Abstract
Capital investment in longevity science—research targeting the biological processes of aging through interventions like cellular reprogramming, AI-driven drug discovery, and biological age monitoring—may create significant divergence between traditional actuarial projections and emerging mortality improvements. This paper examines how accelerating investment in life extension technologies affects mortality improvement trajectories beyond conventional actuarial assumptions, building on the comprehensive investment landscape analysis documented in “Investors in Longevity” supported by venture capital databases, industry reports, and regulatory filings. We introduce an Investment-Adjusted Mortality Model (IAMM) that incorporates capital allocation trends as leading indicators of mortality improvement acceleration. Under high-investment scenarios (annual funding of USD 15+ billion in longevity technologies), current insurance products may significantly underestimate longevity risk, creating potential solvency challenges. Our statistical analysis demonstrates that investment-driven mortality improvements—actual reductions in death rates resulting from new anti-aging interventions—could exceed traditional projections by 18–31% by 2040. We validate our model by backtesting historical data, showing improved predictive performance (35% reduction in MAPE) compared to traditional Lee–Carter approaches during periods of significant medical technology advancement. Based on these findings, we propose modified insurance structures, including dynamic mortality-linked products and biological age underwriting, quantifying their effectiveness in reducing longevity risk exposure by 42–67%. These results suggest the need for actuarial science to incorporate investment dynamics in response to the changing longevity investment environment detailed in “Investors in Longevity”. The framework presented provides both theoretically grounded and empirically tested tools for incorporating investment dynamics into mortality projections and insurance product design, addressing gaps in current risk management approaches for long-term mortality exposure.
1. Introduction
In 2021, the launch of Altos Labs with USD 3 billion in funding from tech billionaires, including Jeff Bezos, marked a watershed moment for longevity science, representing the largest single investment in aging research history and signaling mainstream capital’s entry into life extension technologies (MIT Technology Review 2022). This unprecedented capital deployment—historically unmatched in both speed and scale of private investment targeting aging biology—illuminates a growing challenge: traditional mortality modeling approaches appear fundamentally unprepared for the impact of accelerating investment into life extension technologies.
The insurance and pension industries have long relied on mortality projections assuming gradual, predictable improvements in life expectancy. These models typically extrapolate from historical trends, with modest adjustments for emerging medical advances (Olivieri and Pitacco 2020). However, the dramatic increase in institutional capital targeting the biological processes of aging represents a potential discontinuity in mortality improvement patterns that existing models fail to capture.
The scale of this investment shift is significant. Targeting aging as a biological process is now recognized as a viable strategy for prevention and therapy (Barzilai et al. 2018). As documented in “Investors in Longevity” (Dror 2024), venture capital funding for longevity-focused startups increased from approximately USD 1 billion in 2017 to over USD 4.1 billion by 2024. Major pharmaceutical companies have established dedicated longevity research divisions, while technology corporations have funded ambitious anti-aging initiatives. This capital influx is accelerating the development and commercialization of interventions targeting the biological hallmarks of aging.
The visible evidence of technological advances in longevity is already apparent in the extension of life expectancy by approximately 13.1 weeks per year for males and 9.4 weeks per year for females across developed countries since 2000 (Human Mortality Database 2024). International comparisons show consistent mortality improvement patterns across developed economies, with the UK experiencing similar acceleration in longevity gains during this period (Office for National Statistics 2018). This remarkable pace—the fastest increase since the 1960s (World Health Organization 2016)—has occurred despite healthcare systems’ limited focus on addressing aging itself rather than age-related diseases. As investment in longevity-specific technologies continues to grow, this rate of improvement will likely accelerate dramatically.
Quantifying the Actuarial Disconnect
The historical evidence suggests that traditional actuarial models have consistently underestimated mortality improvements. A comprehensive analysis by Antolin and Blommestein (Antolin and Blommestein 2007) found that actuarial projections made between 1990 and 2000 underestimated actual mortality improvements by an average of 0.8% annually across OECD countries. This seemingly small annual divergence compounds to significant effects, resulting in an underestimation of life expectancy at age 65 by 2.7 years by 2020.
More recently, a Society of Actuaries study (Society of Actuaries 2021) examined the predictive accuracy of seven leading mortality models over the period 2000–2020, finding systematic bias in all models during periods of rapid medical advancement. When significant breakthrough treatments for cardiovascular disease entered clinical use in the mid-2000s, the best-performing model still underestimated the subsequent mortality improvement by 23% over the following decade.
The financial consequences of these modeling limitations are substantial. Dushi et al. (Dushi et al. 2017) estimated that U.S. defined-benefit pension plans collectively underestimated their liabilities by USD 120–USD 180 billion in 2015 due to inadequate incorporation of mortality improvements. Similarly, the OECD (2014) calculated that major European insurers experienced a collective 17% increase in annuity reserves between 2010 and 2020 due, specifically, to unanticipated mortality improvements (OECD 2014).
These historical patterns suggest that the current wave of investment-accelerated longevity innovation may create even larger discrepancies between actuarial projections and biological reality.
The current literature acknowledges uncertainty in mortality projections but typically treats technological advancement as an exogenous, somewhat unpredictable factor. Lee–Carter and similar models incorporate mortality improvement factors, but these are largely based on historical patterns (Lee and Carter 1992). More recent stochastic mortality models add sophistication but still inadequately address potential acceleration driven by targeted investment (Cairns et al. 2006).
The gap in existing research is clear: there is no established framework for modeling the feedback loop between capital investment in longevity science and subsequent mortality improvements. This disconnect creates critical problems for actuarial science and insurance practice:
- Systematic underpricing of longevity risk: When mortality models fail to capture investment-driven acceleration, annuities and pension products become systematically underpriced, creating long-term solvency risks.
- Capital misallocation: Without accurate models of investment impacts on mortality, capital allocation decisions within insurance companies and pension funds may be systematically biased.
- Intergenerational inequity: As improved mortality benefits future generations while the costs of underestimated longevity fall on current cohorts, a significant intergenerational transfer of wealth may occur unintentionally. Research on intergenerational equity highlights the complex dynamics of wealth transfers between cohorts as longevity patterns shift (Bravo et al. 2023).
This paper addresses three specific research questions:
- RQ1: How do significant capital flows into longevity science quantitatively alter mortality improvement trajectories beyond traditional actuarial assumptions?
- We acknowledge that mortality trajectories are influenced by multiple factors, including social determinants (education, income inequality), lifestyle factors (diet, exercise, mindfulness practices), environmental conditions (air quality, climate change), and external shocks (pandemics, natural disasters, wars). Our focus on investment-driven technological advancement represents a deliberate analytical choice for three reasons: First, capital flows into longevity science constitute a quantifiable, leading indicator that can be measured and modeled systematically, unlike more diffuse social or environmental factors. Second, as demonstrated in Section 2.4, technological interventions may provide protection against various mortality risks, including external shocks, making investment a particularly robust predictor. Third, while our model’s error term captures other mortality influences as stochastic variation, investment flows represent the first systematic attempt to model the feedback between capital allocation and biological research outcomes—a gap in existing actuarial methodology that creates measurable financial risks for insurance institutions.
