A New Mortality Framework to Identify Trends and Structural Changes in Mortality Improvement and Its Application in Forecasting
Abstract
:1. Introduction
- Familiarity and ease of implementation that comes with parametric models;
- Excellent fit; the exemplar of the framework, the DLGC, provides MSEs and AICs that are smaller than the Heligman–Pollard model Heligman and Pollard (1980);
- Good accuracy and stability in forecasting; projecting with the DLGC has comparable or better test MSEs in forecasting than the logit-binomial version of the Lee–Carter model, especially for 10-year-ahead forecasts. The forecasting accuracy is also more stable for DLGC than the logit-binomial Lee–Carter model, especially for those countries we examined with only intermediate overall life expectancy: Russia, Ukraine, and the USA.
- The structural changes in mortality revealed by DLGC provide insights that are useful for analyzing historical mortality and for improving forecasting. For example, parameter time plots not only give clues about historical structural changing times, but also help to select an appropriate historical data period to train the forecasting model, which is one of the main difficulties in mortality forecasting. Observed structural changes can also be used to suggest proper assumptions about parameter time series.
- Clear interpretation of each parameter: using the DLGC, trends are observed in almost all parameters; the trends provide evidence of mortality improvement or compression and can be used to quantify the proportion of improvement due to each parameter (see Section 3.5);
- Visibility of structural changes arising from the data, both transient (e.g., evident changes in mortality among French males during the World Wars) and persistent (e.g., observed sudden structural change in teenage female mortality around the 1950s); this allows objective, data-driven observation of structural changes without the need for the subjectivity that often backs expert opinion;
- Flexibility and interpretability of the framework: The framework is built starting with a heuristic modification of the logistic equation as a model for mortality with two functions and that are, respectively, interpreted as age-dependent mortality-decelerating factors (medical advances, broadly adopted healthy behaviors, etc.) and mortality-accelerating factors (aging, increasing pollution, etc.). The functions can be chosen to fit the context, and the DLGC shown below is the result of just one such choice. It shows stable and clear trends for parameters that match demographic characteristics, and it illustrates the model’s good interpretability.
2. Model Construction
2.1. Model Foundations
- : the maximum value of the contribution to one-year mortality from factors arising during adulthood and old ages. At extreme old age x, we have , which is the overall asymptotic mortality.
- : the age at which the function is half of its maximum. The main process causing to increase is happening fastest near .
- An increasing function of age with range : this represents the effect of natural factors cumulatively accelerating the mortality probability during adulthood and old ages. Such factors include biological aging processes and lifestyle patterns with a harmful effect. This adds nuance not found in the teenage term , which adopts the same model, but with a constant growth factor k. We view this as sensible because the increasing process during teenage years happens in a much shorter time period than that during old ages. The aging effect on mortality is obviously stronger at older ages. Therefore, we assumeed is increasing over age during this period. Without any beneficial factors decelerating mortality, would satisfy the special case of (5) with .
- A function of age with range : this represents the effects of beneficial factors decelerating mortality probabilities. We require that for any age x, in order to keep positive always. Furthermore, we require , since if , the function no longer represents a decelerative effect in the model (5). During ages with , beneficial factors and human actions controlling mortality are contributing to slow the mortality growing. During the ages with , these beneficial factors and actions have no extra beneficial effect on mortality compared to the asymptotic effect counted in g and the processes contributing to .
2.2. The General Framework for Modeling Mortality
2.3. DLGC: Death Probability Model with Chosen and
2.4. MSE Minimization
2.5. Modal Age M Fitting
2.6. Mortality Forecasting with DLGC
- , , , , have values whose logs form random walks with drift, e.g., satisfies , where is the drift term and are normally distributed i.i.d. error terms;
- , , , , , , , have values that form random walks with drift, e.g., satisfies , where is the drift and are normally distributed i.i.d. error terms.
3. Main Results of DLGC
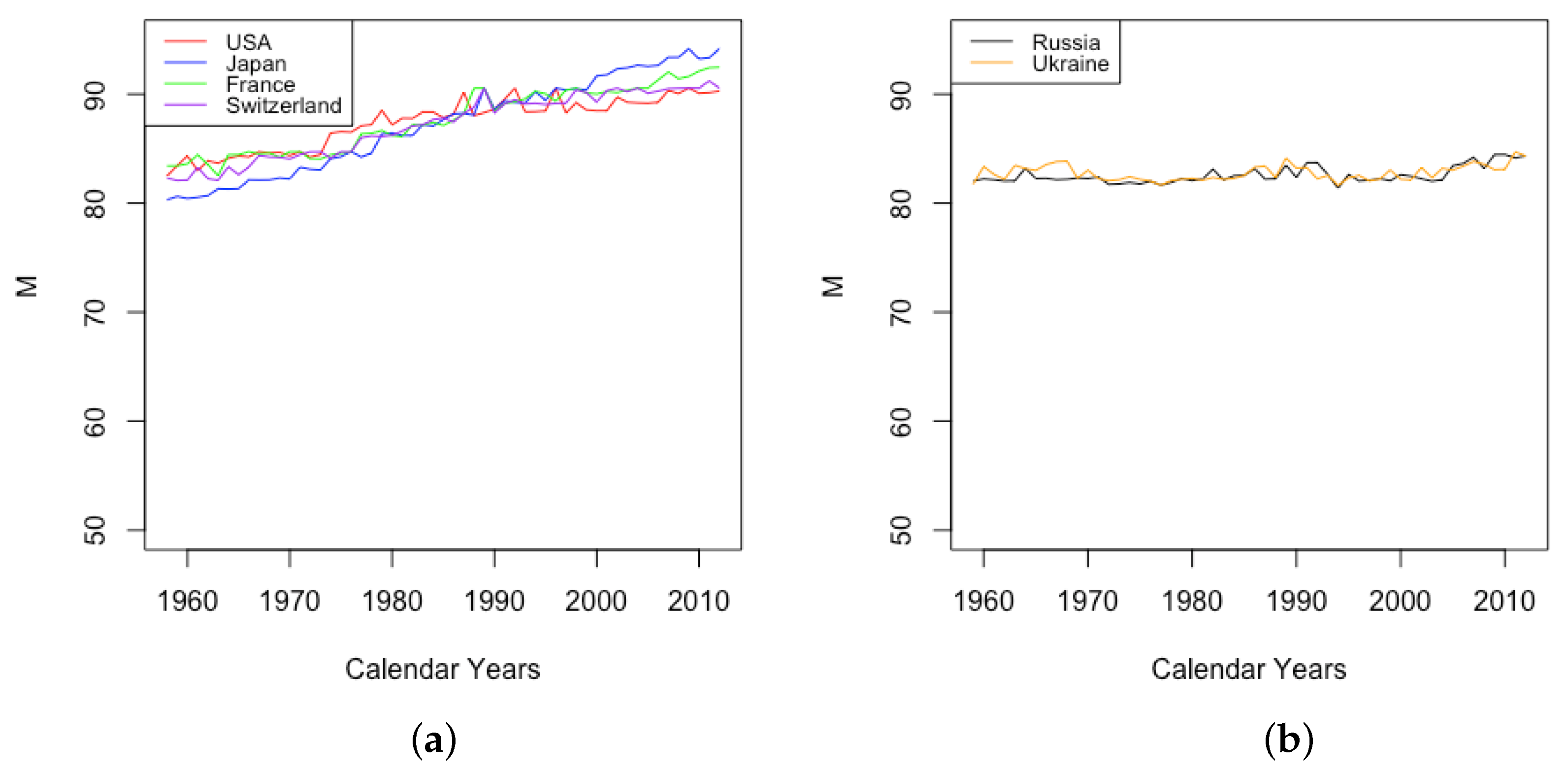
- HLE countries
- France, Japan, Switzerland, and the USA have relatively high life expectancy at birth;
- ILE countries
- Russia and Ukraine have intermediate life expectancy at birth.
3.1. Goodness of Fit for DLGC
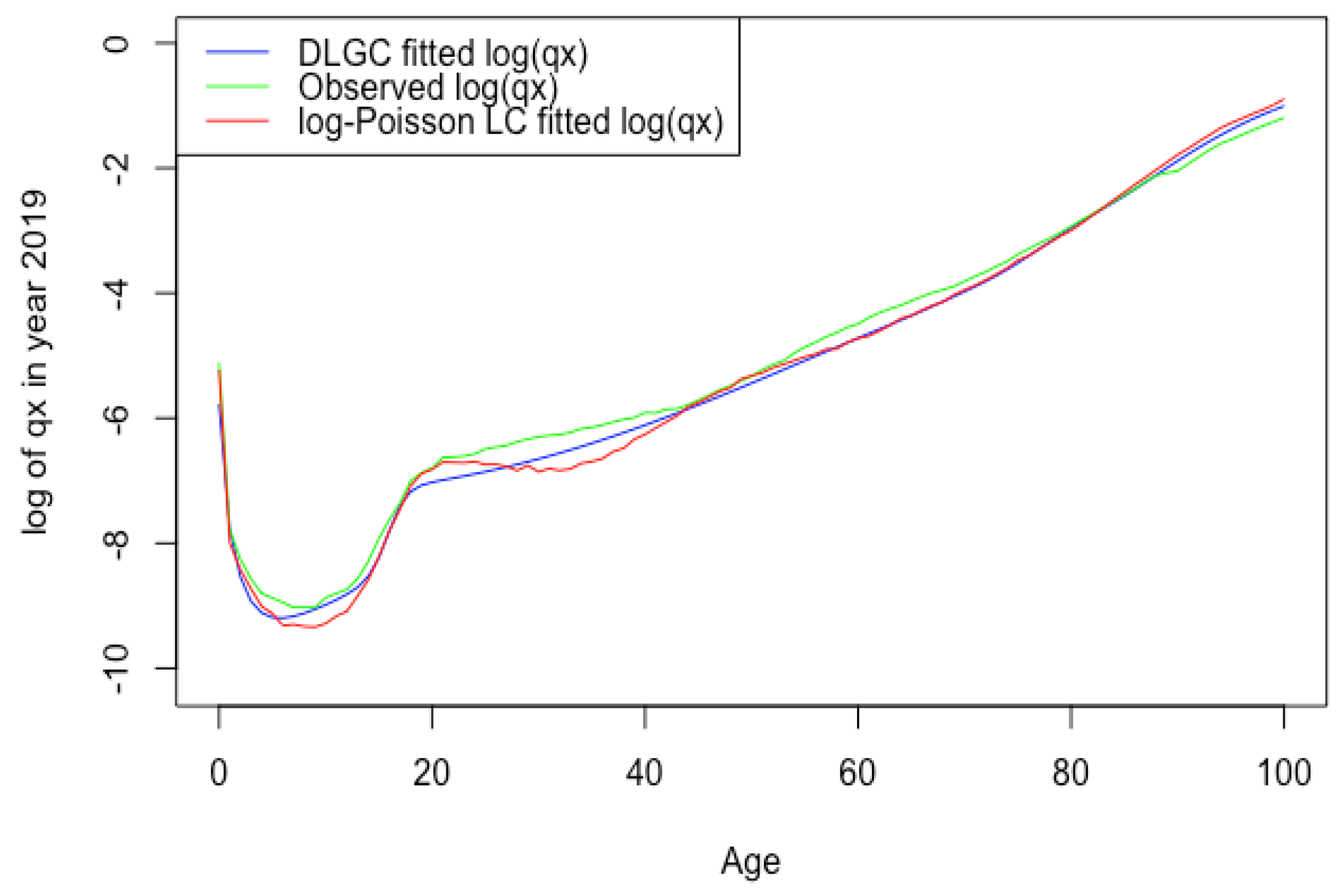
3.1.1. Fits across Age
- Overall, the DLGC gives an excellent fit. For all datasets, we averaged the values across all fitted years for a given country and gender. All means were above 99%. The lowest was 99.55%, occurring for Russian males, and the highest mean was 99.94% for Japanese females.We also fit the Heligman–Pollard model Heligman and Pollard (1980) to the six countries’ data for all available years by maximizing the , to compare the goodness of fit with the DLGC model. (The year period for each country is the same as Table 7.) Table 8 summarizes the fit.The means of for the fitted Heligman–Pollard model were between 93.18% and 96.68% for the six countries, while for DLGC, they were all above 99%. Overall, the DLGC shows much better accuracy than the Heligman–Pollard model does across countries and gender. Several particularly poor fits of the Heligman–Pollard have 71.74% for French male total population in 1915, 76.83% for Russian males in 1994, and 76.43% for Ukrainian males in 2005, while DLGC’s results are all above 91.90%. DLGC fits better than the Heligman–Pollard model on data from both recent years and from those over a century ago.Since our model has more parameters than some others, we also compared it against other models based on the AIC, defined aswhere k is the number of parameters in the model under consideration and n is the number of observations.The DLGC also performs better than the Heligman–Pollard model with respect to the AIC. This is observed universally among the six countries, for both female and male populations, both recent years and older years. For example, as one HLE country, the fitting results of Switzerland female have an AIC −1240.87 (DLGC) compared with −741.88 (Heligman–Pollard) in 2018, −1134.83 (DLGC) compared with −702.34 (Heligman–Pollard) in 1886; in 2013, as one ILE country, the fitting results of Russia have an AIC −1228.57 (DLGC) compared with −962.35 (Heligman–Pollard) for female and −1139.05 (DLGC) compared with −984.77 (Heligman Pollard) for male. We recorded the AIC improvement, i.e., , in each year’s data fitting for both genders in all six countries. Table 1 summarizes the ’s mean among years with available data shown in Table 7. One can notice that, by including 14 parameters, 6 more parameters than the Heligman–Pollard model with 8 parameters, the DLGC obtains great improvement in its fitting accuracy and is not overfitting.
