Determination of Sesquiterpenes in Wines by HS-SPME Coupled with GC-MS
Abstract
:1. Introduction
2. Experimental Section
2.1. Sampling
2.2. Volatiles Extraction: HS-SPME
2.3. Volatiles Analysis: GC-MS
2.4. Quantitative Analyses
3. Results and Discussion
| LOD(µg L−1) | LOQ( µg L−1) | Linear range( µg L−1) | Intraday repeatability(RSD) (%) | Interday Repeatability(RSD) (%) | Recovery (%) | |
|---|---|---|---|---|---|---|
| α-Gurjunene | 0.04 | 0.13 | 0.12–1.02 | 2 | 5 | 94 |
| α-Cedrene | 0.04 | 0.13 | 0.12–1.08 | 1 | 4 | 99 |
| (E)-β-Farnesene | 0.05 | 0.16 | 0.14–0.24 | 2 | 3 | 98 |
| Valencene | 0.03 | 0.10 | 0.10–1.31 | 2 | 3 | 97 |
| (E)-Nerolidol | 0.07 | 0.23 | 0.20–5.98 | 1 | 4 | 104 |
| Compounds | RT | LRI | IUPAC name | Odour | |
|---|---|---|---|---|---|
| 1) | α-Gurjunene * | 35.54 | 1550 | (1aR,4R,4aR,7bS)-1,1,4,7-tetramethyl-1a,2,3,4,4a,5,6,7b-octahydro-1H-cyclopropa[e]azulene | balsamic, woody |
| 2) | α-Cedrene * | 36.60 | 1568 | (3R-(3a,3ab,7b,8aa))-2,3,4,7,8,8a-hexahydro-3,6,8,8-tetramethyl-1H-3a,7-methanoazulene | woody, cedar sweet fresh |
| 3) | α-Aromadendrene | 41.85 | 1658 | (1aR,7S,7bS)-1,1,7-trimethyl-4-methylidene-2,3,4a,5,6,7,7a,7b-octahydro-1aH-cyclopropa[e]azulene | woody |
| 4) | (E)-β-Farnesene * | 42.36 | 1671 | (6E)-7,11-dimethyl-3-methylidenedodeca-1,6,10-triene | woody, citrus, sweet |
| 5) | D Germacrene | 42.67 | 1674 | (S,1Z,6Z)-8-isopropyl-1-methyl-5-methylenecyclodeca-1,6-diene | weak fruity, apple like |
| 6) | Valencene * | 43.12 | 1680 | 4a,5-dimethyl-3-prop-1-en-2-yl-2,3,4,5,6,7-hexahydro-1H-naphthalene | citrus, green, woody |
| 7) | γ-Gurjunene | 43.30 | 1684 | (1R,4R,7R)-1,4-dimethyl-7-prop-1-en-2-yl-1,2,3,3a,4,5,6,7-octahydroazulene | musty |
| 8) | (E)-β-Bisabolene | 44.03 | 1697 | (4R)-1-methyl-4-(6-methylhepta-1,5-dien-2-yl)cyclohexene | balsamic, woody, spicy |
| 9) | δ-Cadinene | 44.89 | 1711 | (1S,8aR)-4,7-dimethyl-1-(propan-2-yl)-1,2,3,5,6,8a-hexahydronaphthalene | woody, weak, medicinal |
| 10) | β-Selinene | 45.26 | 1718 | 3S,4aR,8aS)-8a-methyl-5-methylidene-3-prop-1-en-2-yl-1,2,3,4,4a,6,7,8-octahydronaphthalene | herbal |
| 11) | α-Muurolene | 45.62 | 1721 | (1S,4aS,8aR)-1-isopropyl-4,7-dimethyl-1,2,4a,5,6,8a-hexahydronaphthalene | woody, floral, herbal |
| 12) | α-Selinene | 45.68 | 1725 | 5,8a-dimethyl-3-prop-1-en-2-yl-2,3,4,4a,7,8-hexahydro-1H-naphthalene | amber |
| 13) | (Z)-α-Bisabolene | 48.12 | 1768 | 1-methyl-4-(6-methylhepta-2,5-dien-2-yl)cyclohexene | balsamic, spicy |
| 14) | (E)-Nerolidol * | 61.84 | 2024 | (6E)-3,7,11-trimethyldodeca-1,6,10-trien-3-ol | floral, green, citrus, woody |