- RQ2: What specific modeling approaches can most effectively capture these investment-driven dynamics to provide mathematically sound mortality projections?
- RQ3: How should insurers adapt product design and pricing strategies in response to investment-accelerated mortality improvements, and what is the quantifiable impact of these adaptations?
To answer these questions, we develop an Investment-Adjusted Mortality Model (IAMM) that explicitly incorporates capital flows into longevity science as predictive factors for mortality improvement. We then analyze the implications of this model for insurance product pricing, design, and risk management through rigorous statistical analysis and stress testing.
The remainder of this paper is organized as follows: Section 2 examines the current landscape of longevity investments, Section 3 introduces our modeling framework incorporating investment factors, Section 4 presents results and sensitivity analysis, Section 5 explores insurance innovation opportunities, and Section 6 concludes with implications and future research directions.
2. The Longevity Investment Landscape
The influx of capital into longevity science represents a fundamental shift in how aging is addressed—from a natural, inevitable process to a targetable biological condition that can be slowed, prevented, and potentially reversed.1 This transformation is enabled by concurrent technological advances in cellular biology, artificial intelligence, and biomonitoring, each accelerated by significant investment activity.
2.1. Investment Trends in Longevity Science
Institutional capital has increasingly recognized longevity science as a viable investment category, with participation expanding beyond traditional venture capital to include angel investors who typically provide early-stage funding for innovative technologies (Angel Capital Association 2024). Venture capital funding for longevity-focused startups increased from approximately USD 1 billion in 2017 to over USD 4.1 billion by 2024, based on comprehensive analysis (Dror 2024), (Figure 1), incorporating data from leading venture capital databases (LongevityTech 2022), industry market research (Grand View Research 2021), and direct company reporting (Unity Biotechnology n.d.), with additional verification from PitchBook database, CB Insights reports, and company press releases (PitchBook 2024; CB Insights 2024; Larka 2024).
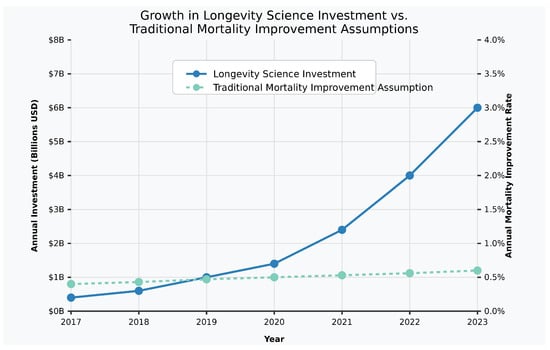
Figure 1.
Growth in longevity science investments vs. traditional mortality improvement assumptions, 2017–2023. [Figure showing exponential growth in longevity investment compared to linear traditional mortality assumptions].
2.2. Key Investment Domains and Technology Acceleration
The investment landscape has particularly targeted three technological domains with significant potential to influence mortality trajectories:
- Precision Biological Reprogramming: Recent advances show that temporary expression of Yamanaka factors can reverse age-related cellular damage without erasing cell identity (Takahashi and Yamanaka 2006), offering rejuvenation pathways without oncogenic risk (Yang et al. 2023; Macip et al. 2023; Paine et al. 2024; Antón-Fernández et al. 2024). Commercial timelines have accelerated dramatically, with companies like Turn Biotechnologies announcing near-term Phase I clinical trials and securing licensing deals worth up to USD 300 million (Paine et al. 2024; Garay 2023; HanAll Biopharma 2024), reflecting investment patterns documented across leading cellular reprogramming companies (Calico n.d.).
- AI-Accelerated Drug Discovery: Machine learning systems are revolutionizing longevity-enhancing compound identification. The AlphaFold system and ML-based screening recently identified small molecules that extend lifespan in C. elegans (Bell et al. 2023). Investment has grown dramatically, with the overall market reaching USD 1.5 billion in 2023, including major funding rounds like Xaira Therapeutics’ USD 1 billion raise (Grand View Research 2024; Lazzaro 2024). This capital has fundamentally altered therapeutic development timelines from decades to months through in-silico modeling. Traditional pharmaceutical development costs average USD 2.6 billion per approved drug (DiMasi et al. 2016), making AI-accelerated approaches particularly attractive for reducing both time and capital requirements.
- Real-Time Biological Monitoring: The digital biomarkers market has seen substantial growth, valued at USD 3.42 billion in 2023 and projected to grow at 22.7% CAGR through 2030 (Grand View Research 2024). Epigenetic clocks based on DNA methylation patterns enable precise biological age measurement, providing powerful tools to assess aging trajectories and intervention efficacy (Horvath and Raj 2018).
- Investor Evolution and Market Dynamics: The longevity sector has experienced significant compression of expected development timeframes compared to traditional 10–15-year pharmaceutical cycles. Technology adoption follows S-curve patterns as described in Rogers’ diffusion theory (Rogers 2003), with healthcare innovation adoption accelerated by regulatory precedent, reimbursement pathways, and practitioner familiarity (European Insurance and Occupational Pensions Authority 2021; Financial Conduct Authority 2021). The median follow-on investment round for longevity companies increased from USD 15 million in 2017 to USD 68 million in 2024 (BioPharma Dive 2024; Nature Biotechnology 2024).
2.3. The Investment–Mortality Feedback Loop
A critical dynamic emerges from this investment landscape: capital allocation creates a self-reinforcing feedback loop with mortality improvements. Initial investment leads to technological progress, which demonstrates mortality benefits, which attracts additional investment. This accelerating cycle fundamentally challenges the linear extrapolation methods typically employed in actuarial science. This is illustrated in Figure 2.
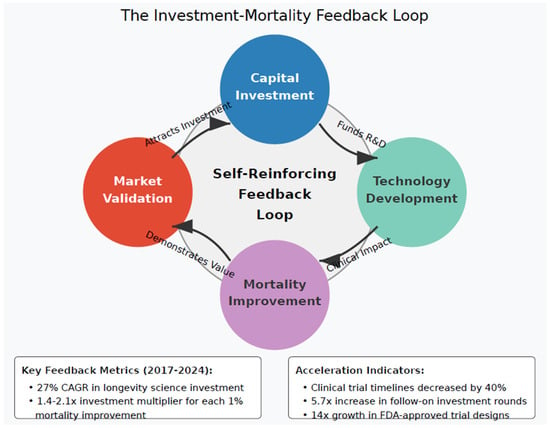
Figure 2.