- The DLGC sometimes gives a noticeably worse fit during a year that a country was at war. This is especially evident for French males during the World Wars. The eight years for which the for French total male population was less than 98.5% were 1914–1918 (exactly the span of World War I) and 1940, 1943, and 1944 (during World War II). By contrast, Switzerland maintained neutrality during the World Wars, and the fit to Swiss male data is not noticeably worse during the war years. Overall, the French civilian male population is fit better than the French total male population in the mean, SD, and range. Excluding the eight war years mentioned above, the fitting results for French total male population in all the remaining years have a mean of 99.75% and SD 0.00185, with a range of 98.94% to 99.94%. These are in line with the French civilian male data, especially if we remove 1919, the single year for which the model produced under 98% (at 96.17%) when fitting French civilian males. Excluding 1919, the French civilian males in all other available years have a mean of 99.75% and SD of 0.00198, with a range of 98.84% to 99.95%, almost identical to the values for French total males with the war years excluded. It is possible that the poorer fit for French civilian males in 1919 compared with other years is a continuation of the anomalous fit seen for total male population during war years: there was likely a number of soldiers who transitioned to civilian life immediately after the war and then died some months later in 1919, from wounds or other factors incurred during service.
- Excluding war years, the DLGC is observed to fit data from HLE countries better than ILE countries. Among the six countries, DLGC gives the best fit for for Japan and the USA, where the is at least 99.57% for all female, male, and total datasets over all available years of data. For Switzerland, over 144 years, the minimum is 99.26%. As mentioned above, for French data over the last 200 years, excluding the war years mentioned above, the minimum is 98.94%. Compared with these high-life-expectancy-at-birth countries, Russia and Ukraine show lower means, higher SDs, and lower minimum (still higher than 97.6%).
- For the four HLE countries, France, Japan, Switzerland, and the USA, the DLGC tends to fit better for female datasets than male datasets. For these countries, the mean for females is higher than that for males during the same time period, and the minimal appears with a male dataset, such as 91.90% for French total male population in 1915 and 96.42% for French civilian male population in 1919. The minimum for female data is at least 98% for all HLE countries and all years. For the two ILE countries, Russia and Ukraine, there is no clear distinction between for females and males.
- The fit is better in more recent years for all countries with data extending into the Nineteenth Century. For French civilian males over the period 1816–1917, the mean is 99.64%, and for females, the mean is 99.77%. For the period 1918–2018, the mean for French civilian males is 99.82%, and for females, the mean is 99.92%.
3.1.2. Forecasting over Years
- In summary, DLGC has better forecasting accuracy than the logit-binomial Lee–Carter model. For the 186 years of death rate data, DLGC performs better on 81.18% of them (151 out of 186). For all test samples, DLGC’s MSE[] is below , while 9.14% (17 out of 186) of the logit-binomial Lee–Carter’s MSE[] are bigger than (and its largest MSE[] is ).
- Table 3 and Table 4 show that DLGC’s superiority to the logit-binomial Lee–Carter model is more pronounced for the longer forecasting period. In 5-year-ahead forecasts, DLGC performs better on 67.24% of test samples (39 out of 58), but in 10-year-ahead forecasts, the proportion rises to 87.50% (112 out of 128). Results for Switzerland and Japan repeat that pattern. For Switzerland, DLGC performs worse in the 5-year-ahead forecasts (2 yellow vs. 8 orange), but forecasts better on almost all 10-year-ahead tests (19 yellow vs. 1 orange). For Japan, DLGC and the logit-binomial Lee–Carter tie (5 yellow vs. 5 orange) for the 5-year-ahead forecasts, but DLGC is much better in the 10-year-ahead tests (19 yellow vs. 1 orange).
- For HLE countries, both models provide very accurate forecasts, but for ILE countries and the USA (which has the lowest life expectancy among the four HLE countries), DLGC’s forecasts are more stable, i.e., the total growth of over time is much smaller for DLGC than for the logit-binomial Lee–Carter, except for Ukraine females, where the comparison is distorted because the latter model has a much worse forecast in the first year.
- DLGC’s superiority to the logit-binomial Lee–Carter model is more pronounced for ILE countries. In both 5-year-ahead and 10-year-ahead forecasts, DLGC forecasts are almost always better (and stabler) for the two ILE countries and the USA. Comparing HLE and ILE countries, there are 21 yellow blocks out of 40 (52.5%) in 5-year-ahead forecasts and 75 yellow blocks out of 90 (83.33%) in 10-year-ahead forecasts for HLE countries, while for all test samples in ILE countries, there are 18 yellow blocks out of 18 (100%) in 5-year-ahead forecasts and 37 yellow blocks out of 38 (97.27%) in 10-year-ahead forecasts.
- DLGC forecasts better than the logit-binomial Lee–Carter model for both males and females, but the superiority appears similar for each gender. In 5-year-ahead forecasts with 29 test samples in each gender, 21 are yellow for female and 17 are yellow for male; in 10-year-ahead forecasts with 64 test samples in each gender, 57 are yellow for female and 55 are yellow for male.