| 15) | Viridiflorol | 68.49 | 2155 | (1aR,4S,4aS,7R,7aS)-1,1,4,7-tetramethyl-2,3,4a,5,6,7,7a,7b-octahydro-1aH-cyclopropa[e]azulen-4-ol | sweet, green |
| 16) | γ-Eudesmol | 69.10 | 2167 | 2-[(2R,4aR)-4a,8-dimethyl-2,3,4,5,6,7-hexahydro-1H-naphthalen-2-yl]propan-2-ol | rose, apple, green, citrus |
| Compounds (µg L−1) | Syrah | CV % | Merlot | CV % | Nero d'Avola | CV % | Frappato | CV % | Petit Verdot | CV % | Refosco | CV % | Barbarossa | CV % | Sagrantino | CV % | Malbec | CV % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Gurjunene | tr | - | - | - | tr | - | tr | - | tr | - | - | - | - | - | - | - | - | - |
| α-Cedrene | - | - | - | - | - | - | - | - | tr | - | - | - | - | - | - | - | - | - |
| α-Aromadendrene | - | - | - | - | - | - | - | - | - | - | 0.21 | 5 | - | - | - | - | - | - |
| (E)-β-Farnesene | tr | - | - | - | tr | - | tr | - | - | - | - | - | - | - | - | - | - | - |
| D Germacrene | 1.01 | 11 | 0.70 | 16 | 0.41 | 6 | 0.20 | 9 | tr | - | 0.13 | 7 | 0.19 | 13 | 0.34 | 13 | 0.19 | 6 |
| Valencene | 0.10 | 7 | - | - | tr | - | - | - | 0.12 | 7 | - | - | - | - | tr | - | tr | - |
| γ-Gurjunene | 1.40 | 14 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| (E)-β-Bisabolene | tr | - | - | - | tr | - | - | - | - | - | - | - | - | - | - | - | - | - |
| δ-Cadinene | tr | - | tr | - | 0.10 | 9 | tr | - | - | - | - | - | - | - | - | - | - | - |
| β-Selinene | - | - | - | - | - | - | - | - | tr | - | - | - | - | - | - | - | - | - |
| α-Muurolene | 1.69 | 12 | 0.11 | 8 | 0.98 | 12 | 0.24 | 12 | 0.97 | 6 | 0.28 | 14 | 0.30 | 9 | tr | - | 1.25 | 4 |
| α-Selinene | - | - | 0.12 | 17 | - | - | - | - | - | - | - | - | 0.31 | 16 | 0.38 | 9 | - | - |
| (Z)-α-Bisabolene | - | - | - | - | - | - | - | - | tr | - | - | - | - | - | tr | - | 0.19 | 14 |
| (E)-Nerolidol | 5.43 | 5 | - | - | - | - | - | - | - | - | - | - | - | - | 2.39 | 6 | - | - |
| Viridiflorol | - | - | - | - | 2.76 | 13 | - | - | - | - | - | - | - | - | - | - | - | - |
| γ-Eudesmol | - | - | - | - | - | - | - | - | - | - | - | - | - | - | tr | - | - | - |
| All | 9.63 | 0.93 | 4.25 | 0.44 | 1.09 | 0.62 | 0.81 | 3.11 | 1.63 |

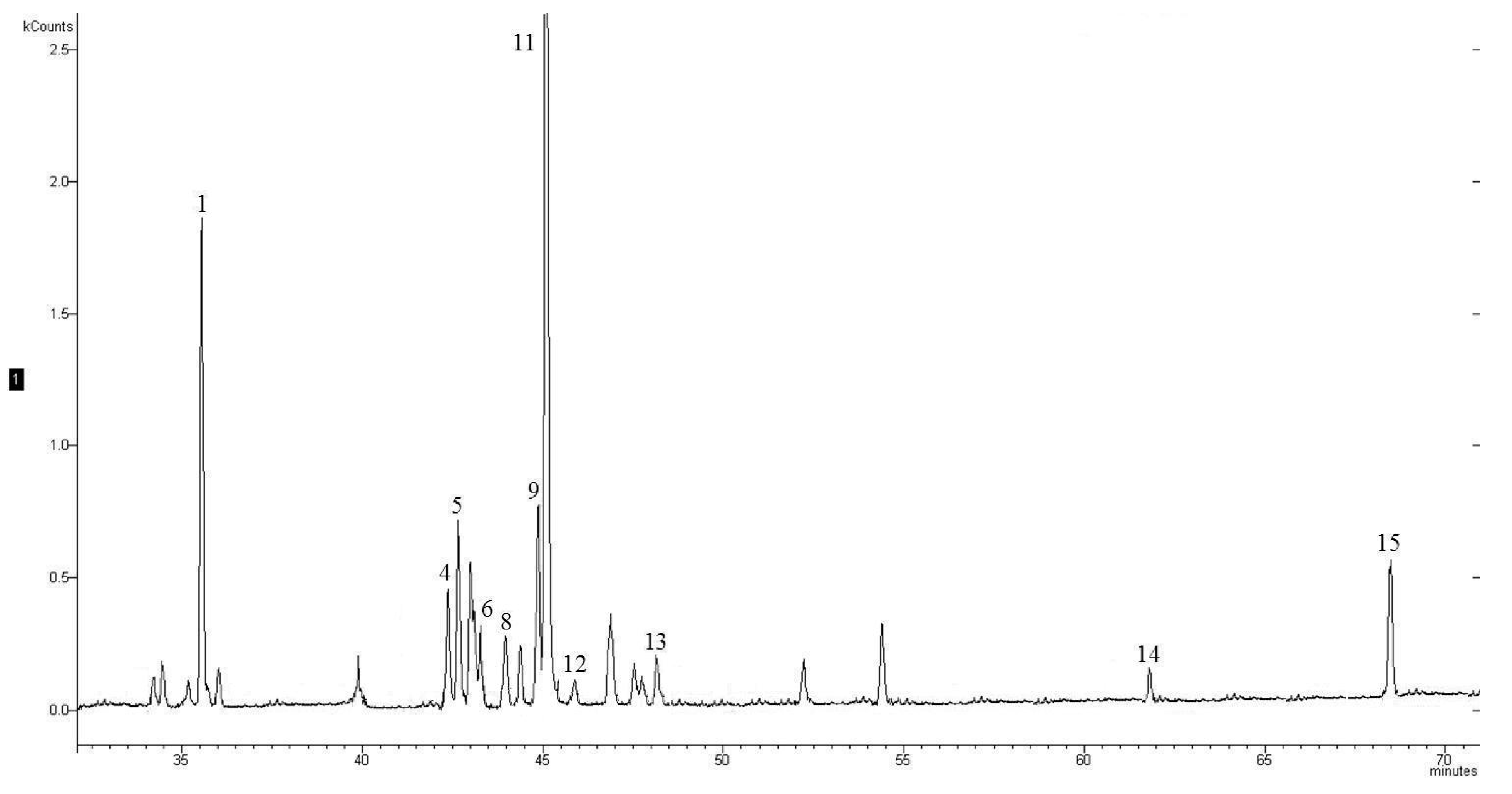
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Scacco, A.; Oliva, D.; Di Maio, S.; Polizzotto, G.; Genna, G.; Tripodi, G.; Verzera, A. Indigenous Saccharomyces Cerevisiae strains and their influence on the quality of Cataratto, Inzolia and Grillo white wines. Food Res. Int. 2012, 46, 1–9. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the aroma of grapes and wines: A review. S. Afr. J. Enol. Vitic. 1983, 4, 49–60. [Google Scholar]
