeneration Diagnosis Among Medicare Beneficiaries with Rheumatoid Arthritis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3520. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Larson, E.B.; Gibbons, L.E.; Latimer, C.S.; Rose, S.E.; Hellstern, L.L.; Keene, C.D.; Crane, P.K.; for the Adult Changes in Thought (ACT) Study. Ophthalmology-Based Neuropathology Risk Factors: Diabetic Retinopathy is Associated with Deep Microinfarcts in a Community-Based Autopsy Study. J. Alzheimer′s Dis. 2019, 68, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Larson, E.B.; Gibbons, L.E.; Lee, A.Y.; McCurry, S.M.; Bowen, J.D.; McCormick, W.C.; Crane, P.K. Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimer′s Dement. 2019, 15, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Almony, A.; Keyloun, K.R.; Shah-Manek, B.; Multani, J.K.; McGuiness, C.B.; Chen, C.-C.; Campbell, J.H. Clinical and economic burden of neovascular age-related macular degeneration by disease status: A US claims-based analysis. J. Manag. Care Spec. Pharm. 2021, 27, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.H.; Longstreth, W.T.; Thielke, S.M.; Francis, C.E.; Carone, M.; Kuller, L.H.; Fitzpatrick, A.L. Ophthalmic conditions associated with dementia risk: The Cardiovascular Health Study. Alzheimer′s Dement. 2021, 17, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Nestler, S.; Kreft, D.; Doblhammer, G.; Guthoff, R.F.; Frech, S. Progression to severe visual impairment and blindness in POAG patients: Pace and risk factors—A cohort study using German health claims data. BMJ Open Ophthalmol. 2022, 7, e000838. [Google Scholar] [CrossRef] [PubMed]
- Loukovaara, S.; Auvinen, A.; Haukka, J. Associations between systemic medications and development of wet age-related macular degeneration. Acta Ophthalmol. (Copenh.) 2022, 100, 572–582. [Google Scholar] [CrossRef]
- Creuzot-Garcher, C.P.; Srour, M.; Baudin, F.; Daien, V.; Dot, C.; Nghiem-Buffet, S.; Girmens, J.-F.; Coulombel, N.; Ponthieux, A.; Delcourt, C. Incidence and Prevalence of Neovascular Age-Related Macular Degeneration in France between 2008 and 2018. Ophthalmol. Sci. 2022, 2, 100114. [Google Scholar] [CrossRef]
- Kido, A.; Miyake, M.; Tamura, H.; Hiragi, S.; Kimura, T.; Yoshida, S.; Takeuchi, M.; Ohtera, S.; Takahashi, A.; Ooto, S.; et al. Incidence and Clinical Practice of Exudative Age-related Macular Degeneration. Ophthalmol. Sci. 2022, 2, 100125. [Google Scholar] [CrossRef]
- Matsumiya, W.; Karaca, I.; Pham, B.H.; Akhavanrezayat, A.; Uludag, G.; Yasar, C.; Ghoraba, H.M.; Mobasserian, A.; Regenold, J.B.; Halim, M.S.; et al. Association of oral montelukast with reduced odds of developing exudative age-related macular degeneration. Retina 2023, 43, 1914–1921. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zou, J.; Yuan, R.; Fan, H.; Hu, H.; Cheng, Y.; Liu, J.; Zou, H.; You, Z. Exploring the Effect of the Gut Microbiome on the Risk of Age-Related Macular Degeneration From the Perspective of Causality. Investig. Opthalmol. Vis. Sci. 2023, 64, 22. [Google Scholar] [CrossRef]
- Moir, J.; Hyman, M.; Wang, J.; Flores, A.; Skondra, D. The Association of Antibiotic Use and the Odds of a New-Onset ICD Code Diagnosis of Age-Related Macular Degeneration: A Large National Case-Control Study. Investig. Opthalmol. Vis. Sci. 2023, 64, 14. [Google Scholar] [CrossRef]
- Rämö, J.T.; Abner, E.; van Dijk, E.H.C.; Wang, X.; Brinks, J.; Nikopensius, T.; Nõukas, M.; Marjonen, H.; Silander, K.; Jukarainen, S.; et al. Overlap of Genetic Loci for Central Serous Chorioretinopathy With Age-Related Macular Degeneration. JAMA Ophthalmol. 2023, 141, 449. [Google Scholar] [CrossRef] [PubMed]
- Javitt, J.C.; Zhou, Z.; Maguire, M.G.; Fine, S.L.; Willke, R.J. Incidence of exudative age-related macular degeneration among elderly americans. Ophthalmology 2003, 110, 1534–1539. [Google Scholar] [CrossRef] [PubMed]
- Moir, J.; Hyman, M.J.; Wang, J.; Shah, A.; Maatouk, C.; Flores, A.; Skondra, D. Associations Between Autoimmune Disease and the Development of Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2023, 64, 45. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/nchs/icd/icd9.htm (accessed on 20 December 2023).