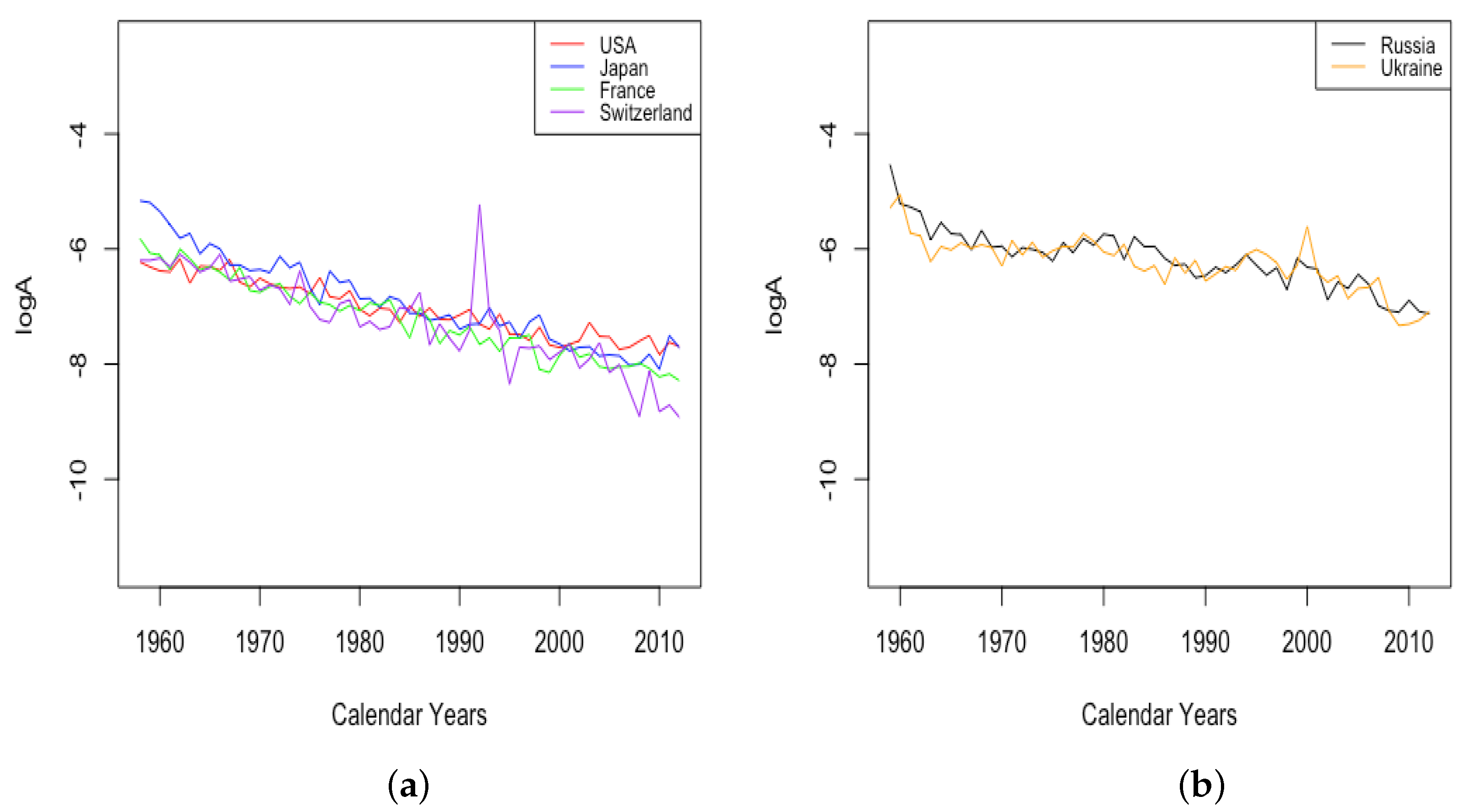
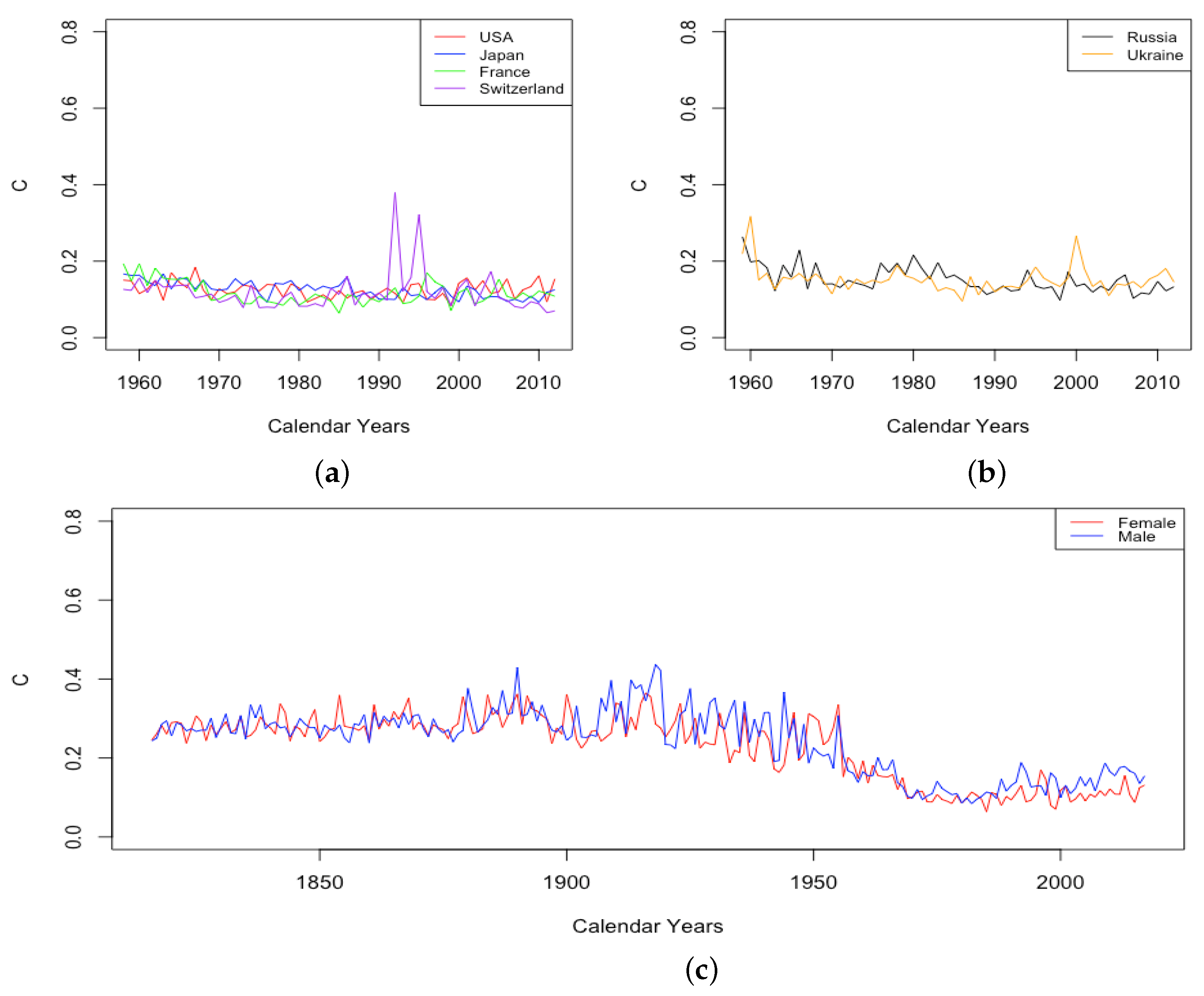
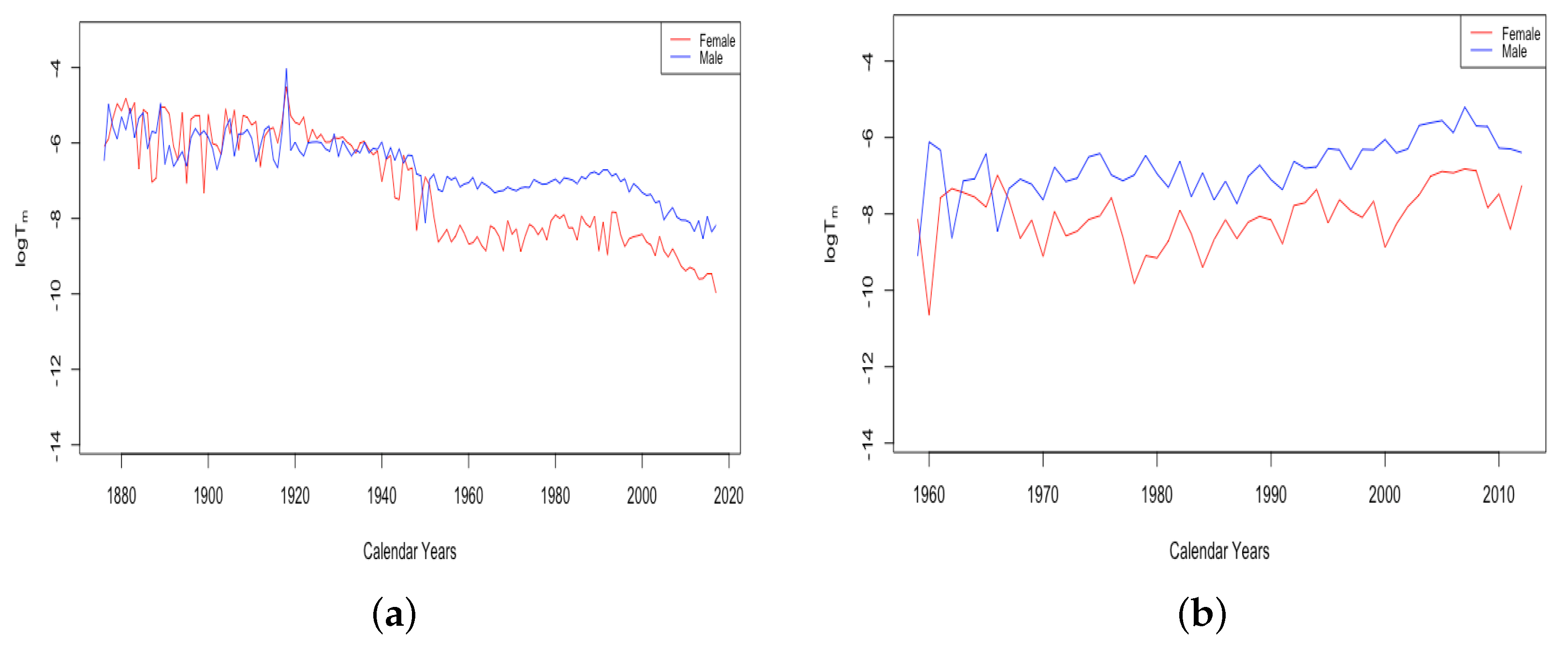
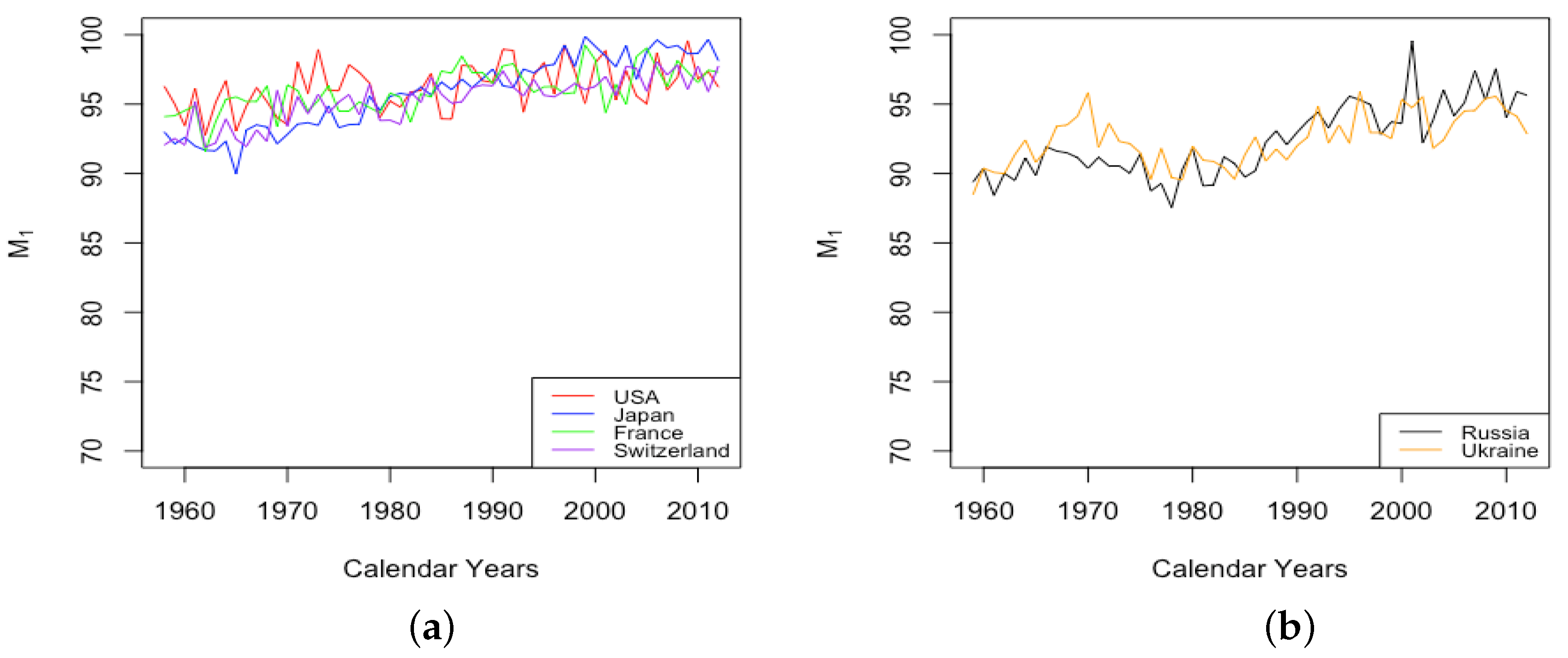
3.2. Variation of Parameters through Time
3.2.1. Mortality Improvement in the Earliest Ages
3.2.2. Mortality Improvement in Teenagers
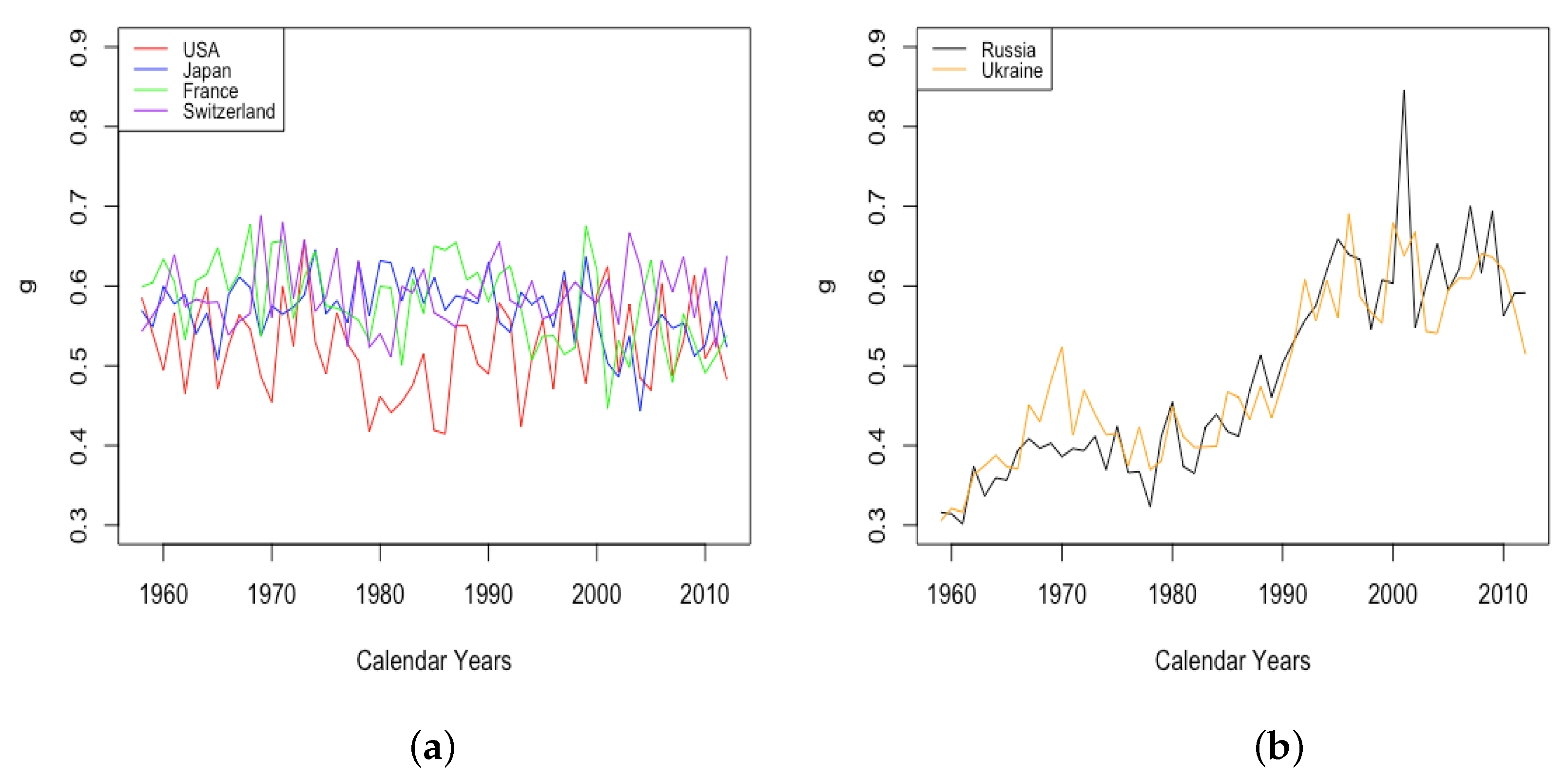
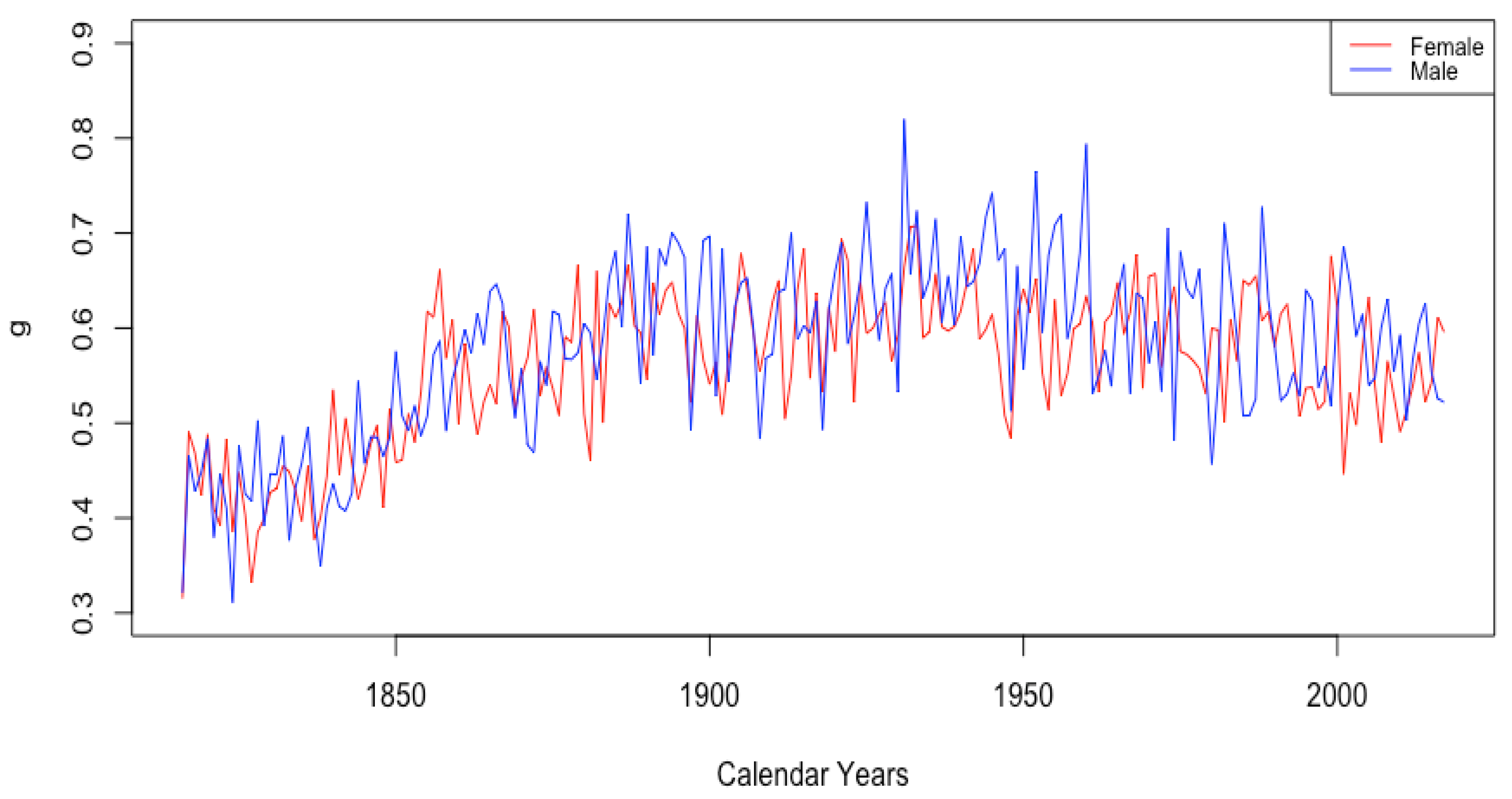
3.2.3. Mortality Improvement in Adulthood and Old Age
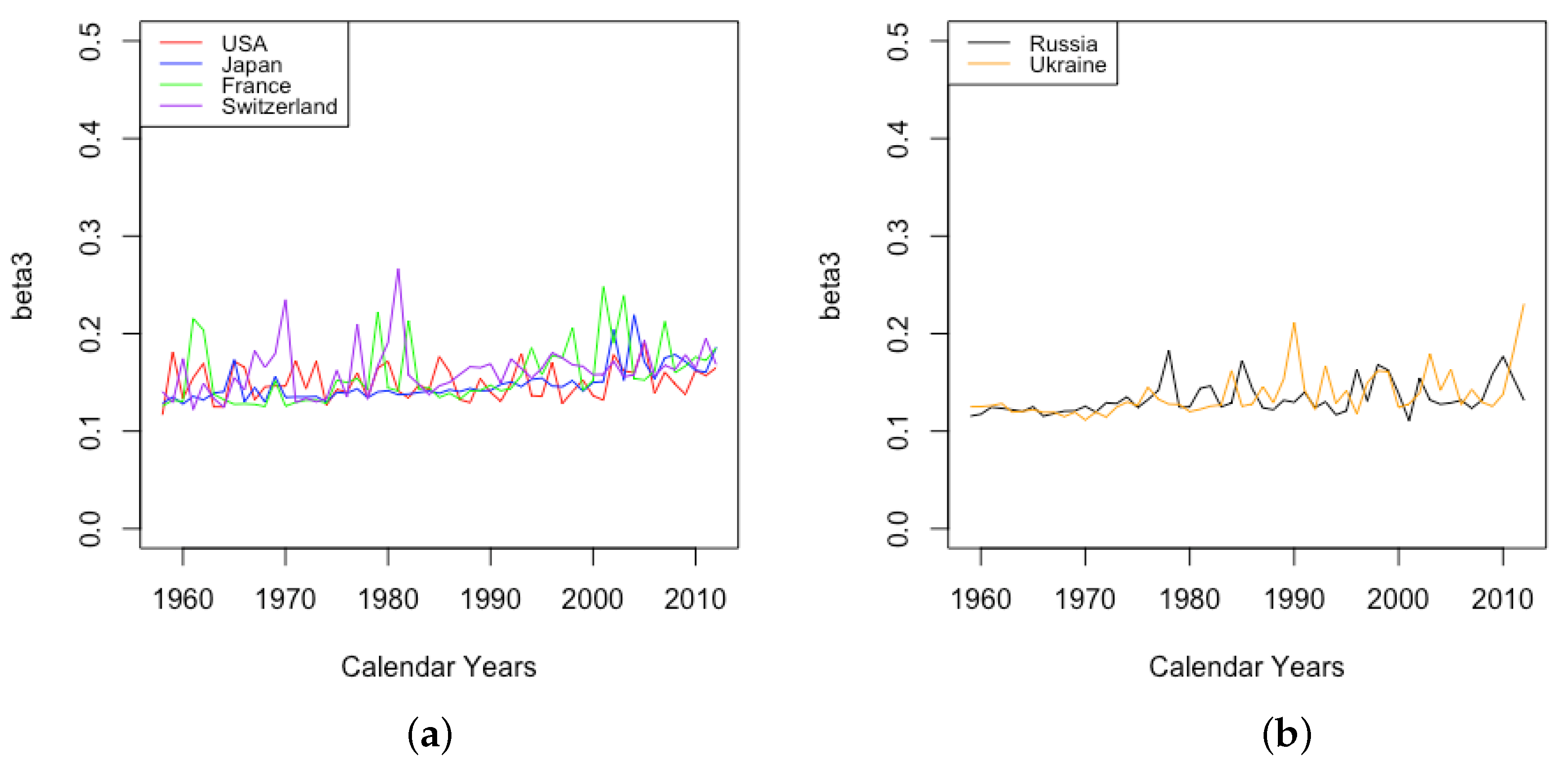
3.3. Events and Inventions—Effects on Parameters
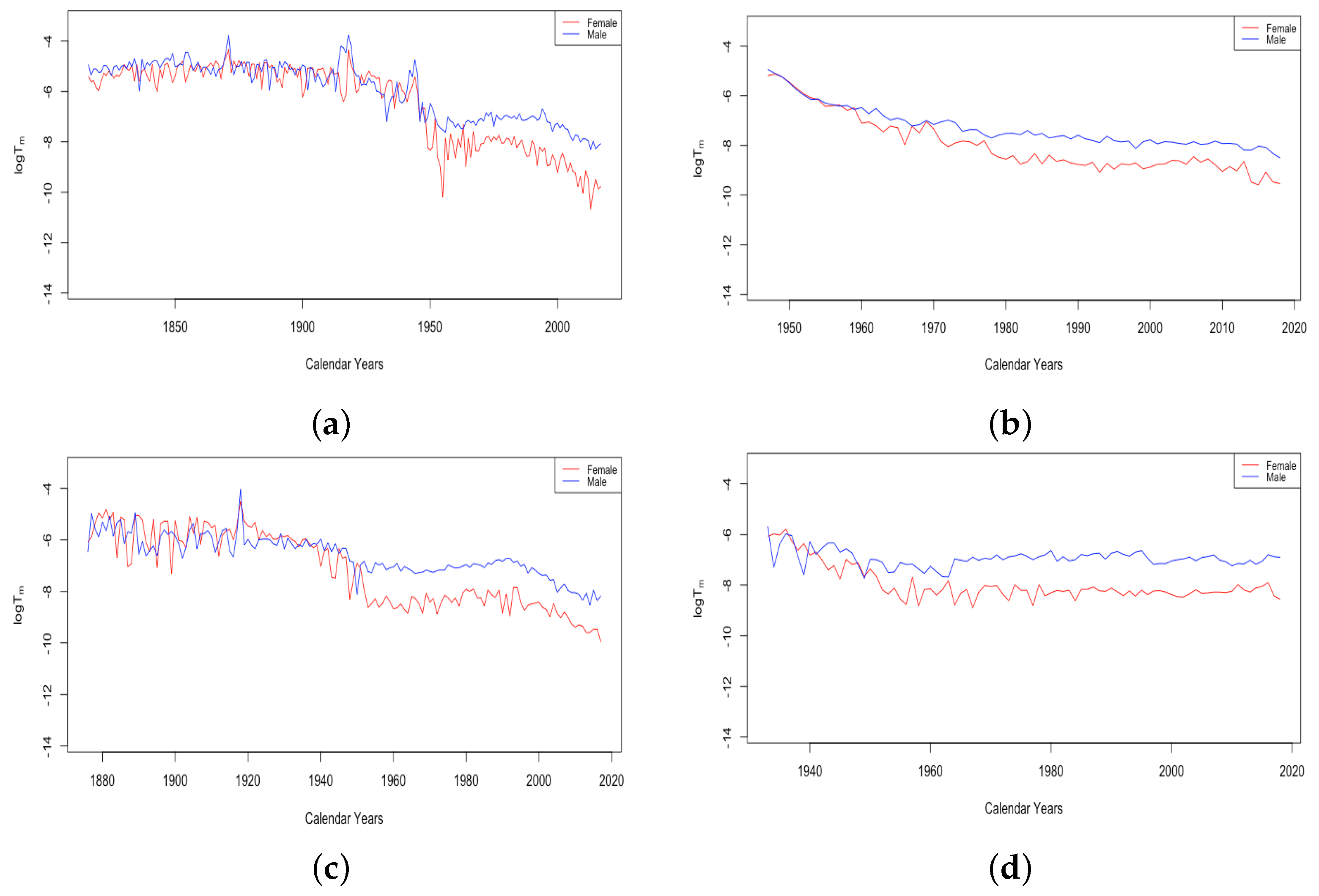