- Tominaga, T.; Murat, M.L.; Dubourdieu, D. Development of a method for analyzing the volatile thiols involved in the characteristic aroma of wines made from Vitis vinifera L. cv. Sauvignon blanc. J. Agric. Food Chem. 1998, 46, 1044–1048. [Google Scholar] [CrossRef]
- Croteau, R. Biosynthesis of monoterpenes and sesquiterpenes. Geruch- und Geschmackstoffe. Verlag Hans Carl Numberg 1975, 153, 153–166. [Google Scholar]
- Meilgaard, M.C. Flavor chemistry of beer. Flavor interaction between principal volatiles. Tech. Q. Mast. Brew Assoc. Am. 1975, 12, 107–117. [Google Scholar]
- Simpson, R.F. Some important aroma components of white wine. Food Technol. 1979, 31, 516–522. [Google Scholar]
- Verzera, A.; Tripodi, G.; Dima, G.; Condurso, C.; Scacco, A.; Cincotta, F.; Giglio, D.M.L.; Sparacio, A. Leaf Removal and Wine Composition of Vitis vinifera L. cv. Nero d'Avola. The volatile aroma constituents. J. Sci. Food Agric. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Herbert, P.; Marques, J.C.; Alves, M.A. Varietal flavour compounds of four grape varieties producing Madeira wines. Anal. Chim. Acta 2004, 513, 203–207. [Google Scholar] [CrossRef]
- Alves, R.F.; Nascimento, A.M.D.; Nogueira, J.M.F. Characterization of the aroma profile of Madeira wine by sorptive extraction techniques. Anal. Chim. Acta 2005, 546, 11–21. [Google Scholar] [CrossRef]
- Rocha, S.M.; Coelho, E.; Vinholes, J.; Coimbra, M.A. Grapes and wine from Vitis vinifera L. as a potential source of sesquiterpenoids. In Natural Products, Series Recent Progress in Medicinal Plants; Editions Studium Press LLC: Huston, TX, USA, 2006; Volume 15, Chapter 12; pp. 253–272. [Google Scholar]
- Robinson, A.L.; Boss, P.K.; Heymann, H.; Solomon, P.S.; Trengove, R.D. Development of a sensitive non-targeted method for characterizing the wine volatile profile using headspace solid-phase microextraction comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (-)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Pereira, J.; Póvoa, P. Antibiotics in critically ill patients: A systematic review of the pharmacokinetics of β-lactams. Crit. Care 2011, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Tatman, D.; Mo, H. Volatile isoprenoid constituents of fruits, vegetables and herbs cumulatively suppress the proliferation of murine B16 melanoma and human HL-60 leukemia cells. Cancer Lett. 2002, 175, 129–139. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Petronilho, S.; Coimbra, M.A.; Rocha, S.M. A critical review on extraction techniques and gas chromatography based determination of grapevine derived sesquiterpenes. Anal. Chim. Acta 2014, 846, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Verzera, A.; Ziino, M.; Scacco, A.; Lanza, C.M.; Mazzaglia, A.; Romeo, V.; Condurso, C. Volatile compound and sensory analysis for the characterization of an Italian white wine from “Inzolia” grapes. Food Anal. Methods 2008, 1, 144–151. [Google Scholar] [CrossRef]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index systemincluding linear temperature programmedgas–liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- McNaught, A.D. Compendium of chemical terminology. In IUPAC Compendium of Chemical Terminology; Blackwell Science: Oxford, UK, 1997; Volume 1669. [Google Scholar]
- Steyer, D.; Ambroset, C.; Brion, C.; Claudel, P.; Delobel, P.; Sanchez, I.; Legras, J.L. QTL mapping of the production of wine aroma compounds by yeast. BMC Genomics 2012, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.O.; Bouwmeester, H.J.; Franke, S.; König, W.A. Mechanisms of the biosynthesis of sesquiterpene enantiomers (+)-and (−)-germacrene D in Solidago Canadensis. Chirality 1999, 11, 353–362. [Google Scholar] [CrossRef]
- Lücker, J.; Bowen, P.; Bohlmann, J. Vitis vinifera terpenoid cyclases: functional identification of two sesquiterpene synthase cDNAs encoding (+)-valencene synthase and (-)-germacrene D synthase and expression of mono-and sesquiterpene synthases in grapevine flowers and berries. Phytochemistry 2004, 65, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao). Molecules 2010, 15, 7603–7698. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.; Siebert, T.E.; Parker, M.; Capone, D.L.; Elsey, G.M.; Pollnitz, A.P.; Egger, M.; Meier, M.; Vossing, T.; Widder, S. From wine to pepper: rotundone, an obscure sesquiterpene, is a potent spicy aroma compound. J. Agric. Food Chem. 2008, 56, 3738–3744. [Google Scholar] [CrossRef] [PubMed]
- Caputi, L.; Carlin, S.; Ghiglieno, I.; Stefanini, M.; Valenti, L.; Vrhovsek, U.; Mattivi, F. Relationship of changes in rotundone content during grape ripening and winemaking to manipulation of the ‘peppery’ character of wine. J. Agric. Food Chem. 2011, 59, 5565–5571. [Google Scholar] [CrossRef] [PubMed]
- Scarlett, N.J.; Bramley, R.G.V.; Siebert, T.E. Within-vineyard variation in the “pepper” compound rotundone is spatially structured and related to variation in the land underlying the vineyard. Aust. J. Grape Wine Res. 2014, 20, 214–222. [Google Scholar] [CrossRef]