- Mues, K.; Liede, A.; Liu, J.; Wetmore, J.B.; Zaha, R.; Bradbury, B.D.; Collins, A.J.; Gilbertson, D.T. Use of the Medicare database in epidemiologic and health services research: A valuable source of real-world evidence on the older and disabled populations in the US. Clin. Epidemiol. 2017, 9, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Centers for Medicare Advocacy. Available online: https://medicareadvocacy.org/?s=medicare+enrollment+numbers (accessed on 20 December 2023).
- Pugely, A.J.; Martin, C.T.; Harwood, J.; Ong, K.L.; Bozic, K.J.; Callaghan, J.J. Database and Registry Research in Orthopaedic Surgery: Part I. J. Bone Jt. Surg. 2015, 97, 1278–1287. [Google Scholar] [CrossRef]
- Pugely, A.J.; Martin, C.T.; Harwood, J.; Ong, K.L.; Bozic, K.J.; Callaghan, J.J. Database and Registry Research in Orthopaedic Surgery: Part 2: Clinical Registry Data. J. Bone Jt. Surg. 2015, 97, 1799–1808. [Google Scholar] [CrossRef]
- Riley, G.F. Administrative and Claims Records as Sources of Health Care Cost Data. Med. Care 2009, 47 (Suppl. 1), S51–S55. [Google Scholar] [CrossRef]
- Stein, J.D.; Lum, F.; Lee, P.P.; Rich, W.L.; Coleman, A.L. Use of Health Care Claims Data to Study Patients with Ophthalmologic Conditions. Ophthalmology 2014, 121, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R. Benefits of using an electronic health record. Nurs. Crit. Care 2017, 12, 9–10. [Google Scholar] [CrossRef]
- Menachemi, N.; Collum, T.H. Benefits and drawbacks of electronic health record systems. Risk Manag. Healthc. Policy 2011, 4, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Sunness, J.S. The Underreporting of Age-related Geographic Atrophy of the Macula See Editorial on page 287. Ophthalmol. Retin. 2023, 7, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.X. International Classification of Disease: Required Specificity when Coding for Age-related Macular Degeneration. Ophthalmol. Retin. 2023, 7, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Krive, J.; Patel, M.; Gehm, L.; Mackey, M.; Kulstad, E.; Li, J.; Lussier, Y.A.; Boyd, A.D. The complexity and challenges of the International Classification of Diseases, Ninth Revision, Clinical Modification to International Classification of Diseases, 10th Revision, Clinical Modification transition in EDs. Am. J. Emerg. Med. 2015, 33, 713–718. [Google Scholar] [CrossRef]
- Yonekawa, Y.; Miller, J.; Kim, I. Age-Related Macular Degeneration: Advances in Management and Diagnosis. J. Clin. Med. 2015, 4, 343–359. [Google Scholar] [CrossRef]
- Silvestri, G.; Williams, M.A.; McAuley, C.; Oakes, K.; Sillery, E.; Henderson, D.C.; Ferguson, S.; Silvestri, V.; Muldrew, K.A. Drusen prevalence and pigmentary changes in Caucasians aged 18–54 years. Eye 2012, 26, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Ophthalmology EyeWiki. Available online: https://eyewiki.org/Process_Overview_for_Submitting_New_Condition_Codes_to_SNOMED_International:_A_Use_Case_in_Retina (accessed on 20 December 2023).