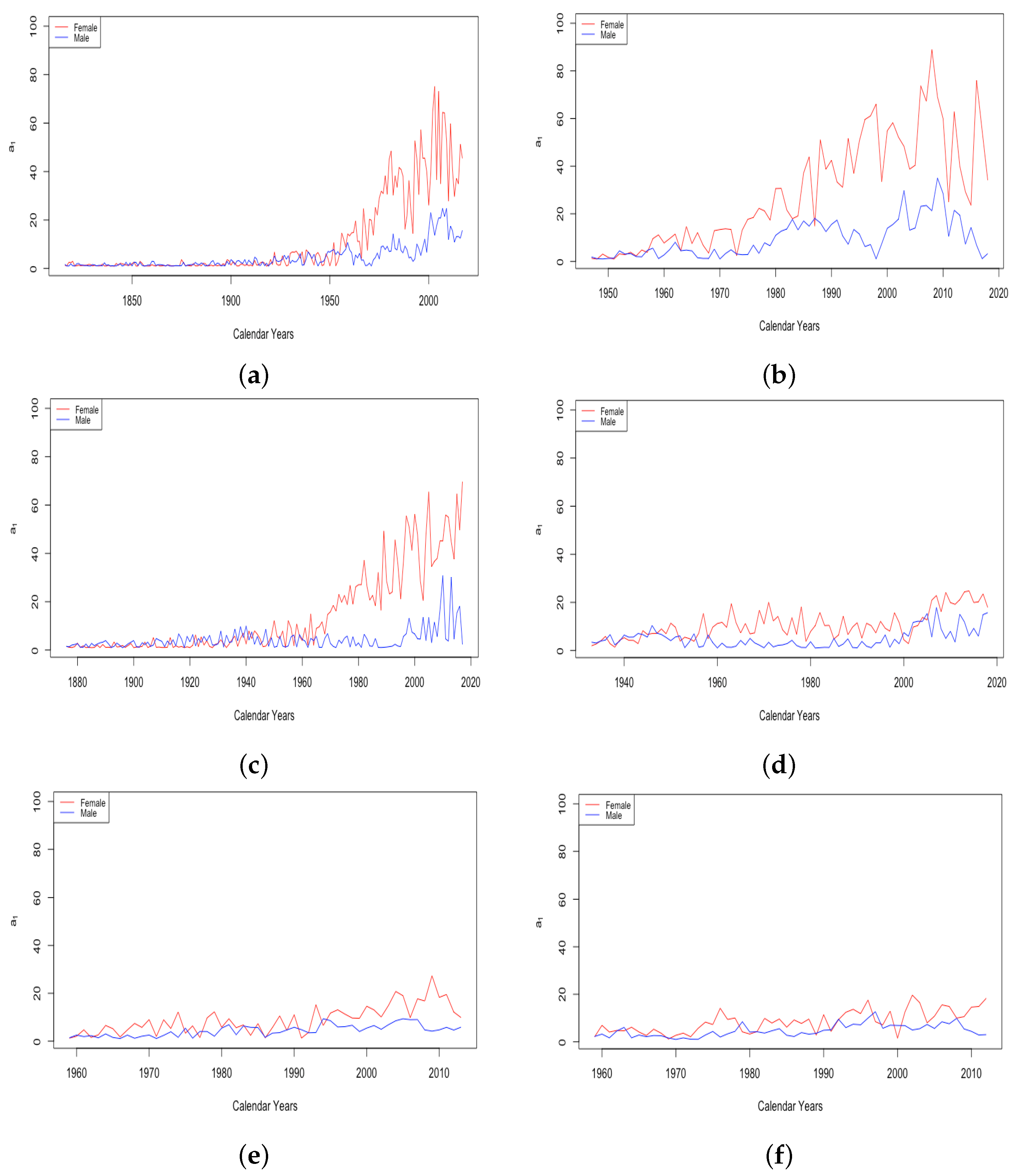
- The effect of World Wars both on the goodness of fit of the model and on the value of , the limiting teenage mortality.
- The spike in in Switzerland in 1918, larger for males than females.
- The sudden and sustained drop in for females in all HLE countries, starting in 1949 in France, 1952 in the USA, 1953 in Switzerland, and less sudden in Japan, but trending down sharply beginning around 1950.
- The sudden and sustained increase for females of , the mortality deceleration factor for adults under 65, staring in 1950 in Switzerland and the USA, 1952 in France, and 1958 in Japan.
- The sudden and sustained increase for males of , the mortality deceleration factor for adults between 65 and 85, starting in 1993 in Russia and 1995 in Ukraine.
- The introduction of penicillin, which came into wide production in the mid-1940s and reduced death in many circumstances Sewell (1993). Deaths due to abortion in the USA dropped by 1950 to a fraction of their level in 1940 Gold (2003), perhaps due to the use of antibiotics to prevent infection after the procedure;
- The widespread introduction of birth control pills starting in 1960.