- Mayr, C.M.; Geue, J.P.; Holt, H.E.; Pearson, W.P.; Jeffery, D.W.; Francis, I.L. Characterization of the key aroma compounds in Shiraz wine by quantitation, aroma reconstitution, and omission studies. J. Agric. Food Chem. 2014, 2014, 62, 4528–4536. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P.; Li, S. The botanical source of Chinese cedarwood oil: Cupressus funebris or Cupressaceae species? J. Essent. Oil Res. 2008, 20, 235–242. [Google Scholar] [CrossRef]
- Martin, D.M.; Aubourg, S.; Schouwey, M.B.; Daviet, L.; Schalk, M.; Toub, O.; Lund, S.T.; Bohlmann, J. Functional annotation, genome organization and phylogeny of the grapevine (Vitis vinifera) terpene synthase gene family based on genome assembly, FLcDNA cloning, and enzyme assays. BMC Plant Biol. 2010, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- May, B.; Wüst, M. Temporal development of sesquiterpene hydrocarbon profiles of different grape varieties during ripening. Flavour Fragrance J. 2012, 27, 280–285. [Google Scholar] [CrossRef]
- Zunin, P.; Boggia, R.; Salvadeo, P.; Evangelisti, F. Geographical traceability of West Liguria extravirgin olive oils by the analysis of volatile terpenoid hydrocarbons. J. Chromatogr. A 2005, 1089, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Favaro, G.; Magno, F.; Boaretto, A.; Bailoni, L.; Mantovani, R. Traceability of Asiago mountain cheese: A rapid, low-cost analytical procedure for its identification based on solid-phase microextraction. J. Dairy Sci. 2005, 88, 3426–3434. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cincotta, F.; Verzera, A.; Tripodi, G.; Condurso, C. Determination of Sesquiterpenes in Wines by HS-SPME Coupled with GC-MS. Chromatography 2015, 2, 410-421. https://doi.org/10.3390/chromatography2030410
Cincotta F, Verzera A, Tripodi G, Condurso C. Determination of Sesquiterpenes in Wines by HS-SPME Coupled with GC-MS. Chromatography. 2015; 2(3):410-421. https://doi.org/10.3390/chromatography2030410
Chicago/Turabian StyleCincotta, Fabrizio, Antonella Verzera, Gianluca Tripodi, and Concetta Condurso. 2015. "Determination of Sesquiterpenes in Wines by HS-SPME Coupled with GC-MS" Chromatography 2, no. 3: 410-421. https://doi.org/10.3390/chromatography2030410
APA StyleCincotta, F., Verzera, A., Tripodi, G., & Condurso, C. (2015). Determination of Sesquiterpenes in Wines by HS-SPME Coupled with GC-MS. Chromatography, 2(3), 410-421. https://doi.org/10.3390/chromatography2030410