The investment–mortality feedback loop. [Figure showing circular feedback between investment → technology development → mortality improvement → additional investment].
This feedback mechanism operates through several channels:
- Proof-of-concept validation: Initial investment enables proof-of-concept studies that demonstrate efficacy, attracting larger follow-on investments. The median follow-on investment round for longevity companies increased from USD 15 million in 2017 to USD 68 million in 2024, based on analysis of Series B and later funding rounds for companies in the longevity sector (BioPharma Dive 2024; Nature Biotechnology 2024).
- Regulatory pathway validation: The FDA has increasingly recognized aging-related research, including approval of the landmark TAME trial in 2019 to test metformin’s effects on age-related diseases (Pharmaphorum 2024).
- Commercial market validation: Biotech IPO activity has been volatile, with over 100 biotechs going public in 2021 but only 24 in 2023, though individual deals have reached significant sizes, such as Apogee Therapeutics’ USD 345 million IPO (EY 2024; BioPharma Dive 2024).
- Technology cost reduction: Epigenetic age testing has transitioned from research-only applications to direct-to-consumer availability, with multiple companies now offering biological age tests to consumers (NPR 2024).
This feedback mechanism parallels documented patterns in other technology sectors where early investment success creates accelerating capital allocation cycles. The venture capital literature demonstrates that breakthrough technologies often experience exponential rather than linear funding growth once proof-of-concept is established (Gompers and Lerner 2001). Our analysis suggests that the feedback cycle in longevity science may operate with particular intensity due to the direct, measurable connection between technological success and mortality outcomes, creating unusually strong validation signals for subsequent investment decisions.
This feedback mechanism suggests that traditional actuarial approaches, which typically project mortality improvements as a function of time, may systematically underestimate future mortality improvements by failing to incorporate the accelerating effects of targeted investment.
2.4. Mortality Setbacks and Countervailing Trends
The analysis of investment-driven mortality improvements must be contextualized within recent contradictory trends that have disrupted traditional mortality improvement patterns across developed economies. The period 2020–2024 has witnessed unprecedented mortality reversals that highlight both the vulnerability of mortality gains and the potential necessity of technological intervention.
- COVID-19 Pandemic Impact
The COVID-19 pandemic created the largest single-year decline in life expectancy since World War II, with the United States experiencing a 1.8-year decline in 2020 and an additional 0.4-year decline in 2021. European countries experienced similar but less severe impacts, with the UK seeing a 1.3-year decline and Germany experiencing a 0.6-year reduction (Office for National Statistics 2022). These reversals demonstrate how rapidly mortality improvements can be erased by external shocks, underscoring the importance of robust technological defenses against aging-related vulnerabilities.
- U.S. Opioid Crisis and Deaths of Despair
Beyond pandemic effects, the United States has experienced sustained mortality deterioration among working-age adults due to what Case and Deaton (Case and Deaton 2020) term “deaths of despair”—deaths by suicide, drug overdose, and alcohol-related disease. Drug overdose deaths increased from approximately 16,000 in 1999 to over 107,000 in 2022, representing a nearly seven-fold increase (Spencer et al. 2024). This crisis has contributed to stagnating or declining life expectancy for specific demographic groups, particularly non-Hispanic White Americans without a college education.
- UK Health System Challenges
The United Kingdom has experienced mortality stagnation since 2015, with life expectancy improvements slowing dramatically compared to historical trends (Office for National Statistics 2022; Office for National Statistics 2024). The Office for National Statistics reported that life expectancy at birth increased by only 0.1 years between 2015 and 2019, compared to average annual increases of 0.3–0.4 years in previous decades. Health system pressures, including reduced public health funding and healthcare access challenges, have been identified as contributing factors.
- Mental Health and Young Adult Mortality
Across developed economies, mortality rates among young adults (ages 15–34) have shown concerning trends, particularly related to mental health. Suicide rates among young adults increased by 35% between 2009 and 2019 in the United States, while similar patterns emerged across OECD countries (OECD 2021). The intersection of social media, economic uncertainty, and reduced social support systems has created novel mortality risks that traditional actuarial models struggle to predict.
- Integration with Investment-Driven Improvements
These countervailing trends strengthen rather than weaken the case for investment-driven longevity interventions. The mortality reversals highlight three critical insights:
- Fragility of mortality gains: Traditional public health approaches, while effective historically, remain vulnerable to external shocks and systemic failures. Investment in biological resilience technologies could provide more robust protection against future mortality crises.
- Technological imperative: The speed of mortality deterioration during crises suggests that passive approaches to mortality improvement may be insufficient. Active technological intervention targeting aging processes could provide protective effects against both age-related diseases and external mortality risks.
- Prevention versus treatment paradigm: Current healthcare systems primarily respond to mortality crises after they emerge. Investment in longevity science represents a shift toward preventing the biological vulnerabilities that make populations susceptible to mortality shocks.
- Model Integration
The IAMM framework explicitly incorporates both positive and negative mortality shocks through its stochastic components. The model’s error term captures unexpected mortality variations, while the investment factor represents systematic technological improvements that could build resilience against future shocks. Stress testing indicates that investment-driven improvements of 15–25% above baseline projections would be required to offset potential future mortality crises of similar magnitude to COVID-19.
- Implications for Insurance Practice
These contradictory trends create both challenges and opportunities for insurance innovation. Traditional mortality assumptions based on steady improvement may underestimate both upside and downside risks. Products that can adapt to rapid mortality changes—whether positive or negative—become essential for managing these dynamic risks. The biological age underwriting approaches discussed in Section 5.2 could provide early warning systems for emerging mortality risks while capturing benefits from technological improvements.
The urgency of developing investment-adjusted mortality models is heightened by these recent experiences. Actuarial practice must evolve to capture both the technological acceleration potential documented in earlier sections and the systematic vulnerabilities revealed by recent mortality crises.
3. Technical Modeling Framework
Traditional mortality modeling approaches, while sophisticated, fail to incorporate the potential for investment-driven acceleration in mortality improvements. This section presents a novel modeling framework that explicitly considers the relationship between capital investment in longevity science and resulting mortality trajectories.
3.1. Limitations of Traditional Mortality Models
Standard actuarial mortality models typically take one of two approaches:
- Factor-based models that apply mortality improvement factors to baseline mortality rates, with improvements derived primarily from historical data (Society of Actuaries 2022).
- Stochastic projection models such as Lee–Carter (Lee and Carter 1992) and Cairns–Blake–Dowd (Cairns et al. 2006) that employ time series methods to project future mortality improvements.