- Tavakoli, K.; Kalaw, F.G.P.; Bhanvadia, S.; Hogarth, M.; Baxter, S.L. Concept Coverage Analysis of Ophthalmic Infections and Trauma among the Standardized Medical Terminologies SNOMED-CT, ICD-10-CM, and ICD-11. Ophthalmol. Sci. 2023, 3, 100337. [Google Scholar] [CrossRef]
- Cai, C.X.; Halfpenny, W.; Boland, M.V.; Lehmann, H.P.; Hribar, M.; Goetz, K.E.; Baxter, S.L. Advancing Toward a Common Data Model in Ophthalmology. Ophthalmol. Sci. 2023, 3, 100391. [Google Scholar] [CrossRef]

| Author | Year of Publication | Dataset Origin | Number of Patients Included |
|---|---|---|---|
| Duan et al. [13] | 2003 | Medicare | 167,034 |
| Sloan et al. [14] | 2004 | Medicare | 4,280 |
| Halpern et al. [15] | 2006 | Medicare | 58,594 |
| Zlateva et al. [16] | 2007 | Medicare | 26,057 |
| Swanson et al. [17] | 2007 | Veterans Affairs | 614 |
| Liao et al. [18] | 2008 | Medicare | 137,838 |
| Day et al. [19] | 2008 | Medicare | 20,671 |
| Latkany et al. [20] | 2010 | Veterans Affairs | 226 |
| Day et al. [21] | 2011 | Medicare | 12,465 |
| Stein et al. [22] | 2011 | Claims database | 2,252,515 |
| French et al. [23] | 2011 | Veterans Affairs | 3,021 |
| Stein et al. [24] | 2011 | Medicare | 23,941 |
| Stein et al. [25] | 2013 | Commercial Claims database | 103 |
| Sloan et al. [26] | 2013 | Medicare | 2,151 |
| Qualls et al. [27] | 2013 | Medicare | 23,133 |
| Kume et al. [28] | 2014 | Commercial Claims database | 3,058 |
| Leisy et al. [29] | 2016 | Institutional EHR | 107 |
| Lee et al. [30] | 2017 | Commercial Claims database | 933 |
| Gower et al. [31] | 2017 | Medicare | 195,812 |
| Chiu et al. [32] | 2018 | Institutional EHR | 579 |
| Rosenfeld et al. [33] | 2018 | Medicare | 3,462,402 |
| Halladay et al. [34] | 2019 | Veterans Affairs | 504,027 |
| Schnabolk et al. [35] | 2019 | Commercial Claims database | 37,252 |
| Lee et al. [36] | 2019 | Institutional EHR | 273 |
| Lee et al. [37] | 2019 | Institutional EHR | 1,036 |
| Almony et al. [38] | 2021 | Commercial Claims database | 6,076 |
| Hwang et al. [39] | 2021 | Medicare | 668 |
| Nestler et al. [40] | 2021 | Commercial Claims database | 1,000 |
| Loukovaara et al. [41] | 2022 | Registry | 2,947 |
| Creuzot-Garcher et al. [42] | 2022 | Commercial Claims database | 432,961 |
| Kido et al. [43] | 2022 | Commercial Claims database | 246,064 |
| Matsumiya et al. [44] | 2023 | Institutional EHR | 1,913 |
| Liu et al. [45] | 2023 | Registry | 6,157 |
| Moi et al. [46] | 2023 | Commercial Claims database | 312,404 |
| Rämö et al. [47] | 2023 | Registry | 8,913 |
| Javitt et al. [48] | 2023 | Medicare | 25,820 |
| Moir et al. [49] | 2023 | Commercial Claims database | 415,027 |
| Article Author | AMD Cohort of Interest | ICD Terminology Used | ICD Codes Used | Correct Codes Used | Missing Codes |
|---|---|---|---|---|---|
| Duan et al. [13] | AMD | 9 | 362.42, 362.43, 362.52, 362.53, 362.5, 362.50, 362.51, 362.57 | 362.52, 362.5, 362.50, 362.51 | |
| Sloan et al. [14] | AMD | 9 | 362.51, 362.57, 362.52, 362.53, 362.5, 362.50 | 362.51, 362.52, 362.5, 362.50 | |
| Swanson et al. [17] | AMD | 9 | 362.50, 362.51, 362.52 | 362.50, 362.51, 362.52 | 362.5 |
| Day et al. [19] | AMD | 9 | 362.50, 362.52, 362.51, 362.57 | 362.50, 362.51, 362.52 | 362.5 |