3.4. Use Observed Structure Change in Mortality to Choose Fitting Data in Forecasting
3.5. Life Expectancy Improvement and Gender Gap Quantitative Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
| 1 | The data are period life tables from Human Mortality Database (n.d.), either female, male, or total. France has both data for the civilian population and the total population. |
| 2 | We use French civilian data instead of French total population data, since the DLGC generally provides a better fit to civilian data. |
References
- Bardoutsos, Anastasios, Joop de Beer, and Fanny Janssen. 2018. Projecting delay and compression of mortality. Genus 74: 17. [Google Scholar] [CrossRef]
- Blackburn, Craig, and Michael Sherris. 2013. Consistent dynamic affine mortality models for longevity risk applications. Insurance: Mathematics and Economics 53: 64–73. [Google Scholar] [CrossRef]
- Booth, Heather, and Leonie Tickle. 2008. Mortality Modelling and Forecasting: A Review of Methods. Annals of Actuarial Science 3: 3–43. [Google Scholar] [CrossRef]
- Booth, Heather, John Maindonald, and Len Smith. 2002. Applying Lee–Carter under conditions of variable mortality decline. Population Studies 56: 325–36. [Google Scholar] [CrossRef] [PubMed]
- Burger, Oskar, Annette Baudisch, and James W. Vaupel. 2012. Human mortality improvement in evolutionary context. Proceedings of the National Academy of Sciences USA 109: 18210–14. [Google Scholar] [CrossRef] [Green Version]
- Callot, Laurent, Neils Haldrup, and Marlene Kallestrup-Lamb. 2016. Deterministic and stochastic trends in the Lee–Carter mortality model. Applied Economics Letters 23: 486–93. [Google Scholar] [CrossRef] [Green Version]
- Carriere, Jacques F. 1992. Parametric models for life tables. Transactions of Society of Actuaries 44: 77–99. [Google Scholar]
- Continuous Mortality Investigation Bureau. 2006. Stochastic Projection Methodologies, Further Progress and p-Spline Model Features, Example Results and Implications. Working Paper 20. London: The Faculty of Actuariaes and Institute of Actuaries. [Google Scholar]
- de Beer, Joop, and Fanny Janssen. 2016. A new parametric model to assess delay and compression of mortality. Population Health Metrics 14: 46. [Google Scholar] [CrossRef] [Green Version]
- Gao, Huan, Rogemar Mamon, Xiaoming Liu, and Anton Tenyakov. 2015. Mortality modelling with regime-switching for the valuation of a guaranteed annuity option. Insurance: Mathematics and Economics 63: 108–20. [Google Scholar] [CrossRef]
- Gold, Rachel B. 2003. Lessons from before Roe: Will past be prologue? Guttmacher Report on Public Policy 5: 1–4. [Google Scholar]
- Gylys, Rokas, and Jonas Šiaulys. 2019. Revisiting Calibration of the Solvency II Standard Formula for Mortality Risk: Does the Standard Stress Scenario Provide and Adequate Approximation of Value-at-Risk? Risks 7: 58. [Google Scholar] [CrossRef] [Green Version]
- Gylys, Rokas, and Jonas Šiaulys. 2020. Estimation of Uncertainty in Mortality Projections Using State-Space Lee–Carter Model. Mathematics 8: 1053. [Google Scholar] [CrossRef]
- Hannerz, Harold. 2001. Presentation and derivation of a five-parameter survival function intended to model mortality in modern female populations. Scandinavian Actuarial Journal, 176–87. [Google Scholar] [CrossRef]
- He, Lingyu, Fei Huang, Jianjie Shi, and Yangrong Yang. 2021. Mortality forecasting using factor models: Time-varying or time-invariant factor loadings? Insurance: Mathematics and Economics 98: 14–34. [Google Scholar] [CrossRef]
- Heligman, Larry, and John Pollard. 1980. The age pattern of mortality. Journal of the Institute of Actuaries 107: 49–80. [Google Scholar] [CrossRef]
- Human Life Table Database. n.d. Available online: Https://www.lifetable.de/cgi-bin/country.php?code=zaf (accessed on 20 March 2022).
- Human Mortality Database. n.d. University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany). Available online: www.mortality.org (accessed on 15 July 2021).
- Hyndman, Rob J., and Yeasmin Khandakar. 2008. Automatic time series forecasting: The forecast package for R. Journal of Statistical Software 26: 1–22. [Google Scholar]
- Ignatieva, Katja, Andrew Song, and Jonathan Ziveyi. 2016. Pricing and hedging of guaranteed minimum benefits under regime-switching and stochastic mortality. Insurance: Mathematics and Economics 70: 286–300. [Google Scholar]
- Kannisto, Väinö. 1994. Development of Oldest-Old Mortality, 1950–1990: Evidence from 28 Developed Countries. Odense: Odense University Press. [Google Scholar]
- Lee, Ronald D., and Lawrence R. Carter. 1992. Modeling and Forecasting U.S. Mortality. Journal of the American Statistical Association 87: 659–71. [Google Scholar] [CrossRef]
- Lee, Ronald D., and Timothy Miller. 2001. Evaluating the performance of the lee-carter method for forecasting mortality. Demography 38: 537–49. [Google Scholar] [CrossRef] [PubMed]
- Lin, X. Sheldon, and Xiaoming Liu. 2007. Markov Aging Process and Phase-Type Law of Mortality. North American Actuarial Journal 11: 92–109. [Google Scholar] [CrossRef]
- McNown, Robert, and Andrei Rogers. 1989. Forecasting mortality: A parameterized time series approach. Demography 26: 645–60. [Google Scholar] [CrossRef] [PubMed]
- Milidonis, Andreas, Yijia Lin, and Samuel H. Cox. 2011. Mortality Regimes and Pricing. North American Actuarial Journal 15: 266–89. [Google Scholar] [CrossRef]
- Mullen, Katherine M., David Ardia, David L. Gil, Donald Windover, and James Cline. 2011. DEoptim: An R Package for Global Optimization by Differential Evolution. Journal of Statistical Software 40: 1–26. [Google Scholar] [CrossRef] [Green Version]
- Njenga, Carolyn Ndigwako, and Michael Sherris. 2020. Modeling mortality with a Bayesian vector autoregression. Insurance: Mathematics and Economics 94: 40–57. [Google Scholar] [CrossRef]
- Perks, Wilfred. 1932. On some experiments in the graduation of mortality statistics. Journal of the Institute of Actuaries 63: 12–57. [Google Scholar] [CrossRef]
- Pongou, Roland. 2013. Why is infant mortality higher in boys than in girls? A new hypothesis based on preconception environment and evidence from a large sample of twins. Demography 50: 421–44. [Google Scholar] [CrossRef]
- R Core Team. 2021. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, Available online: Https://www.R-project.org/ (accessed on 31 December 2021).
- Renshaw, Arthur E., and Steven Haberman. 2003. Lee–Carter mortality forecasting with age-specific enhancement. Insurance: Mathematics and Economics 33: 255–72. [Google Scholar] [CrossRef]
- Renshaw, Arthur E., and Steven Haberman. 2006. A cohort-based extension to the Lee–Carter model for mortality reduction factors. Insurance: Mathematics and Economics 38: 556–70. [Google Scholar] [CrossRef]
- Rogers, Andrei, and Friedrich Planck. 1983. MODEL: A General Program for Estimating Parametrized Model Schedules of Fertility, Mortality, Migration, and Marital and Labor Force Status Transitions. IIASA Working Paper. Available online: https://pure.iiasa.ac.at/id/eprint/2210/ (accessed on 31 December 2021).
- Schrager, David F. 2006. Affine stochastic mortality. Insurance: Mathematics and Economics 38: 81–97. [Google Scholar] [CrossRef] [Green Version]
- Sewell, Jane Eliot. 1993. Cesarean section—A brief history. A Brochure to Accompany an Exhibition on the History of Cesarean Section at the National Library of Medicine Part 3 30. [Google Scholar]
- Sharrow, David, Samuel J. Clark, Mark Collinson, Kathleen Kahn, and Stephen Tollman. 2013. The Age-Pattern of Increases in Mortality Affected by HIV: Bayesian Fit of the Heligman–Pollard Model to Data from the Agincourt HDSS Field Site in Rural Northeast South Africa. Demographic Research 29: 1039–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Yang, and Tak Siu. 2013. Longevity bond pricing under stochastic interest rate and mortality with regime-switching. Insurance: Mathematics and Economics 52: 114–23. [Google Scholar] [CrossRef]
- Siler, William. 1979. Competing-Risk Model for Animal Mortality. Ecology 60: 750–57. [Google Scholar] [CrossRef]
- SriDaran, Dilan, Michael Sherris, Andrés M. Villegas, and Jonathan Ziveyi. 2022. A group regularization approach for constructing generalized age-period-cohort mortality projection models. ASTIN Bulletin 52: 247–89. [Google Scholar] [CrossRef]