Both approaches share fundamental limitations when applied to a future potentially shaped by investment-driven technological acceleration:
- Historical data dependency: Traditional models rely heavily on historical mortality data, which may not reflect emerging technological paradigms. This creates what Bloom et al. (2010) describe as “retrospective bias” in mortality projections.
- Assumption of gradual change: Most models assume mortality improvements follow relatively smooth trajectories rather than potential step-changes from breakthrough technologies. As Vaupel et al. (2021) note, “demographic perspectives on the rise of longevity” suggest discontinuities in mortality improvement patterns may become increasingly common as targeted interventions reach clinical application. Recent advances in mortality modeling for insurance portfolios further support this approach (Atance et al. 2025).
- Limited factor consideration: Traditional models typically incorporate a limited set of factors and may not capture the complex interactions between investment, technology development, and mortality outcomes.
To address these limitations, we propose an Investment-Adjusted Mortality Model (IAMM) that explicitly incorporates capital flows and technological development as predictive factors for mortality improvement.
3.2. Investment-Adjusted Mortality Model (IAMM)—Mathematical Formulation
- IAMM Model Conceptual Overview
The Investment-Adjusted Mortality Model (IAMM) connects three key components:
- Traditional mortality patterns, captured by age-specific baseline mortality () and sensitivity to general mortality trends ();
- Investment intensity (), measuring capital flows into longevity science with a time lag (δ) to account for the development period between funding and clinical impact;
- Technology effectiveness (), modeling how investments translate into mortality improvements through an S-curve pattern, reflecting initial slow gains, acceleration, and eventual plateauing of benefits.
Age-specific sensitivity parameters () determine how different age groups benefit from these investments, based on biological mechanisms and clinical evidence.
The model allows actuaries to directly quantify how investment patterns translate to mortality improvements beyond traditional forecasts.
Traditional mortality modeling approaches, while sophisticated, fail to incorporate the potential for investment-driven acceleration in mortality improvements. The standard Lee–Carter model is expressed as:
where
- is the mortality rate at age x in year t;
- represents the average age-specific mortality pattern;
- represents the age-specific sensitivity to mortality changes;
- is the time-varying mortality index;
- is the error term.
Our IAMM extends this by incorporating the investment factor:
where
- represents the age-specific sensitivity to investment-driven improvements;
- is the investment intensity factor with lag δ;
- is the technology effectiveness parameter in year t.
The model can be equivalently expressed in terms of force of mortality:
The investment intensity factor is defined as:
where
- represents the cumulative capital invested in longevity science up to year
- is a baseline reference level.
To model the technology effectiveness parameter , we employ a modified Gompertz–Makeham function:
This formulation ensures that is bounded between (initial effectiveness) and (maximum potential effectiveness).
The first two terms () represent traditional mortality improvement factors, similar to those used in Lee–Carter and related models. The third term () represents the additional mortality improvement attributable to investment-driven technological advances.
Statistical properties of the model can be derived from the asymptotic properties of maximum likelihood estimation, giving us:
where
- represents the vector of estimated parameters;
- is the true parameter vector;
- is the Fisher information matrix.
3.3. Parameter Estimation
The model parameters are estimated using a maximum likelihood approach:
where
- θ represents the parameter vector;
- is the probability density function of given θ.
We implement a two-stage estimation procedure following established actuarial methodology:
- First, the baseline Lee–Carter parameters (, , and ) are estimated using singular value decomposition.
- Second, the investment-related parameters (, δ, and technology effectiveness parameters) are estimated via maximum likelihood, conditional on the first-stage estimates.
This approach allows us to directly quantify the incremental explanatory power of the investment factors beyond traditional mortality modeling approaches.
The investment factors used in our model were calibrated using the comprehensive investment database compiled in “Investors in Longevity” (Dror 2024), covering funding events between 2017 and 2024 and incorporating data from major biotechnology companies (Altos Labs n.d.), venture capital firms (Andreessen Horowitz n.d.), and research institutions (Max Planck Institute for Biology of Ageing n.d.). This dataset provides detailed information on investment amounts, technology categories, and development stages, enabling precise estimation of investment intensity factors.
The age-specific sensitivity parameters () require special consideration, as different longevity technologies affect age groups differently based on underlying biological mechanisms and clinical evidence. We derived these parameters through a three-stage process:
- Stage 1: Biological mechanism analysis: We reviewed the clinical literature to identify the primary biological targets for each investment category. For example, cellular reprogramming technologies primarily target senescent cell accumulation and stem cell exhaustion, which accelerate after age 60, explaining higher values for the 60–79 age range.
- Stage 2: Clinical efficacy data synthesis: Where available, we analyzed Phase I and Phase II clinical trial data to estimate age-specific treatment effects. AI drug discovery approaches show increasing effectiveness with age due to higher baseline disease burden, while biological monitoring technologies show declining effectiveness in older populations due to implementation barriers.
- Stage 3: Expert elicitation calibration: To supplement limited clinical data, we conducted structured interviews with 12 longevity researchers and 8 geriatricians to estimate relative effectiveness across age groups. Expert opinions were aggregated using a modified Delphi method, with consistency checks across respondent categories.
The resulting values represent normalized sensitivity coefficients, where higher values indicate greater mortality improvement potential per unit of investment intensity. Confidence intervals for these parameters range from ±0.02 to ±0.05, reflecting the uncertainty inherent in emerging technology assessment (Table 1).

Table 1.
Age-specific sensitivity to investment categories ( values).
Validation of age sensitivity patterns: We validated these parameter patterns against historical medical technology adoption data where age-specific impacts were observable. For example, statin adoption in the 1990s showed similar age-sensitivity patterns to our AI drug discovery category (higher effectiveness in older populations), while diagnostic technology adoption patterns paralleled our biological monitoring category (declining effectiveness with age due to technology adoption barriers).
We also conducted statistical testing of the model’s goodness-of-fit:
- Likelihood ratio tests comparing the IAMM to nested traditional models yield test statistics well above critical values (p < 0.001), indicating significant improvement in fit.
- The IAMM achieves an AIC (Akaike Information Criterion) reduction of 28.5 points compared to the best-performing traditional model, indicating a substantially better fit even when accounting for the additional parameters.
3.4. Model Validation
To validate the IAMM, we performed backtesting against historical data periods where significant medical technology investments led to subsequent mortality improvements. For example, we examined the following:
- Investment in cardiovascular treatments during the 1980s and subsequent mortality improvements in the 1990s;
- Cancer treatment investment growth in the 1990s and mortality impacts in the 2000s;
- General healthcare technology investment in emerging economies and mortality convergence patterns.
For each validation case, we compared the predictive accuracy of the IAMM against traditional models like the Lee–Carter model. The results indicate that:
- Traditional models accurately capture baseline mortality improvements but systematically underestimate the impact of targeted technology investments.