| Leisy et al. [29] | AMD | 9 | 362.5, 362.51, 362.52 | 362.5, 362.51, 362.52 | 362.5 |
| Chiu et al. [32] | AMD | 9 | 362.50, 362.51, 362.52 | 362.50, 362.51, 362.52 | 362.5 |
| Rosenfeld et al. [33] | AMD | 9 | 362.50, 362.51, 362.52 | 362.50, 362.51, 362.52 | 362.5 |
| Schnabolk et al. [35] | AMD | 9 | 362.50, 362.51, 362.52 | 362.50, 362.51, 362.52 | 362.5 |
| Lee et al. [36] | AMD | 9 | 3625A, 3625B | 362.5 | 362.50, 362.51, 362.52 |
| Lee et al. [37] | AMD | 9 | 362.50, 362.51, 362.52 | 362.50, 362.51, 362.52 | 362.5 |
| Hwang et al. [39] | AMD | 9 | 3625A, 3625B | 362.5 | 362.50, 362.51, 362.52 |
| Liu et al. [45] | neovascular AMD | 9 | 362.52, 362.42, 362.43 | 362.52 | 362.5, 362.50, 362.51 |
| Moi et al. [46] | neovascular AMD | 9 | 362.52 | 362.52 | 362.5, 362.50, 32.51 |
| Rämö et al. [47] | neovascular AMD | 9 | 362.52, 362.42, 362.43 | 362.52 | 362.5, 362.50, 362.51 |
| Javitt et al. [48] | non-neovascular AMD | 9 | 362.51, 362.57 | 362.51 | 362.5, 362.50, 362.52 |
| Moir et al. [49] | AMD | 9 | 362.50, 362.51, 362.52 | 362.50, 362.51, 362.52 | |
| Kume et al. [28] | AMD | 10 | H35.3 | H35.3 | |
| Nestler et al. [40] | AMD | 10 | H35.3 | H35.3 | |
| Loukovaara et al. [41] | AMD | 10 | H35.30 | H35.3 | |
| Creuzot-Garcher et al. [42] | AMD | 10 | H35.31, H35.32 | H35.3 | |
| Kido et al. [43] | AMD | 10 | H35.30 | H35.3 | |
| Matsumiya et al. [44] | neovascular AMD | 10 | H353 | H35.3 | |
| Liu et al. [45] | neovascular AMD | 10 | H35.3 | H35.3 | |
| Moi et al. [46] | neovascular AMD | 10 | H35.3 | H35.3 | |
| Rämö et al. [47] | non-neovascular AMD | 10 | H35.32 | H35.3 | |
| Moir et al. [49] | AMD | 10 | H35.30, H35.31, H35.32 | H35.3 | |
| Halladay et al. [34] | AMD | 10-CM | H35.31, H35.32 | H35.31, H35.32 | H35.30, H35.311, H35.3110, H35.3111, H35.3112, H35.3113, H35.3114, H35.312, H35.3120, H35.3121, H35.3122, H35.3123, H35.3124, H35.313, H35.3130, H35.3131, H35.3132, H35.3133, H35.3134, H35.319, H35.3190, H35.3191, H35.3192, H35.3193, H35.3194, H35.321, H35.3210, H35.3211, H35.3212, H35.3213, H35.322, H35.3220, H35.3221, H35.3222, H35.3223, H35.323, H35.3233, H35.35.329, H35.3290, H35.3292, H35.3293 |
| Almony et al. [38] | neovascular AMD | 10-CM | H35.3210, H35.3211, H35.3212, H35.3213, H35.3220, H35.3221, H35.3222, H35.3223, H35.3230, H35.3231, H35.3232, H35.3233, H35.3290, H35.3291, H35.3292, H35.3293 | H35.3210, H35.3211, H35.3212, H35.3213, H35.3220, H35.3221, H35.3222, H35.3223, H35.3230, H35.3231, H35.3232, H35.3233, H35.3290, H35.3291, H35.3292, H35.3293 | |
| Halpern et al. [15] | AMD | 9-CM | 362.51, 362.52, 362.57 | 362.51, 362.52 | 362.5, 362.50 |
| Zlateva et al. [16] | AMD | 9-CM | 362.42-362.43, 362.52, 362.53, 362.5, 362.50, 362.51, 362.57 | 362.5, 362.51, 362.52 | 362.5, 362.50 |
| Liao et al. [18] | AMD | 9-CM | 362.50, 362.51, 362.57, 362.52 | 362.50, 362.51, 362.52 | 362.5 |
| Latkany et al. [20] | AMD | 9-CM | 362.51-362.52 | 362.51, 362.52 | 362.5, 362.50 |
| Day et al. [21] | AMD | 9-CM | 362.50-52, 362.57 | 362.51, 362.52 | 362.5 |
| Stein et al. [22] | AMD | 9-CM | 362.51, 362.52 | 362.51, 362.52 | 362.5, 362.50 |
| French et al. [23] | AMD | 9-CM | 362.51, 362.52, 362.5, 362.50, 362.53, 362.57 | 362.5, 362.51, 362.52 | 362.5 |
