Automated Analysis of Oxytocin by On-Line in-Tube Solid-Phase Microextraction Coupled with Liquid Chromatography-Tandem Mass Spectrometry
Abstract
:1. Introduction
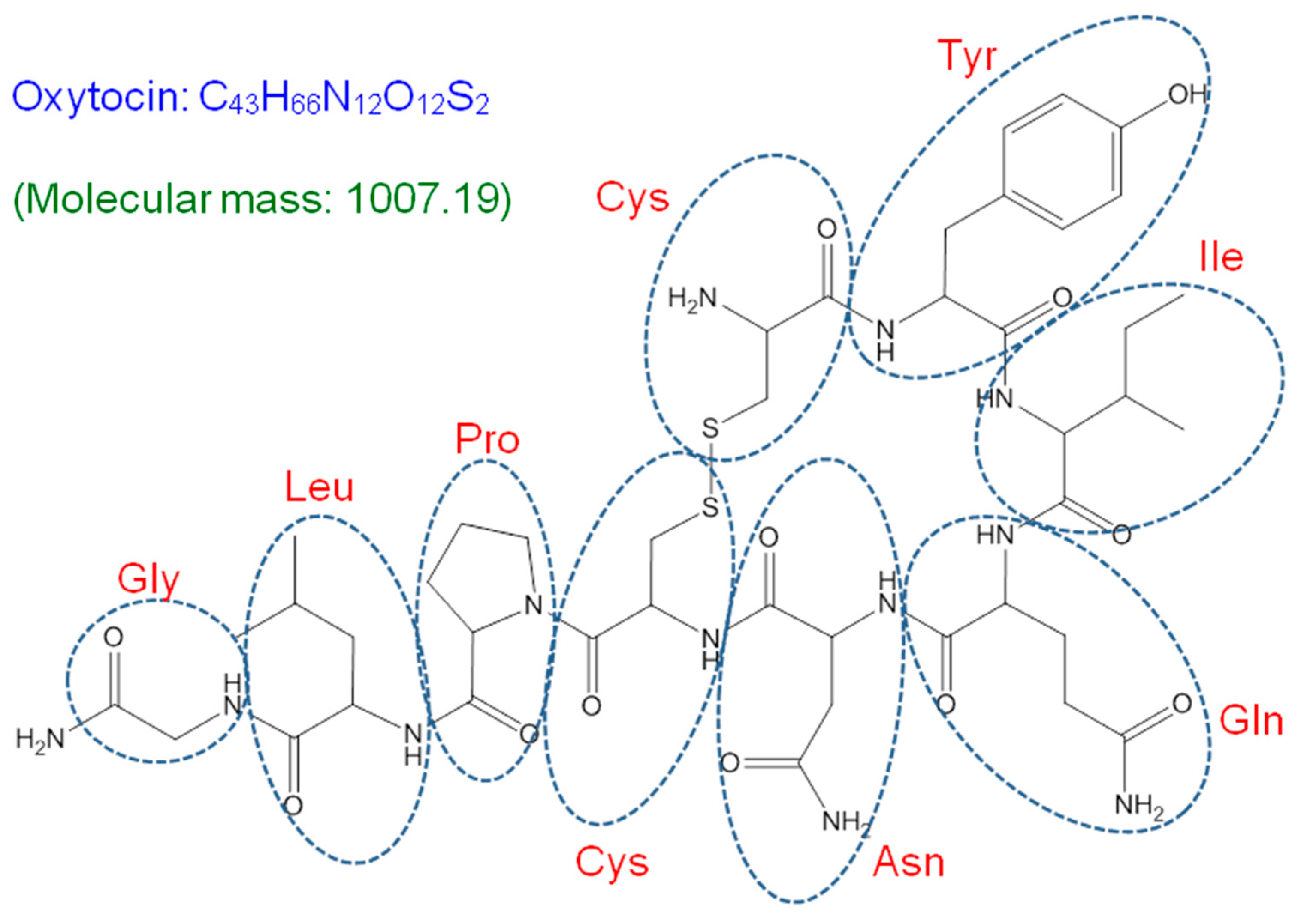
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instruments and Analytical Conditions
2.3. In-tube Solid-Phase Microextraction
2.4. Preparation of Saliva Samples
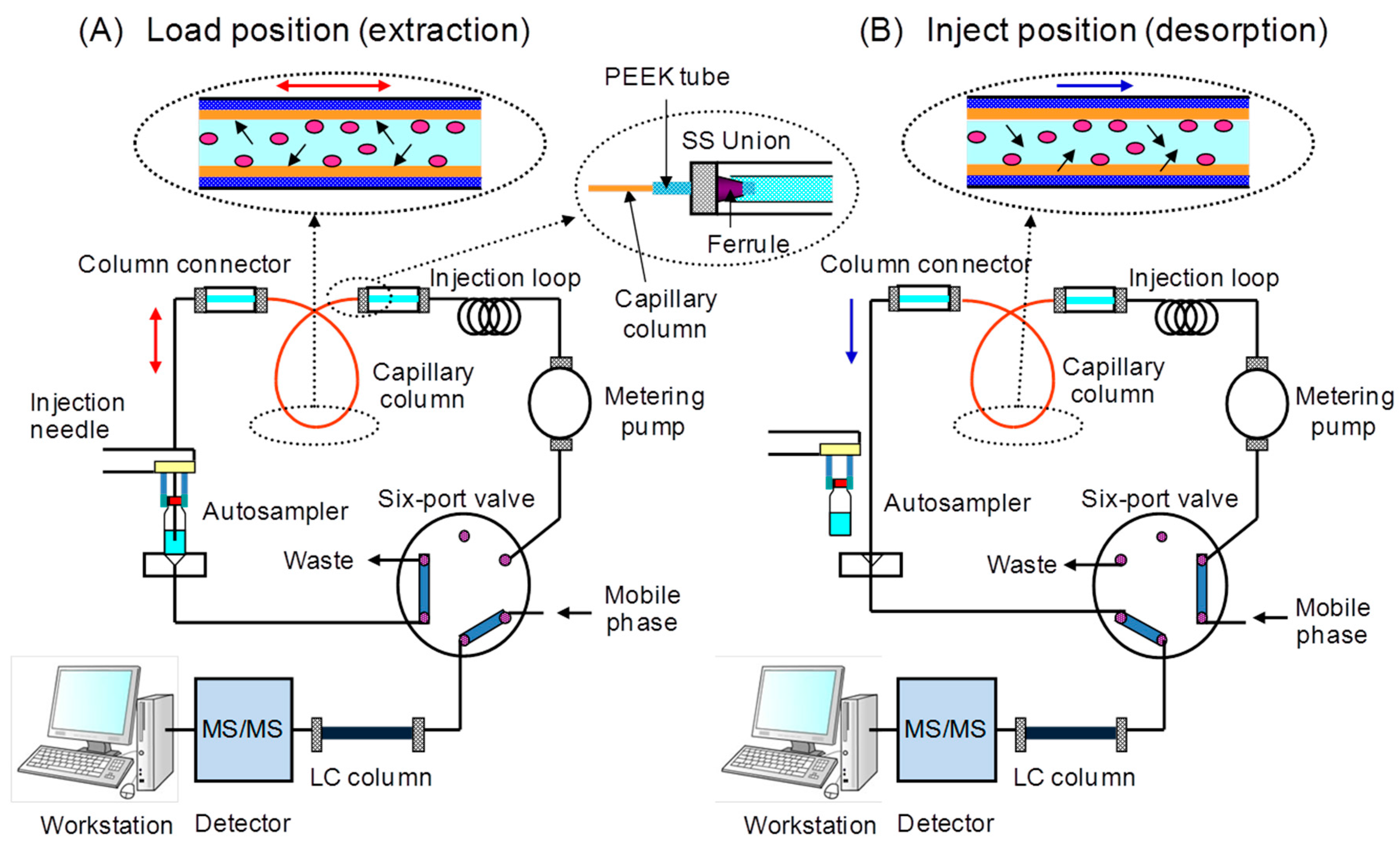
3. Results and Discussion
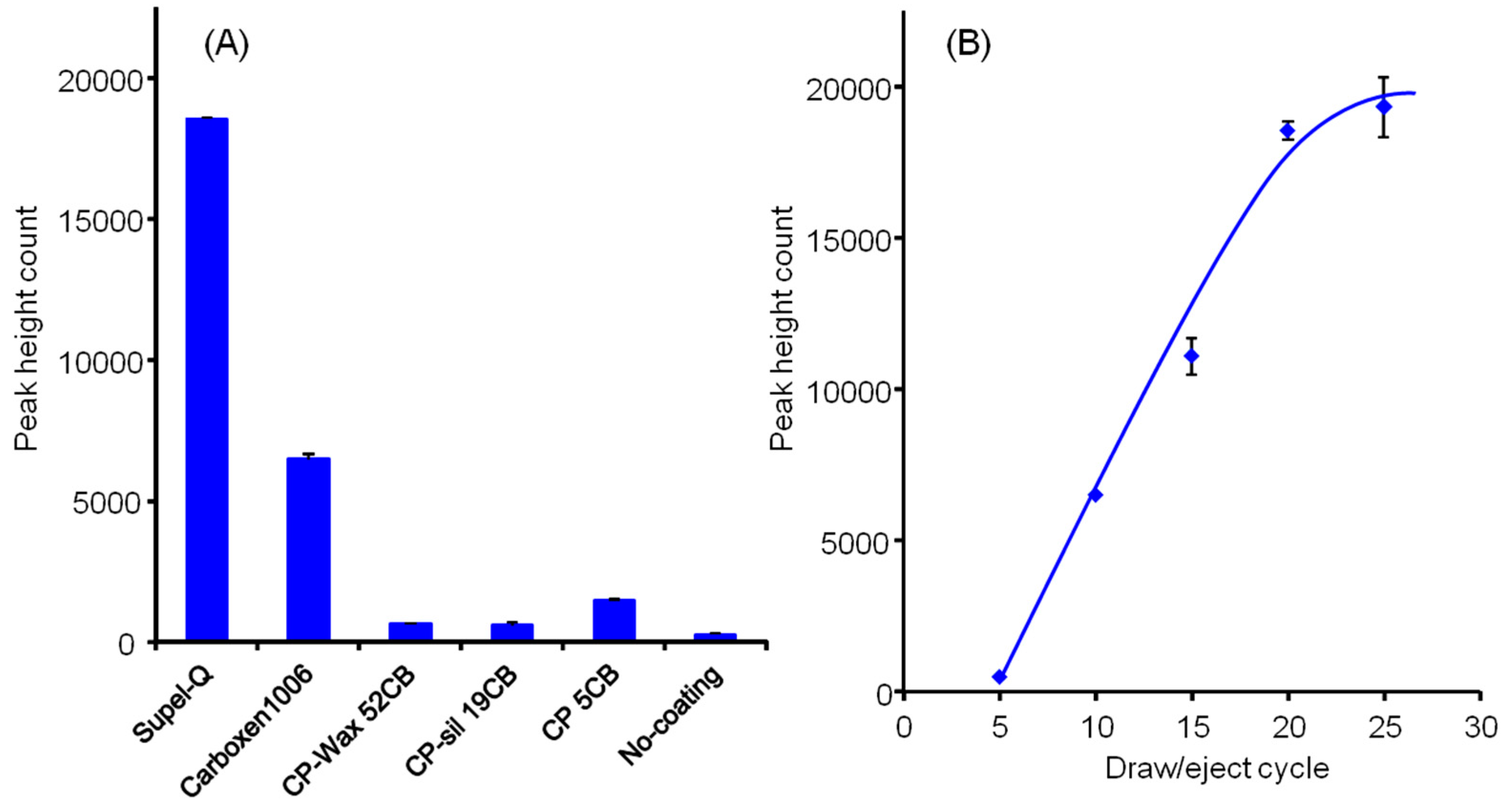
3.1. Optimization of In-tube Solid-phase Microextraction and Desorption
3.2. Linearity, Reproducibility and Detection Limit
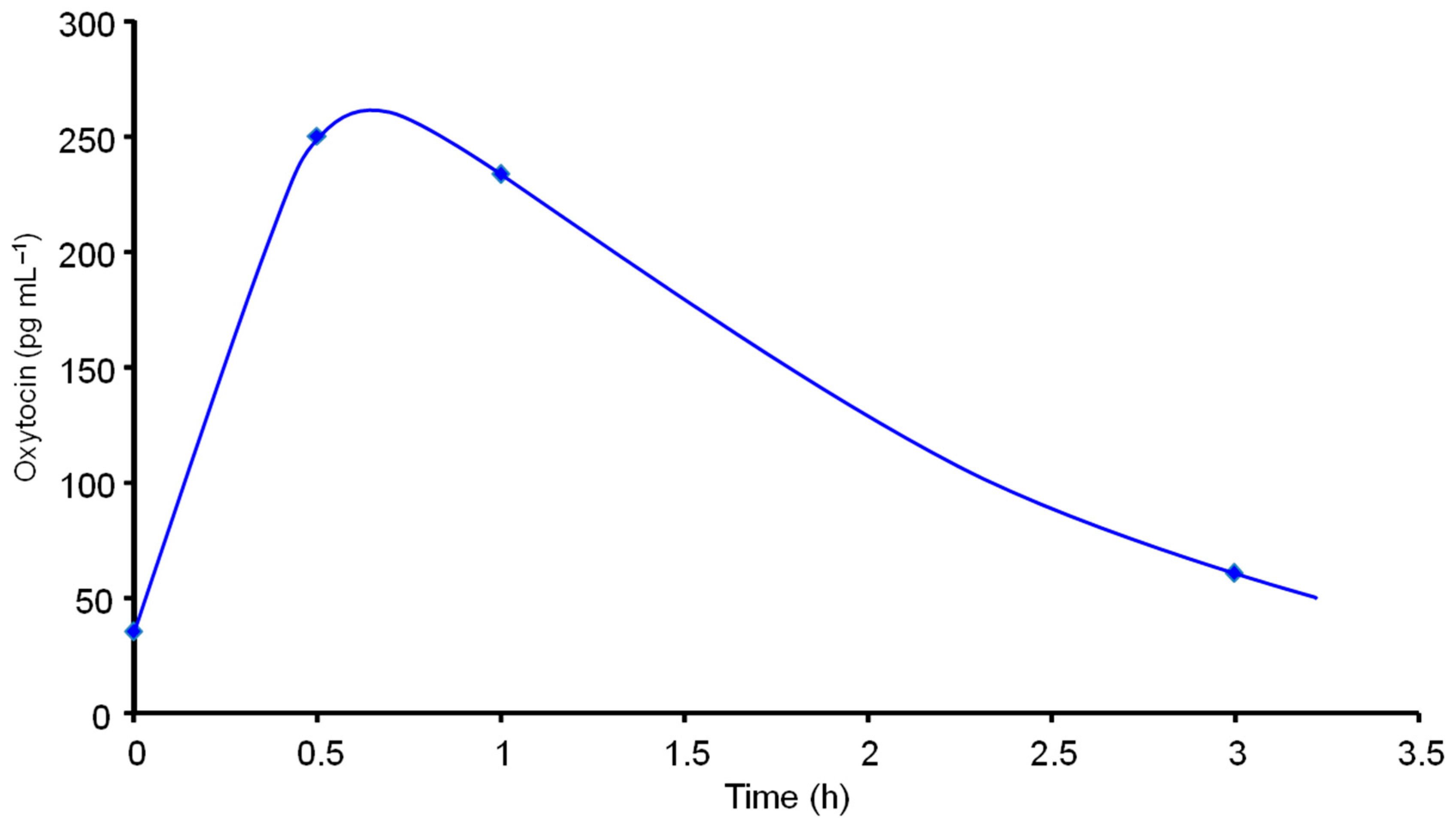
3.3. Application to the Analysis of Saliva Samples

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ross, H.E.; Young, L.J. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 2009, 30, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Macbeth, A.H.; Pagani, J.H.; Young, W.S., 3rd. Oxytocin: The great facilitator of life. Prog. Neurobiol. 2009, 88, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Viero, C.; Shibuya, I.; Kitamura, N.; Verkhratsky, A.; Fujihara, H.; Katoh, A.; Ueta, Y.; Zingg, H.H.; Chvatal, A.; Sykova, E.; et al. Oxytocin: Crossing the bridge between basic science and pharmacotherapy. CNS Neurosci. Ther. 2010, 16, e138–e156. [Google Scholar] [CrossRef] [PubMed]