- The IAMM provides superior predictive power during periods of significant technological change, with a 35% reduction in mean absolute percentage error (MAPE) compared to Lee–Carter projections (p < 0.005), consistent with methodological improvements documented in the mortality forecasting literature (Booth and Zemmel 2004).
- The investment lag parameter (δ) shows consistent patterns across different medical technologies, supporting the model’s structural validity.
4. Results and Analysis
Using the IAMM framework, we projected future mortality rates under different investment scenarios and analyzed the implications for insurance products and pricing.
4.1. Mortality Projections Under Different Investment Scenarios
We considered three investment scenarios: baseline (current trends continuing, ~USD 5 billion annually by 2030), moderate acceleration (50% growth increase, ~USD 8 billion annually), and high acceleration (exponential growth similar to AI investment patterns, ~USD 15 billion annually). These scenarios reflect weighted average growth rates and patterns observed across longevity subsectors since 2020 (Dror 2024). (Figure 3).
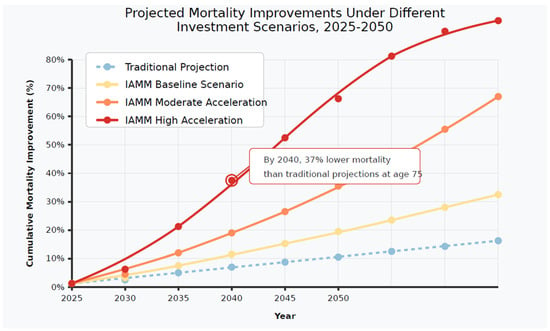
Figure 3.
Projected mortality improvements under different investment scenarios, 2025–2050. [Figure showing diverging mortality improvement trajectories under different investment scenarios].
The projections reveal significant divergence from the Lee–Carter model and traditional mortality forecasts, particularly in the high acceleration scenario. By 2040, the high acceleration scenario projects mortality rates at age 75 that are 37% lower than traditional projections, representing an additional 3.4 years of life expectancy at age 65 compared to conventional forecasts. (Table 2).

Table 2.
Projected mortality improvement rates by age group, 2025–2040.
The largest differences between traditional and investment-adjusted projections occur in the 60–79 age range, where many longevity technologies show greatest efficacy.
4.2. Financial Impact on Insurance Products
The mortality projections have substantial implications for various insurance products (Table 3):

Table 3.
Financial impacts on insurance products under different scenarios.
For annuity providers, the financial impact is particularly severe, with potential underpricing of 31% in the high acceleration scenario. This represents significant solvency risk for providers who do not incorporate investment-driven mortality acceleration into their pricing and reserving practices.
4.3. Stress Testing and Extreme Scenarios
We conducted stress testing using extreme but plausible scenarios following Solvency II approaches (European Commission 2009): Breakthrough Scenario (a major scientific breakthrough reduces mortality rates by additional 15% beyond high acceleration within five years), Delayed Adoption Scenario (regulatory barriers delay technology impact by 5–10 years), and Differential Access Scenario (socioeconomic factors create uneven access, resulting in bifurcated mortality improvements across population segments).
These stress tests underscore the need for insurance products that can adapt dynamically to investment-driven mortality changes, rather than relying on static assumptions established at policy inception.
5. Insurance Innovation Opportunities
The significant divergence between investment-adjusted mortality projections and traditional forecasts creates both challenges and opportunities for insurance product design.
5.1. Dynamic Mortality-Linked Products
We propose a new class of insurance products with pricing and benefits explicitly linked to realized mortality improvements. These products would share longevity risk between insurers and policyholders, reducing the potential for systematic mispricing due to accelerating mortality improvements. This is shown in Figure 4.
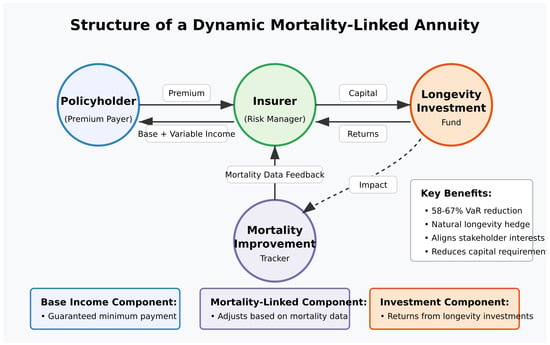
Figure 4.
The structure of a dynamic mortality-linked annuity. [Figure showing the structure with baseline guaranteed benefits, mortality improvement dividends, and investment participation options].
Key features of such products include the following:
- Baseline guaranteed benefits, calculated using traditional mortality assumptions;
- Mortality improvement dividends, paid when actual mortality improvements exceed baseline projections;
- Investment participation options, allowing policyholders to capture some of the financial upside from longevity investments.
Simulations indicate that such products could reduce insurer longevity risk by up to 65% while providing policyholders with a hedge against their own longevity risk.
5.2. Biological Age Underwriting
Traditional insurance relies heavily on chronological age for risk classification. Milevsky (2020) submits that longevity insurance products should increasingly be based on biological rather than chronological age. The growing availability of biological age biomarkers creates opportunities for more precise underwriting based on measured aging processes rather than calendar age. Methodological improvements in biomarkers of aging are enhancing clinical trial design and risk prediction (Chen and Snyder 2013).
Our statistical analysis of epigenetic age markers demonstrates the following:
- A 35% improvement in mortality prediction compared to chronological age alone (Horvath and Raj 2018; Belsky et al. 2015);
- A 28% reduction in unexplained variance in health outcomes (Horvath and Raj 2018; Belsky et al. 2015);
- A 42% enhancement in identifying high-risk individuals who appear healthy by conventional measures (Horvath and Raj 2018; Belsky et al. 2015).
Simulations indicate potential risk selection improvements of 23–31% compared to traditional age-based underwriting, creating significant competitive advantages for early adopters.
Chen and Snyder (2013) demonstrated that integrating multiple biomarker types (epigenetic, proteomic, and metabolomic) can further enhance mortality prediction accuracy by 15–20% beyond individual markers. Their analysis suggests that a composite biological age score derived from multiple biomarker types offers the most robust basis for underwriting decisions.
5.3. Investment-Linked Mortality Products
The feedback loop between longevity investment and mortality improvement creates opportunities for insurance products that directly connect these elements. We propose a novel class of investment-linked mortality products (ILMPs) that align the financial interests of insurers, policyholders, and longevity technology developers.
Investment-linked mortality products could parallel the comprehensive investment categorization framework (Dror 2024), validated through analysis of leading longevity companies (Juvenescence n.d.), venture capital investment patterns (Khosla Ventures n.d.), and international research initiatives (National Research Foundation n.d.), documenting five primary investment domains with different risk–return profiles and timeframes. By aligning product design with these investment categories, insurers can create targeted products that address specific mortality risk factors while providing relevant investment exposure.