- Tuljapulkar, Shripad, Nan Li, and Carl Boe. 2000. A universal pattern of mortality decline in the G7 countries. Nature 405: 789–92. [Google Scholar] [CrossRef]
- Villegas, Andrés M., Pietro Millossovich, and Vladimir K. Kaishev. 2018. StMoMo: An R Package for Stochastic Mortality Modeling. Journal of Statistical Software 84: 1–38. [Google Scholar] [CrossRef] [Green Version]
- Wilmoth, John R., Kirill F. Andreev, Dmitri Jdanov, and Dana A. Glei. 2007. Methods Protocol for the Human Mortality Database. Berkeley: University of California, Berkeley, and Max Planck Institute for Demographic Research, Rostock. [Google Scholar]








| Country | Japan | USA | Switzerland | France Civilian | France Total | Russia | Ukraine | |
|---|---|---|---|---|---|---|---|---|
| Female | Mean | −406.65 | −420.87 | −313.41 | −317.789 | −310.412 | −239.52 | −176.95 |
| Min | −741.89 | −750.52 | −613.61 | −737.326 | −703.706 | −674.01 | −591.33 | |
| Male | Mean | −340.25 | −228.55 | −226.31 | −216.72 | −208.65 | −35.18 | −84.2982 |
| Min | −701.01 | −712.33 | −586.28 | −739.29 | −680.55 | −552.21 | −544.9558 | |
| Population | Female 1925/27 | Male 1925/27 | Female 2006/08 | Male 2006/08 | |
|---|---|---|---|---|---|
| DLGC | 99.84% | 99.87% | 99.44% | 99.62% | |
| Heligman–Pollard | 98.77% | 99.29% | 88.44% | 99.18% | |
| AIC | DLGC | −1096.79 | −1118.36 | −1101.22 | −1177.63 |
| Heligman–Pollard | −920.81 | −912.88 | −997.63 | −1075.69 | |
| Gender | Female | Male | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DLGC MSE[] in | Forecasting Year | 2016 | 2017 | 2018 | 2019 | 2020 | 2016 | 2017 | 2018 | 2019 | 2020 | |
| HLE countries | France Total | 5.381 | 1.246 | 2.694 | 2.273 | 1.251 | 1.572 | |||||
| France Civil | 2.349 | 1.487 | 0.905 | 3.799 | 2.124 | 3.820 | ||||||
| Switzerland | 41.88 | 17.58 | 31.10 | 28.35 | 7.395 | 36.33 | 17.99 | 33.45 | 59.03 | 61.26 | ||
| Japan | 0.502 | 1.943 | 2.225 | 3.353 | 2.526 | 6.038 | 12.75 | 8.896 | 5.062 | 3.279 | ||
| USA | 14.39 | 14.99 | 32.46 | 71.17 | 13.09 | 20.02 | 50.92 | 109.0 | ||||
| Forecasting Year | 2010 | 2011 | 2012 | 2013 | 2014 | 2010 | 2011 | 2012 | 2013 | 2014 | ||
| ILE countries | Russia Ukraine | 10.76 | 34.09 | 31.59 | 41.60 | 44.77 | 9.417 | 61.32 | 57.18 | 108.3 | 126.7 | |
| 13.90 | 14.96 | 38.09 | 58.87 | 10.20 | 67.62 | 124.8 | 136.1 | |||||
| logit-binomial Lee–Carter MSE[] in | Forecasting Year | 2016 | 2017 | 2018 | 2019 | 2020 | 2016 | 2017 | 2018 | 2019 | 2020 | |
| HLE countries | France Total | 1.561 | 6.746 | 2.652 | 2.395 | 1.774 | 2.077 | |||||
| France Civil | 1.560 | 6.746 | 2.651 | 2.395 | 1.774 | 2.077 | ||||||
| Switzerland | 13.03 | 11.03 | 10.75 | 12.48 | 50.44 | 17.76 | 4.834 | 15.38 | 30.25 | 84.35 | ||
| Japan | 2.701 | 5.968 | 5.819 | 6.649 | 0.981 | 2.686 | 6.516 | 3.021 | 1.018 | 10.36 | ||
| USA | 27.78 | 26.17 | 45.45 | 87.17 | 106.4 | 130.9 | 206.8 | 321.0 | ||||
| Forecasting Year | 2010 | 2011 | 2012 | 2013 | 2014 | 2010 | 2011 | 2012 | 2013 | 2014 | ||
| ILE countries | Russia Ukraine | 204.4 | 126.9 | 139.7 | 151.0 | 174.6 | 64.32 | 173.9 | 171.4 | 251.7 | 287.0 | |
| 208.3 | 113.2 | 102.9 | 95.86 | 27.72 | 117.0 | 182.6 | 200.8 | |||||
| DLGC MSE[] in | |||||||||
| Gender | Year | HLE Countries | Year | ILE Countries | |||||
| France Total | France Civil | Switzerland | Japan | USA | Russia | Ukraine | |||
| Female | 2011 | 3.403 | 6.564 | 3.784 | 5.352 | 0.868 | 2005 | 8.306 | 5.229 |
| 2012 | 8.578 | 8.121 | 5.179 | 13.85 | 0.766 | 2006 | 5.155 | 5.734 | |
| 2013 | 3.081 | 4.232 | 9.332 | 15.44 | 0.963 | 2007 | 14.46 | 22.39 | |
| 2014 | 4.208 | 12.33 | 3.107 | 10.34 | 8.706 | 2008 | 13.50 | 35.17 | |
| 2015 | 12.58 | 11.14 | 25.28 | 17.40 | 3.864 | 2009 | 36.29 | 62.45 | |
| 2016 | 4.422 | 8.271 | 5.051 | 19.68 | 21.19 | 2010 | 24.90 | 35.75 | |
| 2017 | 16.16 | 16.92 | 3.568 | 31.99 | 19.99 | 2011 | 136.1 | 158.9 | |
| 2018 | 9.705 | 17.93 | 2.783 | 35.81 | 37.23 | 2012 | 124.7 | 227.8 | |
| 2019 | 1.805 | 42.45 | 75.72 | 2013 | 142.7 | 283.5 | |||
| 2020 | 52.82 | 18.41 | 2014 | 139.2 | |||||
| Male | 2011 | 11.75 | 1.861 | 9.813 | 3.483 | 1.336 | 2005 | 30.53 | 33.13 |
| 2012 | 6.386 | 18.53 | 6.912 | 5.293 | 1.409 | 2006 | 36.51 | 21.31 | |
| 2013 | 3.502 | 11.55 | 8.328 | 2.654 | 4.150 | 2007 | 59.87 | 19.31 | |
| 2014 | 19.33 | 10.72 | 5.661 | 1.945 | 14.33 | 2008 | 72.78 | 35.34 | |
| 2015 | 5.135 | 16.73 | 15.25 | 2.426 | 16.87 | 2009 | 95.73 | 31.43 | |
| 2016 | 5.155 | 22.07 | 9.030 | 3.305 | 47.92 | 2010 | 81.89 | 36.12 | |
| 2017 | 7.510 | 29.60 | 2.707 | 8.845 | 62.26 | 2011 | 231.9 | 140.4 | |
| 2018 | 6.715 | 30.36 | 7.305 | 6.557 | 111.7 | 2012 | 211.9 | 218.8 | |
| 2019 | 15.84 | 3.847 | 191.2 | 2013 | 277.6 | 241.3 | |||
| 2020 | 123.1 | 4.302 | 2014 | 275.9 | |||||
| LC MSE[] in | |||||||||
| Gender | Year | HLE Countries | Year | ILE Countries | |||||
| France Total | France Civil | Switzerland | Japan | USA | Russia | Ukraine | |||
| Female | 2011 | 2.987 | 2.987 | 6.821 | 23.95 | 7.003 | 2005 | 60.48 | 62.01 |
| 2012 | 14.77 | 14.76 | 17.31 | 38.51 | 14.08 | 2006 | 69.59 | 55.57 | |
| 2013 | 6.704 | 6.704 | 20.77 | 39.23 | 18.24 | 2007 | 99.34 | 62.03 | |
| 2014 | 3.615 | 3.615 | 9.626 | 29.11 | 43.05 | 2008 | 109.3 | 72.10 | |
| 2015 | 17.60 | 17.60 | 43.69 | 38.59 | 32.93 | 2009 | 163.3 | 94.28 | |
| 2016 | 7.613 | 7.613 | 8.567 | 40.14 | 73.86 | 2010 | 152.7 | 85.08 | |
| 2017 | 20.98 | 20.98 | 11.17 | 54.81 | 74.35 | 2011 | 345.4 | 175.7 | |
| 2018 | 13.28 | 13.28 | 8.859 | 57.79 | 108.8 | 2012 | 355.8 | 232.5 | |
| 2019 | 10.27 | 63.76 | 174.4 | 2013 | 418.3 | 281.3 | |||
| 2020 | 68.02 | 31.35 | 2014 | 457.7 | |||||
| Male | 2011 | 11.03 | 11.03 | 16.82 | 21.70 | 29.75 | 2005 | 81.73 | 77.33 |
| 2012 | 8.394 | 8.394 | 13.32 | 30.61 | 41.19 | 2006 | 136.0 | 130.0 | |
| 2013 | 5.731 | 5.732 | 17.62 | 16.28 | 59.59 | 2007 | 212.4 | 171.5 | |
| 2014 | 22.01 | 22.01 | 14.97 | 11.79 | 103.0 | 2008 | 247.7 | 251.6 | |
| 2015 | 7.547 | 7.547 | 23.68 | 16.67 | 117.1 | 2009 | 324.2 | 243.7 | |
| 2016 | 9.418 | 9.419 | 19.41 | 20.09 | 202.7 | 2010 | 323.1 | 242.2 | |
| 2017 | 11.02 | 11.02 | 12.40 | 31.45 | 243.5 | 2011 | 666.8 | 559.5 | |
| 2018 | 10.92 | 10.92 | 19.18 | 23.93 | 352.7 | 2012 | 675.4 | 733.3 | |
| 2019 | 33.32 | 15.68 | 507.5 | 2013 | 856.4 | 789.4 | |||
| 2020 | 106.2 | 1.772 | 2014 | 935.9 | |||||
| Training Period | Testing Year | DLGC MSE[] | LC MSE[] | Training Period | Testing Year | DLGC MSE[] | LC MSE[] |
|---|---|---|---|---|---|---|---|
| 1975–2000 | 2005 | 30.28 | 13.38 | 1975–2008 | 2013 | 10.18 | 78.34 |
| 1975–2001 | 2006 | 45.12 | 40.27 | 1975–2009 | 2014 | 3.324 | 86.22 |
| 1975–2002 | 2007 | 63.80 | 66.16 | 1975–2010 | 2015 | 1.654 | 60.94 |
| 1975–2003 | 2008 | 25.05 | 57.60 | 1975–2011 | 2016 | 6.429 | 83.95 |
| 1975–2004 | 2009 | 52.94 | 113.8 | 1975–2012 | 2017 | 6.205 | 73.69 |
| 1975–2005 | 2010 | 35.50 | 97.85 | 1975–2013 | 2018 | 15.33 | 95.36 |
| 1975–2006 | 2011 | 32.95 | 96.22 | 1975–2014 | 2019 | 15.08 | 130.0 |
| 1975–2007 | 2012 | 6.573 | 84.26 |
| Pars | Meaning | Range | Other Constraints | |
|---|---|---|---|---|
| Youngest age | A | the level of child mortality, approximate to | (0,1) | |
| B | the mortality probabilities’ difference between age 0 and 1, i.e., | (0,1) | ||
| C | the decline in mortality during childhood | (0,1) | ||
| Teenage years | k | the growth rate of death probability only caused by teenage years factors | ||
| z | the “accident hump” age | |||
| the maximum death probability only caused by teenage years factors | (0,1) | |||
| Adulthood and old ages | the main increasing process of happens near | |||
| g | the maximum death probability only caused by adulthood and old ages factors | (0,1) | ||
| b(x) | growth rate of considering only natural and basic factors | (0,1) | continuously increasing | |
| a(x) | a controlling factor on the increasing of death probability | continuous; satisfying , for x after a certain old age |
| Gender | Country Code | Years | Mean | SD | Range | Portion of |
|---|---|---|---|---|---|---|
| Female | Japan | 1947–2019 | 0.9994 | 0.00032 | [0.9980,0.9998] | All |
| USA | 1933–2019 | 0.9993 | 0.00029 | [0.9986,0.9998] | All | |
| Switzerland | 1876–2018 | 0.9972 | 0.00086 | [0.9936,0.9984] | All | |