- Galbally, M.; Lewis, A.J.; van Ijzendoorn, M.; Permezel, M. The role of oxytocin in mother-infant relations: A systematic review of human studies. Harv. Rev. Psychiatry 2011, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.; Martin, C.; Feldman, R.; Leckman, J.F. Oxytocin and social motivation. Dev. Cogn. Neurosci. 2011, 1, 471–493. [Google Scholar] [CrossRef] [PubMed]
- Bisagno, V.; Cadet, J.L. Stress, sex, and addiction: potential roles of corticotropin-releasing factor, oxytocin, and arginine-vasopressin. Behav. Pharmacol. 2014, 25, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Martínez, F.; Uvnäs-Moberg, K.; Petersson, M.; Olausson, H.A.; Jiménez-Estrada, I. Neuropeptides as neuroprotective agents: Oxytocin a forefront developmental player in the mammalian brain. Prog. Neurobiol. 2014, 123C, 37–78. [Google Scholar] [CrossRef] [PubMed]
- Kumsta, R.; Heinrichs, M. Oxytocin, stress and social behavior: neurogenetics of the human oxytocin system. Curr. Opin. Neurobiol. 2013, 23, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Arrowsmith, S.; Wray, S. Oxytocin: Its mechanism of action and receptor signalling in the myometrium. J. Neuroendocrinol. 2014, 26, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Bartz, J.A.; Hollander, E. Oxytocin and experimental therapeutics in autism spectrum disorders. Prog. Brain Res. 2008, 170, 451–462. [Google Scholar] [PubMed]
- Matsuzaki, M.; Matsushita, H.; Tomizawa, K.; Matsui, H. Oxytocin: A therapeutic target for mental disorders. J. Physiol. Sci. 2012, 62, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Olff, M.; Frijling, J.L.; Kubzansky, L.D.; Bradley, B.; Ellenbogen, M.A.; Cardoso, C.; Bartz, J.A.; Yee, J.R.; van