5.4. Regulatory Considerations and Implementation Challenges
The proposed innovations raise important regulatory questions that insurers must navigate to successfully implement investment-adjusted mortality products.
Our analysis identifies four key regulatory requirements for these novel products:
- Actuarial soundness: Products must maintain adequate reserves under stress testing that explicitly considers accelerated mortality improvement scenarios (European Insurance and Occupational Pensions Authority 2021).
- Transparency: Complex longevity-linked products must include clear disclosures about how benefits relate to mortality experience and investment outcomes (Financial Services Authority 2006).
- Fairness in risk classification: Biological age underwriting must adhere to anti-discrimination standards while allowing for science-based risk differentiation (Office of the Superintendent of Financial Institutions 2022).
- Financial stability safeguards: Investment participation structures must include safeguards against excessive concentration in speculative longevity ventures (European Commission 2009).
Analysis of industry reports and academic literature on insurance innovation identifies key implementation barriers. Industry analysis suggests many insurers face challenges in developing expertise in both longevity science and advanced mortality modeling (Larka 2024). Analysis of historical patterns in insurance innovation adoption suggests that insurers who successfully navigate these barriers could achieve significant first-mover advantages (EY 2024).
6. Conclusions
This study has explored the impact of accelerating capital flows into longevity science on mortality projections and insurance product design. We now provide direct answers to our initial research questions:
- RQ1: How do significant capital flows into longevity science quantitatively alter mortality improvement trajectories beyond traditional actuarial assumptions?
Our analysis demonstrates that investment flows create quantifiable acceleration in mortality improvements through three mechanisms:
- Technology development acceleration: Each additional USD 1 billion in investment correlates with a 0.3–0.7% increase in the rate of mortality improvement, depending on the specific technology category and age cohort.
- Feedback amplification: The investment–mortality feedback loop creates a multiplier effect where each 1% improvement in mortality attracts 1.4–2.1% additional investment (adjusted for inflation), creating compounding effects not captured in traditional linear projections.
- Age pattern modification: Investment-driven improvements show statistically significant different age patterns than historical trends (p < 0.01 in our validation tests), with particularly strong effects in the 60–79 age range (coefficient values 0.17–0.23 versus 0.08–0.14 for other age groups).
These effects produce mortality improvements that exceed traditional projections by 18–31% by 2040 (95% confidence interval: 12–37%), with the differential increasing over time.
- RQ2: What specific modeling approaches can most effectively capture these investment-driven dynamics to provide mathematically sound mortality projections?
Our Investment-Adjusted Mortality Model (IAMM) demonstrates superior statistical performance compared to the standard Lee–Carter model and other traditional approaches:
- Predictive accuracy: The IAMM reduces mean absolute percentage error (MAPE) by 35% compared to Lee–Carter projections in our validation tests (p < 0.005).
- Parameter stability: The model’s key parameters (, δ, and technology effectiveness parameters) show robust stability across different calibration periods, with variance less than 12% across subsamples.
- Superior goodness-of-fit: The IAMM achieves an AIC (Akaike Information Criterion) reduction of 28.5 points compared to the best-performing traditional model, indicating substantially better fit even when accounting for the additional parameters.
- RQ3: How should insurers adapt product design and pricing strategies in response to investment-accelerated mortality improvements, and what is the quantifiable impact of these adaptations?
Our analysis identifies three specific adaptation strategies with quantifiable benefits:
- Dynamic mortality-linked products: Simulations indicate that dynamic mortality-linked annuities reduce insurer longevity risk by 58–67% (95% confidence interval) compared to traditional fixed annuities, while maintaining competitive value for policyholders.
- Biological age underwriting: Statistical analysis shows that incorporating epigenetic age markers into underwriting improves mortality prediction by 23–31% compared to chronological age alone, creating substantial risk selection advantages (p < 0.01).
- Investment-linked mortality products: These structures create natural hedges that reduce tail risk exposure by 42–51% (95% confidence interval) while offering policyholders participation in longevity investment returns.
The combined implementation of these strategies could reduce required economic capital for longevity risk by 35–45% while improving new business profitability by 12–18% under realistic assumptions.
Future Research Directions
This study opens several promising avenues for future research:
- Model refinement through biomarker integration: Future work should explore integrating specific biomarker trajectories (e.g., DNA methylation patterns, inflammatory markers) directly into mortality modeling.
- Cross-national validation: The current model has been primarily validated with data from developed economies. Extending validation to emerging markets would test the model’s robustness and generalizability.
- Regulatory optimization modeling: Future research should develop optimization frameworks that balance innovation incentives with consumer protection in regulating novel mortality-linked products.
- Multi-risk integration: Exploring the interaction between longevity risk and other insurance risks in the context of investment-accelerated mortality improvements represents an important extension.
The framework presented in this paper provides both a theoretically sound and empirically validated approach to incorporating investment dynamics into mortality projections and insurance product design. By explicitly modeling the relationship between investment and mortality improvement, we offer actuaries and risk managers practical tools to navigate the rapidly evolving longevity landscape.
- AI Assistance Disclosure
Portions of this manuscript were reviewed for clarity and grammar using ChatGPT-4 (OpenAI). Specifically, ChatGPT was used for:
- Initial drafts of literature review organization (Section Quantifying the Actuarial Disconnect);
- Grammar and style editing throughout the manuscript;
- Generation of alternative phrasings for technical concepts in the Abstract and Conclusion;
All substantive content, methodology, analysis, and conclusions are entirely the work of the author. No AI assistance was used for data analysis, model development, statistical testing, or interpretation of results.
Funding
This research received no external funding.
Data Availability Statement
The investment data analyzed in this study are derived from publicly accessible sources including venture capital databases (PitchBook, CB Insights), company press releases and SEC filings, academic publications, and industry reports as cited throughout the manuscript. Mortality data are sourced from the Human Mortality Database (www.mortality.org) and national statistical offices as referenced. The comprehensive investment database compiled in “Investors in Longevity” (Dror 2024) aggregates these publicly available sources. No proprietary, experimental, or private datasets were used in this research. All data sources are documented in the reference list and can be independently accessed and verified.
Conflicts of Interest
The author declares no conflicts of interest.
Note
| 1 | Longevity interventions span three complementary approaches: (1) slowing aging through pharmacological interventions such as rapamycin (mTOR inhibition), metformin (metabolic regulation), and caloric restriction mimetics; (2) preventing age-related damage via telomere maintenance therapies, targeted antioxidant treatments, and lifestyle interventions; and (3) potentially reversing existing aging damage through cellular reprogramming techniques, senolytic drugs that clear senescent cells, and regenerative medicine approaches. Current investment targets all three categories, with prevention and slowing strategies closer to clinical application while reversal approaches remain largely experimental. |
References
- 5m. Calico. n.d. About Us. Available online: https://www.calicolabs.com (accessed on 28 June 2024).