| France Civilian | 1816–2018 | 0.9984 | 0.00109 | [0.9948,0.9996] | All | |
| France Total | 1816–2018 | 0.9983 | 0.00138 | [0.9893,0.9996] | All | |
| Russia | 1959–2014 | 0.9961 | 0.00559 | [0.9704,0.9995] | 55 out of 56 | |
| Ukraine | 1959–2013 | 0.9957 | 0.00552 | [0.9717,0.9992] | 53 out of 55 | |
| Male | Japan | 1947–2019 | 0.9991 | 0.00046 | [0.9973,0.9996] | All |
| USA | 1933–2019 | 0.9983 | 0.00082 | [0.9957,0.9994] | All | |
| Switzerland | 1876–2018 | 0.9970 | 0.00090 | [0.9926,0.9989] | All | |
| France Civilian | 1816–2018 | 0.9973 | 0.00306 | [0.9642,0.9995] | 202 out of 203 | |
| France Total | 1816–2018 | 0.9959 | 0.00922 | [0.9190,0.9994] | 196 out of 203 | |
| Russia | 1959–2014 | 0.9955 | 0.00408 | [0.9769,0.9987] | 55 out of 56 | |
| Ukraine | 1959–2013 | 0.9956 | 0.00430 | [0.9767,0.9988] | 54 out of 55 | |
| Total | Japan | 1947–2019 | 0.9993 | 0.00033 | [0.9983,0.9997] | All |
| USA | 1933–2019 | 0.9989 | 0.00045 | [0.9978,0.9996] | All | |
| Switzerland | 1876–2018 | 0.9982 | 0.00058 | [0.9946,0.9992] | All | |
| France Civilian | 1816–2018 | 0.9982 | 0.00118 | [0.9940,0.9997] | All | |
| France Total | 1816–2018 | 0.9974 | 0.00496 | [0.9543,0.9996] | 199 out of 203 |
| Country | Japan | USA | Switzerland | France Civilian | France Total | Russia | Ukraine | |
|---|---|---|---|---|---|---|---|---|
| Female | Mean | 0.9348 | 0.9318 | 0.9495 | 0.9570 | 0.9590 | 0.9431 | 0.9560 |
| Min | 0.8604 | 0.8281 | 0.8535 | 0.8419 | 0.8412 | 0.8518 | 0.8613 | |
| Male | Mean | 0.9422 | 0.9455 | 0.9537 | 0.9601 | 0.9560 | 0.9668 | 0.9571 |
| Min | 0.8453 | 0.8019 | 0.8318 | 0.7936 | 0.7174 | 0.7683 | 0.7643 | |
| Pars | Meaning | Range | Other Constraints | |
|---|---|---|---|---|
| Youngest age | A | the level of child mortality, approximately equal to | (0,1) | |
| B | approximately the difference in mortalities between age 0 and 1, i.e., | (0,1) | ||
| C | the decline in mortality during childhood | (0,1) | ||
| Teenage years | k | the growth rate of the mortality contribution from factors arising during teenage years | ||
| z | the “accident hump” age | |||
| the limiting mortality contribution from factors arising during teenage years | (0,1) | |||
| Adulthood and old ages | the main increase in the mortality contribution from factors arising during adulthood happens near | |||
| g | the limiting mortality contribution from factors arising during adulthood and old age | (0,1) | ||
| the lower bound of the accelerative factor (i.e., ) incorporated into | (0,1) | |||
| the upper bound of the accelerative factor incorporated into | (0,1) | |||
| the rate of growth of the accelerative factor in between its two levels | ||||
| the main increasing process of the accelerative factor happens near | ||||
| the main level of the controlling factor before age 65 | [1,∞) | |||
| the main level of the controlling factor between ages 65 and 85 | [1,∞) |
| Population | France Civil Female | Switzerland Female | USA Female | Japan Female | Russia Female | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Years for Training | 1950–1985 | 1960–1985 | 1940–1975 | 1950–1975 | 1945–1980 | 1955–1980 | 1950–1990 | 1970–1995 | 1975–2004 | 1985–2004 | |
| MSE in for 10-year-ahead forecasts | 1st | 1.048 | 1.017 | 60.74 | 55.03 | 4.985 | 4.378 | 27.41 | 22.36 | 4.200 | 5.003 |
| 2nd | 27.42 | 23.83 | 23.22 | 27.97 | 17.60 | 14.94 | 27.18 | 17.56 | 5.239 | 4.022 | |
| 3th | 31.35 | 25.73 | 9.854 | 6.705 | 3.889 | 1.958 | 45.53 | 27.35 | 20.36 | 14.20 | |
| 4th | 10.74 | 7.467 | 11.22 | 8.027 | 4.949 | 1.978 | 14.57 | 3.401 | 15.27 | 11.50 | |
| 5th | 12.56 | 8.518 | 51.97 | 29.55 | 4.214 | 3.046 | 67.78 | 32.24 | 43.17 | 27.06 | |
| 6th | 33.04 | 22.89 | 26.93 | 12.70 | 5.575 | 2.996 | 78.17 | 32.96 | 29.80 | 20.35 | |
| 7th | 43.07 | 29.52 | 19.18 | 5.969 | 9.249 | 7.298 | 99.10 | 40.31 | 160.6 | 105.2 | |
| 8th | 25.16 | 14.90 | 73.62 | 34.36 | 15.21 | 16.72 | 76.85 | 21.60 | 151.9 | 92.41 | |
| 9th | 70.10 | 47.38 | 18.48 | 14.11 | 12.73 | 9.607 | 86.36 | 21.50 | 177.9 | 101.6 | |
| 10th | 36.95 | 21.21 | 17.10 | 13.67 | 15.49 | 9.649 | 41.88 | 2.235 | 180.6 | 93.11 | |
| Population | France Civil Male | Switzerland Male | USA Male | ||||
|---|---|---|---|---|---|---|---|
| Years for Training | 1950–1985 | 1960–1985 | 1940–1975 | 1950–1975 | 1945–1980 | 1955–1980 | |
| MSE in for 10-year-ahead forecasts | 1st | 4.409 | 7.637 | 72.96 | 73.35 | 19.63 | 19.51 |
| 2nd | 17.66 | 40.79 | 32.40 | 40.08 | 33.25 | 33.06 | |
| 3th | 28.94 | 78.20 | 17.33 | 21.81 | 15.49 | 15.17 | |
| 4th | 20.27 | 102.6 | 25.62 | 31.72 | 11.73 | 11.91 | |
| 5th | 9.499 | 140.6 | 25.25 | 32.95 | 10.36 | 10.50 | |
| 6th | 22.41 | 184.4 | 22.94 | 38.77 | 14.02 | 14.96 | |
| 7th | 33.26 | 236.9 | 25.42 | 45.28 | 18.70 | 21.41 | |
| 8th | 20.78 | 267.0 | 74.30 | 90.95 | 26.24 | 30.91 | |
| 9th | 38.49 | 329.4 | 19.12 | 65.38 | 32.79 | 41.88 | |
| 10th | 34.22 | 386.5 | 59.24 | 90.27 | 44.43 | 58.23 | |
| Country | Japan | Switzerland | France | USA | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gender | Female | Male | Female | Male | Female | Male | Female | Male | |
| Year Period | 1947–2019 | 1947–2019 | 1876–2018 | 1876–2018 | 1816–2018 | 1816–2018 | 1933–2019 | 1933–2019 | |
| data | 33.80000 | 31.61000 | 43.45000 | 43.12000 | 44.37000 | 40.48000 | 18.92000 | 17.40000 | |
| Explained | 33.86071 | 31.73058 | 43.59133 | 43.33996 | 44.36348 | 40.29138 | 19.00995 | 17.74775 | |
| Unexplained | −0.06071 | −0.12058 | −0.14133 | −0.21996 | 0.00652 | 0.18862 | −0.08995 | −0.34775 | |
| Earliest Ages | Total | 10.05750 | 9.92492 | 13.57758 | 15.55131 | 18.52742 | 19.78979 | 4.64870 | 5.88425 |
| A | 10.11580 | 10.06337 | 12.80801 | 13.75968 | 18.14703 | 18.92012 | 4.46785 | 5.68005 | |
| Teenage years | Total | 8.23280 | 8.56301 | 1.875668 | 1.09767 | 5.49789 | 8.40810 | 2.88721 | 3.23289 |
| 8.24115 | 8.56713 | 1.90180 | 1.12202 | 5.50277 | 8.41084 | 2.86904 | 3.20340 | ||
| Adulthood and old ages | Total | 15.57041 | 13.24264 | 28.13808 | 26.69097 | 20.33817 | 12.09349 | 11.47403 | 8.63061 |
| g | 1.61761 | 1.74418 | 4.52138 | 3.39971 | −4.82562 | −5.68736 | −0.93074 | 0.19970 | |
| 8.49795 | 6.68156 | 8.19871 | 11.68489 | 18.55857 | 18.10215 | 9.14870 | 5.87759 | ||
| Country | Japan F | France F | Switzerland F | USA F | Russia F | Ukraine F | |
|---|---|---|---|---|---|---|---|
| Year Period | 1959–2013 | 1959–2013 | 1959–2013 | 1959–2013 | 1959–2013 | 1959–2013 | |
| data | 16.79000 | 11.74000 | 10.63000 | 8.03000 | 5.13000 | 4.00000 | |
| Explained | 16.90217 | 11.84723 | 10.85521 | 8.05072 | 5.12731 | 4.02174 | |
| Unexplained | −0.11217 | −0.10723 | −0.22521 | −0.02072 | 0.00269 | −0.02174 | |
| Earliest Ages | Total | 3.35330 | 2.20683 | 1.73275 | 1.63774 | 3.17531 | 2.43769 |
| A | 3.22393 | 1.70185 | 1.81240 | 1.38536 | 3.35867 | 2.39839 | |
| Teenage years | Total | 2.17992 | 0.51989 | 0.29626 | 0.07502 | −0.36757 | 0.25968 |
| 2.19159 | 0.52466 | 0.30013 | 0.05499 | −1.18033 | 0.09941 | ||
| Adulthood and old ages | Total | 11.36895 | 9.12050 | 8.82619 | 6.33796 | 2.31958 | 1.32437 |
| g | −0.42729 | 0.47768 | −0.57203 | 0.17951 | −4.22517 | −8.45819 | |
| 9.28527 | 5.66350 | 4.96354 | 3.38443 | 3.55595 | 11.44060 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.; Smith, B.R.; Brewer, P.; Droms, S. A New Mortality Framework to Identify Trends and Structural Changes in Mortality Improvement and Its Application in Forecasting. Risks 2022, 10, 161. https://doi.org/10.3390/risks10080161
Fu W, Smith BR, Brewer P, Droms S. A New Mortality Framework to Identify Trends and Structural Changes in Mortality Improvement and Its Application in Forecasting. Risks. 2022; 10(8):161. https://doi.org/10.3390/risks10080161
Chicago/Turabian StyleFu, Wanying, Barry R. Smith, Patrick Brewer, and Sean Droms. 2022. "A New Mortality Framework to Identify Trends and Structural Changes in Mortality Improvement and Its Application in Forecasting" Risks 10, no. 8: 161. https://doi.org/10.3390/risks10080161
APA StyleFu, W., Smith, B. R., Brewer, P., & Droms, S. (2022). A New Mortality Framework to Identify Trends and Structural Changes in Mortality Improvement and Its Application in Forecasting. Risks, 10(8), 161. https://doi.org/10.3390/risks10080161