Zuiden, M. The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 2013, 38, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.M.; Fallon, D.; Hill, M.; Frazier, J.A. The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv. Rev. Psychiatry 2013, 21, 219–247. [Google Scholar] [CrossRef] [PubMed]
- Preti, A.; Melis, M.; Siddi, S.; Vellante, M.; Doneddu, G.; Fadda, R. Oxytocin and autism: A systematic review of randomized controlled trials. J. Child Adolesc. Psychopharmacol. 2014, 24, 54–68. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, R.J.; McInnis, O.A.; Abizaid, A.; Anisman, H. Making room for oxytocin in understanding depression. Neurosci. Biobehav. Rev. 2014, 45, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.B.; van Zuiden, M.; Nawijn, L.; Frijling, J.L.; Veltman, D.J.; Olff, M. Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: Salience processing and fear inhibition processes. Psychoneuroendocrinology 2014, 40, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Guastella, A.J.; MacLeod, C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm. Behav. 2012, 61, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Guastella, A.J.; Hickie, I.B.; McGuinness, M.M.; Otis, M.; Woods, E.A.; Disinger, H.M.; Chan, H.K.; Chen, T.F.; Banati, R.B. Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology 2013, 38, 612–625. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, D.; Marini, S.; Iasevoli, F.; Tomasetti, C.; de Bartolomeis, A.; Mazza, M.; Valchera, A.; Fornaro, M.; Cavuto, M.; Srinivasan, V.; et al. The role of intranasal oxytocin in the treatment of patients with schizophrenia: A systematic review. CNS Neurol. Disord. Drug Targets 2013, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Vecsernyés, M.; Török, A.; Jójárt, I.; Laczi, F.; Penke, B.; Julesz, J. Specific radioimmunoassay of oxytocin in rat plasma. Endocr. Regul. 1994, 28, 145–150. [Google Scholar] [PubMed]
- Szeto, A.; McCabe, P.M.; Nation, D.A.; Tabak, B.A.; Rossetti, M.A.; McCullough, M.E.; Schneiderman, N.; Mendez, A.J. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom. Med. 2011, 73, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.S.; Metten, M.; Schams, D.; Wuttke, W. Development of a sensitive enzymeimmunoassay for oxytocin determination in bovine plasma. Anim. Reprod. Sci. 1998, 51, 185–194. [Google Scholar] [CrossRef]
- Carter, C.S.; Pournajafi-Nazarloo, H.; Kramer, K.M.; Ziegler, T.E.; White-Traut, R.; Bello, D.; Schwertz, D. Oxytocin: Behavioral associations and potential as a salivary biomarker. Ann. NY Acad. Sci. 2007, 1098, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Ali, S.; Das, M. Analysis of oxytocin in milk samples and intake pattern in different age groups of Indian population. Toxicol. Mech. Methods 2014, 24, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Kukucka, M.A.; Misra, H.P. Determination of oxytocin in biological samples by isocratic high-performance liquid chromatography with coulometric detection using C18 solid-phase extraction and polyclonal antibody-based immunoaffinity column purification. J. Chromatogr. B 1994, 653, 139–145. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Fast, D.M.; Lin, Z.; Steenwyk, R. Ultra sensitive quantitation of endogenous oxytocin in rat and human plasma using a two-dimensional liquid chromatography-tandem mass spectrometry assay. Anal. Biochem. 2011, 416, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, O.S.; Kennedy, R.T. Simultaneous oxytocin and arg-vasopressin measurements in microdialysates using capillary liquid chromatography-mass spectrometry. J. Neurosci. Methods 2012, 209, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Inoue, R.; Yagi, K.; Saito, K. Determination of nicotine, cotinine, and related alkaloids in human urine and saliva by automated in-tube solid-phase microextraction coupled with liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Ehara, K.; Yasuhara, R.; Saito, K. Simultaneous determination of testosterone, cortisol and dehydroepiandrosterone in saliva by stable isotope dilution on-line in-tube solid-phase microextraction coupled with liquid chromatography−tandem mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. Automated sample preparation using in-tube solid-phase microextraction and its application—A review. Anal. Bioanal. Chem. 2002, 373, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Ishizaki, A.; Nonaka, Y.; Saito, K. Developments and applications of capillary microextraction techniques: A review. Anal. Chim. Acta 2009, 655, 8–29. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. Recent developments and applications of microextraction techniques in drug analysis. Anal. Bioanal. Chem. 2010, 396, 339–364. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Saito, K. Recent advances in SPME techniques in biomedical analysis. J. Pharm. Biomed. Anal. 2011, 54, 926–950. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriyama, E.; Kataoka, H. Automated Analysis of Oxytocin by On-Line in-Tube Solid-Phase Microextraction Coupled with Liquid Chromatography-Tandem Mass Spectrometry. Chromatography 2015, 2, 382-391. https://doi.org/10.3390/chromatography2030382
Moriyama E, Kataoka H. Automated Analysis of Oxytocin by On-Line in-Tube Solid-Phase Microextraction Coupled with Liquid Chromatography-Tandem Mass Spectrometry. Chromatography. 2015; 2(3):382-391. https://doi.org/10.3390/chromatography2030382
Chicago/Turabian StyleMoriyama, Eri, and Hiroyuki Kataoka. 2015. "Automated Analysis of Oxytocin by On-Line in-Tube Solid-Phase Microextraction Coupled with Liquid Chromatography-Tandem Mass Spectrometry" Chromatography 2, no. 3: 382-391. https://doi.org/10.3390/chromatography2030382
APA StyleMoriyama, E., & Kataoka, H. (2015). Automated Analysis of Oxytocin by On-Line in-Tube Solid-Phase Microextraction Coupled with Liquid Chromatography-Tandem Mass Spectrometry. Chromatography, 2(3), 382-391. https://doi.org/10.3390/chromatography2030382