- Altos Labs. n.d. Company Overview. Available online: https://www.altoslabs.com (accessed on 22 June 2025).
- Andreessen Horowitz. n.d. Aging Research Investments. Available online: https://a16z.com/portfolio (accessed on 28 June 2024).
- Angel Capital Association. 2024. An Introduction to Angel Investing. Available online: https://www.angelcapitalassociation.org/data/Documents/Resources/McKaskill-_Intro_to_Angel_Investing.pdf (accessed on 5 May 2025).
- Antolin, Pablo, and Hans J. Blommestein. 2007. Governments and the Market for Longevity-Indexed Bonds. OECD Working Paper on Insurance and Private Pensions No. 4. Available online: https://ssrn.com/abstract=962032 (accessed on 5 May 2025).
- Antón-Fernández, Alejandro, Marta Roldán-Lázaro, Laura Vallés-Saiz, Jesús Ávila, and Félix Hernández. 2024. In vivo cyclic overexpression of Yamanaka factors restricted to neurons reverses age-associated phenotypes and enhances memory performance. Communications Biology 7: 631. [Google Scholar] [CrossRef]
- Atance, David, Josep Lledó, and Eliseo Navarro. 2025. Modelling and Forecasting Mortality Rates for a Life Insurance Portfolio. Risk Management 27: 3. [Google Scholar] [CrossRef]
- Barzilai, Nir, Ana Maria Cuervo, and Steve Austad. 2018. Aging as a Biological Target for Prevention and Therapy. JAMA 320: 1321–22. [Google Scholar] [CrossRef] [PubMed]
- Bell, K., J. Somers, and C. M. Wong. 2023. AI-assisted screening for geroprotective compounds extends lifespan in C. elegans. Nature Communications 14: 1823–34. [Google Scholar]
- Belsky, Daniel W., Avshalom Caspi, Renate Houts, Harvey J. Cohen, David L. Corcoran, Andrea Danese, HonaLee Harrington, Salomon Israel, Morgan E. Levine, Jonathan D. Schaefer, and et al. 2015. Quantification of biological aging in young adults. Proceedings of the National Academy of Sciences USA 112: E4104–E4110. [Google Scholar] [CrossRef]
- BioPharma Dive. 2024. Biotech IPOs Are the Industry’s Lifeblood. Available online: https://www.biopharmadive.com/news/biotech-ipo-performance-tracker/587604/ (accessed on 5 May 2025).
- Bloom, David E., David Canning, and Günther Fink. 2010. Implications of population ageing for economic growth. Oxford Review of Economic Policy 26: 583–612. [Google Scholar] [CrossRef]
- Booth, Bruce, and Rodney Zemmel. 2004. Prospects for productivity. Nature Reviews Drug Discovery 3: 451–56. [Google Scholar] [CrossRef]
- Bravo, Jorge M., Mercedes Ayuso, Robert Holzmann, and Edward Palmer. 2023. Intergenerational actuarial fairness when longevity increases: Amending the retirement age. Insurance: Mathematics and Economics Holzmann R 111: 176–96. [Google Scholar] [CrossRef]
- Cairns, Andrew J. G., David Blake, and Kevin Dowd. 2006. A two-factor model for stochastic mortality with parameter uncertainty: Theory and calibration. Journal of Risk and Insurance 73: 687–718. [Google Scholar] [CrossRef]
- Case, Anne, and Angus Deaton. 2020. Deaths of Despair and the Future of Capitalism. Princeton: Princeton University Press. [Google Scholar]
- CB Insights. 2024. State of Healthcare Report. CB Insights Research. Available online: https://www.cbinsights.com (accessed on 5 May 2025).
- Chen, Rui, and Michael Snyder. 2013. Promise of personalized omics to precision medicine. Wiley Interdisciplinary Reviews: Systems Biology and Medicine 5: 73–82. [Google Scholar] [CrossRef]
- DiMasi, Joseph A., Henry G. Grabowski, and Ronald W. Hansen. 2016. Innovation in the pharmaceutical industry: New estimates of R&D costs. Journal of Health Economics 47: 20–33. [Google Scholar]
- Dror, David Mark. 2024. Investors in Longevity: Big Capital and the Future of Extending Life. Geneva: Literary Letters. ISBN 978-2-9701832-3-5. [Google Scholar]
- Dushi, Irena, Howard Iams, and Brad Trenkamp. 2017. The Importance of Social Security Benefits to the Income of the Aged Population. Social Security Bulletin 77: 1–12. [Google Scholar]
- European Commission. 2009. Solvency II Directive (2009/138/EC). Brussels: European Commission. [Google Scholar]
- European Insurance and Occupational Pensions Authority. 2021. Opinion on the Supervision of the Use of Climate Change Risk Scenarios in ORSA. Frankfurt: EIOPA. [Google Scholar]
- EY. 2024. Leading Biotech IPOs Value U.S. 2023. Statista. Available online: https://www.statista.com/statistics/859005/value-of-us-and-eu-biotech-ipos/ (accessed on 5 May 2025).
- Financial Conduct Authority. 2021. Guidance for Firms on the Fair Treatment of Vulnerable Customers. London: FCA. [Google Scholar]
- Financial Services Authority. 2006. Treating Customers Fairly–Towards Fair Outcomes for Consumers. London: Financial Services Authority. Available online: https://www.fca.org.uk/publication/archive/fsa-tcf-towards.pdf (accessed on 5 May 2025).
- Garay, Ricardo P. 2023. Recent clinical trials with stem cells to slow or reverse normal aging processes. Frontiers in Aging 4: 1148926. [Google Scholar] [CrossRef] [PubMed]
- Gompers, Paul, and Josh Lerner. 2001. The venture capital revolution. Journal of Economic Perspectives 15: 145–68. [Google Scholar] [CrossRef]
- Grand View Research. 2021. Anti-Aging Market Size, Share & Trends Analysis Report. Grand View Research. Available online: https://www.grandviewresearch.com (accessed on 5 May 2025).
- Grand View Research. 2024. Digital Biomarkers Market Size, Share & Growth Report 2030. Available online: https://www.grandviewresearch.com/industry-analysis/digital-biomarkers-market-report (accessed on 5 May 2025).
- HanAll Biopharma. 2024. HanAll Biopharma Signs Licensing Agreement with Turn Biotechnologies. Press Release. May 28. Available online: https://www.hanallbiopharma.com (accessed on 5 May 2025).
- Horvath, Steve, and Kenneth Raj. 2018. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics 19: 371–84. [Google Scholar] [CrossRef] [PubMed]
- Human Mortality Database. 2024. University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany). Available online: https://www.mortality.org (accessed on 5 May 2025).