| Stein et al. [24] | neovascular AMD | 9-CM | 362.42, 362.43, or 362.52 | 362.52 | 362.5, 362.50, 362.51 |
| Stein et al. [25] | neovascular AMD | 9-CM | 362.52 | 362.52 | 362.5, 362.50, 362.53 |
| Sloan et al. [26] | neovascular AMD | 9-CM | 362.52, 362.42, 362.43 | 362.52 | 362.5, 362.50, 362.51 |
| Qualls et al. [27] | AMD | 9-CM | 362.52 | 362.52 | 362.5, 362.50, 362.51 |
| Lee et al. [30] | neovascular AMD | 9-CM | 362.52 | 362.52 | 362.5, 362.50, 362.51 |
| Gower et al. [31] | neovascular AMD | 9-CM | 362.52 | 362.52 | 362.5, 362.50, 362.51 |
| Halladay et al. [34] | non-neovascular AMD | 9-CM | 362.50, 362.51, 362.57 | 362.5, 362.51 | 362.50, 362.52 |
| Matsumiya et al. [44] | non-neovascular AMD | 9-CM | 362.52 | 362.52 | 362.5, 362.50, 362.51 |
| Article Number | Additional Codes | Codified Diagnosis |
|---|---|---|
| Duan et al. [13] | 362.53 362.57 362.42 362.43 | Cystoid macular degeneration of retina Drusen (degenerative) of retina Serous detachment of retinal pigment epithelium Hemorrhagic detachment of retinal pigment epithelium |
| Sloan et al. [14] | 362.53 362.57 | Cystoid macular degeneration of retina Drusen (degenerative) of retina |
| Halpern et al. [15] | 362.57 | Drusen (degenerative) of retina |
| Zlateva et al. [16] | 362.42 362.43 | Serous detachment of retinal pigment epithelium Hemorrhagic detachment of retinal pigment epithelium |
| Liao et al. [18] | 362.42 362.43 362.53 362.57 | Serous detachment of retinal pigment epithelium Hemorrhagic detachment of retinal pigment epithelium Cystoid macular degeneration of retina Drusen (degenerative) of retina |
| Day et al. [19] | 362.43 | Hemorrhagic detachment of retinal pigment epithelium |
| Day et al. [21] | 362.42 362.43 | Serous detachment of retinal pigment epithelium Hemorrhagic detachment of retinal pigment epithelium |
| Stein et al. [22] | 362.57 | Drusen (degenerative) of retina |
| Stein et al. [25] | 362.57 | Drusen (degenerative) of retina |
| Sloan et al. [26] | 362.53 362.57 | Cystoid macular degeneration of retina Drusen (degenerative) of retina |
| Lee et al. [30] | 362.57 | Drusen (degenerative) of retina |
| Chiu et al. [32] | 362.57 | Drusen (degenerative) of retina |
| Javitt et al. [48] | 362.42 362.43 | Serous detachment of retinal pigment epithelium Hemorrhagic detachment of retinal pigment epithelium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalaw, F.G.P.; Chen, J.S.; Baxter, S.L. Variations in Using Diagnosis Codes for Defining Age-Related Macular Degeneration Cohorts. Informatics 2024, 11, 28. https://doi.org/10.3390/informatics11020028
Kalaw FGP, Chen JS, Baxter SL. Variations in Using Diagnosis Codes for Defining Age-Related Macular Degeneration Cohorts. Informatics. 2024; 11(2):28. https://doi.org/10.3390/informatics11020028
Chicago/Turabian StyleKalaw, Fritz Gerald Paguiligan, Jimmy S. Chen, and Sally L. Baxter. 2024. "Variations in Using Diagnosis Codes for Defining Age-Related Macular Degeneration Cohorts" Informatics 11, no. 2: 28. https://doi.org/10.3390/informatics11020028
APA StyleKalaw, F. G. P., Chen, J. S., & Baxter, S. L. (2024). Variations in Using Diagnosis Codes for Defining Age-Related Macular Degeneration Cohorts. Informatics, 11(2), 28. https://doi.org/10.3390/informatics11020028