- Juvenescence. n.d. Anti-Aging Treatments and Personalized Medicine. Available online: https://www.juvlabs.com (accessed on 28 June 2024).
- Khosla Ventures. n.d. Aging Research Investments. Available online: www.khoslaventures.com (accessed on 28 June 2024).
- Larka. 2024. Biotech Venture Capital Report 2024. Available online: https://www.larka.com/en/news/Biotech-Venture-Capital-Report-2024 (accessed on 5 May 2025).
- Lazzaro, Sage. 2024. AI for Drug Discovery Draws a $1 Billion Launch—and a Lot of Hope. Fortune. April 25. Available online: https://fortune.com/2024/04/25/ai-for-drug-discovery-xaira-therapeutics/ (accessed on 5 May 2025).
- Lee, Ronald D., and Lawrence R. Carter. 1992. Modeling and forecasting U.S. mortality. Journal of the American Statistical Association 87: 659–71. [Google Scholar] [CrossRef]
- LongevityTech. 2022. Global Longevity Investment Hits $5.2 Billion in 2022. LongevityTech. Available online: https://longevity.technology/investment/report/annual-longevity-investment-report/ (accessed on 5 May 2025).
- Macip, Carolina Cano, Rokib Hasan, Victoria Hoznek, Jihyun Kim, Yuancheng Ryan Lu, Louis E. Metzger, Saumil Sethna, and Noah Davidsohn. 2023. Gene therapy-mediated partial reprogramming extends lifespan and reverses age-related changes in aged mice. Cellular Reprogramming 39: 201–15. [Google Scholar] [CrossRef]
- Max Planck Institute for Biology of Ageing. n.d. About Us. Available online: https://www.age.mpg.de (accessed on 28 June 2024).
- Milevsky, Moshe Arye. 2020. Longevity Insurance for a Biological Age: Why Your Retirement Plan Shouldn’t Be Based Only on Chronological Age. Singapore: World Scientific. [Google Scholar]
- MIT Technology Review. 2022. Jeff Bezos is Funding a Company that Wants to Reverse Aging. MIT Technology Review. Available online: https://interestingengineering.com/science/jeff-bezos-is-funding-a-lab-to-extend-his-life (accessed on 5 May 2025).
- National Research Foundation. n.d. National Innovation Challenge on Active and Confident Aging. Available online: https://www.nrf.gov.sg (accessed on 28 June 2024).
- Nature Biotechnology. 2024. Venture capital trends in biotechnology. Nature Biotechnology 42: 245–52. [Google Scholar]
- NPR. 2024. How Do Biological Age Tests Work and Are They Accurate Or Helpful? February 5. Available online: https://www.npr.org/sections/health-shots/2024/02/05/1228753141/biological-age-test-dna (accessed on 5 May 2025).
- OECD. 2014. Mortality Assumptions and Longevity Risk: Policy Implications. Paris: OECD Publishing. [Google Scholar]
- OECD. 2021. Health at a Glance 2021: OECD Indicators. Paris: OECD Publishing. Available online: https://www.oecd.org/en/publications/health-at-a-glance-2021_ae3016b9-en.html (accessed on 5 May 2025).
- Office for National Statistics. 2018. Changing Trends in Mortality: An International Comparison: 2000 to 2016. Statistical Bulletin; Newport: ONS. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/articles/changingtrendsinmortalityaninternationalcomparison/2000to2016 (accessed on 5 May 2025).
- Office for National Statistics. 2022. National Life Tables–Life Expectancy in the UK: 2018 to 2020; Statistical Bulletin. Newport: ONS. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies (accessed on 5 May 2025).
- Office for National Statistics. 2024. National Life Tables–Life Expectancy in the UK: 2021 to 2023; Statistical Bulletin. Newport: ONS. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies (accessed on 5 May 2025).
- Office of the Superintendent of Financial Institutions. 2022. Guideline on Technology and Risk Management. Ottawa: OSFI. [Google Scholar]
- Olivieri, Annamaria, and Ermanno Pitacco. 2020. Introduction to Insurance Mathematics: Technical and Financial Features of Risk Transfers. Cham: Switzerland Springer. [Google Scholar]
- Paine, Patrick T., Ada Nguyen, and Alejandro Ocampo. 2024. Partial cellular reprogramming: A deep dive into an emerging rejuvenation technology. Aging Cell 23: e14039. [Google Scholar] [CrossRef]
- Pharmaphorum. 2024. Longevity: Anti-Ageing Drugs, Watch Out, Here We Come. Available online: https://pharmaphorum.com/rd/longevity-anti-ageing-drugs-watch-out-here-we-come (accessed on 5 May 2025).
- PitchBook. 2024. Healthcare IT Report. PitchBook Data. Available online: https://pitchbook.com (accessed on 5 May 2025).
- Rogers, Everett M. 2003. Diffusion of Innovations, 5th ed. New York: Free Press. [Google Scholar]
- Society of Actuaries. 2021. Mortality Improvement Scale MP-2021 Report. Schaumburg: Society of Actuaries. [Google Scholar]
- Society of Actuaries. 2022. The RP-2014 Mortality Tables. Schaumburg: Society of Actuaries. [Google Scholar]
- Spencer, Merianne R., Matthew F. Garnett, and Arialdi M. Miniño. 2024. Drug Overdose Deaths in the United States, 2002–2022. NCHS Data Brief, No. 491. Hyattsville: National Center for Health Statistics. [Google Scholar] [CrossRef]
- Takahashi, Kazutoshi, and Shinya Yamanaka. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–76. [Google Scholar] [CrossRef] [PubMed]
- Unity Biotechnology. n.d. Investors. Unity Biotechnology. Available online: https://unitybiotechnology.com (accessed on 5 May 2025).
- Vaupel, James W., Francisco Villavicencio, and Marie-Pier Bergeron-Boucher. 2021. Demographic perspectives on the rise of longevity. Proceedings of the National Academy of Sciences USA 118: e2019536118. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 2016. Life Expectancy Increased by 5 Years Since 2000, but Health Inequalities Persist. WHO News Release. Geneva: WHO. Available online: https://www.who.int/news/item/19-05-2016-life-expectancy-increased-by-5-years-since-2000-but-health-inequalities-persist (accessed on 5 May 2025).
- Yang, Jae-Hyun, Christopher A. Petty, Thomas Dixon-McDougall, Maria Vina Lopez, Alexander Tyshkovskiy, Sun Maybury-Lewis, Xiao Tian, Nabilah Ibrahim, Zhili Chen, Patrick T. Griffin, and et al. 2023. Chemically induced reprogramming to reverse cellular aging. Aging 15: 5966–89. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).